Stress Response and Cognitive Performance Modulation in Classroom versus Natural Environments: A Quasi-Experimental Pilot Study with Children
Abstract
1. Introduction
1.1. Natural Learning Environments to Reduce Early-Life Stress
1.2. The Present Study
1.2.1. Situational Autonomic Indexes of Stress
1.2.2. Neurobiological Connections between Stress and Attention
1.2.3. Aims of the Present Study
2. Materials and Methods
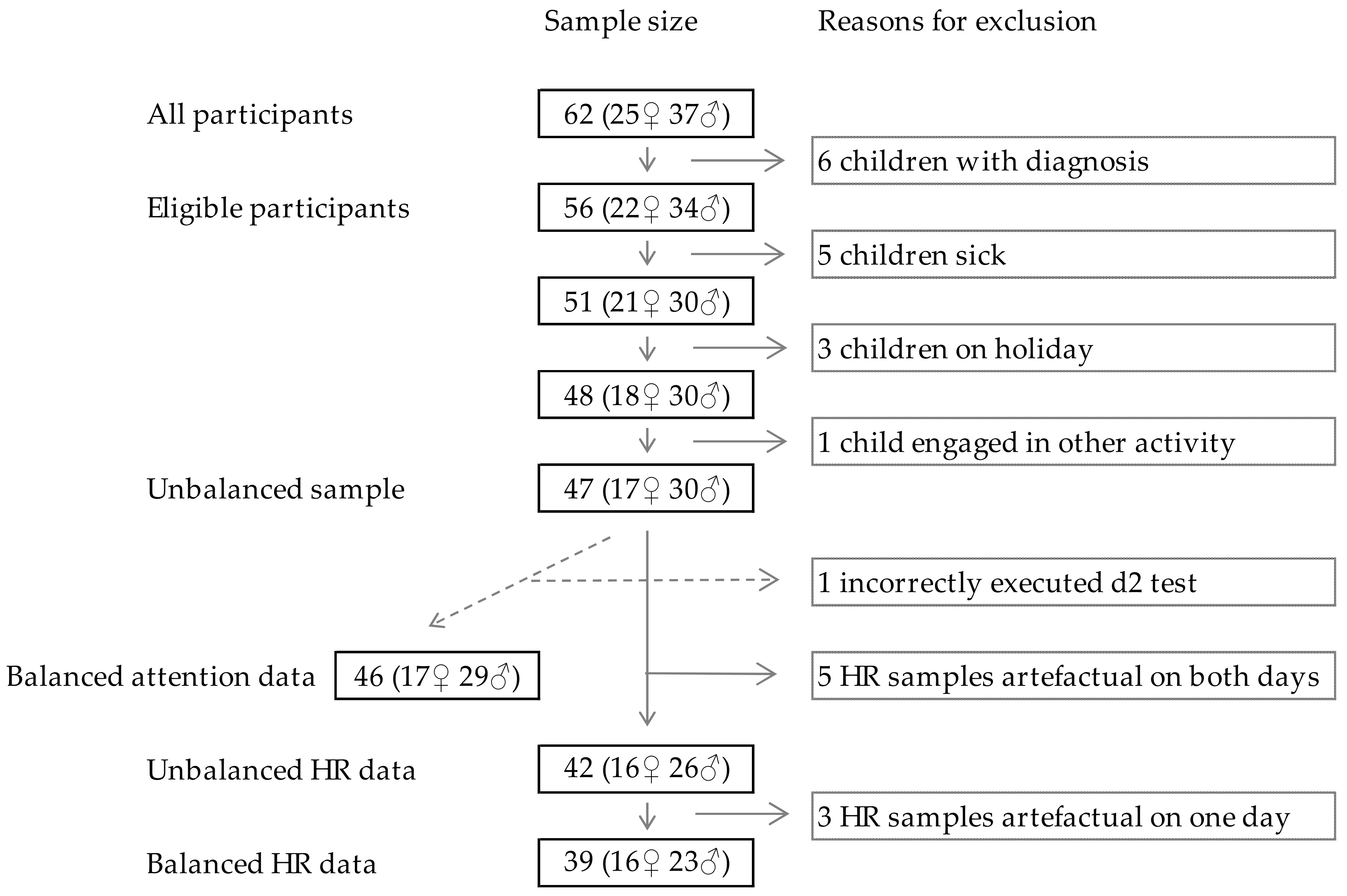
2.1. Sampling and Population
2.2. Learning Environments
2.3. Measures and Instruments
2.3.1. Stress Response
2.3.2. Cognitive Performance
2.4. Procedure
2.5. Statistical Analysis
3. Results
3.1. Observed Distributions on Primary Measures
3.2. Stress Response in the Natural and Classroom Environments
3.3. Cognitive Performance in the Natural and Classroom Environments
4. Discussion
Strengths, Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Report on Urban Health-Equitable, Healthier Cities for Sustainable Development; World Health Organization: Geneva, Switzerland, 2016; p. 241. [Google Scholar]
- Tost, H.; Champagne, F.A.; Meyer-Lindenberg, A. Environmental influence in the brain, human welfare and mental health. Nat. Neurosci. 2015, 18, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Lederbogen, F.; Kirsch, P.; Haddad, L.; Streit, F.; Tost, H.; Schuch, P.; Wüst, S.; Pruessner, J.C.; Rietschel, M.; Deuschle, M.; et al. City living and urban upbringing affect neural social stress processing in humans. Nature 2011, 474, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Düzel, S.; Eibich, P.; Krekel, C.; Wüstemann, H.; Kolbe, J.; Martensson, J.; Goebel, J.; Gallinat, J.; Wagner, G.G.; et al. In search of features that constitute an “enriched environment” in Humans: Associations between geographical properties and brain structure. Sci. Rep. 2017, 7, 11920. [Google Scholar] [CrossRef] [PubMed]
- Peen, J.; Dekker, J.; Schoevers, R.A.; Have, M.T.; de Graaf, R.; Beekman, A.T. Is the prevalence of psychiatric disorders associated with urbanization? Soc. Psychiatry Psychiatr. Epidemiol. 2007, 42, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Van Os, J.; Kenis, G.; Rutten, B.P.F. The environment and schizophrenia. Nature 2010, 468, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Haddad, L.; Schäfer, A.; Streit, F.; Lederbogen, F.; Grimm, O.; Wüst, S.; Deuschle, M.; Kirsch, P.; Tost, H.; Meyer-Lindenberg, A. Brain Structure Correlates of Urban Upbringing, an Environmental Risk Factor for Schizophrenia. Schizophr. Bull. 2015, 41, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Lupien, S.J.; McEwen, B.S.; Gunnar, M.R.; Heim, C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009, 10, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.E. Mechanisms linking early life stress to adult health outcomes. PNAS 2010, 107, 8507–8512. [Google Scholar] [CrossRef] [PubMed]
