Distribution of Arsenic and Risk Assessment of Activities on Soccer Pitches Irrigated with Arsenic-Contaminated Water
Abstract
1. Introduction
2. Materials and Methods
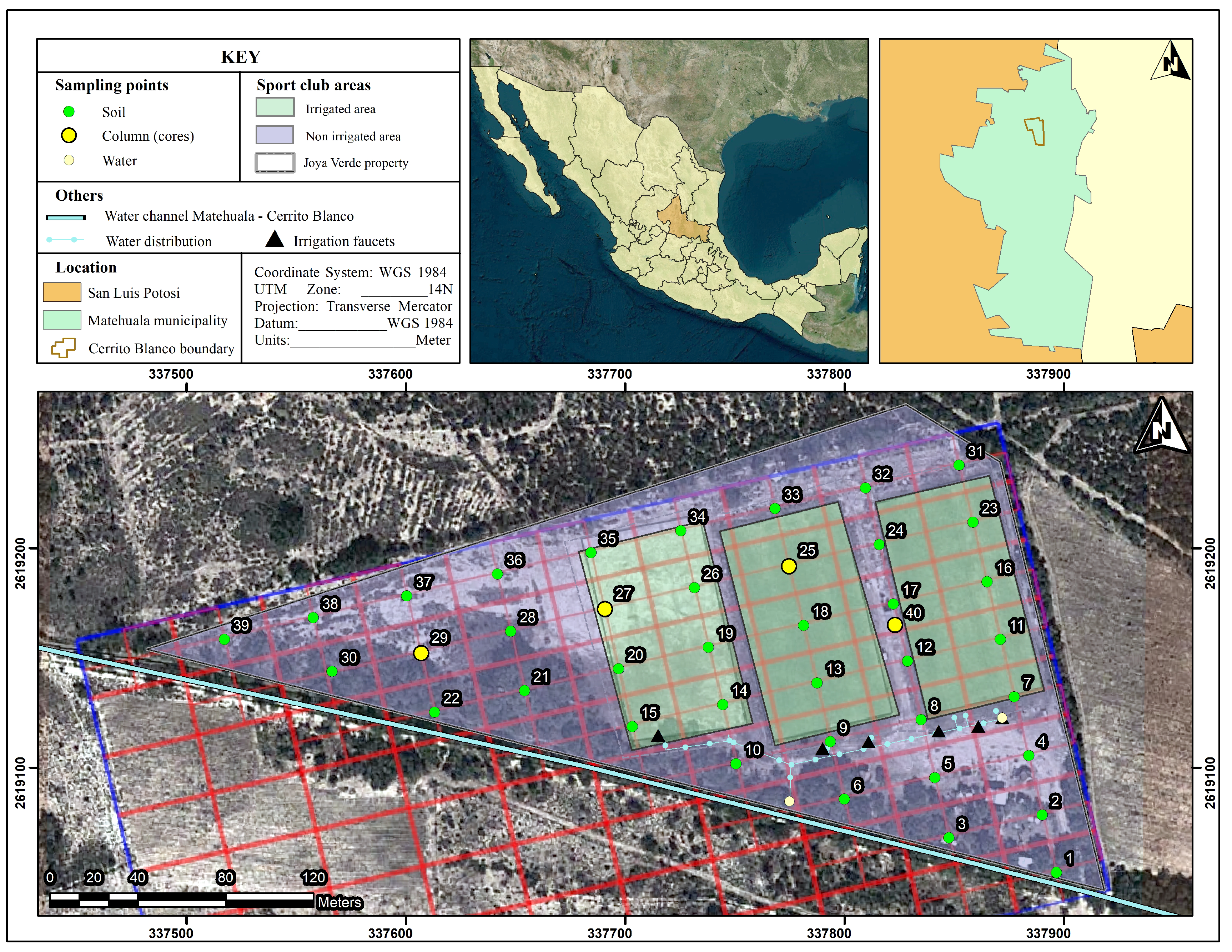
2.1. Study Site
2.2. Soil and Surface Water Sampling and Analysis
2.3. Spatial Analysis Using GIS
2.4. Risk Characterization
3. Results and Discussion
3.1. Arsenic Concentration in Irrigation Water
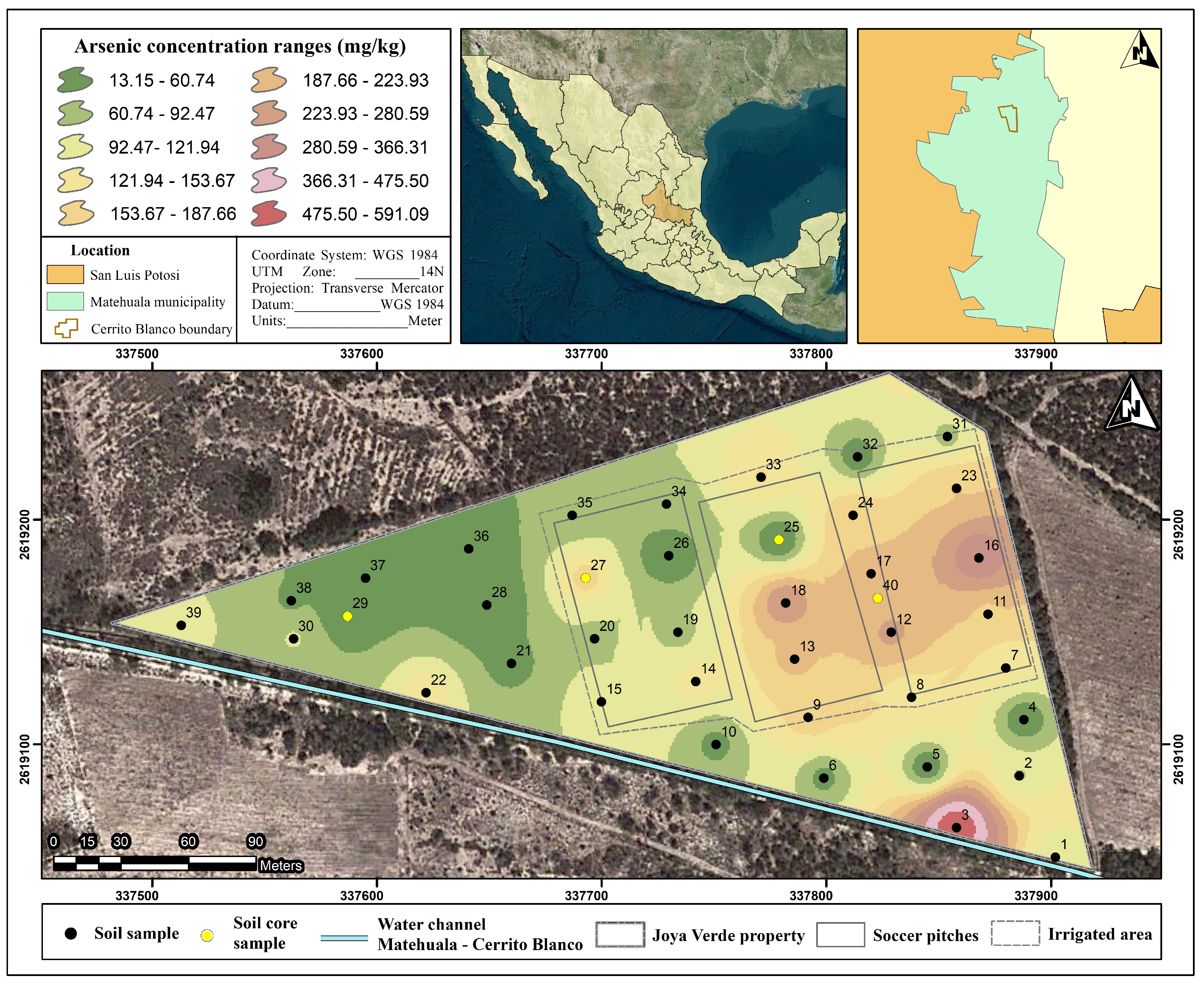
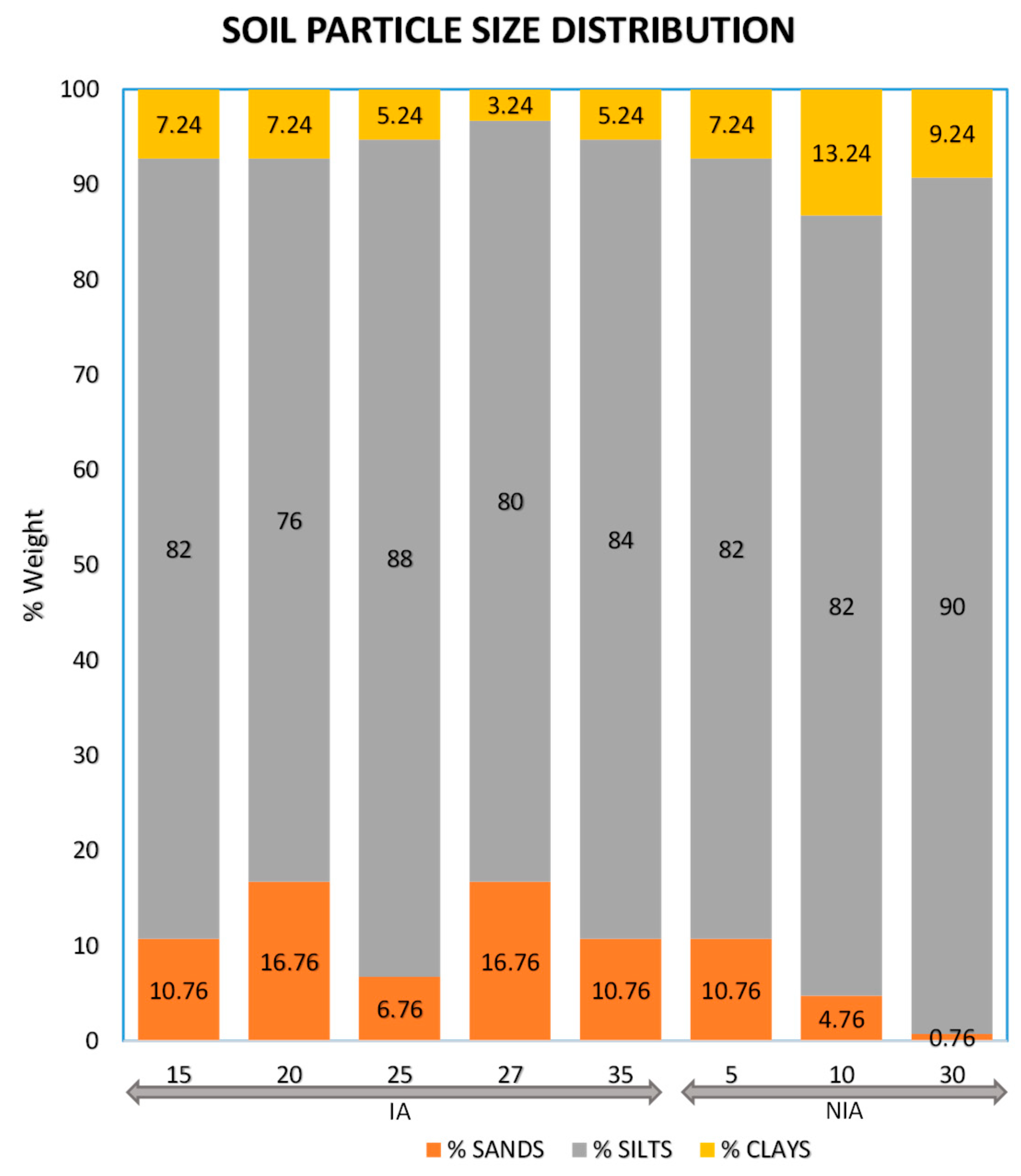
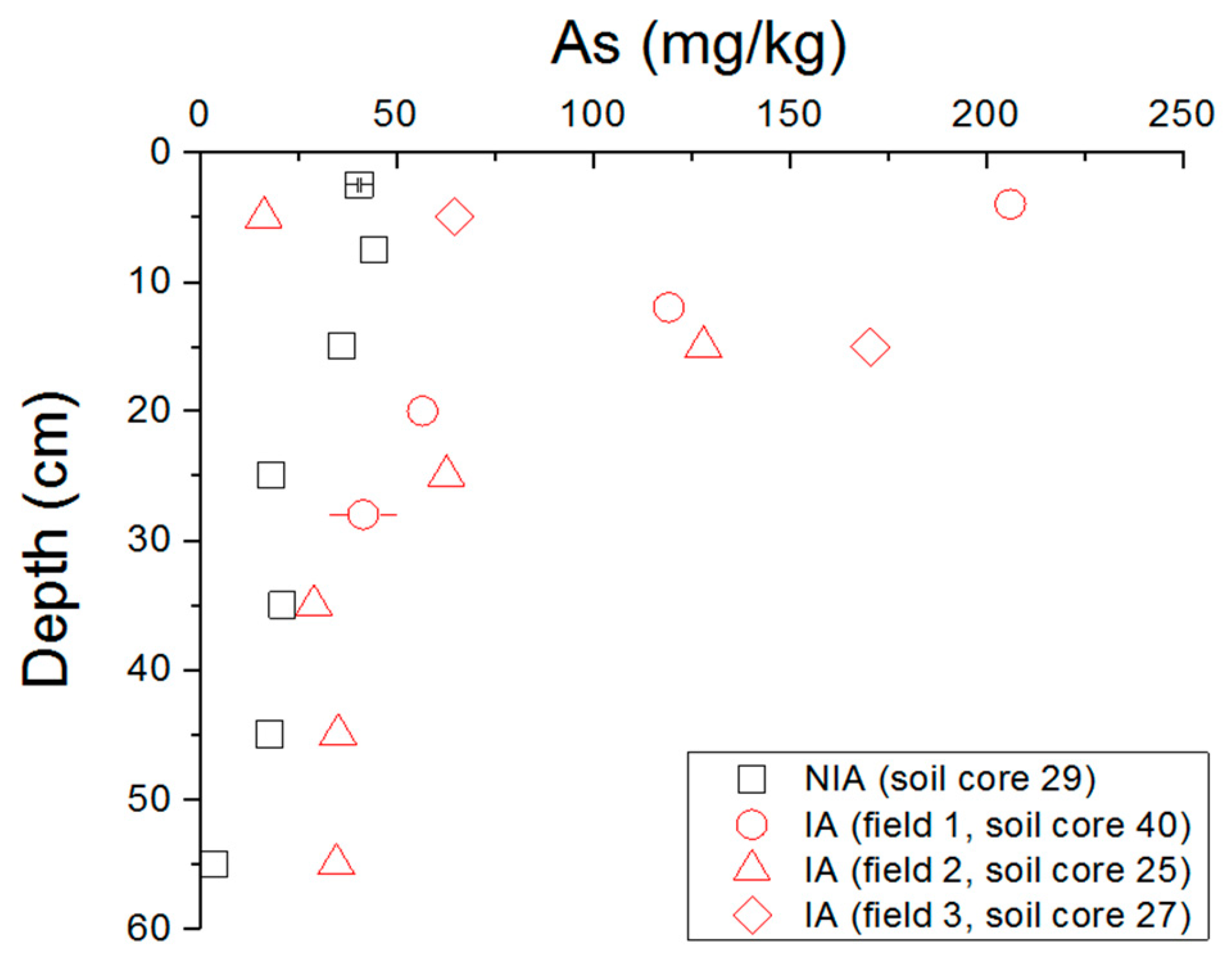
3.2. Arsenic Concentration in Soils
3.3. Risk Assessment on Soccer Pitches
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- OMS Arsénico. Available online: http://www.who.int/mediacentre/factsheets/fs372/es/ (accessed on 23 March 2018).
- Ravenscroft, P.; Brammer, H.; Richards, K. Arsenic Pollution: A Global Synthesis, 1st ed.; John Wiley & Sons: Singapore, 2009; Volume 28, ISBN 978-1-4051-8602-5. [Google Scholar]
- Kabata Pendias, A. Trace Elements in Soils and Plants, 5th ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-4200-9368-1. [Google Scholar]
- Dittmar, J.; Voegelin, A.; Roberts, L.C.; Hug, S.J.; Saha, G.C.; Ali, M.A.; Badruzzaman, A.B.M.; Kretzschmar, R. Spatial distribution and temporal variability of arsenic in irrigated rice fields in Bangladesh. 2. Paddy soil. Environ. Sci. Technol. 2007, 41, 5967–5972. [Google Scholar] [CrossRef] [PubMed]
- Duxbury, J.M.; Zavala, Y.J. What are safe levels of arsenic in food and soils? In Proceedings of the International Symposium Behavior of Arsenic in Aquifers, Soils and Plants, Dhaka, Bangladesh, 16–28 January 2005. [Google Scholar]
- Hossain, M.A.; Sengupta, M.K.; Ahamed, S.; Rahman, M.M.; Mondal, D.; Lodh, D.; Das, B.; Nayak, B.; Roy, B.K.; Mukherjee, A.; et al. Ineffectiveness and poor reliability of arsenic removal plants in West Bengal, India. Environ. Sci. Technol. 2005, 39, 4300–4306. [Google Scholar] [CrossRef] [PubMed]
- Huq, S.M.I.; Rahman, A.; Sultana, N.; Naidu, R. Extent and Severity of Arsenic Contamination in Soils of Bangladesh; Bangladesh University of Engineering and Technology: Dhaka, Bangladesh, 2003. [Google Scholar]
