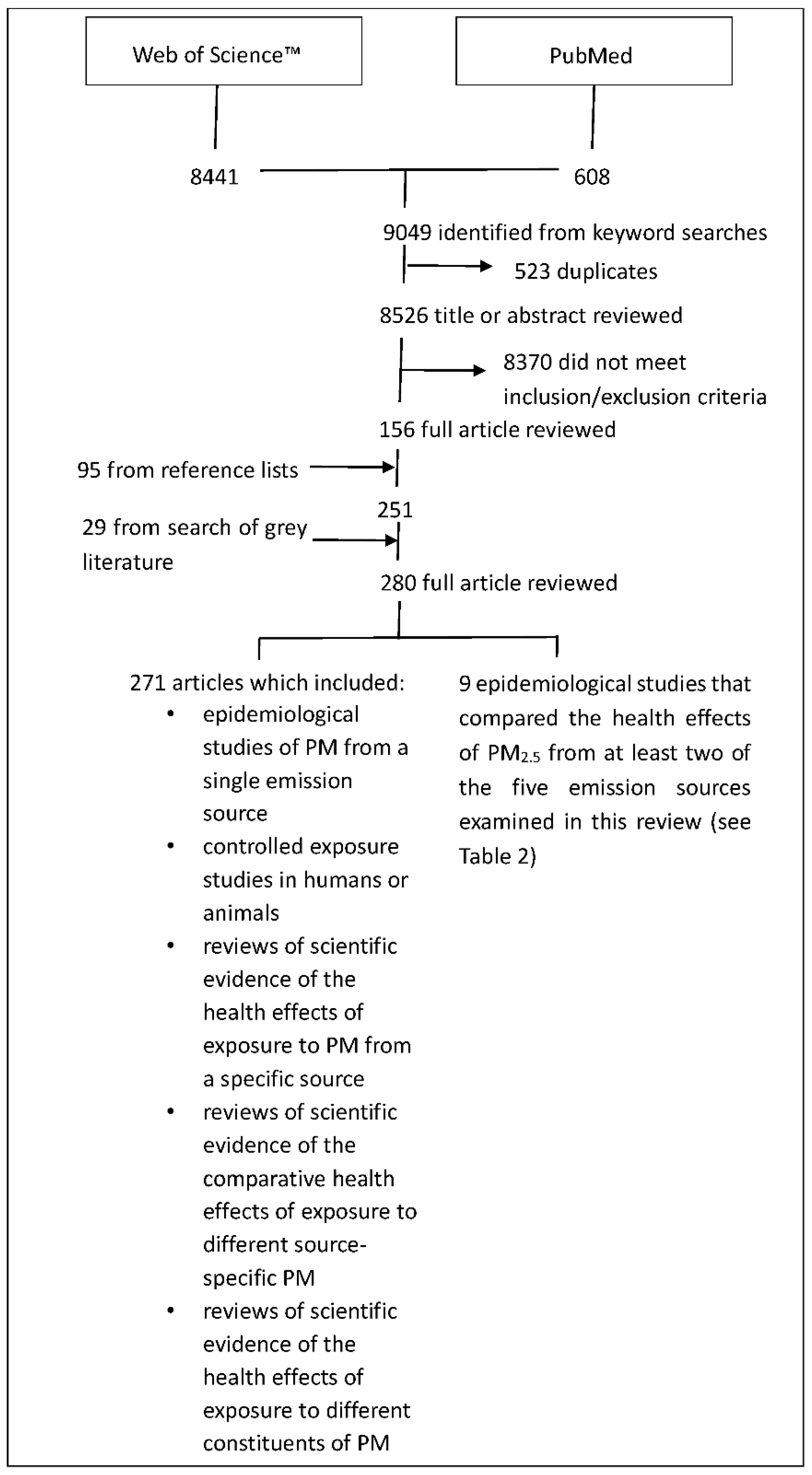
A Comparison of the Health Effects of Ambient Particulate Matter Air Pollution from Five Emission Sources
Abstract
1. Health Effects of Particulate Matter (PM) Air Pollution
1.1. Observations of the Health Effects of PM Air Pollution Particle Mass
1.2. Towards an Understanding of the Health Effects Specific to PM Air Pollution from Different Sources
- Epidemiological studies that gave due consideration to measured and unmeasured confounders of the exposure–health response relationship
- Controlled exposure studies in humans or animals, where exposure was by way of inhalation of concentrated or ambient levels of air pollution particles
- Reviews of scientific evidence
- Air pollution exposure studies and source speciation data without health outcome data
- Epidemiological studies that did not consider PM air pollution from at least one of the five sources examined in this review
- Epidemiological studies in which the study cohort and/or pollution exposure data was deemed too small to have external validity
- Quantitative studies that did not characterize the uncertainty of effect estimates (i.e., lacking confidence intervals or standard errors)
- Studies of the effects of gas/particle mixtures without consideration of the effects of the PM component were generally excluded
- Cell culture or molecular studies that provided no mechanistic insight into health effects observed in epidemiological studies
- Review the evidence of the health effects associated with PM air pollution from traffic, coal-fired power stations, diesel exhaust, domestic wood combustion heaters, and crustal dust and qualitatively compare the weight of evidence of health effects associated with PM from those emission sources. Both investigations that examined the health effects associated with PM from a single source and investigations that compared health effects from PM from different sources were included in this review of evidence.
- Conduct a quantitative comparison of the health effects of source-specific PM using epidemiological studies that compared the health effects associated with PM from different sources within the same study.
- Use the findings of this review to conclude whether PM from some emission sources are clearly and consistently associated with worse health outcomes than PM from other sources.