- Shonkoff, J.P.; Boyce, W.T.; McEwen, B.S. Neuroscience, Molecular Biology, and the Childhood Roots of Health Disparities: Building a New Framework for Health Promotion and Disease Prevention. JAMA 2009, 301, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Konijnendijk, C.C.; Annerstedt, M.; Nielsen, A.B.; Maruthaveeran, S. Benefits of Urban Parks: A Systematic Review; A Report for IPFRA; International Federation of Park and Recreation Administration (IFPRA): Copenhagen, Denmark; Alnarp, Sweden, 2013. [Google Scholar]
- Cassarino, M.; Setti, A. Environment as ‘Brain Training’: A review of geographical and physical environmental influences on cognitive ageing. Ageing Res. Rev. 2015, 23, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Nakamura, K.; Watanabe, M. Urban residential environments and senior citizens’ longevity in megacity Areas: The importance of walkable green spaces. J. Epidemiol. Community Health 2002, 56, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.; Popham, F. Effect of exposure to natural environment on health Inequalities: An observational population study. Lancet 2008, 372, 1655–1660. [Google Scholar] [CrossRef]
- Alcock, I.; White, M.P.; Wheeler, B.W.; Fleming, L.E.; Depledge, M.H. Longitudinal Effects on Mental Health of Moving to Greener and Less Green Urban Areas. Environ. Sci. Technol. 2014, 48, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Roe, J.J.; Thompson, C.W.; Aspinall, P.A.; Brewer, M.J.; Duff, E.I.; Miller, D.; Mitchell, R.; Clow, A. Green Space and Stress: Evidence from Cortisol Measures in Deprived Urban Communities. Int. J. Environ. Res. Public Health 2013, 10, 4086–4103. [Google Scholar] [CrossRef] [PubMed]
- Haluza, D.; Schönbauer, R.; Cervinka, R. Green Perspectives for Public Health: A Narrative Review on the Physiological Effects of Experiencing Outdoor Nature. Int. J. Environ. Res. Public Health 2014, 11, 5445–5461. [Google Scholar] [CrossRef] [PubMed]
- Berto, R.; Pasini, M.; Barbiero, G. How does Psychological Restoration Work in Children? An Exploratory Study. J. Child Adolesc. Behav. 2015, 2015. [Google Scholar] [CrossRef]
- Faber Taylor, A.; Kuo, F.E. Is contact with nature important for healthy child development? State of the evidence. In Children and Their Environments; Spencer, C., Blades, M., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 124–140. [Google Scholar]
- Schutte, A.R.; Torquati, J.C.; Beattie, H.L. Impact of Urban Nature on Executive Functioning in Early and Middle Childhood. Environ. Behav. 2017, 49, 3–30. [Google Scholar] [CrossRef]
- Clements, R. An investigation of the status of outdoor play. Contemp. Issues Early Child. 2004, 5, 68–80. [Google Scholar] [CrossRef]
- Natural England Childhood and Nature: A Survey on Changing Relationships with Nature across Generations. England Marketing: Cambridgeshire, 2009. Available online: http://publications.naturalengland.org.uk/publication/5853658314964992 (accessed on 16 June 2017).
- Strife, S.; Downey, L. Childhood Development and Access to Nature. Organ. Environ. 2009, 22, 99–122. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Organization School Policy Framework Implementation of the WHO Global Strategy on Diet, Physical Activity and Health; World Health Organization: Geneva, Switzerland, 2008; ISBN 978-92-4-159686-2. [Google Scholar]