- Norra, S.; Berner, Z.A.; Agarwala, P.; Wagner, F.; Chandrasekharam, D.; Stüben, D. Impact of irrigation with As rich groundwater on soil and crops: A geochemical case study in West Bengal Delta Plain, India. Appl. Geochem. 2005, 20, 1890–1906. [Google Scholar] [CrossRef]
- Roberts, L.C.; Hug, S.J.; Dittmar, J.; Voegelin, A.; Saha, G.C.; Ali, M.A.; Badruzzanian, A.B.M.; Kretzschniar, R. Spatial distribution and temporal variability of arsenic in irrigated rice fields in Bangladesh. 1. Irrigation water. Environ. Sci. Technol. 2007, 41, 5960–5966. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, T.; Tokunaga, H.; Uchino, T.; Ando, M. Effect of arsenic-contaminated irrigation water on agricultural land soil and plants in West Bengal, India. Chemosphere 2005, 58, 799–810. [Google Scholar] [CrossRef] [PubMed]
- DOF. Norma Oficial Mexicana NOM-147-SEMARNAT/SSA1-2004-Establece Criterios Para la Caracterización y Determinación de Concentraciones de Remediación de Suelos Contaminados Por Arsénico, Bario, Berilio, Cadmio, Cromo Hexavalente, Mercurio, Níquel, Plata, Plomo, Selenio, Talio, Vanadio; SEMARNAT/SSA1; DOF: México City, México, 2007; p. 69. [Google Scholar]
- Ruby, M.V.; Lowney, Y.W. Selective soil particle adherance to hands: Implications for understanding oral exposure to soil contaminants. Environ. Sci. Technol. 2012, 46, 12759–12771. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-T.; Wang, M.C.; Li, G.-C. Complexation of arsenate with humic substance in water extract of compost. Chemosphere 2004, 56, 1105–1112. [Google Scholar] [CrossRef] [PubMed]
- Thanabalasingam, P.; Pickering, W.F. Arsenic sorption by humic acids. Environ. Pollut. Ser. B Chem. Phys. 1986, 12, 233–246. [Google Scholar] [CrossRef]
- Gomez, J.J.; Lillo, J.; Sahun, B. Naturally occurring arsenic in groundwater and identification of the geochemical sources in the Duero Cenozoic Basin, Spain. Environ. Geol. 2006, 50, 1151–1170. [Google Scholar] [CrossRef]
