Neighborhood Socioeconomic Deprivation and Allostatic Load: A Scoping Review
Abstract
1. Introduction
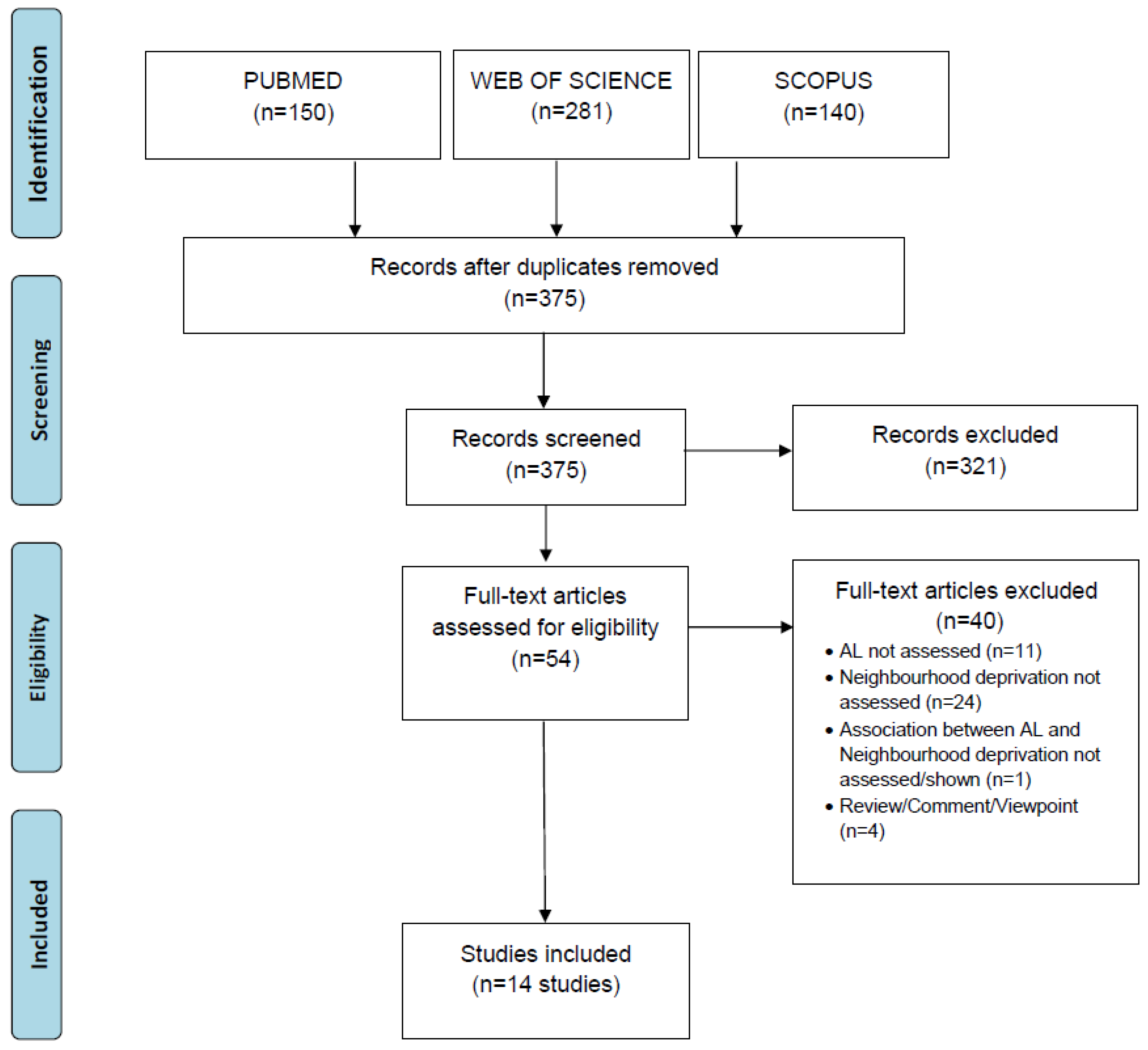
2. Materials and Methods
3. Results
3.1. General Characteristics
3.2. Measurement of Allostatic Load
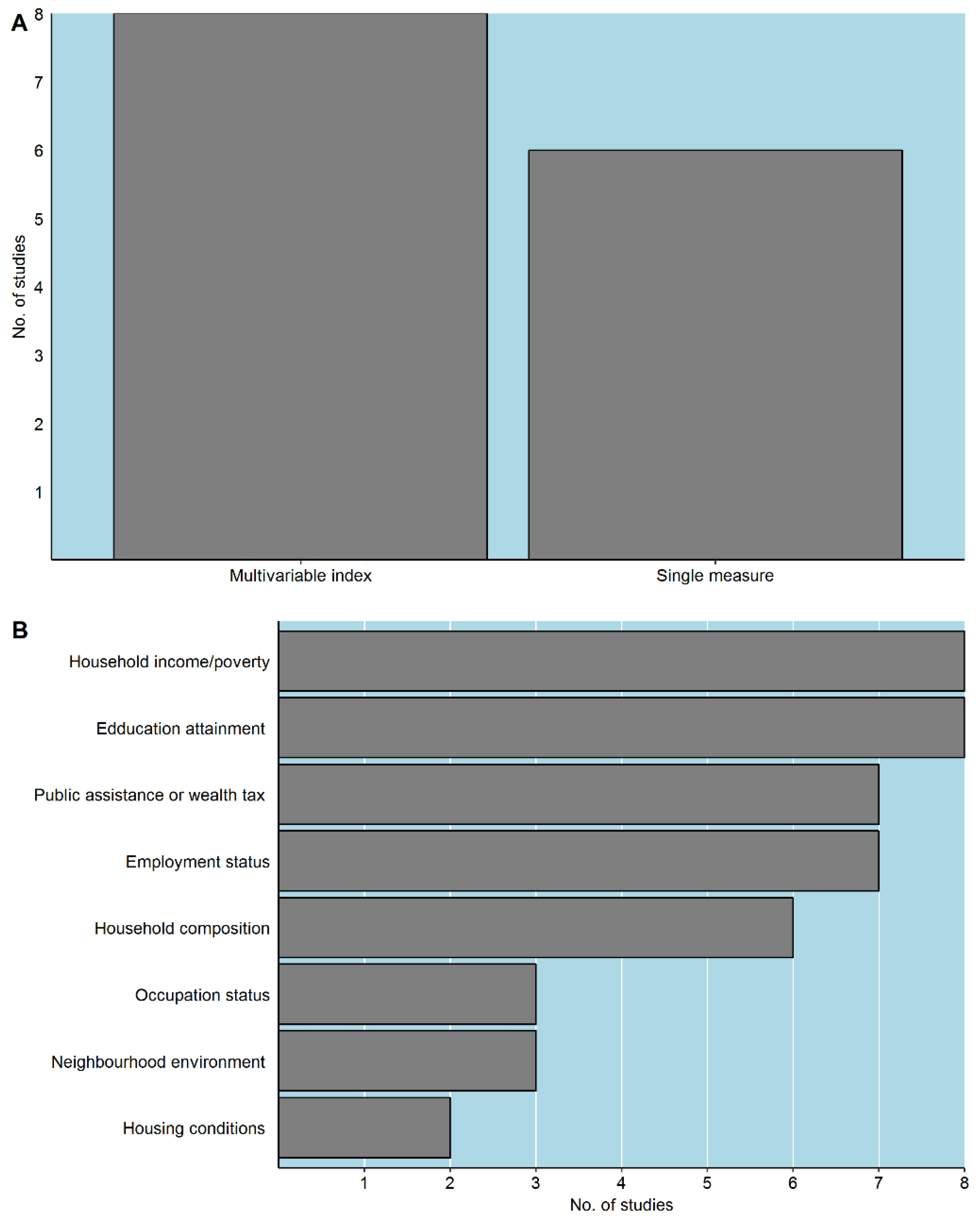
3.3. Definition of Neighbourhood and Measurement of Neighbourhood Socioeconomic Deprivation
3.4. Associations
3.5. Confounding and Robustness Checks
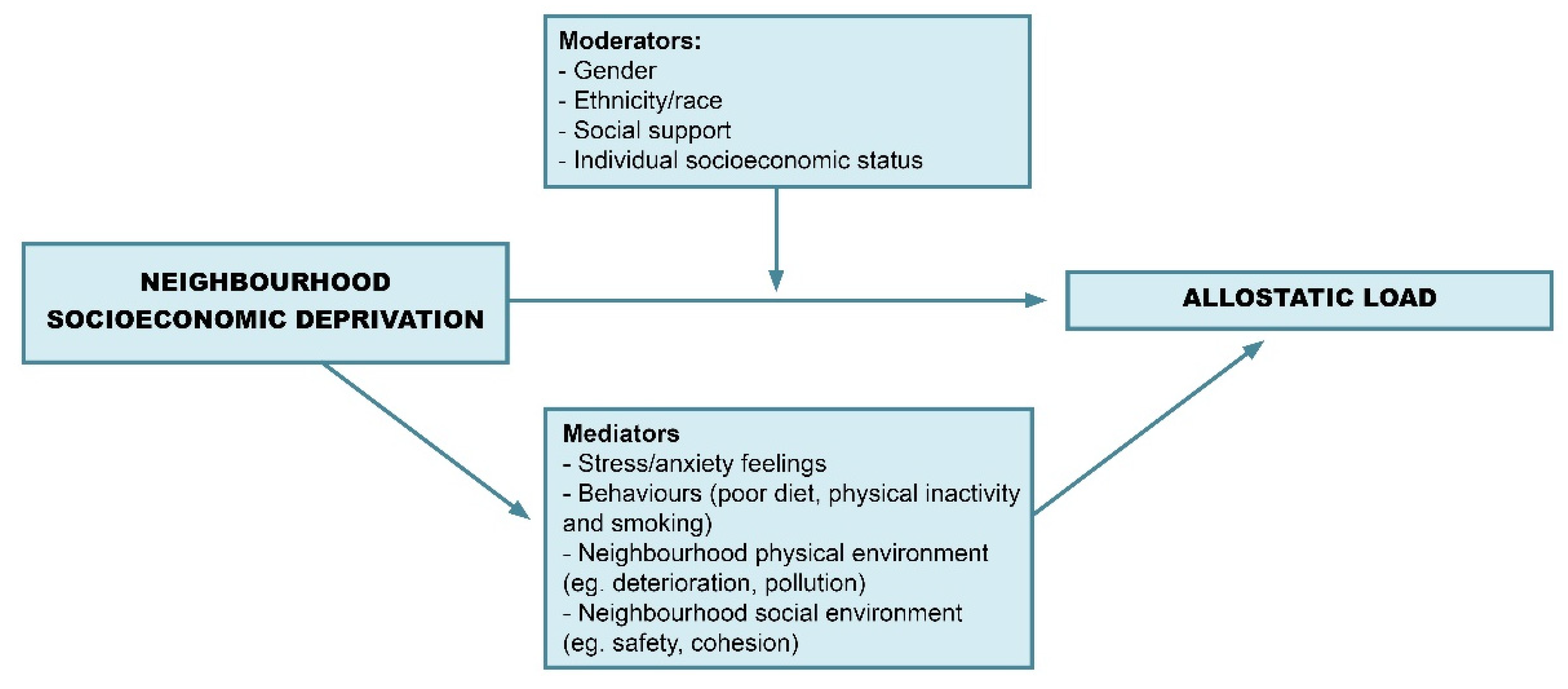
3.6. Moderators
3.7. Mediators
3.8. Longitudinal Studies
3.9. Framework for Future Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sundquist, K.; Malmström, M.; Johansson, S.E. Neighbourhood deprivation and incidence of coronary heart disease: A multilevel study of 2.6 million women and men in Sweden. J. Epidemiol. Community Health 2003, 58, 71–77. [Google Scholar] [CrossRef]
- Meijer, M.; Röhl, J.; Bloomfield, K.; Grittner, U. Do neighborhoods affect individual mortality? A systematic review and meta-analysis of multilevel studies. Soc. Sci. Med. 2012, 74, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Major, J.M.; Doubeni, C.A.; Freedman, N.D.; Park, Y.; Lian, M.; Hollenbeck, A.R.; Schatzkin, A.; Graubard, B.I.; Sinha, R. Neighborhood Socioeconomic Deprivation and Mortality: NIH-AARP Diet and Health Study. PLoS ONE 2010, 5, e15538. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.R.; Burgoine, T.; Monsivais, P. Area deprivation and the food environment over time: A repeated cross-sectional study on takeaway outlet density and supermarket presence in Norfolk, UK, 1990–2008. Health Place 2015, 33, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Hoffimann, E.; Barros, H.; Ribeiro, I.A. Socioeconomic Inequalities in Green Space Quality and Accessibility—Evidence from a Southern European City. Int. J. Environ. Res. Public Health 2017, 14, 916. [Google Scholar] [CrossRef] [PubMed]
- Padilla, C.M.; Kihal-Talantikite, W.; Vieira, V.M.; Rosselo, P.; LeNir, G.; Zmirou-Navier, D.; Deguen, S. Air quality and social deprivation in four French metropolitan areas—A localized spatiotemporal environmental inequality analysis. Environ. Res. 2014, 134, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Martuzzi, M.; Mitis, F.; Forastiere, F. Inequalities, inequities, environmental justice in waste management and health. Eur. J. Public Health 2010, 20, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, S.; Ellaway, A.; Cummins, S. Place effects on health: How can we conceptualise, operationalise and measure them? Soc. Sci. Med. 2002, 55, 125–139. [Google Scholar] [CrossRef]
