A Muscarinic Antagonist Reduces Airway Inflammation and Bronchoconstriction Induced by Ambient Particulate Matter in a Mouse Model of Asthma
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Preparation of Ambient PM
2.3. Experimental Protocol
2.4. BALF Procedure
2.5. Quantitative Determination of Cytokine Concentrations
2.6. Histological Examination
2.7. Measurement of Airway Resistance
2.8. Oxidative Stress Measurements
2.9. Statistical Analysis
3. Results
3.1. Cell Counts in BALF
3.2. Cytokine Profile of BALF
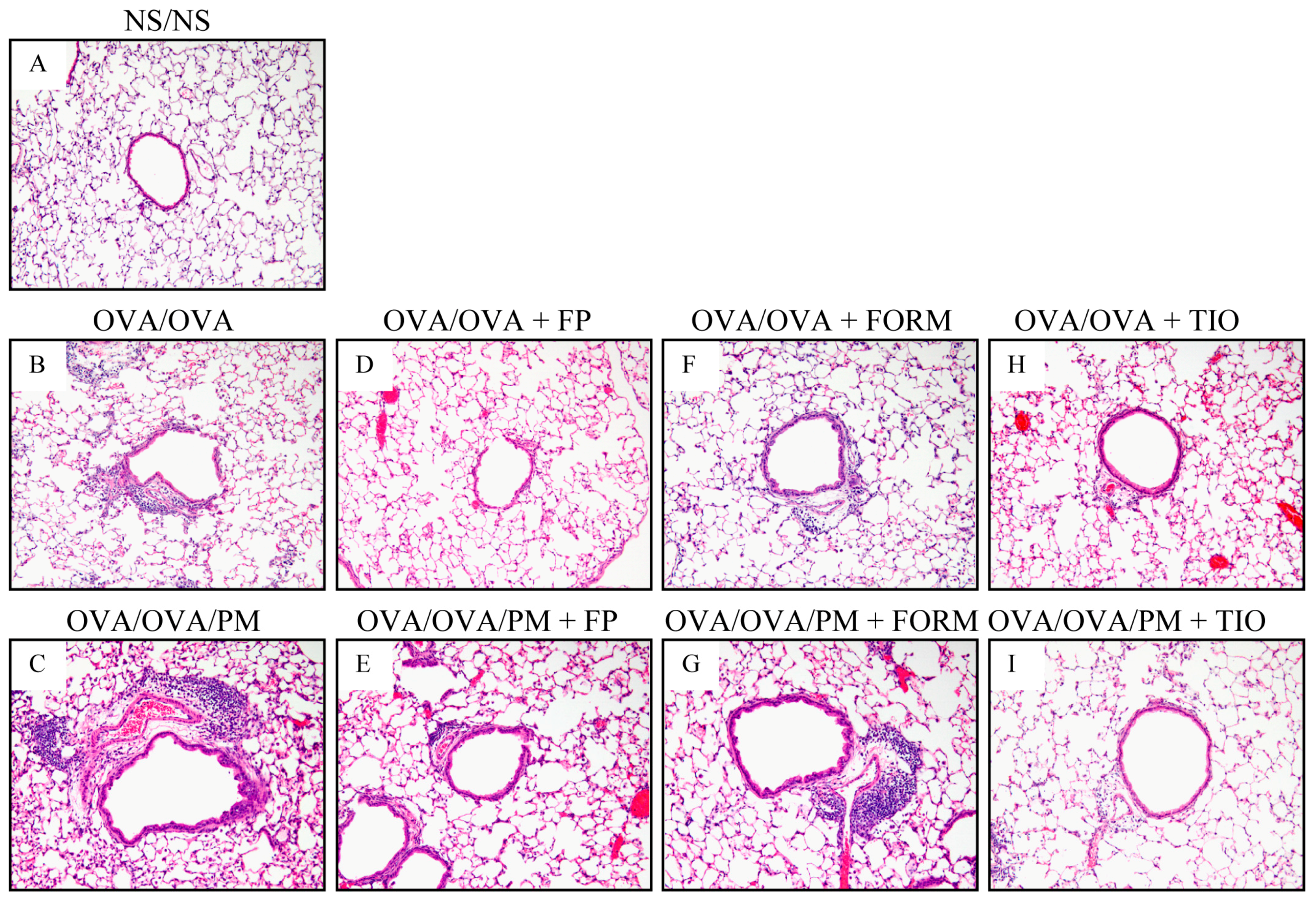
3.3. Histopathological Changes in the Lung
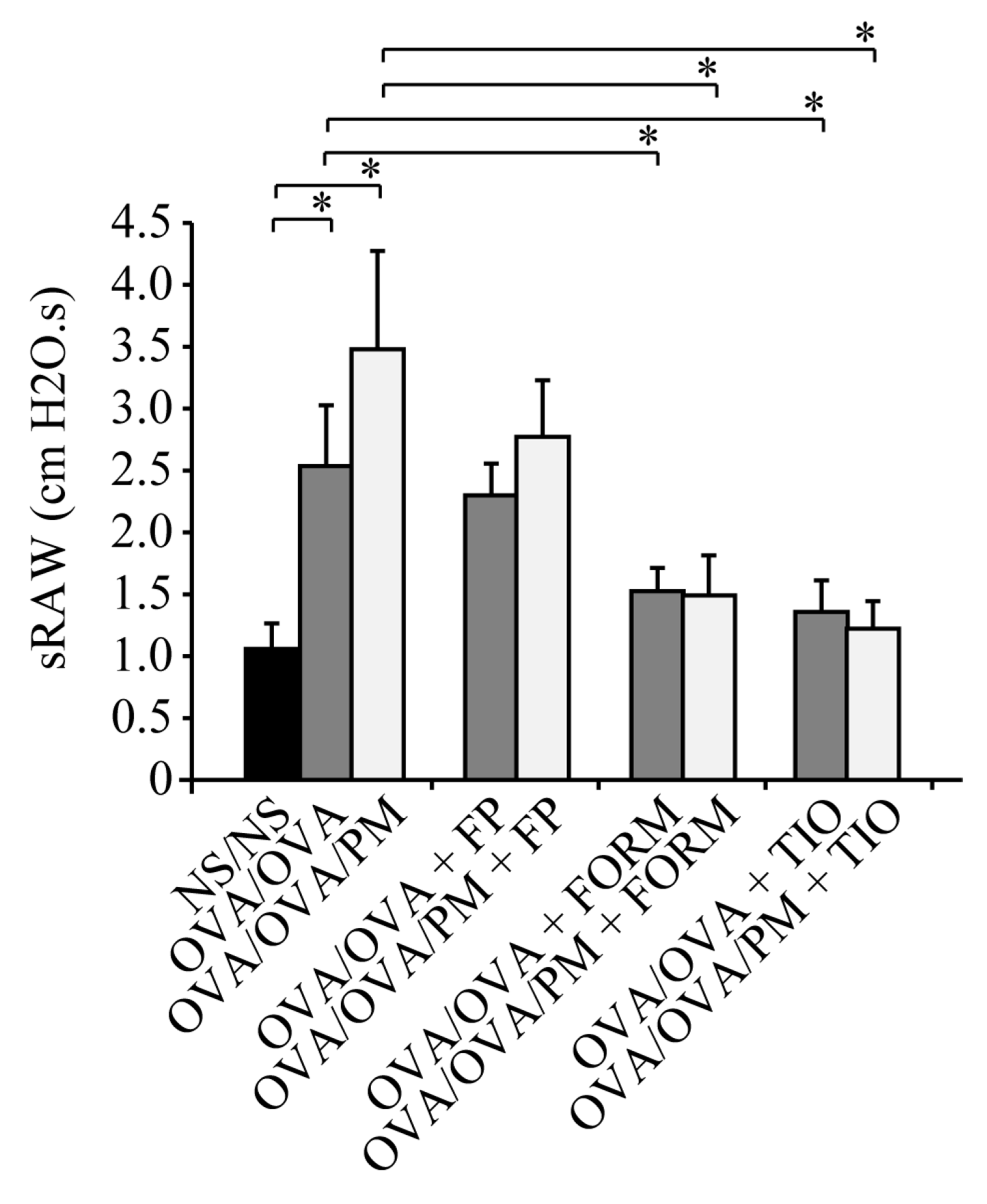
3.4. Measurement of Airway Resistance
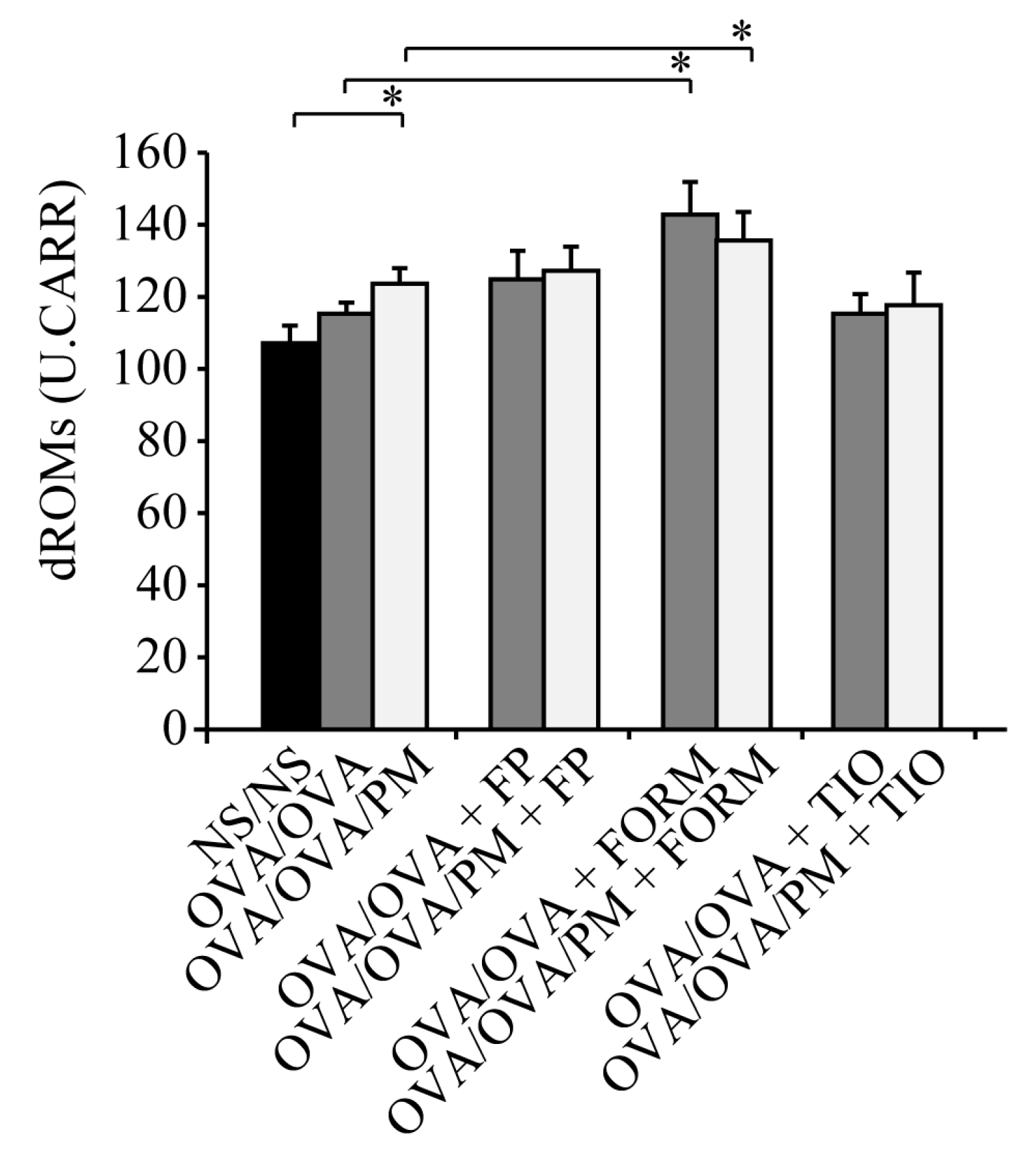
3.5. Measurement of dROMs in the Serum
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| PM | particulate matter |
| OVA | ovalbumin |
| IFN | interferon |
| IL | interleukin |
| KC/CXCL1 | keratinocyte-derived chemokine |
| BALF | bronchoalveolar lavage fluid |
| dROMs | diacron reactive oxygen metabolites |
| MIP-2/CXCL2 | macrophage inflammatory protein |
| NS | normal saline |
| FORM | formoterol fumarate |
| FP | fluticasone propionate |
| TIO | tiotropium bromide |
| sRAW | specific airway resistance |
| EIA | enzyme immunoassay |
| H&E | hematoxylin and eosin |
| SD | standard deviation |
| ROS | reactive oxygen species |
References
- Ware, J.H.; Thibodeau, L.A.; Speizer, F.E.; Colome, S.; Ferris, B.G., Jr. Assessment of the health effects of atmospheric sulfur oxides and particulate matter: Evidence from observational studies. Environ. Health Perspect. 1981, 41, 255–276. [Google Scholar] [CrossRef] [PubMed]
- Dockery, D.W.; Pope, C.A., 3rd; Xu, X.; Spengler, J.D.; Ware, J.H.; Fay, M.E.; Ferris, B.G., Jr.; Speizer, F.E. An association between air pollution and mortality in six U.S. Cities. N. Engl. J. Med. 1993, 329, 1753–1759. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Shima, M.; Yoda, Y.; Oka, K.; Kurosaka, F.; Shimizu, S.; Takahashi, H.; Nakatani, Y.; Nishikawa, J.; Fujiwara, K.; et al. Association between PM2.5 and primary care visits due to asthma attack in Japan: Relation to Beijing’s air pollution episode in January 2013. Environ. Health Prev. Med. 2014, 19, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Yamasaki, A.; Burioka, N.; Kurai, J.; Yoneda, K.; Yoshida, A.; Igishi, T.; Fukuoka, Y.; Nakamoto, M.; Takeuchi, H.; et al. Correlation between asian dust storms and worsening asthma in western Japan. Allergol. Int. 2011, 60, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kurai, J.; Igishi, T.; Yamasaki, A.; Burioka, N.; Takeuchi, H.; Sako, T.; Touge, H.; Nakamoto, M.; Hasegawa, Y.; et al. Influence of asian desert dust on lower respiratory tract symptoms in patients with asthma over 4 years. Yonago Acta Med. 2012, 55, 41–48. [Google Scholar] [PubMed]
- Pope, C.A., 3rd; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Mustafic, H.; Jabre, P.; Caussin, C.; Murad, M.H.; Escolano, S.; Tafflet, M.; Perier, M.C.; Marijon, E.; Vernerey, D.; Empana, J.P.; et al. Main air pollutants and myocardial infarction: A systematic review and meta-analysis. JAMA 2012, 307, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Williams, G.; Jalaludin, B.; Baker, P. Panel studies of air pollution on children’s lung function and respiratory symptoms: A literature review. J. Asthma Off. J. Assoc. Care Asthma 2012, 49, 895–910. [Google Scholar] [CrossRef] [PubMed]
- McCreanor, J.; Cullinan, P.; Nieuwenhuijsen, M.J.; Stewart-Evans, J.; Malliarou, E.; Jarup, L.; Harrington, R.; Svartengren, M.; Han, I.K.; Ohman-Strickland, P.; et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N. Engl. J. Med. 2007, 357, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Y.; Ding, H.; Jiang, L.N.; Chen, S.W.; Zheng, J.P.; Qiu, M.; Zhou, Y.X.; Chen, Q.; Guan, W.J. Association between air pollutants and asthma emergency room visits and hospital admissions in time series studies: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0138146. [Google Scholar] [CrossRef] [PubMed]
- Kurai, J.; Watanabe, M.; Tomita, K.; Yamasaki, H.S.; Shimizu, E. Influence of asian dust particles on immune adjuvant effects and airway inflammation in asthma model mice. PLoS ONE 2014, 9, e111831. [Google Scholar] [CrossRef] [PubMed]