- Dettweiler, U.; Becker, C.; Auestad, B.H.; Simon, P.; Kirsch, P. Stress in School. Some Empirical Hints on the Circadian Cortisol Rhythm of Children in Outdoor and Indoor Classes. Int. J. Environ. Res. Public Health 2017, 14, 475. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. The Polyvagal Theory: Neurophysiological Foundations of Emotions, Attachment, Communication, and SELF-Regulation, 1st ed.; W. W. Norton & Company: New York, NY, USA; London, UK, 2011. [Google Scholar]
- Thayer, J.F.; Hansen, A.L.; Saus-Rose, E.; Johnsen, B.H. Heart Rate Variability, Prefrontal Neural Function, and Cognitive Performance: The Neurovisceral Integration Perspective on Self-regulation, Adaptation, and Health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research–Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Guastella, A.J.; Outhred, T.; Hickie, I.B.; Kemp, A.H. Heart rate variability is associated with emotion recognition: Direct evidence for a relationship between the autonomic nervous system and social cognition. Int. J. Psychophysiol. 2012, 86, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.A.; Quintana, D.S.; Abbott, M.J.-A.; Kemp, A.H. Anxiety Disorders are Associated with Reduced Heart Rate Variability: A Meta-Analysis. Front. Psychiatry 2014, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- Kemp, K.; Kemp, A.H. Impact of depression and antidepressant treatment on heart rate Variability: A review and meta-analysis. Biol. Psychiatry 2010, 67, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; McGregor, I.S.; Guastella, A.J.; Malhi, G.S.; Kemp, A.H. A Meta-Analysis on the Impact of Alcohol Dependence on Short-Term Resting-State Heart Rate Variability: Implications for Cardiovascular Risk. Alcohol. Clin. Exp. Res. 2013, 37, E23–E29. [Google Scholar] [CrossRef] [PubMed]
- Umetani, K.; Singer, D.H.; McCraty, R.; Atkinson, M. Twenty-four hour time domain heart rate variability and heart Rate: Relations to age and gender over nine decades. J. Am. Coll. Cardiol. 1998, 31, 593–601. [Google Scholar] [CrossRef]
- Silvetti, M.S.; Drago, F.; Ragonese, P. Heart rate variability in healthy children and adolescents is partially related to age and gender. Int. J. Cardiol. 2001, 81, 169–174. [Google Scholar] [CrossRef]
- Kazuma, N.; Otsuka, K.; Wakamatsu, K.; Shirase, E.; Matsuoka, I. Heart rate variability in normotensive healthy children with aging. Clin. Exp. Hypertens. 2002, 24, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Association for Supervision and Curriculum Development (ASCD). Statement for the Integration of Health and Education; ASCD: Alexandria, VA, USA, 2015. [Google Scholar]
- Hamre, B.K.; Pianta, R.C. Can instructional and emotional support in the first-grade classroom make a difference for children at risk of school failure? Child Dev. 2005, 76, 949–967. [Google Scholar] [CrossRef] [PubMed]