- Kuipers, M.A.G.; van Poppel, M.N.M.; van den Brink, W.; Wingen, M.; Kunst, A.E. The association between neighborhood disorder, social cohesion and hazardous alcohol use: A national multilevel study. Drug Alcohol Depend. 2012, 126, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Slopen, N.; Dutra, L.M.; Williams, D.R.; Mujahid, M.S.; Lewis, T.T.; Bennett, G.G.; Ryff, C.D.; Albert, M.A. Psychosocial Stressors and Cigarette Smoking Among African American Adults in Midlife. Nicotine Tob. Res. 2012, 14, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Macintyre, S. Deprivation amplification revisited; or, is it always true that poorer places have poorer access to resources for healthy diets and physical activity? Int. J. Behav. Nutr. Phys. Act. 2007, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Kawachi, I.; Berkman, L. Neighborhoods and Health; Oxford University Press: New York, NY, USA, 2003. [Google Scholar]
- Daniel, M.; Moore, S.; Kestens, Y. Framing the biosocial pathways underlying associations between place and cardiometabolic disease. Health Place 2008, 14, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Nazmi, A.; Roux, A.D.; Ranjit, N.; Seeman, T.E.; Jenny, N.S. Cross-sectional and longitudinal associations of neighborhood characteristics with inflammatory markers: Findings from the Multi-Ethnic Study of Atherosclerosis. Health Place 2010, 16, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Barrington, W.E.; Stafford, M.; Hamer, M.; Beresford, S.A.A.; Koepsell, T.; Steptoe, A. Socioeconomic deprivation, perceived neighborhood factors, and cortisol responses to induced stress among healthy adults. Health Place 2014, 27, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.J.; Mentz, G.; Lachance, L.; Johnson, J.; Gaines, C.; Israel, B.A. Associations Between Socioeconomic Status and Allostatic Load: Effects of Neighborhood Poverty and Tests of Mediating Pathways. Am. J. Public Health 2012, 102, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Stellar, E. Stress and the individual: Mechanisms leading to disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.E.; Singer, B.H.; Rowe, J.W.; Horwitz, R.I.; McEwen, B.S. Price of