- Ichinose, T.; Yoshida, S.; Sadakane, K.; Takano, H.; Yanagisawa, R.; Inoue, K.; Nishikawa, M.; Mori, I.; Kawazato, H.; Yasuda, A.; et al. Effects of Asian sand dust, arizona sand dust, amorphous silica and aluminum oxide on allergic inflammation in the murine lung. Inhal. Toxicol. 2008, 20, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Vargas, M.P.; Guzman-Grenfell, A.M.; Blanco-Jimenez, S.; Sepulveda-Sanchez, J.D.; Bernabe-Cabanillas, R.M.; Cardenas-Gonzalez, B.; Ceballos, G.; Hicks, J.J. Airborne particulate matter PM2.5 from mexico city affects the generation of reactive oxygen species by blood neutrophils from asthmatics: An in vitro approach. J. Occup. Med. Toxicol. 2009, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Frampton, M.W.; Utell, M.J.; Zareba, W.; Oberdorster, G.; Cox, C.; Huang, L.S.; Morrow, P.E.; Lee, F.E.; Chalupa, D.; Frasier, L.M.; et al. Effects of exposure to ultrafine carbon particles in healthy subjects and subjects with asthma. Res. Rep. Health Eff. Inst. 2004, 126, 1–47; discussion 49–63. [Google Scholar]
- Kim, Y.S.; Choi, E.J.; Lee, W.H.; Choi, S.J.; Roh, T.Y.; Park, J.; Jee, Y.K.; Zhu, Z.; Koh, Y.Y.; Gho, Y.S.; et al. Extracellular vesicles, especially derived from gram-negative bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both th1 and th17 cell responses. Clin. Exp. Allergy 2013, 43, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Kay, A.B. The role of eosinophils in the pathogenesis of asthma. Trends Mol. Med. 2005, 11, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Drews, A.C.; Pizzichini, M.M.; Pizzichini, E.; Pereira, M.U.; Pitrez, P.M.; Jones, M.H.; Sly, P.D.; Stein, R.T. Neutrophilic airway inflammation is a main feature of induced sputum in nonatopic asthmatic children. Allergy 2009, 64, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Bosmann, M.; Grailer, J.J.; Zhu, K.; Matthay, M.A.; Sarma, J.V.; Zetoune, F.S.; Ward, P.A. Anti-inflammatory effects of beta2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2012, 26, 2137–2144. [Google Scholar]
- Bos, I.S.; Gosens, R.; Zuidhof, A.B.; Schaafsma, D.; Halayko, A.J.; Meurs, H.; Zaagsma, J. Inhibition of allergen-induced airway remodelling by tiotropium and budesonide: A comparison. Eur. Respir. J. 2007, 30, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Gosens, R.; Bos, I.S.; Zaagsma, J.; Meurs, H. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. Am. J. Respir. Crit. Care Med. 2005, 171, 1096–1102. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Oda, N.; Yokoe, T.; Tanaka, A.; Yamamoto, Y.; Watanabe, Y.; Minoguchi, K.; Ohnishi, T.; Hirose, T.; Nagase, H.; et al. Effect of tiotropium bromide on airway inflammation and remodelling in a mouse model of asthma. Clin. Exp. Allergy 2010, 40, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Kimura, G.; Ueda, K.; Eto, S.; Watanabe, Y.; Masuko, T.; Kusama, T.; Barnes, P.J.; Ito, K.; Kizawa, Y. Toll-like receptor 3 stimulation causes corticosteroid-refractory airway neutrophilia and hyperresponsiveness in mice. Chest 2013, 144, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Hatchwell, L.; Girkin, J.; Dun, M.D.; Morten, M.; Verrills, N.; Toop, H.D.; Morris, J.C.; Johnston, S.L.; Foster, P.S.; Collison, A.; et al. Salmeterol attenuates chemotactic responses in rhinovirus-induced exacerbation of allergic airways disease by modulating protein phosphatase 2a. J. Allergy Clin. Immunol. 2014, 133, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Glaab, T.; Taube, C.; Braun, A.; Mitzner, W. Invasive and noninvasive methods for studying pulmonary function in mice. Respir. Res. 2007, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Cornelli, U.; Terranova, R.; Luca, S.; Cornelli, M.; Alberti, A. Bioavailability and antioxidant activity of some food supplements in men and women using the d-roms test as a marker of oxidative stress. J. Nutr. 2001, 131, 3208–3211. [Google Scholar] [CrossRef] [PubMed]
- Disse, B.; Speck, G.A.; Rominger, K.L.; Witek, T.J., Jr.; Hammer, R. Tiotropium (spiriva): Mechanistical considerations and clinical profile in obstructive lung disease. Life Sci. 1999, 64, 457–464. [Google Scholar] [CrossRef]
- Cui, Y.; Devillier, P.; Kuang, X.; Wang, H.; Zhu, L.; Xu, Z.; Xia, Z.; Zemoura, L.; Advenier, C.; Chen, H. Tiotropium reduction of lung inflammation in a model of chronic gastro-oesophageal reflux. Eur. Respir. J. 2010, 35, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Kondo, M.; Izumo, T.; Tamaoki, J.; Nagai, A. Inhibition of neutrophil elastase-induced goblet cell metaplasia by tiotropium in mice. Eur. Respir. J. 2010, 35, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Wollin, L.; Pieper, M.P. Tiotropium bromide exerts anti-inflammatory activity in a cigarette smoke mouse model of copd. Pulm. Pharmacol. Ther. 2010, 23, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Poon, R.; Chen, L.; Frescura, A.M.; Montuschi, P.; Ciabattoni, G.; Wheeler, A.; Dales, R. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ. Health Perspect. 2009, 117, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Bellido-Casado, J.; Plaza, V.; Perpina, M.; Picado, C.; Bardagi, S.; Martinez-Bru, C.; Torrejon, M. (inflammatory response of rapid onset asthma exacerbation). Arch. Bronconeumol. 2010, 46, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Wittkopp, S.; Staimer, N.; Tjoa, T.; Gillen, D.; Daher, N.; Shafer, M.; Schauer, J.J.; Sioutas, C.; Delfino, R.J. Mitochondrial genetic background modifies the relationship between traffic-related air pollution exposure and systemic biomarkers of inflammation. PLoS ONE 2013, 8, e64444. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Baines, K.J.; Fu, J.; Scott, H.A.; Gibson, P.G. The neutrophilic inflammatory phenotype is associated with systemic inflammation in asthma. Chest 2012, 142, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Pepe, C.; Foley, S.; Shannon, J.; Lemiere, C.; Olivenstein, R.; Ernst, P.; Ludwig, M.S.; Martin, J.G.; Hamid, Q. Differences in airway remodeling between subjects with severe and moderate asthma. J. Allergy Clin. Immunol. 2005, 116, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Shannon, J.; Ernst, P.; Yamauchi, Y.; Olivenstein, R.; Lemiere, C.; Foley, S.; Cicora, L.; Ludwig, M.; Hamid, Q.; Martin, J.G. Differences in airway cytokine profile in severe asthma compared to moderate asthma. Chest 2008, 133, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Todokoro, M.; Mochizuki, H.; Tokuyama, K.; Utsugi, M.; Dobashi, K.; Mori, M.; Morikawa, A. Effect of ozone exposure on intracellular glutathione redox state in cultured human airway epithelial cells. Inflammation 2004, 28, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Peden, D.B.; Boehlecke, B.; Horstman, D.; Devlin, R. Prolonged acute exposure to 0.16 ppm ozone induces eosinophilic airway inflammation in asthmatic subjects with allergies. J. Allergy Clin. Immunol. 1997, 100, 802–808. [Google Scholar] [CrossRef]