2. A Comparison of the Health Effects Associated with Five Different Source-Specific PM
2.1. Traffic
2.2. Coal-Fired Power Stations
2.3. Diesel Exhaust
2.4. Domestic Wood Combustion Heaters
2.5. Crustal Dust
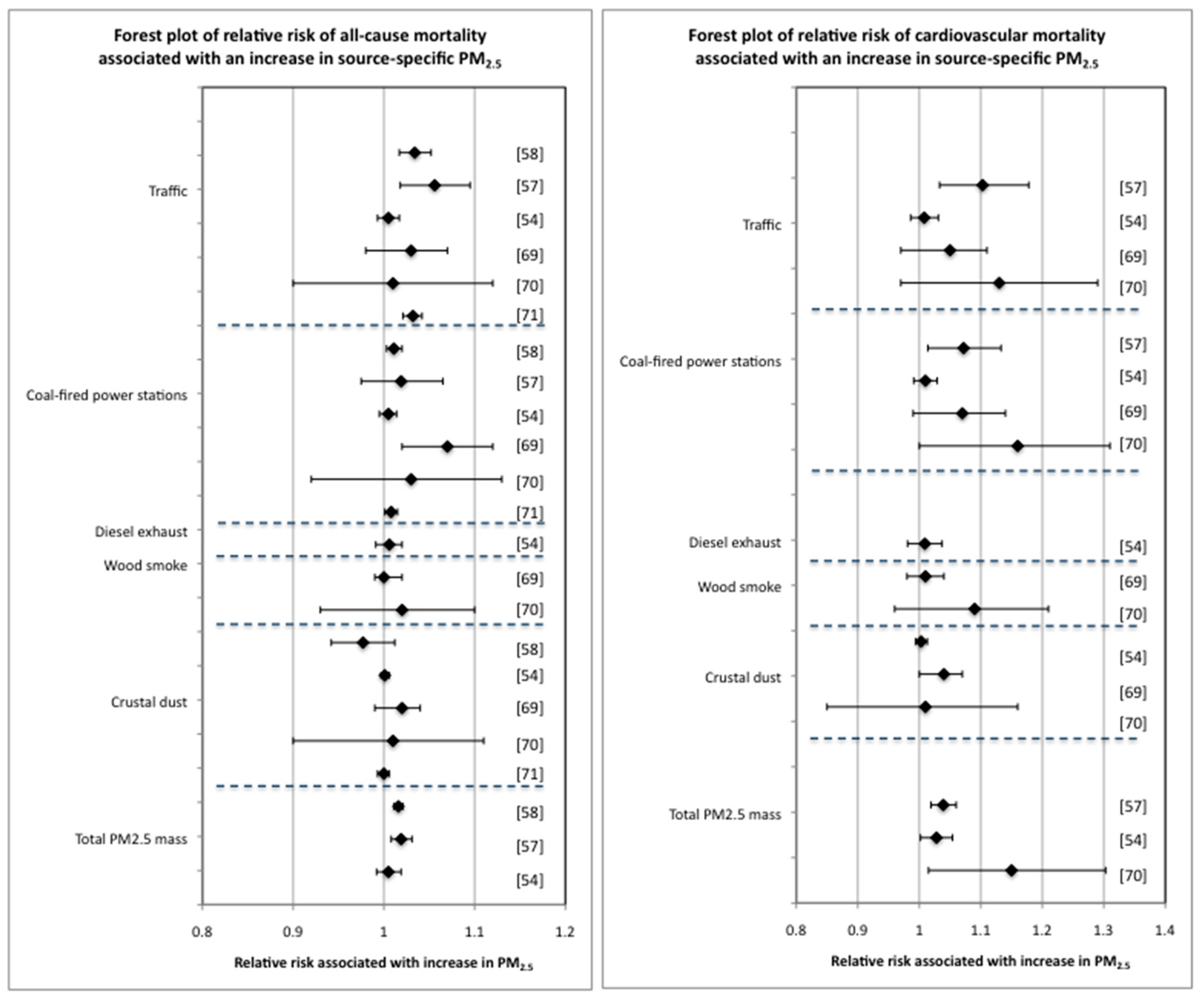
2.6. Comparison of the Effect on All-Cause and Cardiovascular Mortality of Increases in Different Source-Specific PM2.5
3. Discussion—Challenges in Differentiating Health Effects Associated with Exposure to PM from Different Emission Sources
4. Conclusions on the Comparative Health Effects of Source-Specific PM Air Pollution from This Review
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Dockery, D.W.; Pope, C.A.; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G.; Speizer, F.E. An association between air pollution and mortality in six U.S. cities. N. Eng. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Lepeule, J.; Laden, F.; Dockery, D.; Schwartz, J. Chronic exposure to fine particles and mortality: An extended follow-up of the Harvard Six Cities Study from 1974 to 2009. Environ. Health Perspect. 2012, 120, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.A.; Thun, M.J.; Namboodiri, M.M.; Dockery, D.W.; Evans, J.S.; Speizer, F.E.; Heath, C.W. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am. J. Respir. Crit. Care Med. 1995, 151, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Beelen, R.; Raaschou-Nielsen, O.; Stafoggia, M.; Andersen, Z.J.; Weinmayr, G.; Hoffmann, B.; Wolf, K.; Samoli, E.; Fischer, P.; Nieuwenhuijsen, M.; et al. Effects of long-term exposure to air pollution on natural-cause mortality: An analysis of 22 European cohorts within the multicentre ESCAPE Project. Lancet 2014, 383, 785–795. [Google Scholar] [CrossRef]
- Hoek, G.; Krishnan, R.M.; Beelen, R.; Peters, A.; Ostro, B.; Brunekreef, B.; Kaufman, J.D. Long-term air pollution exposure and cardio-respiratory mortality: A review. Environ. Health 2013, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO); Regional Office for Europe. Health Risks of Air Pollution in Europe—HRAPIE Project: Recommendations for Concentration-Response Functions for Cost-Benefit Analysis of Particulate Matter, Ozone and Nitrogen Dioxide; WHO Regional Office for Europe: Copenhagen, Denmark, 2013. [Google Scholar]
- Cesaroni, G.; Forastiere, F.; Stafoggia, M.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; de Faire, U.; Erbel, R.; Eriksen, K.T.; et al. Long term exposure to ambient air pollution and incidence of acute coronary events: Prospective cohort study and meta-analysis in 11European cohorts from the ESCAPE Project. BMJ 2014, 348, f7412. [Google Scholar] [CrossRef] [PubMed]
- Stafoggia, M.; Cesaroni, G.; Peters, A.; Andersen, Z.J.; Badaloni, C.; Beelen, R.; Caracciolo, B.; Cyrys, J.; de Faire, U.; de Hoogh, K.; et al. Long-term exposure to ambient air pollution and incidence of cerebrovascular events: Results from 11 European cohorts within the ESCAPE Project. Environ. Health Perspect. 2014, 122, 919–925. [Google Scholar] [CrossRef] [PubMed]
- Gehring, U.; Gruzieva, O.; Agius, R.M.; Beelen, R.; Custovic, A.; Cyrys, J.; Eeftens, M.; Flexeder, C.; Fuertes, E.; Heinrich, J.; et al. Air pollution exposure and lung function in children: The ESCAPE Project. Environ. Health Perspect. 2013, 121, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.; Schikowski, T.; Carsin, A.E.; Cai, Y.; Jacquemin, B.; Sanchez, M.; Vierkotter, A.; Marcon, A.; Keidel, D.; Sugiri, D.; et al. Adult lung function and long-term air pollution. ESCAPE: A multicentre cohort study and meta-analysis. Eur. Respir. J. 2015, 45, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Rice, M.B.; Ljungman, P.L.; Wilker, E.H.; Dorans, K.S.; Gold, D.R.; Schwartz, J.; Koutrakis, P.; Washko, G.R.; O’Connor, G.T.; Mittleman, M.A. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am. J. Respir. Crit. Care Med. 2015, 191, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Schikowski, T.; Adam, M.; Marcon, A.; Cai, Y.; Vierkotter, A.; Carsin, A.E.; Jacquemin, B.; Al Kanani, Z.; Beelen, R.; Birk, M.; et al. Association of ambient air pollution with the prevalence and incidence of COPD. Eur. Respir. J. 2014, 44, 614–626. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, E.A.; Gehring, U.; Molter, A.; Fuertes, E.; Klumper, C.; Kramer, U.; Quass, U.; Hoffmann, B.; Gascon, M.; Brunekreef, B.; et al. Air pollution and respiratory infections during early childhood: An analysis of 10 European birth cohorts within the ESCAPE Project. Environ. Health Perspect. 2014, 122, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zijlema, W.L.; Doiron, D.; Blangiardo, M.; Burton, P.R.; Fortier, I.; Gaye, A.; Gulliver, J.; de Hoogh, K.; Hveem, K.; et al. Ambient air pollution, traffic noise and adult asthma prevalence: A BioSHaRE approach. Eur. Respir. J. 2017, 49, 1502127. [Google Scholar] [CrossRef] [PubMed]
- Raaschou-Nielsen, O.; Andersen, Z.J.; Beelen, R.; Samoli, E.; Stafoggia, M.; Weinmayr, G.; Hoffmann, B.; Fischer, P.; Nieuwenhuijsen, M.J.; Brunekreef, B.; et al. Air pollution and lung cancer incidence in 17 European cohorts: Prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013, 14, 813–822. [Google Scholar] [CrossRef]
- Pedersen, M.; Giorgis-Allemand, L.; Bernard, C.; Aguilera, I.; Andersen, A.M.; Ballester, F.; Beelen, R.M.; Chatzi, L.; Cirach, M.; Danileviciute, A.; et al. Ambient air pollution and low birthweight: A European cohort study (ESCAPE). Lancet Respir. Med. 2013, 1, 695–704. [Google Scholar] [CrossRef]
- Dai, L.; Zanobetti, A.; Koutrakis, P.; Schwartz, J.D. Associations of fine particulate matter species with mortality in the United States: A multicity time-series analysis. Environ. Health Perspect. 2014, 122, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Katsouyanni, K.; Samet, J.M.; Anderson, H.R.; Atkinson, R.; Le Tertre, A.; Medina, S.; Samoli, E.; Touloumi, G.; Burnett, R.T.; Krewski, D.; et al. Air Pollution and Health: A European and North American Approach (APHENA); HEI Research Report 142; Health Effects Institute: Boston, MA, USA, 2009. [Google Scholar]