- Porges, S.W. The polyvagal perspective. Biol. Psychol. 2007, 74, 116–143. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F.T. Stress weakens prefrontal Networks: Molecular insults to higher cognition. Nat. Neurosci. 2015, 18, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Kane, M.J.; Engle, R.W. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid Intelligence: An individual-differences perspective. Psychon. Bull. Rev. 2002, 9, 637–671. [Google Scholar] [CrossRef] [PubMed]
- Posner, M.I.; Rothbart, M.K. Research on attention networks as a model for the integration of psychological science. Annu. Rev. Psychol. 2007, 58, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Duschek, S.; Muckenthaler, M.; Werner, N.; Reyes del Paso, G.A. Relationships between features of autonomic cardiovascular control and cognitive performance. Biol. Psychol. 2009, 81, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, J.; Richman, R. Heart rate variability during a continuous performance test in children with problems of attention. Isr. J. Psychiatry Relat. Sci. 2011, 48, 19–24. [Google Scholar] [PubMed]
- Hansen, A.L.; Johnsen, B.H.; Thayer, J.F. Vagal influence on working memory and attention. Int. J. Psychophysiol. 2003, 48, 263–274. [Google Scholar] [CrossRef]
- Suess, P.E.; Porges, S.W.; Plude, D.J. Cardiac vagal tone and sustained attention in school-age children. Psychophysiology 1994, 31, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Marcovitch, S.; Leigh, J.; Calkins, S.D.; Leerks, E.M.; O’Brien, M.; Blankson, A.N. Moderate vagal withdrawal in 3.5-year-old children is associated with optimal performance on executive function tasks. Dev. Psychobiol. 2010, 52, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Onwuegbuzie, A.J.; Johnson, R.B. The validity issue in mixed research. Res. Sch. 2006, 13, 48–63. [Google Scholar]
- Bentsen, P.; Jensen, F.S. The nature of Udeskole: Outdoor learning theory and practice in Danish schools. J. Adv. Edu. Outdoor Learn. 2012, 12, 199–219. [Google Scholar] [CrossRef]
- Bentsen, P.; Mygind, E.; Randrup, T.B. Towards an understanding of Udeskole: Education outside the classroom in a Danish context. Education 3–13 2009, 37, 29–44. [Google Scholar]
- Barfod, K.; Ejbye-Ernst, N.; Mygind, L. Bentsen, P. Increased provision of udeskole in Danish schools: An updated national population survey. Urban For. Urban Green. 2016, 20, 277–281. [Google Scholar] [CrossRef]
- Quintana, D.S.; Alvares, G.A.; Heathers, J.A.J. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): Recommendations to advance research communication. Transl. Psychiatry 2016, 6, e803. [Google Scholar] [CrossRef] [PubMed]
- Laukkanen, R.M.; Virtanen, P.K. Heart rate Monitors: State of the art. J. Sports Sci. 1998, 16, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Vanderlei, L.C.M.; Silva, R.A.; Pastre, C.M.; Azevedo, F.M.; Godoy, M.F. Comparison of the Polar S810i monitor and the ECG for the analysis of heart rate variability in the time and frequency domains. Braz. J. Med. Biol. Res. 2008, 41, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Weippert, M.; Kumar, M.; Kreuzfeld, S.; Arndt, D.; Rieger, A.; Stoll, R. Comparison of three mobile devices for measuring R–R intervals and heart rate variability: Polar S810i, Suunto t6 and an ambulatory ECG system. Eur. J. Appl. Physiol. 2010, 109, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Gamelin, F.X.; Berthoin, S.; Bosquet, L. Validity of the polar S810 heart rate monitor to measure RR intervals at rest. Med. Sci. Sports Exerc. 2006, 38, 887. [Google Scholar] [CrossRef] [PubMed]
