Electrode Modification and Optimization in Air-Cathode Single-Chamber Microbial Fuel Cells
Abstract
1. Introduction
2. Experimental
2.1. Preparation of Electrodes
2.1.1. Electrode Pretreatment
2.1.2. Graphene/Carbon Cloth Electrode
2.1.3. Polyaniline-Graphene/Carbon Cloth Electrode
2.1.4. Sulfonated Cobalt Phthalocyanine-Polyaniline-Graphene Composite Electrode
2.2. Microbial Fuel Cells Setup and Operation
2.3. Electrochemical and Chemical Analysis
3. Results and Discussion
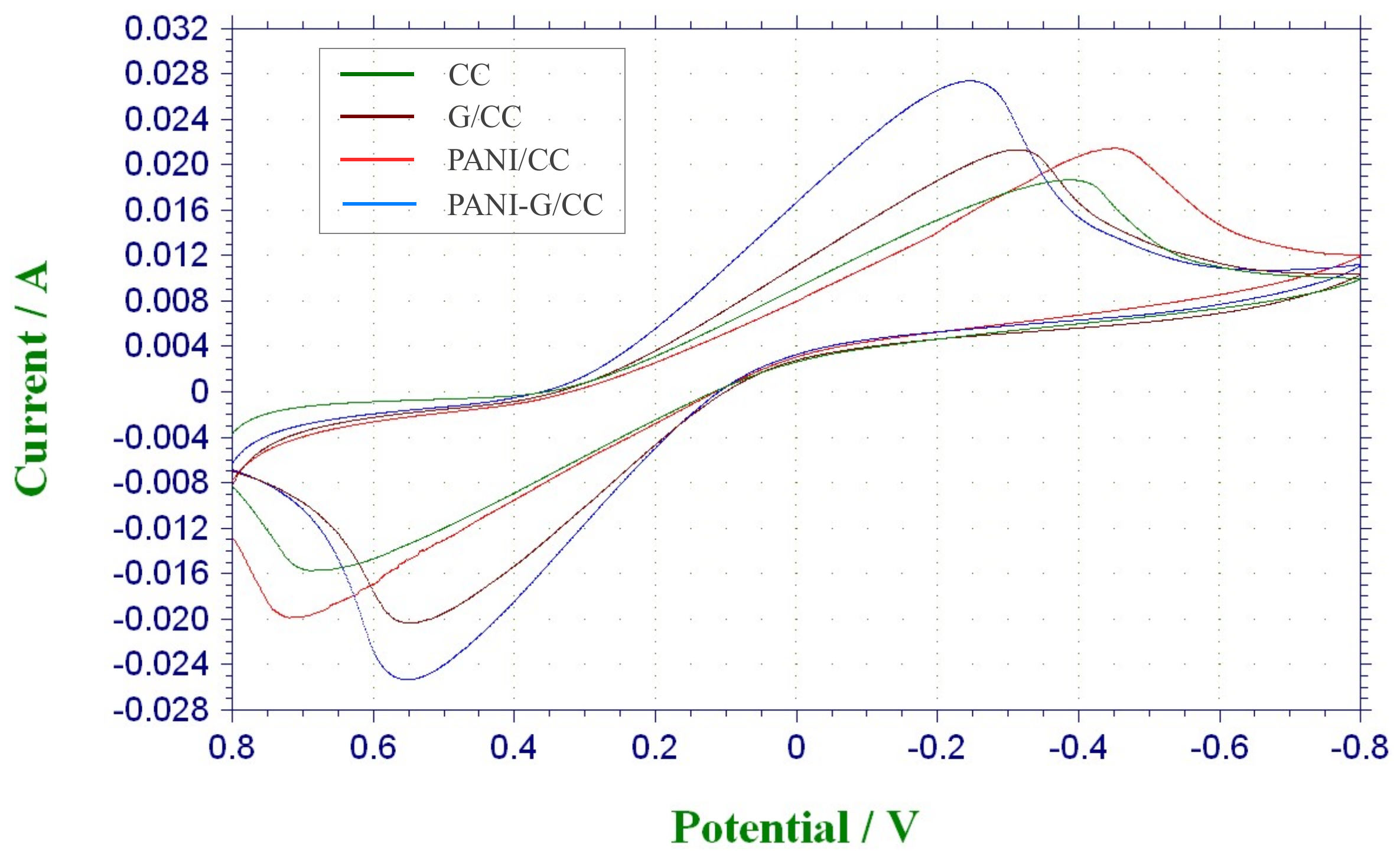
3.1. Graphene/Polyaniline-Modified Cathode
3.1.1. Electrochemical Characterization
LSV Test
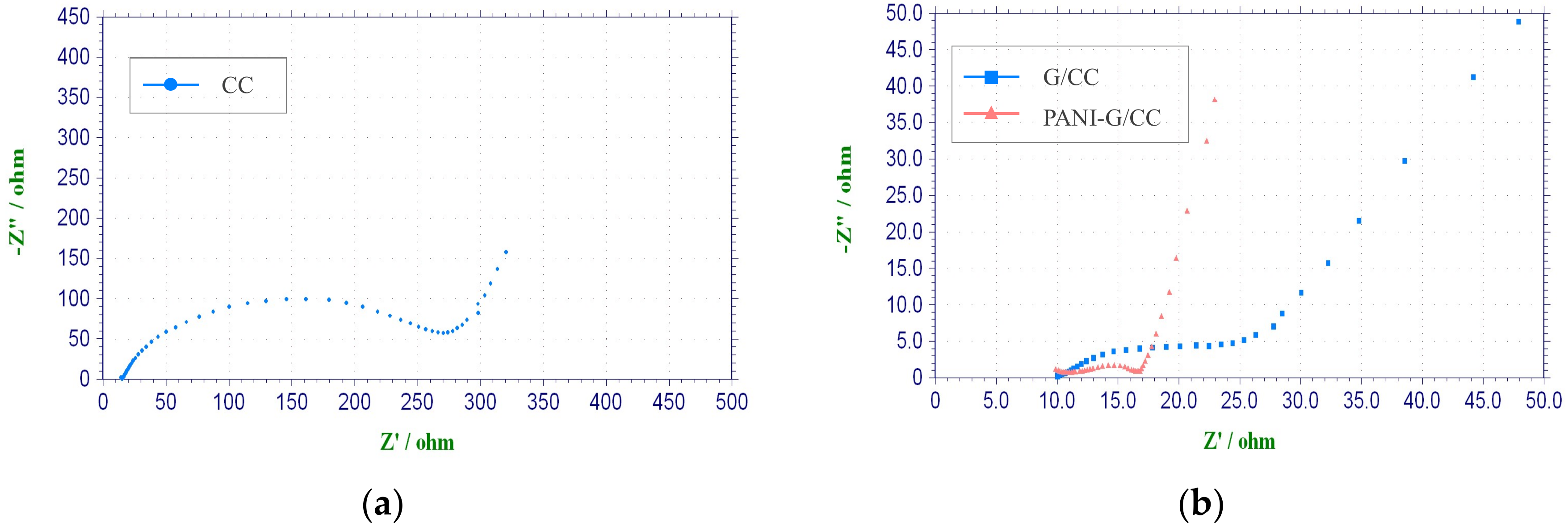
Electrochemical Impedance Spectroscopy Test
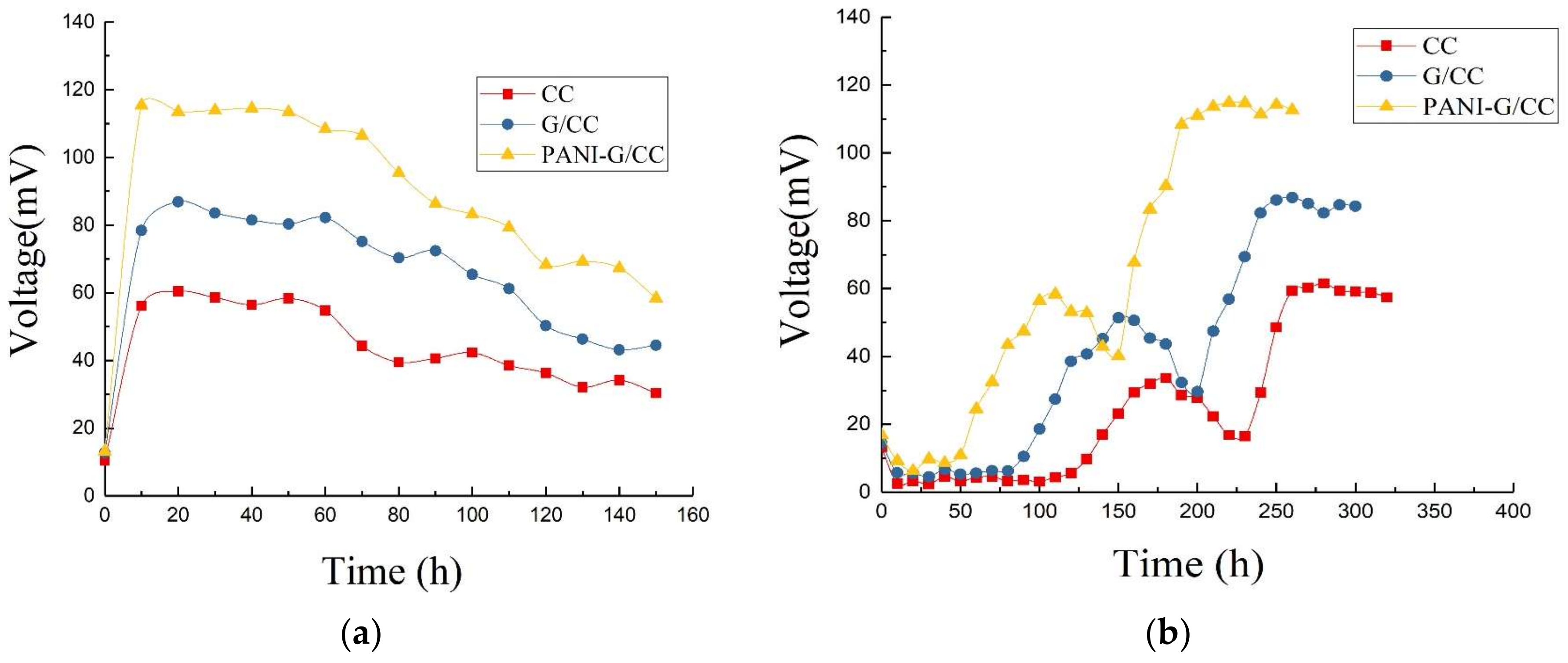
3.1.2. Startup Voltage Curves
3.1.3. Output Voltage Characteristic Curves
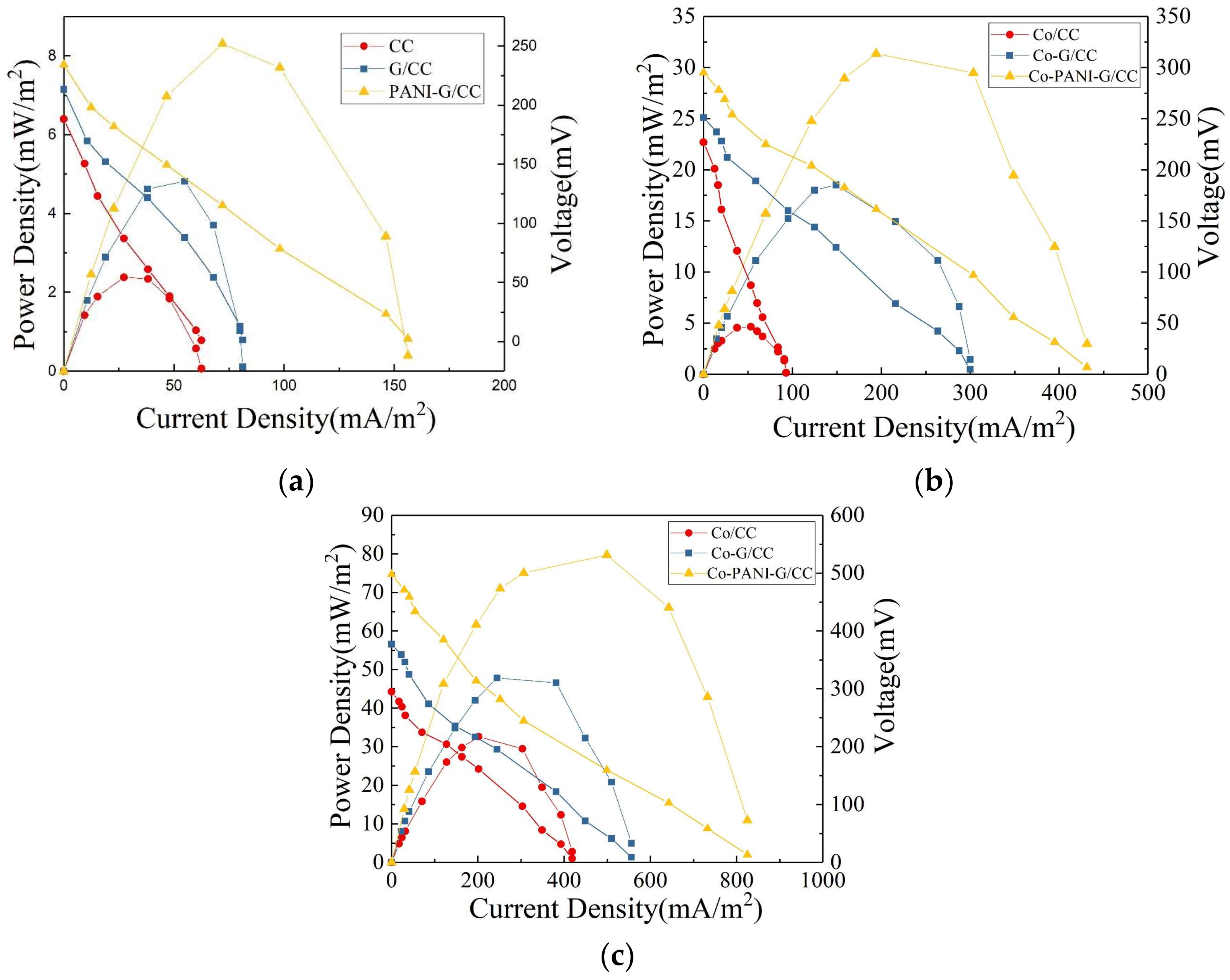
3.1.4. Effect of Cathode Modification on Electricity Generation Performance of Microbial Fuel Cells
3.2. Sulfonated Cobalt Phthalocyanine-Modified of Graphene/Polyaniline Cathodes
3.2.1. Electrochemical Characterization
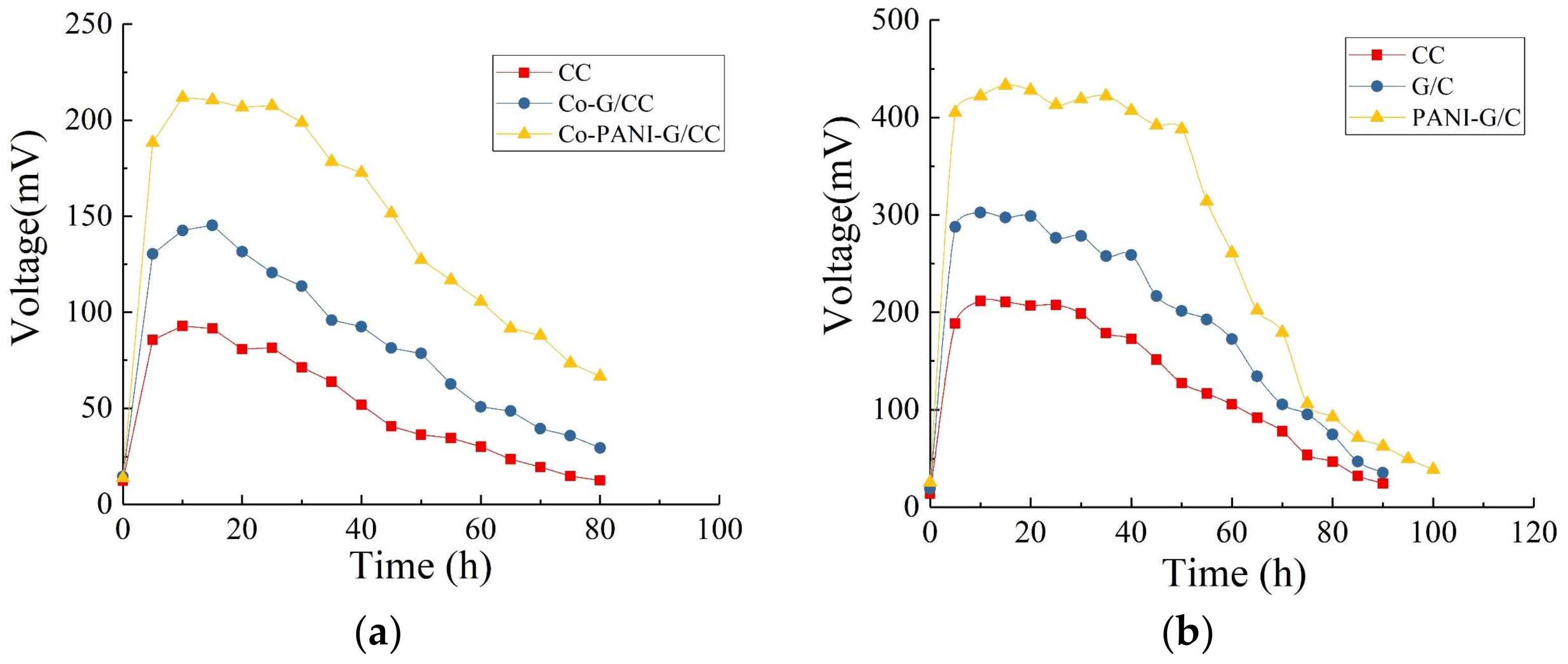
3.2.2. Output Voltage Characteristic Curves
3.2.3. Effect of Cathode Modification on Electricity Generation Performance of Microbial Fuel Cells
3.3. Graphene/Polyaniline-Modified Anodes
3.3.1. Electrochemical Characterization
3.3.2. Output Voltage Characteristic Curves
3.3.3. Effect of Anode Modification on Electricity Generation Performance of Microbial Fuel Cells
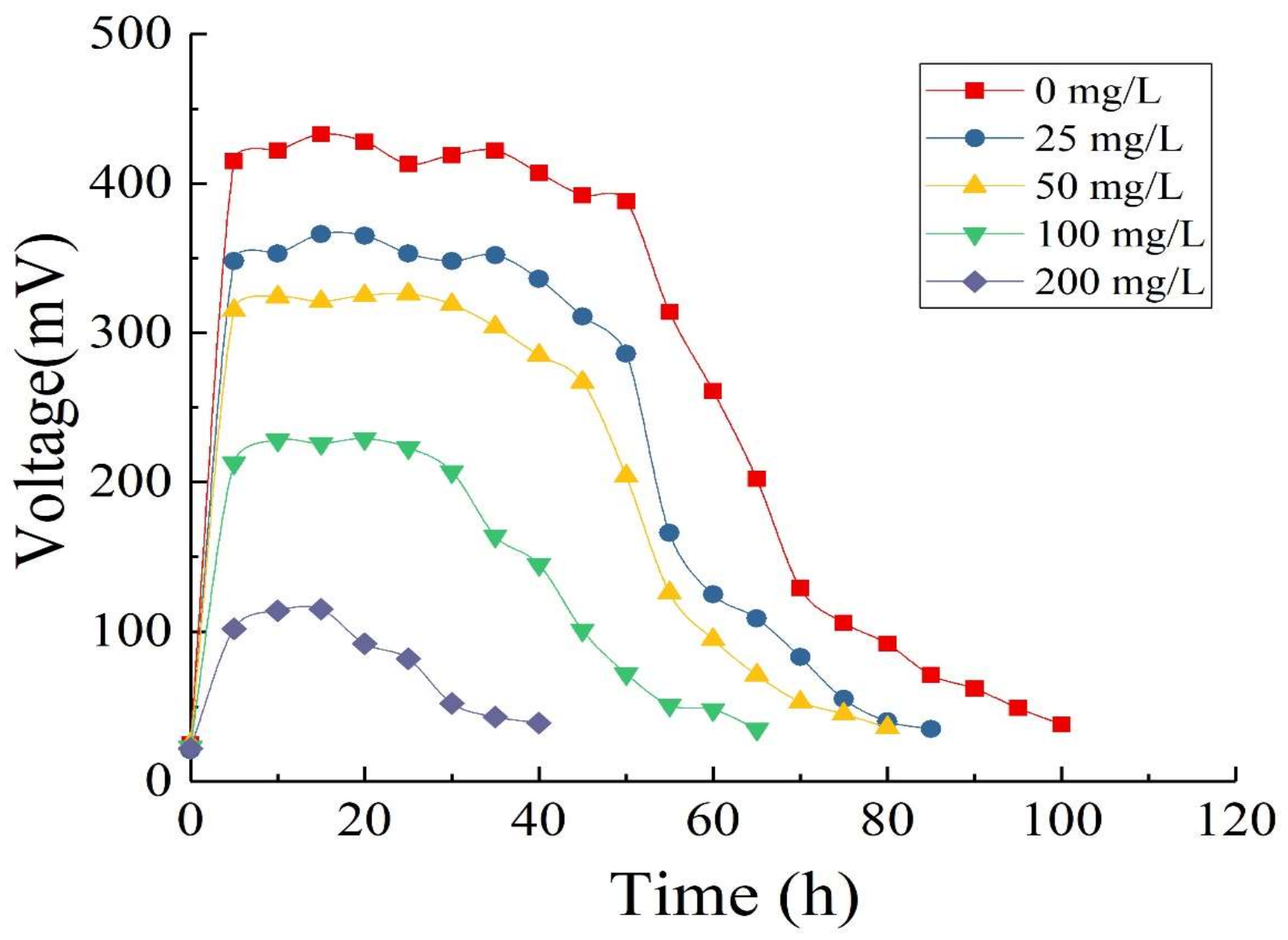
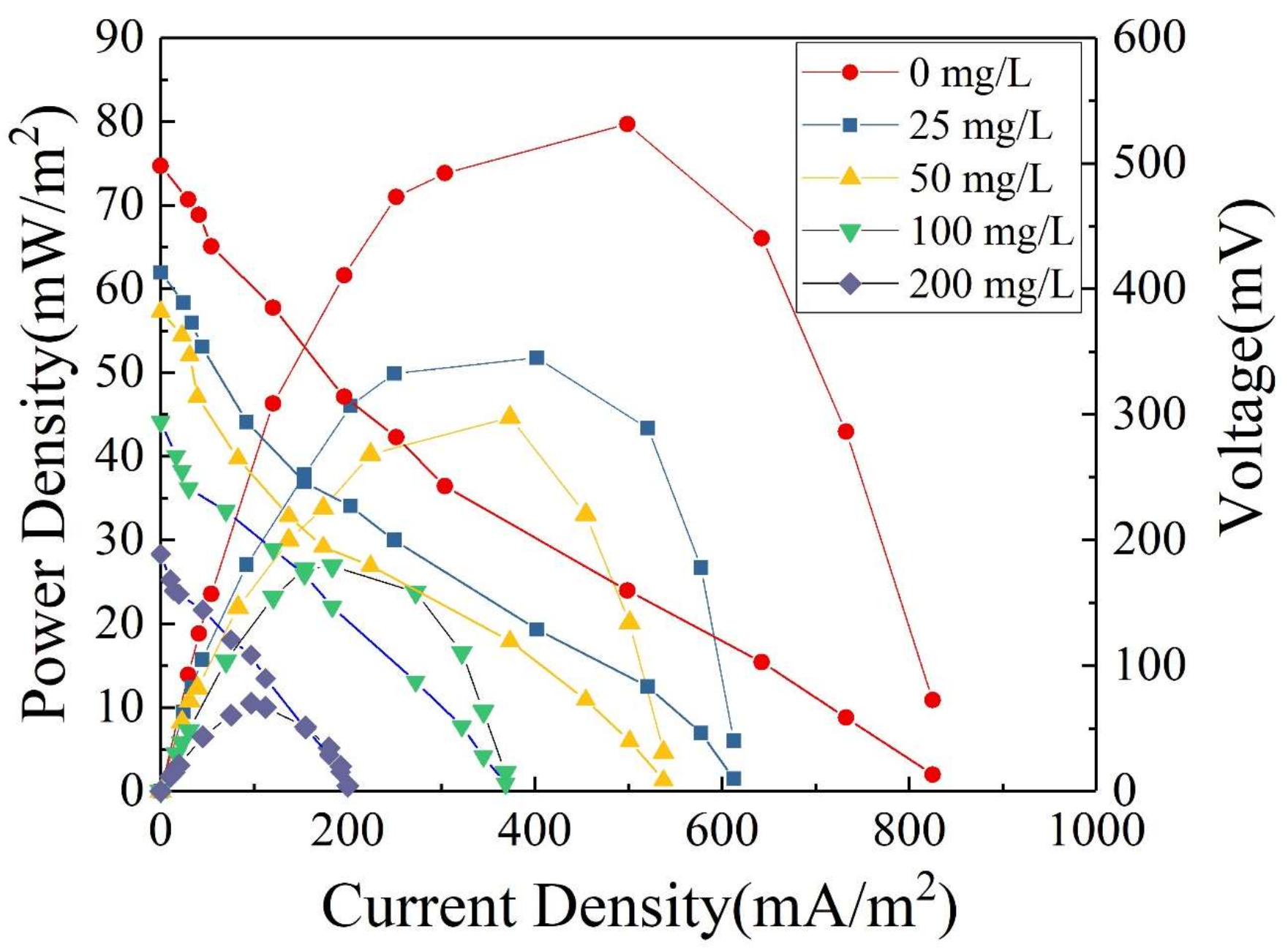
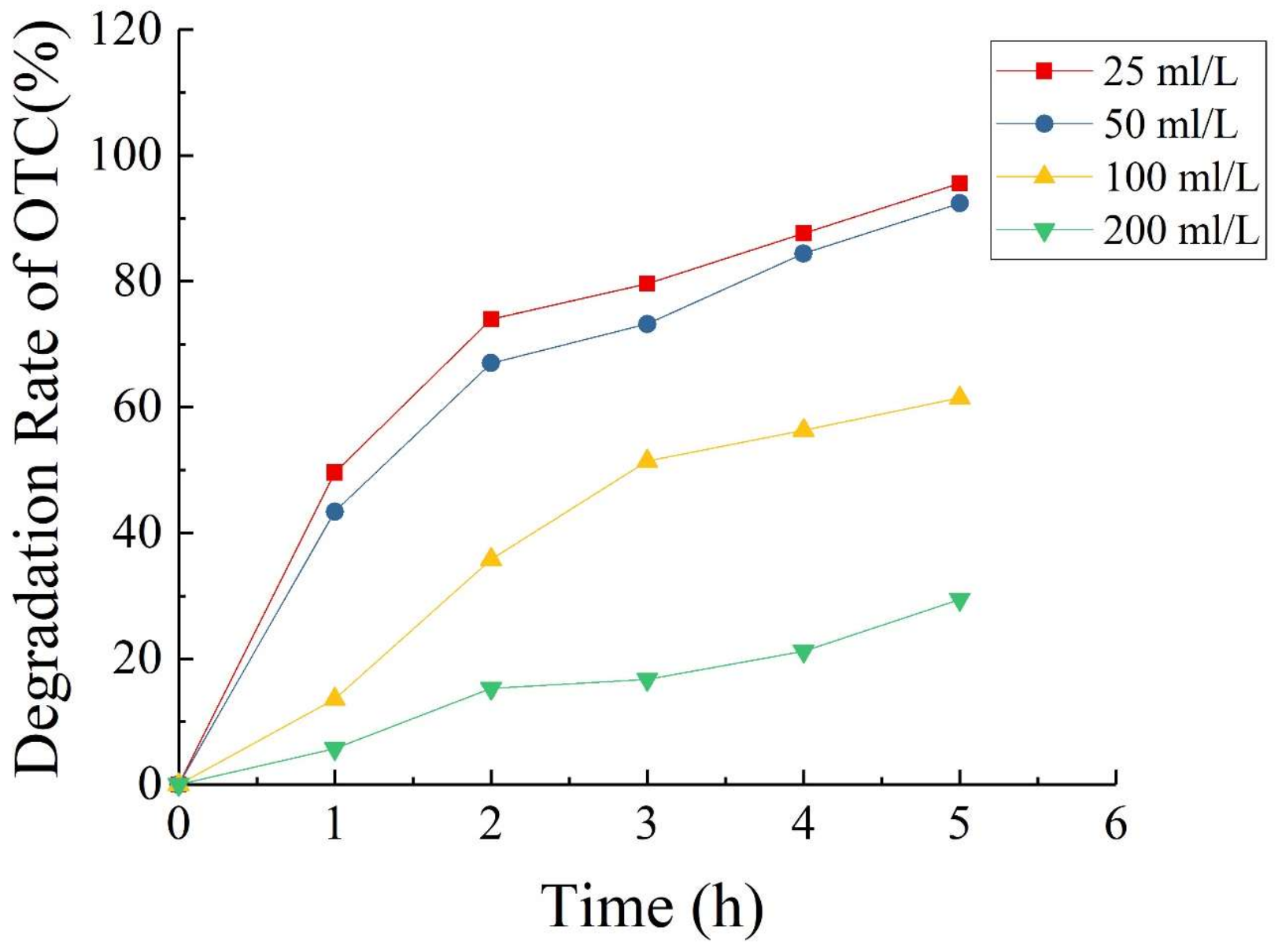
3.4. Oxytetracycline Degradation in Single-Chamber Microbial Fuel Cells