- Ruíz-Huerta, E.A.; de la Garza Varela, A.; Gómez-Bernal, J.M.; Castillo, F.; Avalos-Borja, M.; SenGupta, B.; Martínez-Villegas, N. Arsenic contamination in irrigation water, agricultural soil and maize crop from an abandoned smelter site in Matehuala, Mexico. J. Hazard. Mater. 2017, 339, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Alexandratos, V.G.; Elzinga, E.J.; Reeder, R.J. Arsenate uptake by calcite: Macroscopic and spectroscopic characterization of adsorption and incorporation mechanisms. Geochim. Cosmochim. Acta 2007, 71, 4172–4187. [Google Scholar] [CrossRef]
- Lin, J.; Chen, N.; Nilges, M.J.; Pan, Y. Arsenic speciation in synthetic gypsum (CaSO4·2H2O): A. synchrotron XAS, single-crystal EPR, and pulsed {ENDOR} study. Geochim. Cosmochim. Acta 2013, 106, 524–540. [Google Scholar] [CrossRef]
- Renard, F.; Putnis, C.V.; Montes-Hernandez, G.; Ruiz-Agudo, E.; Hovelmann, J.; Sarret, G. Interactions of arsenic with calcite surfaces revealed by in situ nanoscale imaging. Geochim. Cosmochim. Acta 2015, 159, 61–79. [Google Scholar] [CrossRef]
- Rodríguez-Blanco, J.D.; Jiménez, A.; Prieto, M. Oriented Overgrowth of Pharmacolite (CaHAsO4·2H2O) on Gypsum (CaSO4·2H2O). Cryst. Growth Des. 2007, 7, 2756–2763. [Google Scholar] [CrossRef]
- Román-Ross, G.; Cuello, G.J.; Turrillas, X.; Fernández-Martínez, A.; Charlet, L. Arsenite sorption and co-precipitation with calcite. Chem. Geol. 2006, 233, 328–336. [Google Scholar] [CrossRef]
- Romero, F.M.; Armienta, M.A.; Carrillo-Chavez, A. Arsenic sorption by carbonate-rich aquifer material, a control on arsenic mobility at Zimapán, México. Arch. Environ. Contam. Toxicol. 2004, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sø, H.U.; Postma, D.; Jacobsen, R.; Larsen, F. Sorption and desorption of arsenate and arsenite on calcite. Geochim. Cosmochim. Acta 2008, 72, 5871–5884. [Google Scholar] [CrossRef]