- Samoli, E.; Stafoggia, M.; Rodopoulou, S.; Ostro, B.; Declercq, C.; Alessandrini, E.; Diaz, J.; Karanasiou, A.; Kelessis, A.G.; Le Tertre, A.; et al. Associations between fine and coarse particles and mortality in Mediterranean cities: Results from the MED-PARTICLES Project. Environ. Health Perspect. 2013, 121, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Zanobetti, A.; Schwartz, J. The effect of fine and coarse particulate air pollution on mortality: A national analysis. Environ Health Perspect. 2009, 117, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.W.; Kang, S.; Anderson, H.R.; Mills, I.C.; Walton, H.A. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: A systematic review and meta-analysis. Thorax 2014, 69, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Stafoggia, M.; Samoli, E.; Alessandrini, E.; Cadum, E.; Ostro, B.; Berti, G.; Faustini, A.; Jacquemin, B.; Linares, C.; Pascal, M.; et al. Short-term associations between fine and coarse particulate matter and hospitalizations in Southern Europe: Results from the MED-PARTICLES Project. Environ. Health Perspect. 2013, 121, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Weinmayr, G.; Romeo, E.; De Sario, M.; Weiland, S.K.; Forastiere, F. Short-term effects of PM10 and NO2 on respiratory health among children with asthma or asthma-like symptoms: A systematic review and meta-analysis. Environ. Health Perspect. 2010, 118, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.D.; Kipen, H.; Annesi-Maesano, I.; Balmes, J.; Brook, R.D.; Cromar, K.; De Matteis, S.; Forastiere, F.; Forsberg, B.; Frampton, M.W.; et al. A joint ERA/ATS policy statement: What constitutes an adverse health effect of air pollution? An analytical framework. Eur. Respir. J. 2017, 49, 1600419. [Google Scholar] [CrossRef] [PubMed]
- Putaud, J.-P.; Van Dingenen, R.; Alastuey, A.; Bauer, H.; Birmili, W.; Cyrys, J.; Flentje, H.; Fuzzi, S.; Gehrig, R.; Hansson, H.C.; et al. A European aerosol phenomenology—3: Physical and chemical characteristics of particulate matter from 60 rural, urban, and kerbside sites across Europe. Atmos. Environ. 2010, 44, 1308–1320. [Google Scholar] [CrossRef]
- Bell, M. Assessment of the Health Impacts of Particulate Matter Characteristics; Health Effects Institute: Boston, MA, USA, 2012. [Google Scholar]
- Cassee, F.R.; Heroux, M.E.; Gerlofs-Nijland, M.E.; Kelly, F.J. Particulate matter beyond mass: Recent health evidence on the role of fractions, chemical constituents and sources of emission. Inhal. Toxicol. 2013, 25, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Lipsett, M.; Reynolds, P.; Goldberg, D.; Hertz, A.; Garcia, C.; Henderson, K.D.; Bernstein, L. Long-term exposure to constituents of fine particulate air pollution and mortality: Results from the California Teachers Study. Environ. Health Perspect. 2010, 118, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Stanek, L.W.; Sacks, J.D.; Dutton, S.J.; Dubois, J.-J.B. Attributing health effects to apportioned components and sources of particulate matter: An evaluation of collective results. Atmos. Environ. 2011, 45, 5655–5663. [Google Scholar] [CrossRef]
- Burnett, R.T.; Brook, J.; Dann, T.; Delocla, C.; Philips, O.; Cakmak, S.; Vincent, R.; Goldberg, M.S.; Krewski, D. Association between particulate- and gas-phase components of urban air pollution and daily mortality in eight Canadian cities. Inhal. Toxicol. 2000, 12, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.; Koutrakis, P.; Schwartz, P. The role of particle composition on the association between PM2.5 and mortality. Epidemiology 2008, 19, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.R.; Jerrett, M.; Anderson, H.R.; Burnett, R.T.; Stone, V.; Derwent, R.; Atkinson, R.W.; Cohen, A.; Shonkoff, S.B.; Krewski, D.; et al. Public health benefits of strategies to reduce greenhouse-gas emissions: Health implications of short-lived greenhouse pollutants. Lancet 2009, 374, 2091–2103. [Google Scholar] [CrossRef]
- Adams, K.; Greenbaum, D.S.; Shaikh, R.; van Erp, A.M.; Russell, A.G. Particulate matter components, sources, and health: Systematic approaches to testing effects. J Air Waste Manag. Assoc. 2015, 65, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Hampel, R.; Peters, A.; Beelen, R.; Brunekreef, B.; Cyrys, J.; de Faire, U.; de Hoogh, K.; Fuks, K.; Hoffmann, B.; Huls, A.; et al. Long-term effects of elemental composition of particulate matter on inflammatory blood markers in European cohorts. Environ. Int. 2015, 82, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Beelen, R.; Stafoggia, M.; Raaschou-Nielsen, O.; Andersen, Z.J.; Hoffmann, B.; Fischer, P.; Houthuijs, D.; Nieuwenhuijsen, M.; Weinmayr, G.; et al. Long-term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 European cohorts: Results from the ESCAPE and TRANSPHORM projects. Environ. Int. 2014, 66, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Grahame, T.J.; Klemm, R.; Schlesinger, R.B. Public health and components of particulate matter: The changing assessment of black carbon. J. Air Waste Manag. Assoc. 2014, 64, 620–660. [Google Scholar] [CrossRef] [PubMed]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Levy, J.I.; Diez, D.; Dou, Y.; Barr, C.D.; Dominici, F. A meta-analysis and multisite time-series analysis of the differential toxicity of major fine particulate matter constituents. Am. J. Epidemiol. 2012, 175, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Rohr, A.C.; Wyzga, R.E. Attributing health effects to individual particulate matter constituents. Atmos. Environ. 2012, 62, 130–152. [Google Scholar] [CrossRef]
- Chen, L.C.; Lippmann, M. Effects of metals within ambient air particulate matter (PM) on human health. Inhal. Toxicol. 2009, 21, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Schlensinger, R.B. The health impact of common inorganic components of fine particulate matter (PM2.5) in ambient air: A critical review. Inhal. Toxicol. 2007, 19, 811–832. [Google Scholar] [CrossRef] [PubMed]
- Grahame, T.J.; Schlesinger, R.B. Health effects of airborne particulate matter: Do we know enough to consider regulating specific particle types or sources? Inhal. Toxicol. 2007, 19, 457–481. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.M.; Yin, J. Particulate matter in the atmosphere: Which particle properties are important for its effects on health? Sci. Total Environ. 2000, 249, 85–101. [Google Scholar] [CrossRef]
- Health Effects Institute (HEI). Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects; HEI: Boston, MA, USA, 2010. [Google Scholar]
- Morawska, L.; Ristovski, Z.; Jayaratne, E.R.; Keogh, D.U.; Ling, X. Ambient nano and ultrafine particles from motor vehicle emissions: Characteristics, ambient processing and implications on human exposure. Atmos. Environ. 2008, 42, 8113–8138. [Google Scholar] [CrossRef]
- World Health Organization (WHO); Regional Office for Europe. Review of Evidence on Health Aspects of Air Pollution-REVIHAAP Project; WHO Regional Office for Europe: Bonn, Germany, 2013. [Google Scholar]
- United States Environmental Protection Agency (U.S. EPA). Integrated Science Assessment for Particulate Matter; U.S. EPA: Research Triangle Park, NC, USA, 2009.