- Noah, J.A.; Ono, Y.; Shimada, S.; Tachibana, A.; Bronner, S. Changes in Sympathetic Tone during Cooperative Game Play. Soc. Behav. Personal. Int. J. 2015, 43, 1123–1134. [Google Scholar] [CrossRef]
- Silva-Urra, J.A.; Núñez-Espinosa, C.A.; Niño-Mendez, O.A.; Gaitán-Peñas, H.; Altavilla, C.; Toro-Salinas, A.; Torrella, J.R.; Pagès, T.; Javierre, C.F.; Behn, C.; et al. Circadian and Sex Differences After Acute High-Altitude Exposure: Are Early Acclimation Responses Improved by Blue Light? Wilderness Environ. Med. 2015, 26, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-aho, P.O.; Karjalainen, P.A. Kubios HRV–Heart rate variability analysis software. Comput. Methods Prog. Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Michels, N.; Sioen, I.; Clays, E.; De, B.; Ahrens, W.; Huybrechts, I.; Vanaelst, B.; De, H. Children’s heart rate variability as stress indicator: Association with reported stress and cortisol. Biol. Psychol. 2013, 94, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Brickenkamp, R. Test d2: Aufmerksamkeits-Belastungs-Test Hogrefe; Testzentrale: Göttingen, Germany, 1994. [Google Scholar]
- Zillmer, E.A.; Kennedy, C.H. Construct validity for the D2 test of attention. Arch. Clin. Neuropsychol. 1999, 14, 728. [Google Scholar] [CrossRef]
- IBM Corporation. IBM SPSS Advanced Statistics 25; IBM: Armonk, NY, USA, 2017. [Google Scholar]
- Hardin, J.W.; Hilbe, J.M. Generalized Estimating Equations. In Wiley Encyclopedia of Clinical Trials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 978-0-471-46242-2. [Google Scholar]
- Bowler, D.E.; Buyung-Ali, L.M.; Knight, T.M.; Pullin, A.S. A systematic review of evidence for the added benefits to health of exposure to natural environments. BMC Public Health 2010, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.F.; Kuo, F.E. Children With Attention Deficits Concentrate Better after Walk in the Park. J. Attent. Disord. 2009, 12, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Faber Taylor, A.; Kuo, F.E.; Sullivan, W.C. Views of nature and self-discipline: Evidence from inner city children. J. Environ. Psychol. 2002, 22, 49–63. [Google Scholar] [CrossRef]
- Van den Berg, A.E.; van den Berg, C.G. A comparison of children with ADHD in a natural and built setting: Nature and ADHD. Child Care Health Dev. 2011, 37, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Ohly, H.; White, M.P.; Wheeler, B.W.; Bethel, A.; Ukoumunne, O.C.; Nikolaou, V.; Garside, R. Attention Restoration Theory: A systematic review of the attention restoration potential of exposure to natural environments. J. Toxicol. Environ. Health B Crit. Rev. 2016, 19, 305–343. [Google Scholar] [CrossRef] [PubMed]