adaptation—Allostatic load and its health consequences: Macarthur studies of successful aging. Arch. Intern. Med. 1997, 157, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Mauss, D.; Li, J.; Schmidt, B.; Angerer, P.; Jarczok, M.N. Measuring allostatic load in the workforce: A systematic review. Ind. Health 2015, 53, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Beckie, T.M. A Systematic Review of Allostatic Load, Health, and Health Disparities. Biol. Res. Nurs. 2012, 14, 311–346. [Google Scholar] [CrossRef] [PubMed]
- Barboza Solís, C.; Fantin, R.; Kelly-Irving, M.; Delpierre, C. Physiological wear-and-tear and later subjective health in mid-life: Findings from the 1958 British birth cohort. Psychoneuroendocrinology 2016, 74, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Seeman, T.; Epel, E.; Gruenewald, T.; Karlamangla, A.; McEwen, B.S. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Ann. N. Y. Acad. Sci. 2010, 1186, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339. [Google Scholar] [CrossRef] [PubMed]
- Merkin, S.S.; Basurto-Dávila, R.; Karlamangla, A.; Bird, C.; Lurie, N.; Escarce, J.; Seeman, T. Neighborhoods and Cumulative Biological Risk Profiles by Race/Ethnicity in a National Sample of U.S. Adults: NHANES III. Ann. Epidemiol. 2009, 19, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.E.; Seeman, T.; Escarce, J.J.; Basurto-Dávila, R.; Finch, B.K.; Dubowitz, T.; Heron, M.; Hale, L.; Merkin, S.S.; Weden, M.; et al. Neighbourhood Socioeconomic Status and Biological “Wear & Tear” in a Nationally Representative Sample of US Adults. J. Epidemiol. Community Health 2010, 64, 860–865. [Google Scholar] [PubMed]
- King, K.E.; Morenoff, J.D.; House, J.S. Neighborhood Context and Social Disparities in Cumulative Biological Risk Factors. Psychosom. Med. 2011, 73, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.J.; Mentz, G.; Lachance, L.; Zenk, S.N.; Johnson, J.; Stokes, C.; Mandell, R. Do observed or perceived characteristics of the neighborhood environment mediate associations between neighborhood poverty and cumulative biological risk? Health Place 2013, 24. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Harville, E.; Theall, K.; Webber, L.; Chen, W.; Berenson, G. Neighborhood Poverty, Allostatic Load, and Birth Outcomes in African American and White Women: Findings from the Bogalusa Heart Study. Health Place 2013, 24, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Brody, G.H.; Lei, M.