- Lippmann, M. Toxicological and epidemiological studies of cardiovascular effects of ambient air fine particulate matter (PM2.5) and its chemical components: Coherence and public health implications. Crit. Rev. Toxicol. 2014, 44, 299–347. [Google Scholar] [CrossRef] [PubMed]
- Morales, E.; Garcia-Esteban, R.; de la Cruz, O.A.; Basterrechea, M.; Lertxundi, A.; de Dicastillo, M.D.; Zabaleta, C.; Sunyer, J. Intrauterine and early postnatal exposure to outdoor air pollution and lung function at preschool age. Thorax 2015, 70, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Fuks, K.B.; Weinmayr, G.; Foraster, M.; Dratva, J.; Hampel, R.; Houthuijs, D.; Oftedal, B.; Oudin, A.; Panasevich, S.; Penell, J.; et al. Arterial blood pressure and long-term exposure to traffic-related air pollution: An analysis in the European Study of Cohorts for Air Pollution Effects (ESCAPE). Environ. Health Perspect. 2014, 122, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Janssen, N.A.H.; Brunekreef, B.; van Vliet, P.; Aarts, F.; Meliefste, K.; Harssema, H.; Fischer, P. The relationship between air pollution from heavy traffic and allergic sensitization, bronchial hyperresponsiveness, and respiratory symptoms in Dutch schoolchildren. Environ. Health Perspect. 2003, 111, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Schauer, J.J.; Yi, O.; Paek, D.; Kim, H.; Yi, S.M. Fine particle air pollution and mortality: Importance of specific sources and chemical species. Epidemiology 2014, 25, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Lall, R.; Ito, K.; Thurston, G.D. Distributed lag analyses of daily hospital admissions and source-apportioned fine particle air pollution. Environ. Health Perspect. 2011, 119, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Laden, F.; Zanobetti, A. The concentration-response relation between PM2.5 and daily deaths. Environ. Health Perspect. 2002, 110, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Tobias, A.; Querol, X.; Alastuey, A.; Amato, F.; Pey, J.; Perez, N.; Sunyer, J. The effects of particulate matter sources on daily mortality: A case-crossover study of Barcelona, Spain. Environ. Health Perspect. 2011, 119, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Laden, F.; Neas, L.M.; Dockery, D.W.; Schwartz, J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ. Health Perspect. 2000, 108, 941–947. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO); Regional Office for Europe. Health Effects of Transport-Related Air Pollution; Krzyzanowski, M., Kuna-Dibbert, B., Schneider, J., Eds.; WHO Regional Office for Europe: Bonn, Germany, 2005. [Google Scholar]
- Vedal, S.; Campen, M.J.; McDonald, J.D.; Kaufman, J.D.; Larson, T.V.; Sampson, P.D.; Sheppard, L.; Simpson, C.D.; Szpiro, A.A. National Particle Component Toxicity (NPACT) Initiative Report on Cardiovascular Effects; Report 178; Health Effects Institute (HEI): Boston, MA, USA, 2013. [Google Scholar]
- De Kok, T.M.; Driece, H.A.; Hogervorst, J.G.; Briede, J.J. Toxicological assessment of ambient and traffic-related particulate matter: A review of recent studies. Mutat. Res. 2006, 613, 103–122. [Google Scholar] [CrossRef] [PubMed]
- Gerlofs-Nijland, M.E.; Dormans, J.A.; Bloemen, H.J.; Leseman, D.L.; John, A.; Boere, F.; Kelly, F.J.; Mudway, I.S.; Jimenez, A.A.; Donaldson, K.; et al. Toxicity of coarse and fine particulate matter from sties with contrasting traffic profiles. Inhal. Toxicol. 2007, 19, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, M.I.; McGee, J.; Duvall, R.M.; Dailey, L.; Daniels, M.; Boykin, E.; Cho, S.H.; Doerfler, D.; Gordon, T.; Devlin, R.B. Comparative toxicity of size-fractionated airborne particulate matter obtained from different cities in the United States. Inhal. Toxicol. 2007, 19 (Suppl. 1), 7–16. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Mathes, R.; Ross, Z.; Nadas, A.; Thurston, G.; Matte, T. Fine particulate matter constituents associated with cardiovascular hospitalizations and mortality in New York City. Environ. Health Perspect. 2011, 119, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, R.W.; Fuller, G.W.; Anderson, H.R.; Harrison, R.M.; Armstrong, B. Urban ambient particle metrics and health: A time-series analysis. Epidemiology 2010, 21, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Peel, J.; Hannigan, M.; Dutton, S.; Sheppard, L.; Clark, M.L.; Vedal, S. The temporal lag structure of short-term associations of fine particulate matter chemical constituents and cardiovascular and respiratory hospitalizations. Environ. Health Perspect. 2012, 120, 1094–1099. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Roth, L.; Malig, B.; Marty, M. The effects of fine particle components on respiratory hospital admissions in children. Environ. Health Perspect. 2009, 117, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Committee on the Medical Effects of Air Pollutants (COMEAP). Long-Term Exposure to Air Pollution: Effect on Mortality; Department of Health, United Kingdom Government: London, UK, 2009.
- Ito, K.; Christensen, W.F.; Eatough, D.J.; Henry, R.C.; Kim, E.; Laden, F.; Lall, R.; Larson, T.V.; Neas, L.; Hopke, P.K.; et al. PM source apportionment and health effects: 2. An investigation of intermethod variability in associations between source-apportioned fine particle mass and daily mortality in Washington, DC. J. Expo. Sci. Environ. Epidemiol. 2006, 16, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Mar, T.F.; Ito, K.; Koenig, J.Q.; Larson, T.V.; Eatough, D.J.; Henry, R.C.; Kim, E.; Laden, F.; Lall, R.; Neas, L.; et al. PM source apportionment and health effects. 3. Investigation of inter-method variations in associations between estimated source contributions of PM2.5 and daily mortality in Phoenix, AZ. J. Expo. Sci. Environ. Epidemiol. 2006, 16, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.D.; Ito, K.; Lall, R.; Burnett, R.T.; Turner, M.C.; Kriewski, D.; Shi, Y.; Jerrett, M.; Gapstur, S.M.; Diver, W.R.; et al. National Particle Component Toxicity (NPACT) Study 4. Mortality and Long-Term Exposure to PM2.5 and Its Components in the American Cancer Society’s Cancer Prevention Study II Cohort; Report 177; Health Effects Institute (HEI): Boston, MA, USA, 2013. [Google Scholar]
- Sarnat, J.A.; Marmur, A.; Klein, M.; Kim, E.; Russell, A.G.; Sarnat, S.E.; Mulholland, J.A.; Hopke, P.K.; Tolbert, P.E. Fine particle sources and cardiorespiratory morbidity: An application of chemical mass balance and factor analytical source-apportionment methods. Environ. Health Perspect. 2008, 116, 459–466. [Google Scholar] [CrossRef] [PubMed]
- BeruBe, K.; Balharry, D.; Sexton, K.; Koshy, L.; Jones, T. Combustion-derived nanoparticles: Mechanisms of pulmonary toxicity. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Possamai, F.P.; Junior, S.A.; Parisotto, E.B.; Moratelli, A.M.; Inacio, D.B.; Garlet, T.R.; Dal-Pizzol, F.; Filho, D.W. Antioxidant intervention compensates oxidative stress in blood of subjects exposed to emissions from a coal electric-power plant in South Brazil. Environ. Toxicol. Pharmacol. 2010, 30, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.-H.; Kuo, C.-Y.; Hsu, M.-L.; Wang, T.-Y.; Chang, P.-I.; Wu, T.H.; Huang, S. Increased levels of 8-hydroxy-2-deoxyguanosine attributable to carcinogenic metal exposure among schoolchildren. Environ. Health Perspect. 2005, 113, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (U.S. EPA). Provisional Assessment of Recent Studies on Health Effects of Particulate Matter Exposure; U.S. EPA: Research Triangle Park, NC, USA, 2012.
- Wichmann, H.E. Diesel exhaust particles. Inhal. Toxicol. 2007, 19 (Suppl. 1), 241–244. [Google Scholar] [CrossRef] [PubMed]
- Maricq, M. Chemical characterization of particulate emissions from diesel engines: A review. J. Aerosol. Sci. 2007, 38, 1079–1118. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (U.S. EPA). Health Assessment Document for Diesel Engine Exhaust; U.S. EPA: Washington DC, USA, 2002.