- Sariñana-Ruiz, Y.A.; Vazquez-Arenas, J.; Sosa-Rodríguez, F.S.; Labastida, I.; Armienta, M.A.; Aragón-Piña, A.; Escobedo-Bretado, M.A.; González-Valdez, L.S.; Ponce-Peña, P.; Ramírez-Aldaba, H.; et al. Assessment of arsenic and fluorine in surface soil to determine environmental and health risk factors in the Comarca Lagunera, Mexico. Chemosphere 2017, 178, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Armienta, M.A.; Segovia, N. Arsenic and fluoride in the groundwater of Mexico. Environ. Geochem. Health 2008, 30, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Herrera, M.T.; Bundschuh, J.; Nath, B.; Nicolli, H.B.; Gutierrez, M.; Reyes-Gomez, V.M.; Nuñez, D.; Martín-Dominguez, I.R.; Sracek, O. Co-occurrence of arsenic and fluoride in groundwater of semi-arid regions in Latin America: Genesis, mobility and remediation. J. Hazard. Mater. 2013, 262, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.; Masuda, H.; Firdous, N. Toxic fluoride and arsenic contaminated groundwater in the Lahore and Kasur districts, Punjab, Pakistan and possible contaminant sources. Environ. Pollut. 2007, 145, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Brahman, K.D.; Kazi, T.G.; Baig, J.A.; Afridi, H.I.; Khan, A.; Arain, S.S.; Arain, M.B. Fluoride and arsenic exposure through water and grain crops in Nagarparkar, Pakistan. Chemosphere 2014, 100, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-X.; Wang, Z.-H.; Cheng, X.-T.; Li, J.; Sang, Z.-P.; Zhang, X.-D.; Han, L.-L.; Mao, X.-Y.; Wu, Z.-M.; Wang, Z.-Q. Arsenic and fluoride exposure in drinking water: Children’s IQ and growth in Shanyin County, Shanxi Province, China. Environ. Health Perspect. 2007, 115, 643–647. [Google Scholar] [CrossRef] [PubMed]
- ATSDR Public Health Statement: Arsenic. Available online: http://www.atsdr.cdc.gov/phs/phs.asp?id=18&tid=3 (accessed on 21 February 2016).
- Selinus, O.; Alloway, B.; Centeno, J.; Finkelman, R.; Fuge, R.; Lindh, U.; Smedley, P. Essentials of Medical Geology: Revised Edition; Olle, S., Ed.; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-4375-5. [Google Scholar]