3.4.1. Output Voltage
3.4.2. Effect of Substrate Concentration on Electricity Generation Performance of Microbial Fuel Cells
3.4.3. Oxytetracycline Degradation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| CV | Cyclic voltammograms |
| CC | Carbon cloth electrode |
| CoPcS | Sulfonated cobalt phthalocyanine |
| Co-PANI-G/CC | CoPcS-polyaniline-graphene composite electrode |
| EIS | Electrochemical impedance spectroscopy |
| GLU | glucose |
| G | graphene |
| G/CC | graphene/carbon cloth electrode |
| LSV | linear sweep voltammetry |
| MFCs | microbial fuel cells |
| OTC | oxytetracycline |
| PANI | polyaniline |
| PANI-G | polyaniline-graphene |
| PANI-G/CC | polyaniline-graphene/carbon cloth electrode |
| PBS | phosphate buffer solution |
| Pt | platinum |
References
- Cao, X.X.; Huang, X.; Liang, P.; Boon, N.; Fan, M.Z.; Zhang, L.; Zhang, X.Y. A completely anoxic microbial fuel cell using a photo-biocathode for cathodic carbon dioxide reduction. Energy Environ. Sci. 2009, 2, 498–501. [Google Scholar] [CrossRef]
- Yuan, Y.; Ahmed, J.; Kim, S. Polyaniline/carbon black composite-supported iron phthalocyanine as an oxygen reduction catalyst for microbial fuel cells. J. Power Sources 2011, 196, 1103–1106. [Google Scholar] [CrossRef]
- Jin, T.; Luo, J.M.; Yang, J.; Zhao, Y.Y.; Zhou, L.; Zhou, M.H. Coupling of anodic and cathodic modification for increased power generation in microbial fuel cells. J. Power Sources 2012, 219, 358–363. [Google Scholar] [CrossRef]
- Du, Z.W.; Li, H.R.; Gu, T.Y. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef] [PubMed]