- Zielinska, B.; Samy, S.; McDonald, J.D.; Seagrave, J. Atmospheric Transformation of Diesel Emissions; Health Effects Institute (HEI): Boston, MA, USA, 2010. [Google Scholar]
- California Environmental Protection Agency (EPA); Air Resources Board. The Report on Diesel Exhaust; California EPA Air Resources Board: Sacramento, CA, USA, 1998.
- Olsson, A.C.; Gustavsson, P.; Krombout, H.; Peters, S.; Vermeulen, R.; Bruske, I.; Pesch, B.; Siemiatycki, J.; Pintos, J.; Bruning, T.; et al. Exposure to diesel motor exhaust and lung cancer risk in a pooled analysis from case-control studies in Europe and Canada. Am. J. Respir. Crit. Care Med. 2011, 183, 941–948. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Diesel and Gasoline Engine Exhausts and Some Nitroarenes; IARC: Lyson, France, 2012; Volume 105. [Google Scholar]
- Attfield, M.D.; Schleiff, P.L.; Lubin, J.H.; Blair, A.; Stewart, P.A.; Vermeulen, R.; Coble, J.B.; Silverman, D.T. The Diesel Exhaust in Miners study: A cohort mortality study with emphasis on lung cancer. J. Natl. Cancer Inst. 2012, 104, 869–883. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.T.; Samanic, C.M.; Lubin, J.H.; Blair, A.E.; Stewart, P.A.; Vermuelen, R.; Coble, J.B.; Rothman, N.; Schleiff, P.L.; Travis, W.D.; et al. The Diesel Exhaust in Miners study: A nested case-control study of lung cancer and diesel exhaust. J. Natl. Cancer Inst. 2012, 104, 855–868. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Bochmann, F.; Nold, A.; Mattenklott, M. Diesel exhaust exposure and the risk of lung cancer-a review of the epidemiological evidence. Int. J. Environ. Res. Public Health 2014, 11, 1312–1340. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.F.; Nicolich, M.J.; Boffetta, P. Lung cancer and diesel exhaust: An updated critical review of the occupational epidemiology literature. Crit. Rev. Toxicol. 2012, 42, 549–598. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M.; Chen, L.-C.; Gordon, T.; Ito, K.; Thurston, G.D. National Particle Component Toxicity (NPACT) Initiative: Integrated Epidemiologic and Toxicologic Studies of the Health Effects of Particulate Matter Components; Research Report 177; Health Effects Institute: Boston, MA, USA, 2013. [Google Scholar]
- Peng, R.D.; Bell, M.L.; Geyh, A.S.; McDermott, A.; Zeger, S.L.; Samet, J.M.; Dominici, F. Emergency admissions for cardiovascular and respiratory diseases and the chemical composition of fine particle air pollution. Environ. Health Perspect. 2009, 117, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Hesterberg, T.W.; Long, C.M.; Bunn, W.B.; Sax, S.N.; Lapin, C.A.; Valberg, P.A. Non-cancer health effects of diesel exhaust: A critical assessment of recent human and animal toxicological literature. Crit. Rev. Toxicol. 2009, 39, 195–227. [Google Scholar] [CrossRef] [PubMed]
- Lucking, A.J.; Lundback, M.; Barath, S.L.; Mills, N.L.; Sidhu, M.K.; Langrish, J.P.; Boon, N.A.; Pourazar, J.; Badimon, J.J.; Gerlofs-Nijland, M.E.; et al. Paticle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation 2011, 123, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Mills, N.L.; Miller, M.R.; Lucking, A.J.; Beveridge, J.; Flint, L.; Boere, A.J.; Fokkens, P.H.; Boon, N.A.; Sandstrom, T.; Blomberg, A.; et al. Combustion-derived nanoparticulate induces the adverse vascular effects of diesel exhaust inhalation. Eur. Heart J. 2011, 32, 2660–2671. [Google Scholar] [CrossRef] [PubMed]
- Kodavanti, U.P.; Thomas, R.; Ledbetter, A.D.; Schladweiler, M.C.; Shannahan, J.H.; Wallenborn, J.G.; Lund, A.K.; Campen, M.J.; Butler, E.O.; Gottipolu, R.R.; et al. Vascular and cardiac impairments in rats inhaling ozone and diesel exhaust particles. Environ. Health Perspect. 2011, 119, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R.; McLean, S.G.; Duffin, R.; Lawal, A.O.; Araujo, J.A.; Shaw, C.A.; Mills, N.L.; Donaldson, K.; Newby, D.E.; Hadoke, P.W. Diesel exhaust particulate increases the size and complexity of lesions in atherosclerotic mice. Part. Fibre Toxicol. 2013, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Poss, J.; Lorenz, D.; Werner, C.; Pavlikova, V.; Gensch, C.; Speer, T.; Alessandrini, F.; Berezowski, V.; Kuntz, M.; Mempel, M.; et al. Diesel exhaust particles impair endothelial progenitor cells, compromise endothelial integrity, reduce neoangiogenesis, and increase atherogenesis in mice. Cardiovasc. Toxicol. 2013, 13, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.Y.; Park, M.K.; Leikauf, G.D.; Park, C.S.; Jang, A.S. Diesel exhaust particle-induced airway responses are augmented in obese rats. Int. J. Toxicol. 2014, 33, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Subramaniyan, D.; Yasin, J.; Ali, B.H. Impact of experimental type 1 diabetes mellitus on systemic and coagulation vulnerability in mice acutely exposed to diesel exhaust particles. Part. Fibre Toxicol. 2013, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Acciani, T.; Brandt, E.; Khurana Hershey, G.; Le Cras, T. Diesel exhaust particle exposure increases severity of allergic asthma in young mice. Clin. Exp. Allergy 2013, 43, 1406–1418. [Google Scholar] [CrossRef] [PubMed]
- Noah, T.L.; Zhou, H.; Zhang, H.; Horvath, K.; Robinette, C.; Kesic, M.; Meyer, M.; Diaz-Sanchez, D.; Jaspers, I. Diesel exhaust exposure and nasal response to attenuated influenza in normal and allergic volunteers. Am. J. Respir. Crit. Care Med. 2012, 185, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, G.; Tanaka, H.; Wakahara, K.; Nasu, R.; Hashimoto, M.; Miyoshi, K.; Takano, H.; Yamashita, H.; Inagaki, N.; Nagai, H. Effect of diesel exhaust particles on house dust mite-induced airway eosinophilic inflammation and remodeling in mice. J. Pharmacol. Sci. 2010, 112, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Ema, M.; Naya, M.; Horimoto, M.; Kato, H. Developmental toxicity of diesel exhaust: A review of studies in experimental animals. Reprod. Toxicol. 2013, 42, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Manners, S.; Alam, R.; Schwartz, D.A.; Gorska, M.M. A mouse model links asthma susceptibility to prenatal exposure to diesel exhaust. J. Allergy Clin. Immunol. 2014, 134, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Weldy, C.S.; Liu, Y.; Liggitt, H.D.; Chin, M.T. In utero exposure to diesel exhaust air pollution promotes adverse intrauterine conditions, resulting in weight gain, altered blood pressure, and increased susceptibility to heart failure in adult mice. PLoS ONE 2014, 9, e88582. [Google Scholar] [CrossRef] [PubMed]
- Ancelet, T.; Davy, P.K.; Trompetter, W.J.; Markwitz, A.; Weatherburn, D.C. Carbonaceous aerosols in a wood burning community in rural New Zealand. Atmos. Pollut. Res. 2013, 4, 245–249. [Google Scholar] [CrossRef]
- Glasius, M.; Ketzel, M.; Wahlin, P.; Jensen, B.; Monster, J.; Berkowicz, R.; Palmgren, F. Impact of wood combustion on particle levels in a residential area in Denmark. Atmos. Environ. 2006, 40, 7115–7124. [Google Scholar] [CrossRef]
- Grange, S.K.; Salmond, J.A.; Trompetter, W.J.; Davy, P.K.; Ancelet, T. Effect of atmospheric stability on the impact of domestic wood combustion to air quality of a small urban township in winter. Atmos. Environ. 2013, 70, 28–38. [Google Scholar] [CrossRef]