- O’Neill, A.; Sen Gupta, B.; Phillips, D.H. Distribution of arsenic and risk assessment of activities on a golf course fertilised with arsenic-containing Ascophyllum nodosum seaweed. Sci. Total Environ. 2014, 482–483, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Rovira, J.; Nadal, M.; Schuhmacher, M.; Domingo, J.L. Alternative fuel implementation in a cement plant: Human health risks and economical valuation. Arch. Environ. Contam. Toxicol. 2016, 71, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Li, Z.; Liu, T.; Chen, J.; Wu, T.; Feng, X. Metal exposure and associated health risk to human beings by street dust in a heavily industrialized city of Hunan Province, Central China. Int. J. Environ. Res. Public Health 2017, 14, 261. [Google Scholar] [CrossRef] [PubMed]
- Lowney, Y.W.; Wester, R.C.; Schoof, R.A.; Cushing, C.A.; Edwards, M.; Ruby, M.V. Dermal absorption of arsenic from soils as measured in the Rhesus Monkey. Toxicol. Sci. 2007, 100, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Wester, R.C.; Hui, X.; Barbadillo, S.; Maibach, H.I.; Lowney, Y.W.; Schoof, R.A.; Holm, S.E.; Ruby, M.V. In vivo percutaneous absorption of arsenic from water and CCA-treated wood residue. Toxicol. Sci. 2004, 79, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Hostynek, J.J.; Hinz, R.S.; Lorence, C.R.; Price, M.; Guy, R.H. Metals in the skin. Crit. Rev. Toxicol. 1993, 23, 171–235. [Google Scholar] [CrossRef] [PubMed]
- Turkall, R.M.; Skowronski, G.A.; Suh, D.H.; Abdel-Rahman, M.S. Effect of a chemical mixture on dermal penetration of arsenic and nickel in male pig in vitro. J. Toxicol. Environ. Health A 2003, 66, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Orlando, G.; Selicato, F.; Torre, C.M. The use of GIS as tool to support risk assessment. In Geo-Information for Disaster Management; Springer: Berlin, Germany, 2005; pp. 1381–1399. ISBN 978-3-540-24988-7. [Google Scholar]
- Hossain, M.M.; Piantanakulchai, M. Groundwater arsenic contamination risk prediction using GIS and classification tree method. Eng. Geol. 2013, 156, 37–45. [Google Scholar] [CrossRef]