- Bullen, R.A.; Arnot, T.C.; Lakeman, J.B.; Walsh, F.C. Bio-fuel cells and their development. Biosens. Bioelectron. 2006, 21, 2015–2045. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.X.; Liu, Z.L.; Li, Y.X.; Yang, S.Q.; Zhou, Y. A comparative study of graphene-coated stainless steel fiber felt. Bioprocess Biosyst. Eng. 2015, 38, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiao, Y.; Guo, C.X.; Lim, S.; Song, H.; Li, C.M. Graphene/carbon cloth anode for high-performance mediatorless microbial fuel cells. Bioresour. Technol. 2012, 114, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Yong, Y.C.; Dong, X.C.; Chan-Park, M.B.; Song, H.; Chen, P. Macroporous and Monolithic Anode Based on Polyaniline Hybridized Three-Dimensional Graphene for High-Performance Microbial Fuel Cells. ACS Nano 2012, 6, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.H.; Chen, S.S.; Yuan, Y.; Cai, X.X.; Zhou, S.J. In situ formation of graphene layers on graphite surfaces for efficient anodes of microbial fuel cells. Biosens. Bioelectron. 2015, 71, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; He, G.; Liu, Q.; Harnisch, F.; Zhou, Y.; Chen, Y.; Hanif, M.; Wang, S.; Peng, X.; Hou, H. Layered corrugated electrode macrostructures boost microbial bioelectrocatalysis. Energy Environ. Sci. 2012, 5, 9769–9772. [Google Scholar] [CrossRef]
- Deeke, A.; Sleutels, T.H.; Hamelers, H.V.; Buisman, C.J. Capacitive Bioanodes Enable Renewable Energy Storage in Microbial Fuel Cells. Environ. Sci. Technol. 2012, 46, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.X.; Liu, Z.L.; Zhang, P.Y. A new method for fabrication of graphene/polyaniline nanocomplex modified microbial fuel cell anodes. J. Power Sources 2013, 224, 139–144. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, C.M.; Bao, S.J.; Bao, Q.L. Carbon Nanotube/Polyaniline Composite as Anode Material for Microbial Fuel Cells. J. Power Sources 2007, 170, 79–84. [Google Scholar] [CrossRef]