- Molnar, P.; Sallsten, G. Contribution to PM(2.5) from domestic wood burning in a small community in Sweden. Env. Sci. Process Impacts 2013, 15, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Trompetter, W.J.; Grange, S.K.; Davy, P.K.; Ancelet, T. Vertical and temporal variations of black carbon in New Zealand urban areas during winter. Atmos. Environ. 2013, 75, 179–187. [Google Scholar] [CrossRef]
- Boman, B.C.; Forsberg, A.B.; Jarvholm, B.G. Adverse health effects from ambient air pollution in relation to residential wood combustion in modern society. Scand. J. Work Environ. Health 2003, 29, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Lipsett, M.; Hurley, S.; Ostro, B. Air pollution and emergency room visits for asthma in Santa Clara County, California. Environ. Health Perspect. 1997, 105, 216–222. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.A.; Hider, P.N.; Chacko, E.; Town, G.I. Particulate air pollution and hospital admissions in Christchurch, New Zealand. Aust. N. Z. J. Public Health 2002, 26, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Naeher, L.P.; Brauer, M.; Lipsett, M.; Zelikoff, J.T.; Simpson, C.D.; Koenig, J.Q.; Smith, K.R. Woodsmoke health effects: A review. Inhal. Toxicol. 2007, 19, 67–106. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.; Slater, D.; Larson, T.V.; Pierson, W.E.; Koenig, J.Q. Particulate air pollution and hospital emergency room visits for asthma in Seattle. Am. Rev. Respir. Dis. 1993, 147, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Town, G.I. The health effects of particulate air pollution-a Christchurch perspective. Biomarkers 2001, 6, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Andersen, Z.J.; Wahlin, P.; Raaschou-Nielsen, O.; Scheike, T.; Loft, S. Ambient particle source apportionment and daily hospital admissions among children and elderly in Copenhagen. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Zelikoff, J.T.; Chen, L.C.; Cohen, M.D.; Schlesinger, R.B. The toxicology of inhaled woodsmoke. J. Toxicol. Environ. Health B Crit. Rev. 2002, 5, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.D.; Campen, M.J.; Gigliotti, A.P.; Harrod, K.S.; McDonald, J.D.; Seagrave, J.C.; Mauderly, J.L.; Seilkop, S.K. Health effects of subchronic exposure to environmental levels of hardwood smoke. Inhal. Toxicol. 2006, 18, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Seagrave, J.; McDonald, J.D.; Reed, M.D.; Seilkop, S.K.; Mauderly, J.L. Responses to subchronic inhalation of low concentrations of diesel exhaust and hardwood smoke measured in rat bronchoalveolar lavage fluid. Inhal. Toxicol. 2005, 17, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, H.D., 3rd; Boffetta, P.; Greenland, S.; Lee, Y.C.; McLaughlin, J.; Seow, A.; Duell, E.J.; Andrew, A.S.; Zaridze, D.; Szeszenia-Dabrowska, N.; et al. In-home coal and wood use and lung cancer risk: A pooled analysis of the International Lung Cancer Consortium. Environ. Health Perspect. 2010, 118, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- De Longueville, F.; Ozer, P.; Doumbia, S.; Henry, S. Desert dust impacts on human health: An alarming worldwide reality and a need for studies in West Africa. Int. J. Biometeorol. 2013, 57, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Johnston, F.; Hanigan, I.; Henderson, S.; Morgan, G.; Bowman, D. Extreme air pollution events from bushfires and dust storms and their association with mortality in Sydney, Australia 1994–2007. Environ. Res. 2011, 111, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Honda, Y.; Lim, Y.-H.; Guo, Y.L.; Hashizume, M.; Kim, H. Effect of Asian dust storms on mortality in three Asian cities. Atmos. Environ. 2014, 89, 309–317. [Google Scholar] [CrossRef]
- Lee, H.; Kim, H.; Honda, Y.; Lim, Y.-H.; Yi, S. Effect of Asian dust storms on daily mortality in seven metropolitan cities of Korea. Atmos. Environ. 2013, 79, 510–517. [Google Scholar] [CrossRef]
- Chan, C.C.; Ng, H.C. A case-crossover analysis of Asian dust storms and mortality in the downwind areas using 14-year data in Taipei. Sci. Total Environ. 2011, 410–411, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Levy, J.K.; Lin, Z. The effect of sandstorms and air pollution on cause-specific hospital admissions in Taipei, Taiwan. Occup. Environ. Med. 2008, 65, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Liu, T.C.; Keller, J.; Lin, H.C. Asian dust storm events are associated with an acute increase in stroke hospitalisation. J. Epidemiol. Community Health 2013, 67, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, A.; Schindeler, S.; Jalaludin, B.; Smith, W. Health effects of the September 2009 dust storm in Sydney, Australia: Did emergency department visits and hospital admissions increase? Environ. Health 2013, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Middleton, N.; Yiallouros, P.; Kleanthous, S.; Kolokotroni, O.; Schwartz, J.; Dockery, D.W.; Demokritou, P.; Koutrakis, P. A 10-year time-series analysis of respiratory and cardiovascular morbidity in Nicosia, Cyprus: The effect of short-term changes in air pollution and dust storms. Environ. Health 2008, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.; Wong, T.; Wong, A. Effect of dust storm events on daily emergency admissions for cardiovascular diseases. Circ. J. 2012, 76, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Chen, Y.S.; Chiu, H.F.; Goggins, W.B. Effects of Asian dust storm events on daily stroke admissions in Taipei, Taiwan. Environ. Res. 2005, 99, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Cheng, M.H.; Chen, C.C. Effects of Asian dust storm events on hospital admissions for congestive heart failure in Taipei, Taiwan. J. Toxicol. Environ. Health A 2009, 72, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-W.; Lee, K.-K. Effects of Asian dust events on daily asthma patients in Seoul, Korea. Meteorol. Appl. 2014, 21, 202–209. [Google Scholar] [CrossRef]
- Park, J.W.; Lim, Y.H.; Kyung, S.Y.; An, C.H.; Lee, S.P.; Jeong, S.H.; Ju, Y.S. Effects of ambient particulate matter on peak expiratory flow rates and respiratory symptoms of asthmatics during Asian dust periods in Korea. Respirology 2005, 10, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Chen, C.S.; Lin, C.L. The threat of Asian dust storms on asthma patients: A population-based study in Taiwan. Glob. Public Health 2014, 9, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, K.T.; Ito, I.; Al-Delaimy, W.K.; Adachi, Y.; Mathews, W.C.; Ramsdell, J.W. Toyama Asian Desert Dust and Asthma Study Team. Desert dust exposure is associated with increased risk of asthma hospitalization in children. Am. J. Respir. Crit. Care Med. 2010, 182, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.; Choung, J.T.; Yu, J.; Kim do, K.; Koh, Y.Y. Acute effects of Asian dust events on respiratory symptoms and peak expiratory flow in children with mild asthma. J. Korean Med. Sci. 2008, 23, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.F.; Tiao, M.M.; Ho, S.C.; Kuo, H.W.; Wu, T.N.; Yang, C.Y. Effects of Asian dust storm events on hospital admissions for chronic obstructive pulmonary disease in Taipei, Taiwan. Inhal. Toxicol. 