- Chakraborti, D.; Das, B.; Rahman, M.M.; Nayak, B.; Pal, A.; Sengupta, M.K.; Ahamed, S.; Hossain, M.A.; Chowdhury, U.K.; Biswas, B.K.; et al. Arsenic in groundwater of the Kolkata Municipal Corporation (KMC), India: Critical review and modes of mitigation. Chemosphere 2017, 180, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Dummer, T.J.; Yu, Z.M.; Nauta, L.; Murimboh, J.D.; Parker, L. Geostatistical modelling of arsenic in drinking water wells and related toenail arsenic concentrations across Nova Scotia, Canada. Sci. Total Environ. 2015, 505, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Akopyan, K.; Petrosyan, V.; Grigoryan, R.; Melkomian, D.M. Assessment of residential soil contamination with arsenic and lead in mining and smelting towns of northern Armenia. J. Geochem. Explor. 2018, 184, 97–109. [Google Scholar] [CrossRef]
- Martínez-Villegas, N.; Briones-Gallardo, R.; Ramos-Leal, J.A.; Avalos-Borja, M.; Castañón-Sandoval, A.D.; Razo-Flores, E.; Villalobos, M. Arsenic mobility controlled by solid calcium arsenates: A case study in Mexico showcasing a potentially widespread environmental problem. Environ. Pollut. 2013, 176, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Razo, I.; Carrizales, L.; Castro, J.; Díaz-Barriga, F.; Monroy, M. Arsenic and heavy metal pollution of soil, water and sediments in a semi-arid climate mining area in Mexico. Water. Air Soil Pollut. 2004, 152, 129–152. [Google Scholar] [CrossRef]
- Bueno Pedroza, A. Interpretación Hidrogeoquímica de los Sistemas de Flujo de la Parte Norte del Altiplano Potosino. Master’s Thesis, Universidad Autónoma De Nuevo León, San Nicolás de los, México, 2005. [Google Scholar]
- INEGI. Prontuario de Información Geográfica Municipal de los Estados Unidos Mexicanos. Matehuala, San Luis Potosí; Instituto Nacional de Estadística Geografía e Informática: Aguascalientes, México, 2009. [Google Scholar]
- Secretaria de Economía (SE). NMX-AA-132-SCFI-2001—Muestreo de Suelos Para la Identificación y la Cuantificación de Metales y Metaloides, y Manejo de la Muestra; Secretaria de Economía: México City, México, 2006; p. 32. [Google Scholar]
- Vercoutere, K.; Fortunati, U.; Muntau, B.; Griepink, B.; Maier, E.A. The certified reference materials CRM 142 R light sandy soils, CRM 143 R sewage sludge amended soil and CRM 145 R sewage sludge for quality control in monitoring environmental and soil pollution. Fresenius’ J. Anal. Chem. 1995, 352, 197–202. [Google Scholar] [CrossRef]