- Lv, Z.S.; Xie, D.H.; Yue, X.J.; Feng, C.H.; Wei, C.H. Ruthenium oxide-coated carbon felt electrode: A highly active anode for microbial fuel cell applications. J. Power Sources 2012, 210, 26–31. [Google Scholar] [CrossRef]
- Guo, J.; Wang, R.; Tjiu, W.W.; Pan, J.S.; Liu, T.X. Synthesis of Fe nanoparticles@graphene composites for environmental applications. J. Hazard. Mater. 2012, 225–226, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.X.; Dou, X.M.; Mohan, D.; Charles, P.; Luo, J.M. Synthesis of graphene oxide/schwertmannite nanocomposites and their application in Sb(V) adsorption from water. Chem. Eng. J. 2015, 270, 205–214. [Google Scholar] [CrossRef]
- Li, Z.J.; Chen, F.; Yuan, L.Y.; Liu, Y.L.; Zhao, Y.L.; Chai, Z.F.; Shi, W.Q. Uranium adsorption on graphene oxide nanosheets from aqueous solutions. Chem. Eng. J. 2012, 210, 539–546. [Google Scholar] [CrossRef]
- Li, Z.J.; Wang, L.; Yuan, L.Y.; Xiao, C.L.; Mei, L.; Zheng, L.R.; Zhang, J.; Yang, J.H.; Zhao, Y.L.; Zhu, Z.T.; et al. Efficient removal of uranium from aqueous solution by zero-valent iron nanoparticle and its graphene composite. J. Hazard. Mater. 2015, 290, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, H.; Chandra, V.; Jung, S.; Lee, J.W.; Kim, K.S.; Kim, S.B. Enhanced Cr(VI) removal using iron nanoparticle decorated graphene. Nanoscale 2011, 3, 3583–3585. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.Q.; Li, Z.J.; Wang, C.Z.; Yuan, L.Y.; Liu, Z.R.; Zhang, J.; Zheng, L.R.; Zhao, Y.L.; Chai, Z.F.; Shi, W.Q. Interactions between Th(IV) and graphene oxide: Experimental and density functional theoretical investigations. RSC Adv. 2014, 4, 3340–3347. [Google Scholar] [CrossRef]