2008, 20, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.W.; Wong, T.W.; Wong, A.H.; Hui, D.S. Effect of dust storm events on daily emergency admissions from respiratory diseases. Respirology 2012, 17, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H. Effects of the Asian dust events on daily mortality in Seoul, Korea. Environ. Res. 2002, 90, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Sheen, P.C.; Chen, E.R.; Liu, Y.K.; Wu, T.N.; Yang, C.Y. Effects of Asian dust storm events on daily mortality in Taipei, Taiwan. Environ. Res. 2004, 95, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Zauli Sajani, S.; Miglio, R.; Bonasoni, P.; Cristofanelli, P.; Marinoni, A.; Sartini, C.; Goldoni, C.A.; De Girolamo, G.; Lauriola, P. Saharan dust and daily mortality in Emilia-Romagna (Italy). Occup. Environ. Med. 2011, 68, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.F.; Ho, S.C.; Chiu, H.F.; Wu, T.N.; Chen, P.S.; Yang, C.Y. Consequences of exposure to Asian dust storm events on daily pneumonia hospital admissions in Taipei, Taiwan. J. Toxicol. Environ. Health A 2008, 71, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.W. Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin. Microbiol. Rev. 2007, 20, 459–477. [Google Scholar] [CrossRef] [PubMed]
- Goudie, A.S. Desert dust and human health disorders. Environ. Int. 2014, 63, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Sprigg, W.A.; Nickovic, S.; Galgiani, J.N.; Pejanovic, G.; Petkovic, S.; Vujadinovic, M.; Vukovic, A.; Dacic, M.; DiBiase, S.; Prasad, A.; et al. Regional dust storm modeling for health services: The case of valley fever. Aeolian Res. 2014, 14, 53–73. [Google Scholar] [CrossRef]
- Yang, C.Y. Effects of Asian dust storm events on daily clinical visits for conjunctivitis in Taipei, Taiwan. J. Toxicol. Environ. Health A 2006, 69, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Kummarapurugu, S.T.; Tong, H.; Soukup, J.M.; Dailey, L.A.; Boykin, E.; Ian Gilmour, M.; Ingram, P.; Roggli, V.L.; Goldstein, H.L.; et al. Biological effects of desert dust in respiratory epithelial cells and a murine model. Inhal. Toxicol. 2014, 26, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.C.; Chan, C.C.; Wang, P.Y.; Lee, C.T.; Cheng, T.J. Effects of Asian dust event particles on inflammation markers in peripheral blood and bronchoalveolar lavage in pulmonary hypertensive rats. Envion. Res. 2004, 95, 71–76. [Google Scholar] [CrossRef]
- Kim, W.; Doh, S.J.; Yu, Y. Asian dust storm as conveyance media of anthropogenic pollutants. Atmos. Environ. 2012, 49, 41–50. [Google Scholar] [CrossRef]
- Van Pelt, R.S.; Zobeck, T.M. Chemical constituents of fugitive dust. Environ. Monit. Assess. 2007, 130, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.C.; Pan, X.C.; Kim, S.Y.; Park, K.; Park, E.J.; Jin, X.; Yi, S.M.; Kim, Y.H.; Park, C.H.; Song, S.; et al. Asian dust storm and pulmonary function of school children in Seoul. Sci. Total Environ. 2010, 408, 754–759. [Google Scholar] [CrossRef] [PubMed]
- Wyzga, R.E.; Rohr, A.C. Long-term particulate matter exposure: Attributing health effects to individual PM components. J. Air Waste Manag. Assoc. 2015, 65, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Molitor, J.; Coker, E.; Jerrett, M.; Ritz, B.; Li, A. Part 3. Modeling of Multipollutant Profiles and Spatially Varying Health Effects with Applications to Indicators of Adverse Birth Outcomes. In Development of Statistical Methods for Multipollutant Research; Research Report 183; Health Effects Institute: Boston, MA, USA, 2016. [Google Scholar]
- Viana, M.; Kuhlbusch, T.A.J.; Querol, X.; Alastuey, A.; Harrison, R.M.; Hopke, P.K.; Winiwarter, W.; Vallius, M.; Szidat, S.; Prevot, A.S.H.; et al. Source apportionment of particulate matter in Europe: A review of methods and results. J. Aerosol Sci. 2008, 39, 827–849. [Google Scholar] [CrossRef]
- Eeftens, M.; Hoek, G.; Gruzieva, O.; Molter, A.; Agius, R.; Beelen, R.; Brunekreef, B.; Custovic, A.; Cyrys, J.; Fuertes, E.; et al. Elemental composition of particulate matter and the association with lung function. Epidemiology 2014, 25, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Fuertes, E.; MacIntyre, E.; Agius, R.; Beelen, R.; Brunekreef, B.; Bucci, S.; Cesaroni, G.; Cirach, M.; Cyrys, J.; Forastiere, F.; et al. Associations between particulate matter elements and early-life pneumonia in seven birth cohorts: Results from the ESCAPE and TRANSPHORM projects. Int. J. Hyg. Environ. Health 2014, 217, 819–829. [Google Scholar] [CrossRef] [PubMed]
| Reference | Study Conclusions in Relation to Health Effects of Source-Specific PM Air Pollution |
|---|---|
| [38] | The black carbon, for which vehicles and particularly diesel vehicles are a major source in urban areas, in PM might make PM from those sources the most harmful. The relative toxicity of wood smoke compared with vehicle exhaust emissions is unclear. |
| [29] | Current evidence does not allow a precise differentiation to be made as to which constituents or sources of PM are most closely related to specific health outcomes. However, three components, black carbon, secondary organic aerosols, and secondary inorganic aerosols may be important contributors to PM toxicity. |
| [39] | Current knowledge does not allow precise quantification or definitive ranking of the health effects of PM from different sources. However, some results suggest that a range of serious health effects are more consistently associated with traffic-related PM and specific metals and elemental carbon in PM. |
| [40] | There is a lack of information by which to differentiate the toxicity of different components of PM. |
| [41] | Evidence suggests that carbon components and several metals in PM are associated with health effects however it is unclear whether these components are responsible for health impacts or they are surrogates for other pollutants. |
| [31] | Cardiovascular health effects may be associated with PM2.5 from crustal or combustion sources, including traffic, but at this time, no consistent relationships have emerged. Collective evidence has not yet isolated factors or sources that would be closely and unequivocally related to specific health outcomes. |
| [42] | There is evidence that metals within PM affect health but considerable uncertainties about causality remain. |
| [43] | Evidence relating to the toxicity of inorganic components of PM2.5 is not consistent. Crustal components of PM2.5 are not likely, by themselves, to be a significant health risk. |
| [44] | Public health will likely be better protected by reduction of various vehicular emissions than by regulation of total PM2.5 mass as if all PM2.5 is equitoxic. However, the knowledge base is incomplete. |
| [45] | There is little support for the idea that any single major or trace component of PM is responsible for the adverse health effects of PM. |
| Ref. | Method Used to Identify Source-Specific PM | Health Outcomes Investigated | Relative Risk Associated with an Increase in PM2.5 (95% Confidence Interval) 1 | |||||
|---|---|---|---|---|---|---|---|---|