-K.; Chen, E.; Miller, G.E. Neighborhood Poverty and Allostatic Load in African American Youth. Pediatrics 2014. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, P.E.; San Sebastian, M.; Janlert, U.; Theorell, T.; Westerlund, H.; Hammarström, A. Life-Course Accumulation of Neighborhood Disadvantage and Allostatic Load: Empirical Integration of Three Social Determinants of Health Frameworks. Am. J. Public Health 2014, 104, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Miller, G.E.; Brody, G.H.; Lei, M. Neighborhood Poverty, College Attendance, and Diverging Profiles of Substance Use and Allostatic Load in Rural African American Youth. Clin. Psychol. Sci. J. Assoc. Psychol. Sci. 2015, 3, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.P.; Osypuk, T.L.; Arevalo, S.; Tucker, K.L.; Falcon, L.M. Neighborhood Socioeconomic Context and Change in Allostatic Load Among Older Puerto Ricans: The Boston Puerto Rican Health Study. Health Place 2015, 33, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.; Hickson, D.A.; Kawachi, I.; Subramanian, S.V.; Earls, F. Neighborhood Disadvantage and Cumulative Biological Risk Among a Socioeconomically Diverse Sample of African American Adults: An Examination in the Jackson Heart Study. J. Racial Ethn. Health Disparities 2016, 3, 444–456. [Google Scholar] [CrossRef] [PubMed]
- Barber, S.; Hickson, D.A.; Kawachi, I.; Subramanian, S.V.; Earls, F. Double-jeopardy: The joint impact of neighborhood disadvantage and low social cohesion on cumulative risk of disease among African American men and women in the Jackson Heart Study. Soc. Sci. Med. 2016, 153, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Robinette, J.W.; Charles, S.T.; Almeida, D.M.; Gruenewald, T.L. Neighborhood Features and Physiological Risk: An Examination of Allostatic Load. Health Place 2016, 41, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Slopen, N.; Non, A.; Williams, D.R.; Roberts, A.L.; Albert, M.A. Childhood Adversity, Adult Neighborhood Context, and Cumulative Biological Risk for Chronic Diseases in Adulthood. Psychosom. Med. 2014, 76, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Porta, M. A Dictionary of Epidemiology, 5th ed.; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Goldman, N.; Turra, C.M.; Glei, D.A.; Seplaki, C.L.; Lin, Y.H.; Weinstein, M. Predicting mortality from clinical and nonclinical biomarkers. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Townsend, P. Deprivation. J. Soc. Policy 1987, 125–146. [Google Scholar] [CrossRef]