- Liu, J.; Liu, G.; Liu, W. Preparation of water-soluble b-cyclodextrin/poly(acrylic acid)/graphene oxide nanocomposites as new adsorbents to remove cationic dyes from aqueous solution. Chem. Eng. J. 2014, 257, 299–308. [Google Scholar] [CrossRef]
- Daniela, C.; Marcano, V.D.; Kosynkin, J.M.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, M.J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Noorden, R.V. Chemistry: The trails of new carbon. Nature 2011, 469, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Myung, S.; Solanki, A.; Kim, C.; Park, J.; Kim, K.S.; Lee, K.B. Graphene-encapsulated nanoparticle-based biosensor for the selective detection of cancer biomarkers. Adv. Mater. 2011, 23, 2221–2225. [Google Scholar] [CrossRef] [PubMed]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Tu, K.H.; Lin, C.C.; Chen, C.W.; Chhowalla, M. Solution-processable graphene oxide as an efficient hole transport layer in polymer solar cells. ACS Nano 2010, 4, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.H.; Li, X.F.; Ren, Y.P.; Wang, X.H. Performance improvement of microbial fuel cell with polyaniline dopped graphene anode. Environ. Sci. 2017, 38, 1717–1725. (In Chinese) [Google Scholar] [CrossRef]
- Bashyam, R.; Zelenay, P. A class of non-precious metal composite catalysts for fuel cells. Nature 2006, 443, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.B.; Liu, D.J.; Kariuki, N.N.; Chen, L.X. Aligned carbon nanotubes with built in FeN4 active sites for electrocatalytic reduction of oxygen. Chem. Commun. 2008, 329–331. [Google Scholar] [CrossRef]