- Studente, S.; Seppala, N.; Sadowska, N. Facilitating creative thinking in the classroom: Investigating the effects of plants and the colour green on visual and verbal creativity. Think. Skills Creat. 2016, 19, 1–8. [Google Scholar] [CrossRef]
- Lee, K.E.; Williams, K.J.H.; Sargent, L.D.; Williams, N.S.G.; Johnson, K.A. 40-second green roof views sustain attention: The role of micro-breaks in attention restoration. J. Environ. Psychol. 2015, 42, 182–189. [Google Scholar] [CrossRef]
- Van den Berg, A.E.; Koole, S.L.; van der Wulp, N.Y. Environmental preference and restoration: (How) are they related? J. Environ. Psychol. 2003, 23, 135–146. [Google Scholar] [CrossRef]
- Greenwood, A.; Gatersleben, B. Let’s go outside! Environmental restoration amongst adolescents and the impact of friends and phones. J. Environ. Psychol. 2016, 48, 131–139. [Google Scholar] [CrossRef]
- Korpela, K.M.; Ylén, M.; Tyrväinen, L.; Silvennoinen, H. Determinants of restorative experiences in everyday favorite places. Health Place 2008, 14, 636–652. [Google Scholar] [CrossRef] [PubMed]
- Gatersleben, B.; Andrews, M. When walking in nature is not restorative?The role of prospect and refuge. Health Place 2013, 20, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Von Lindern, E. Setting-dependent constraints on human restoration while visiting a wilderness park. J. Outdoor Recreat. Tour. 2015, 10, 29–37. [Google Scholar] [CrossRef]
- Southall, L. Using Realistic Evaluation to Evaluate ‘Forest School’with Young People Aged 14–16 with Special Educational Needs. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2014. [Google Scholar]
- Liu, W.; Lian, Z.; Liu, Y. Heart rate variability at different thermal comfort levels. Eur. J. Appl. Physiol. 2008, 103, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; O’Neill, M.S.; Park, S.K.; Sparrow, D.; Vokonas, P.; Schwartz, J. Ambient Temperature, Air Pollution, and Heart Rate Variability in an Aging Population. Am. J. Epidemiol. 2011, 173, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Deng, F.; Liu, Y.; Shima, M.; Niu, J.; Huang, Q.; Guo, X. Temperature, traffic-related air pollution, and heart rate variability in a panel of healthy adults. Environ. Res. 2013, 120, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Miyawaki, T.; Ue, H.; Kanda, T.; Zenji, C.; Moritani, T. Autonomic responsiveness to acute cold exposure in obese and non-obese young women. Int. J. Obes. 1999, 23, 793–800. [Google Scholar] [CrossRef]
- Lively, K.J. Comment on “Methodological Innovations From the Sociology of Emotions – Methodological Advances”. Emotion. Rev. 2015, 7, 181–182. [Google Scholar] [CrossRef]
- Bentsen, P.; Søndergaard Jensen, F.; Mygind, E.; Barfoed Randrup, T. The extent and dissemination of i udeskole in Danish schools. Urban For. Urban Green. 2010, 9, 235–243. [Google Scholar] [CrossRef]
- Christie, B. Outdoor Education Provision in Scottish Schools. Scott. Educ. Rev. 2014, 46, 48–64. [Google Scholar]
- Dillon, J.; Rickinson, M.; Teamey, K.; Morris, M.; Choi, M.Y.; Sanders, D.; Benefield, P. The value of outdoor Learning: Evidence from research in the UK and elsewhere. Sch. Sci. Rev. 2006, 87, 107. [Google Scholar]