- Townsend, P. Poverty in the United Kingdom; Allen Lane and Penguin Books: London, UK, 1979. [Google Scholar]
- Ribeiro, A.I.; Mayer, A.; Miranda, A.; Pina, M.F. The Portuguese Version of the European Deprivation Index: An Instrument to Study Health Inequalities. Acta Méd. Port. 2017, 30, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Take the edge off: A hybrid geographic food access measure. Appl. Geogr. 2017, 87, 149–159. [Google Scholar] [CrossRef]
- Chen, X.; Kwan, M.-P. Contextual Uncertainties, Human Mobility, and Perceived Food Environment: The Uncertain Geographic Context Problem in Food Access Research. Am. J. Public Health 2015, 105, 1734–1737. [Google Scholar] [CrossRef] [PubMed]
- Kwan, M.-P. The Uncertain Geographic Context Problem. Ann. Assoc. Am. Geogr. 2012, 102, 958–968. [Google Scholar] [CrossRef]
- Smith, G.; Gidlow, C.; Davey, R.; Foster, C. What is my walking neighbourhood? A pilot study of English adults’ definitions of their local walking neighbourhoods. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 34. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Kwan, M.-P. Multi-Contextual Segregation and Environmental Justice Research: Toward Fine-Scale Spatiotemporal Approaches. Int. J. Environ. Res. Public Health 2017, 14, 1205. [Google Scholar] [CrossRef]
- Stafford, M.; Cummins, S.; Macintyre, S.; Ellaway, A.; Marmot, M. Gender differences in the associations between health and neighbourhood environment. Soc. Sci. Med. 2005, 60, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, A.M.; Bentley, R.; Turrell, G.; Broom, D.H.; Subramanian, S.V. Does gender modify associations between self rated health and the social and economic characteristics of local environments? J. Epidemiol. Community Health 2006, 60, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.S.; Trinh-Shevrin, C.; Yen, I.H.; Kwon, S.C. Racial/Ethnic Differences in Associations between Neighborhood Social Cohesion and Meeting Physical Activity Guidelines, United States, 2013–2014. Prev. Chronic Dis. 2016, 13, E165. [Google Scholar] [CrossRef] [PubMed]