- Deng, L.; Zhou, M.; Liu, C.; Liu, L.; Liu, C.; Dong, S. Performance of Co/Fe/N/CNT nanocatalyst for oxygen reduction in microbial fuel cells. Talanta 2010, 81, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Wang, Y.H.; Zhou, Y.; Li, H.H.; Wang, W.K. Using graphene/polyaniline-modified electrodes enhance the performance of two-chambered microbial fuel cells. Pol. J. Environ. Stud. 2017, 26, 1233–1243. [Google Scholar] [CrossRef]
- Kim, J.R.; Kim, J.Y.; Han, S.B.; Park, K.W.; Saratale, G.D.; Oh, S.E. Application of Co-naphthalocyanine (CoNPc) as alternative cathode catalyst and support structure for microbial fuel cells. Bioresour. Technol. 2011, 102, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Watson, V.J.; Saito, T.; Hickner, M.A.; Logan, B.E. Polymer coatings as separator layers for microbial fuel cell cathodes. J. Power Sources 2011, 196, 3009–3014. [Google Scholar] [CrossRef]
- Mahmoud, M.; Gad-Allah, T.A.; El-Khatib, K.M.; EIGohary, F. Power generation using spinel manganese-cobalt oxide as a cathode catalyst for microbial fuel cell applications. Bioresour. Technol. 2011, 102, 10459–10464. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Lv, Z.S.; Xu, J.M.; Peng, D.Q.; Liu, Y.X.; Chen, J.X.; Sun, X.B.; Feng, C.H.; Wei, C.H. Stainless steel mesh coated with MnO2/carbon nanotube and polymethylphenyl siloxane as low-cost and high-performance microbial fuel cell cathode materials. J. Power Sources 2012, 201, 136–141. [Google Scholar] [CrossRef]