- Zou, Y.; Jin, C.; Su, Y.; Li, J.; Zhu, B. Water soluble and insoluble components of urban PM2.5 and their cytotoxic effects on epithelial cells (a549) in vitro. Environ. Pollut. 2016, 212, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Nam, M.H.; Lee, H.R.; Hong, C.O.; Lee, K.W. Protective effects of chebulic acid on alveolar epithelial damage induced by urban particulate matter. BMC Complement. Altern. Med. 2017, 17, 373. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Tian, D.; He, J.; Wang, Y.; Zhang, L.; Cui, L.; Jia, L.; Zhang, L.; Li, L.; Shu, Y.; et al. Repeated PM2.5 exposure inhibits beas-2b cell p53 expression through ros-akt-dnmt3b pathway-mediated promoter hypermethylation. Oncotarget 2016, 7, 20691–20703. [Google Scholar] [CrossRef] [PubMed]
- Vacca, G.; Randerath, W.J.; Gillissen, A. Inhibition of granulocyte migration by tiotropium bromide. Respir. Res. 2011, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Maris, N.A.; de Vos, A.F.; Dessing, M.C.; Spek, C.A.; Lutter, R.; Jansen, H.M.; van der Zee, J.S.; Bresser, P.; van der Poll, T. Antiinflammatory effects of salmeterol after inhalation of lipopolysaccharide by healthy volunteers. Am. J. Respir. Crit. Care Med. 2005, 172, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N. Alveolar macrophage in the driver’s seat. Immunity 2006, 24, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Kurai, J.; Watanabe, M.; Sano, H.; Hantan, D.; Shimizu, E. The effect of seasonal variations in airborne particulate matter on asthma-related airway inflammation in mice. Int. J. Environ. Res. Public Health 2016, 13. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; Ebisu, K.; Peng, R.D.; Walker, J.; Samet, J.M.; Zeger, S.L.; Dominici, F. Seasonal and regional short-term effects of fine particles on hospital admissions in 202 US counties, 1999–2005. Am. J. Epidemiol. 2008, 168, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Dominici, F.; McDermott, A.; Zeger, S.L.; Samet, J.M. National maps of the effects of particulate matter on mortality: Exploring geographical variation. Environ. Health Perspect. 2003, 111, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Franklin, M.; Zeka, A.; Schwartz, J. Association between PM2.5 and all-cause and specific-cause mortality in 27 US communities. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hui, Y.; Zhao, L.; Hao, Y.; Guo, H.; Ren, F. Oral administration of lactobacillus paracasei l9 attenuates PM2.5-induced enhancement of airway hyperresponsiveness and allergic airway response in murine model of asthma. PLoS ONE 2017, 12, e0171721. [Google Scholar] [CrossRef] [PubMed]