| Source | Traffic | Coal-Fired Power Stations (Secondary Sulphate) | Diesel Exhaust | Wood Smoke | Crustal Dust (Soil) | All (Total Mass) | ||
| [56] | Factor analysis to identify up to 5 common factors from 15 specified elements | Daily all-cause mortality per 10 µg/m3 increase in PM2.5 | 1.03 (no CI’s) | 1.05 (no CI’s) | ||||
| [58] | Factor analysis to identify up to 5 common factors from 15 specified elements | Daily all-cause mortality per 10 µg/m3 increase in PM2.5 | 1.034 (1.017–1.052) | 1.011 (1.003–1.020) | 0.977 (0.942–1.012) | 1.016 (1.011–1.021) | ||
| [55] | Positive matrix factorization | Daily cardiovascular and respiratory hospital admissions per 5–95th percentile increase in PM2.5 | 1.04 (1.01–1.08) 2 (cardiovascular) 1.01 (0.97–1.06) 2 (respiratory) | 1.01 (0.97–1.05)2 (cardiovascular) 1.03 (0.97–1.09) 2 (respiratory) | 1.00 (0.95–1.04) 2 (cardiovascular) 1.02 (0.96–1.09) 2 (respiratory) | 1.01 (0.98–1.05) 2 (cardiovascular) 1.05 (1.00–1.10) 2 (respiratory) | ||
| [57] | Positive matrix factorization | Daily all-cause and cardiovascular mortality per IQR increase in PM2.5 | 1.056 (1.018–1.095) (all-cause) 1.103 (1.033–1.178) (cardiovascular) | 1.019 (0.975–1.065) (all-cause) 1.072 (1.014–1.133) (cardiovascular) | 1.019 (1.008–1.031) (all-cause) 1.039 (1.019–1.060) (cardiovascular) | |||
| [54] | Positive matrix factorization | Daily all-cause, cardiovascular and respiratory mortality per IQR increase in PM2.5 | 1.005 (0.993–1.017) (all-cause) 1.008 (0.986–1.031) (cardiovascular) 1.055 (1.005–1.107) (respiratory) | 1.005 (0.995–1.014) (all-cause) 1.010 (0.991–1.029) (cardiovascular) 1.021 (0.983–1.061) (respiratory) | 1.006 (0.991–1.020) (all-cause) 1.009 (0.981–1.037) (cardiovascular) 1.067 (1.002–1.137) (respiratory) | 1.001 (0.997–1.006) (all-cause) 1.003 (0.994–1.013) (cardiovascular) 1.016 (0.997–1.035) (respiratory) | 1.005 (0.992–1.019) (all-cause) 1.028 (1.002–1.054) (cardiovascular) 1.021 (0.972–1.071) (respiratory) | |
| [72] | Positive matrix factorization | Daily cardiovascular and respiratory emergency department (ED) visits per IQR increase in PM2.5 | 1.022 (1.012–1.032) 2 (cardiovascular) 0.999 (0.993–1.007)2 (respiratory) | 1.004 (0.992–1.021) 2 (cardiovascular) 1.015 (1.002–1.028) 2 (respiratory) | 1.030 (1.017–1.039) 2 (cardiovascular) 0.997 (0.991–1.005) 2 (respiratory) | 1.029 (1.018–1.037) 2 (cardiovascular) 0.999 (0.993–1.006) 2 (respiratory) | 1.005 (0.998–1.012) 2 (cardiovascular) 0.998 (0.993–1.003) 2 (respiratory) | 1.025 (1.008–1.041) 2 (cardiovascular) 1.007 (0.996–1.019) 2 (respiratory) |
| [69] | Various multivariate factor analysis based receptor models | Daily all-cause and cardiovascular mortality per 5–95th percentile increase in PM2.5 | 1.03 (0.98–1.07) 2 (all-cause) 1.05 (0.97–1.11) 2 (cardiovascular) | 1.07 (1.02–1.12) 2 (all-cause) 1.07 (0.99–1.14) 2 (cardiovascular) | 1.00 (0.99–1.02) 2 (all-cause) 1.01 (0.98–1.04) 2 (cardiovascular) | 1.02 (0.99–1.04) 2 (all-cause) 1.04 (1.00–1.07) 2 (cardiovascular) | ||
| [70] | Various multivariate factor analysis based receptor models | Daily all-cause and cardiovascular mortality per 5–95th percentile increase in PM2.5 | 1.01 (0.90–1.12) 2 (all-cause) 1.13 (0.97–1.29) 2 (cardiovascular) | 1.03 (0.92–1.13) 2 (all-cause) 1.16 (1.00–1.31) 2 (cardiovascular) | 1.02 (0.93–1.10) 2 (all-cause) 1.09 (0.96–1.21) 2 (cardiovascular) | 1.01 (0.90–1.11) 2 (all-cause) 1.01 (0.85–1.16) 2 (cardiovascular) | Not reported (all-cause) 1.150 (1.015–1.303) (cardiovascular) | |
| [71] | Multivariate factor analysis of elemental data with source modeling | All-cause, ischemic heart disease (IHD) and respiratory mortality per IQR increase in PM2.5 | 1.032 (1.021–1.042) 2 (all-cause) 1.013 (0.987–1.039) 2 (IHD) 1.09 (1.05–1.13) 2 (respiratory) | 1.008 (1.001–1.015) 2 (all-cause) 1.042 (1.024–1.060) 2 (IHD) 0.95 (0.92–0.97) 2 (respiratory) | 1.000 (0.993–1.006) 2 (all-cause) 1.000 (0.986–1.012) 2 (IHD) 1.02 (1.00–1.04) 2 (respiratory) | |||
| Emission Source | Health Risk and Reference |
|---|---|
| Traffic | |
| Total traffic-related air pollution (TRAP) | exacerbation and onset of childhood asthma, respiratory symptoms, impaired lung function, all-cause mortality, cardiovascular morbidity [46] |
| myocardial infarction [49] | |
| reduced lung function in children [51] | |
| increased blood pressure [52] | |
| allergic sensitization [53] | |
| premature birth [38] | |
| Specifically traffic PM | all-cause, respiratory and cardiovascular mortality, cardiovascular, stroke and heart failure morbidity [54,55,56,57,58,70,71,72] |
| cardiovascular toxicity and various cardiovascular effects [50,60] | |
| cytotoxicity, pulmonary inflammation [62,63] | |
| Coal-fired power stations | all-cause, cardiovascular, respiratory, ischaemic heart disease, pneumonia, lung cancer mortality [19,34,57,58,69,70,71] |
| respiratory morbidity [48,49,65,66,67,68,72] | |
| cardiovascular morbidity [48,49,68] | |
| Diesel exhaust | respiratory mortality [54] |
| lung and oesophageal cancer mortality [84,85] | |
| allergic inflammation, asthma symptoms, lung cancer [79,81,82,83] | |
| cardiovascular morbidity [72,89] | |
| cardiovascular changes indicative of increased coronary event risk, changes in lung function, nose and throat irritation [48,49,90] | |
| atopy and susceptibility to infection [98,99,100] | |
| effects on offspring from exposure during pregnancy [101,102,103] | |
| Domestic wood combustion heaters (studies of outdoor exposure to heater emissions) | respiratory symptoms and exacerbations [109,110,111,112,113,114] |
| cardiovascular morbidity [72] | |
| respiratory morbidity [115] | |
| compromised lung immunity, airway inflammation [112,116,117,118] | |
| Crustal dust | all-cause and cardiovascular mortality [120,121,122,123,124] |
| respiratory mortality(>75 years of age) [141] | |
| respiratory and COPD morbidity [127,137,138] | |
| asthma exacerbation [125,132,133,134,135,136] | |
| reduced lung function in children [151] | |
| pneumonia [142,143] | |
| lung inflammation [147,148] | |
| infectious disease [144,145,146] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hime, N.J.; Marks, G.B.; Cowie, C.T. A Comparison of the Health Effects of Ambient Particulate Matter Air Pollution from Five Emission Sources. Int. J. Environ. Res. Public Health 2018, 15, 1206. https://doi.org/10.3390/ijerph15061206
Hime NJ, Marks GB, Cowie CT. A Comparison of the Health Effects of Ambient Particulate Matter Air Pollution from Five Emission Sources. International Journal of Environmental Research and Public Health. 2018; 15(6):1206. https://doi.org/10.3390/ijerph15061206
Chicago/Turabian StyleHime, Neil J., Guy B. Marks, and Christine T. Cowie. 2018. "A Comparison of the Health Effects of Ambient Particulate Matter Air Pollution from Five Emission Sources" International Journal of Environmental Research and Public Health 15, no. 6: 1206. https://doi.org/10.3390/ijerph15061206
APA StyleHime, N. J., Marks, G. B., & Cowie, C. T. (2018). A Comparison of the Health Effects of Ambient Particulate Matter Air Pollution from Five Emission Sources. International Journal of Environmental Research and Public Health, 15(6), 1206. https://doi.org/10.3390/ijerph15061206