- Wang, H.M.; Wu, Z.C.; Plaseied, A.; Jenkins, P.; Simpson, L.; Engtrakul, C.; Ren, Z.Y. Carbon nanotube modified air-cathodes for electricity production in microbial fuel cells. J. Power Sources 2011, 196, 7465–7469. [Google Scholar] [CrossRef]
- He, Z.; Mansfeld, F. Exploring the use of electrochemical impedance spectroscopy (EIS) in microbial fuel cell studies. Energy Environ. Sci. 2009, 2, 215–219. [Google Scholar] [CrossRef]
- Papurello, D.; Menichini, D.; Lanzini, A. Distributed relaxation times technique for the determination of fuel cell losses with an equivalent circuit model to identify physicochemical processes. Electrochim. Acta 2017, 258, 98–109. [Google Scholar] [CrossRef]
- Li, S.Z.; Hu, Y.Y.; Xu, Q.; Sun, J.; Hou, B.; Zhang, Y.P. Iron and nitrogen-functionalized graphene as a non-precious metal catalyst for enhanced oxygen reduction in an air-cathode microbial fuel cell. J. Power Sources 2012, 213, 265–269. [Google Scholar] [CrossRef]
- Liang, P.; Fan, M.Z.; Cao, X.X.; Huang, X.; Huang, Z.H.; Wang, C. Electricity Generation Using the Packing-Type Microbial Fuel Cells. Environ. Sci. 2008, 29, 512–517. (In Chinese) [Google Scholar]
- Min, B.; Cheng, S.A.; Logan, B.E. Electricity generation using membrane and salt bridge microbial fuel cells. J. Water Res. 2005, 39, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Yan, H.; Wang, W.; Mench, M.M.; Regan, J.M. Characterization of Microbial Fuel Cells at Microbially and Electrochemically Meaningful Time Scales. Environ. Sci. Technol. 2011, 45, 2435–2441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Mo, G.Q.; Li, X.W.; Zhang, W.D.; Zhang, J.Q.; Ye, J.S.; Huang, X.D.; Yu, C.Z. A graphene modified anode to improve the performance of microbial fuel cells. J. Power Sources 2011, 196, 5402–5407. [Google Scholar] [CrossRef]