- Niskavaara, H.; Reimann, C.; Chekushin, V.; Kashulina, G. Seasonal variability of total and easily leachable element contents in topsoils (0–5 cm) from eight catchments in the European Arctic (Finland, Norway and Russia). Environ. Pollut. 1997, 96, 261–274. [Google Scholar] [CrossRef]
- DOF. Norma Oficial Mexicana NOM-021-SEMARNAT-2000, Que Establece Las Especificaciones de Fertilidad, Salinidad Y Clasificación de Suelos, Estudio, Muestreo y Análisis; SEMARNAT: México City, México, 2002; p. 85. [Google Scholar]
- United States Environmental Protection Agency (EPA). Method 200.7: Revision 4.4, Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emission Spectrometry; United States Environmental Protection Agency: Cincinnati, OH, USA, 1994.
- Isaaks, E.H.; Srivastava, R.M. An Introduction to Applied Geostatistics; Oxford University Press: New York, NY, USA, 1989; p. 590. ISBN 0-19-505012-6. [Google Scholar]
- Goovaerts, P. Geostatistics for Natural Resources Evaluation; Applied Geostatistics Series; Oxford University Press: Oxford, UK, 1997; ISBN 978-0-19-511538-3. [Google Scholar]
- Appleton, J.D.; Adlam, K.A.M. Geogenic control on soil chemistry in urban areas: A novel method for urban geochemical mapping using parent material classified data. Appl. Geochem. 2012, 27, 161–170. [Google Scholar] [CrossRef]
- Qiao, M.; Cai, C.; Huang, Y.; Liu, Y.; Lin, A.; Zheng, Y. Characterization of soil heavy metal contamination and potential health risk in metropolitan region of northern China. Environ. Monit. Assess. 2011, 172, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Guo, H.; Hao, Z. Spatial distribution of heavy metals in Hong Kong’s marine sediments and their human impacts: A GIS-based chemometric approach. Mar. Pollut. Bull. 2007, 54, 1372–1384. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Risk Assessment Guidance for Superfund (RAGS): Part E; United States Environmental Protection Agency: Washington, DC, USA, 2004.