- Penton, P.C.; Wang, X.; Amatullah, H.; Cooper, J.; Godri, K.; North, M.L.; Khanna, N.; Scott, J.A.; Chow, C.W. Spleen tyrosine kinase inhibition attenuates airway hyperresponsiveness and pollution-induced enhanced airway response in a chronic mouse model of asthma. J. Allergy Clin. Immunol. 2013, 131, 512-20.e1-10. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Moreno-Vinasco, L.; Huang, Y.; Lang, G.D.; Linares, J.D.; Goonewardena, S.N.; Grabavoy, A.; Samet, J.M.; Geyh, A.S.; Breysse, P.N.; et al. Murine lung responses to ambient particulate matter: Genomic analysis and influence on airway hyperresponsiveness. Environ. Health Perspect. 2008, 116, 1500–1508. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurai, J.; Watanabe, M.; Sano, H.; Iwata, K.; Hantan, D.; Shimizu, E. A Muscarinic Antagonist Reduces Airway Inflammation and Bronchoconstriction Induced by Ambient Particulate Matter in a Mouse Model of Asthma. Int. J. Environ. Res. Public Health 2018, 15, 1189. https://doi.org/10.3390/ijerph15061189
Kurai J, Watanabe M, Sano H, Iwata K, Hantan D, Shimizu E. A Muscarinic Antagonist Reduces Airway Inflammation and Bronchoconstriction Induced by Ambient Particulate Matter in a Mouse Model of Asthma. International Journal of Environmental Research and Public Health. 2018; 15(6):1189. https://doi.org/10.3390/ijerph15061189
Chicago/Turabian StyleKurai, Jun, Masanari Watanabe, Hiroyuki Sano, Kyoko Iwata, Degejirihu Hantan, and Eiji Shimizu. 2018. "A Muscarinic Antagonist Reduces Airway Inflammation and Bronchoconstriction Induced by Ambient Particulate Matter in a Mouse Model of Asthma" International Journal of Environmental Research and Public Health 15, no. 6: 1189. https://doi.org/10.3390/ijerph15061189
APA StyleKurai, J., Watanabe, M., Sano, H., Iwata, K., Hantan, D., & Shimizu, E. (2018). A Muscarinic Antagonist Reduces Airway Inflammation and Bronchoconstriction Induced by Ambient Particulate Matter in a Mouse Model of Asthma. International Journal of Environmental Research and Public Health, 15(6), 1189. https://doi.org/10.3390/ijerph15061189