- Hassan, R.Y.; Mekawy, M.M.; Ramnani, P.; Mulchandani, A. Monitoring of microbial cell viability using nanostructured electrodes modified with Graphene/Alumina nanocomposite. Biosens. Bioelectron. 2017, 91, 857–862. [Google Scholar] [CrossRef] [PubMed]

| Oxytetracycline (OTC) (mg/L) | 25 | 50 | 100 | 200 |
|---|---|---|---|---|
| R2 | 0.9715 | 0.9829 | 0.9719 | 0.9690 |
| k (d−1) | 0.4717 | 0.4033 | 0.1717 | 0.0567 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, J.; Yang, S.; Li, H.; Li, X. Electrode Modification and Optimization in Air-Cathode Single-Chamber Microbial Fuel Cells. Int. J. Environ. Res. Public Health 2018, 15, 1349. https://doi.org/10.3390/ijerph15071349
Wang Y, Wu J, Yang S, Li H, Li X. Electrode Modification and Optimization in Air-Cathode Single-Chamber Microbial Fuel Cells. International Journal of Environmental Research and Public Health. 2018; 15(7):1349. https://doi.org/10.3390/ijerph15071349
Chicago/Turabian StyleWang, Yanhua, Jiayan Wu, Shengke Yang, Huihui Li, and Xiaoping Li. 2018. "Electrode Modification and Optimization in Air-Cathode Single-Chamber Microbial Fuel Cells" International Journal of Environmental Research and Public Health 15, no. 7: 1349. https://doi.org/10.3390/ijerph15071349
APA StyleWang, Y., Wu, J., Yang, S., Li, H., & Li, X. (2018). Electrode Modification and Optimization in Air-Cathode Single-Chamber Microbial Fuel Cells. International Journal of Environmental Research and Public Health, 15(7), 1349. https://doi.org/10.3390/ijerph15071349