- Fiennes, C.; Oliver, E.; Dickson, K.; Escobar, D.; Romans, A.; Oliver, S. The Existing Evidence-Base about the Effectiveness of Outdoor Learning; Institute of Outdoor Learnong: London, UK, 2015. [Google Scholar]
- O’Brien, L. Learning Outdoors: The Forest School approach. Education 3–13 2009, 37, 45–60. [Google Scholar] [CrossRef]
- O’Brien, L.; Murray, R. Forest School and its impacts on young children: Case studies in Britain. Urban For. Urban Green. 2007, 6, 249–265. [Google Scholar] [CrossRef]
| Natural | Classroom | ||||
|---|---|---|---|---|---|
| Male | Female | Male | Female | n | |
| Tonic vagal tone | 100.74 (58.34–128.16) | 57.45 (39.22–100.4) | 83.15 (61.86–117.71) | 53.94 (31.55–65.93) | 41 |
| Event vagal tone | 52.55 (28.56–76.98) | 28.46 (19.45–37.34) | 42.95 (29.73–60.37) | 29.7 (23.87–38.54) | 41 |
| Phasic vagal tone | 54.92 (42.34–63.0) | 52.28 (39.55–65.18) | 49.92 (46.53–71.54) | 57.6 (47.69–82.49) | 39 |
| TN-E | 373.69 (82.53) | 393.24 (99.42) | 343.27 (67.28) | 354.78 (77.98) | 46 |
| TN | 388.45 (87.27) | 405.0 (101.91) | 357.43 (71.89) | 365.11 (83.83) | 46 |
| E | 14.76 (13.43) | 11.76 (11.23) | 14.17 (11.86) | 10.33 (9.65) | 46 |
| N | C | ||||||
|---|---|---|---|---|---|---|---|
| β | SE | 95% CI | EMM | n | p | ||
| Tonic vagal tone | 1.13 | 1.06 | 1.01–1.26 | 71.38 | 63.27 | 41 | 0.031 |
| Event vagal tone | 1.1 | 1.07 | 0.96–1.25 | 40.27 | 36.74 | 42 | 0.161 |
| Phasic vagal tone | −0.94 | 1.07 | −1.07–0.82 | 53.28 | 56.66 | 40 | 0.366 |
| TN-E | −1.74 | 5.29 | −12.11–8.63 | 361.66 | 363.4 | 48 | 0.691 |
| TN | −2.1 | 5.05 | −11.99–1.79 | 374.5 | 376.6 | 48 | 0.677 |
| E | −0.22 | 1.67 | −3.5-3.07 | 12.98 | 13.18 | 48 | 0.898 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mygind, L.; Stevenson, M.P.; Liebst, L.S.; Konvalinka, I.; Bentsen, P. Stress Response and Cognitive Performance Modulation in Classroom versus Natural Environments: A Quasi-Experimental Pilot Study with Children. Int. J. Environ. Res. Public Health 2018, 15, 1098. https://doi.org/10.3390/ijerph15061098
Mygind L, Stevenson MP, Liebst LS, Konvalinka I, Bentsen P. Stress Response and Cognitive Performance Modulation in Classroom versus Natural Environments: A Quasi-Experimental Pilot Study with Children. International Journal of Environmental Research and Public Health. 2018; 15(6):1098. https://doi.org/10.3390/ijerph15061098
Chicago/Turabian StyleMygind, Lærke, Matt P. Stevenson, Lasse S. Liebst, Ivana Konvalinka, and Peter Bentsen. 2018. "Stress Response and Cognitive Performance Modulation in Classroom versus Natural Environments: A Quasi-Experimental Pilot Study with Children" International Journal of Environmental Research and Public Health 15, no. 6: 1098. https://doi.org/10.3390/ijerph15061098
APA StyleMygind, L., Stevenson, M. P., Liebst, L. S., Konvalinka, I., & Bentsen, P. (2018). Stress Response and Cognitive Performance Modulation in Classroom versus Natural Environments: A Quasi-Experimental Pilot Study with Children. International Journal of Environmental Research and Public Health, 15(6), 1098. https://doi.org/10.3390/ijerph15061098