- Pearl, M.; Braveman, P.; Abrams, B. The Relationship of Neighborhood Socioeconomic Characteristics to Birthweight among 5 Ethnic Groups in California. Am. J. Public Health 2001, 91, 1808–1814. [Google Scholar] [CrossRef] [PubMed]
- Dwan, K.; Gamble, C.; Williamson, P.R.; Kirkham, J.J.; For the Reporting Bias Group. Systematic Review of the Empirical Evidence of Study Publication Bias and Outcome Reporting Bias—An Updated Review. PLoS ONE 2013, 8, e66844. [Google Scholar] [CrossRef] [PubMed]

| Study | Design | Sample Size 1 | Neighborhoods | Association with Neighborhood Deprivation | Physiological System 2 | Moderators | Mediators |
|---|---|---|---|---|---|---|---|
| [36] | CS | 4408 | 100 census tracts | YES | M, N | ||
| [35] | CS | 4410 | 102 census tracts | YES | M, N | G, SS | |
| [37] | CS | 995 | 979 census tracts | YES | S, B | ||
| [33] | L | 452 | 91 census tracts | YES | SES | ||
| [34] | L | 1258 | 318 block groups | NO | |||
| [31] | L | 420 | 41 census tracts | YES | SS | ||
| [32] | L | 818 | 374 SAMS | YES | G | ||
| [38] | CS | 550 | 80 focal neighborhood clusters | YES | |||
| [29] | CS | 919 | 69 block groups | YES | E | ||
| [30] | CS | 866 | 55 block groups | NO | |||
| [16] | CS | 919 | 60 block groups | YES | E | ||
| [28] | CS | 549 | 80 focal neighborhood clusters | YES | |||
| [27] | CS | 13,184 | 1805 neighborhoods | YES | M, CV | ||
| [26] | CS | 13,199 | 1772 census tracts | YES | R |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.I.; Amaro, J.; Lisi, C.; Fraga, S. Neighborhood Socioeconomic Deprivation and Allostatic Load: A Scoping Review. Int. J. Environ. Res. Public Health 2018, 15, 1092. https://doi.org/10.3390/ijerph15061092
Ribeiro AI, Amaro J, Lisi C, Fraga S. Neighborhood Socioeconomic Deprivation and Allostatic Load: A Scoping Review. International Journal of Environmental Research and Public Health. 2018; 15(6):1092. https://doi.org/10.3390/ijerph15061092
Chicago/Turabian StyleRibeiro, Ana Isabel, Joana Amaro, Cosima Lisi, and Silvia Fraga. 2018. "Neighborhood Socioeconomic Deprivation and Allostatic Load: A Scoping Review" International Journal of Environmental Research and Public Health 15, no. 6: 1092. https://doi.org/10.3390/ijerph15061092
APA StyleRibeiro, A. I., Amaro, J., Lisi, C., & Fraga, S. (2018). Neighborhood Socioeconomic Deprivation and Allostatic Load: A Scoping Review. International Journal of Environmental Research and Public Health, 15(6), 1092. https://doi.org/10.3390/ijerph15061092