- Molak, V. Fundamentals of Risk Analysis and Risk Management; Lewis Publishers: Boca Raton, FL, USA, 1996; ISBN 978-1-56670-130-3. [Google Scholar]
- DOF. Norma Oficial Mexicana NOM-001-SEMARNAT-1996, Que Establece los Límites Máximos Permisibles de Contaminantes en las Descargas de Aguas Residuales en Aguas y Bienes Nacionales; SEMARNAT: México City, México, 2003; p. 35. [Google Scholar]
- Chiprés, J.A.; Castro-Larragoitia, J.; Monroy, M.G. Exploratory and spatial data analysis (EDA–SDA) for determining regional background levels and anomalies of potentially toxic elements in soils from Catorce–Matehuala, Mexico. Appl. Geochem. 2009, 24, 1579–1589. [Google Scholar] [CrossRef]
- Ott, R.L.; Longnecker, M. An Introduction to Statistical Methods and Data Analysis; Cengage Learning: Boston, MA, USA, 2015; ISBN 978-1-305-26947-7. [Google Scholar]
- SGM. Carta Geológico–Minera Matehuala F14-A25; SGM: Pachuca, México, 2013. [Google Scholar]
- Castillo, F.; Ávalos-Borja, M.; Jamieson, H.; Hernández-Bárcenas, G.; Martínez-Villegas, N. Identification of diagenetic calcium arsenates using synchrotron-based micro X-ray diffraction. Bol. Soc. Geol. Mex. 2015, 67, 479–491. [Google Scholar] [CrossRef]
- Yolcubal, I.; Akyol, N.H. Adsorption and transport of arsenate in carbonate-rich soils: Coupled effects of nonlinear and rate-limited sorption. Chemosphere 2008, 73, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bárcenas, G.; Castillo, F.; Ávalos-Borja, M.; Martínez-Villegas, N.V. Síntesis de arseniatos de calcio (guerinita, haidingerita y farmacolita) morfológicamente similares a los encontrados en suelos contaminados. Rev. Int. Contam. Ambient. 2017, 33, 153–163. [Google Scholar] [CrossRef]
- Hernández-Bárcenas, L.G. Caracterización Mineralógica de Suelos Impactados Por Residuos Minero-Metalúrgicos en Una Fundición Abandonada en Matehuala, San Luis Potosí. Master’s Thesis, Instituto Potosino de Investigación Científica y Tecnológica, San Luis Potosí, México, 2017. [Google Scholar]
- Cai, Y.; Cabrera, J.C.; Georgiadis, M.; Jayachandran, K. Assessment of arsenic mobility in the soils of some golf courses in South Florida. Sci. Total Environ. 2002, 291, 123–134. [Google Scholar] [CrossRef]
- Ljung, K.; Selinus, O.; Otabbong, E.; Berglund, M. Metal and arsenic distribution in soil particle sizes relevant to soil ingestion by children. Appl. Geochem. 2006, 21, 1613–1624. [Google Scholar] [CrossRef]
- Gerba, C.P. Assessment of enteric pathogen shedding by bathers during recreational activity and its impact on water quality. Quant. Microbiol. 2000, 2, 55–68. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, Z.; Wang, T.; Lian, H.; Sun, Y.; Wu, J. Bioaccessibility and health risk of arsenic and heavy metals (Cd, Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2.5 in Nanjing, China. Atmos. Environ. 2012, 57, 146–152. [Google Scholar] [CrossRef]
- Liang, C.-P.; Liu, C.-W.; Jang, C.-S.; Wang, S.-W.; Lee, J.-J. Assessing and managing the health risk due to ingestion of inorganic arsenic from fish and shellfish farmed in blackfoot disease areas for general Taiwanese. J. Hazard. Mater. 2011, 186, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Polya, D.A. Rice is a major exposure route for arsenic in Chakdha Block, West Bengal: A probabilistic risk assessment. Appl. Geochem. 2008, 23, 2987–2998. [Google Scholar] [CrossRef]
- Zakharova, T.; Tatàno, F.; Menshikov, V. Health cancer risk assessment for arsenic exposure in potentially contaminated areas by fertilizer plants: A possible regulatory approach applied to a case study in Moscow region, Russia. Regul. Toxicol. Pharmacol. 2002, 36, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Carbonell Barrachina, Á.A.; Burló Carbonell, F.M.; Mataix Beneyto, J.J. Arsénico en el Sistema Suelo-Planta, Significado Ambiental, 1st ed.; Espagrafic: Esparreguera, Spain, 1995; ISBN 84-7908-192-9. [Google Scholar]
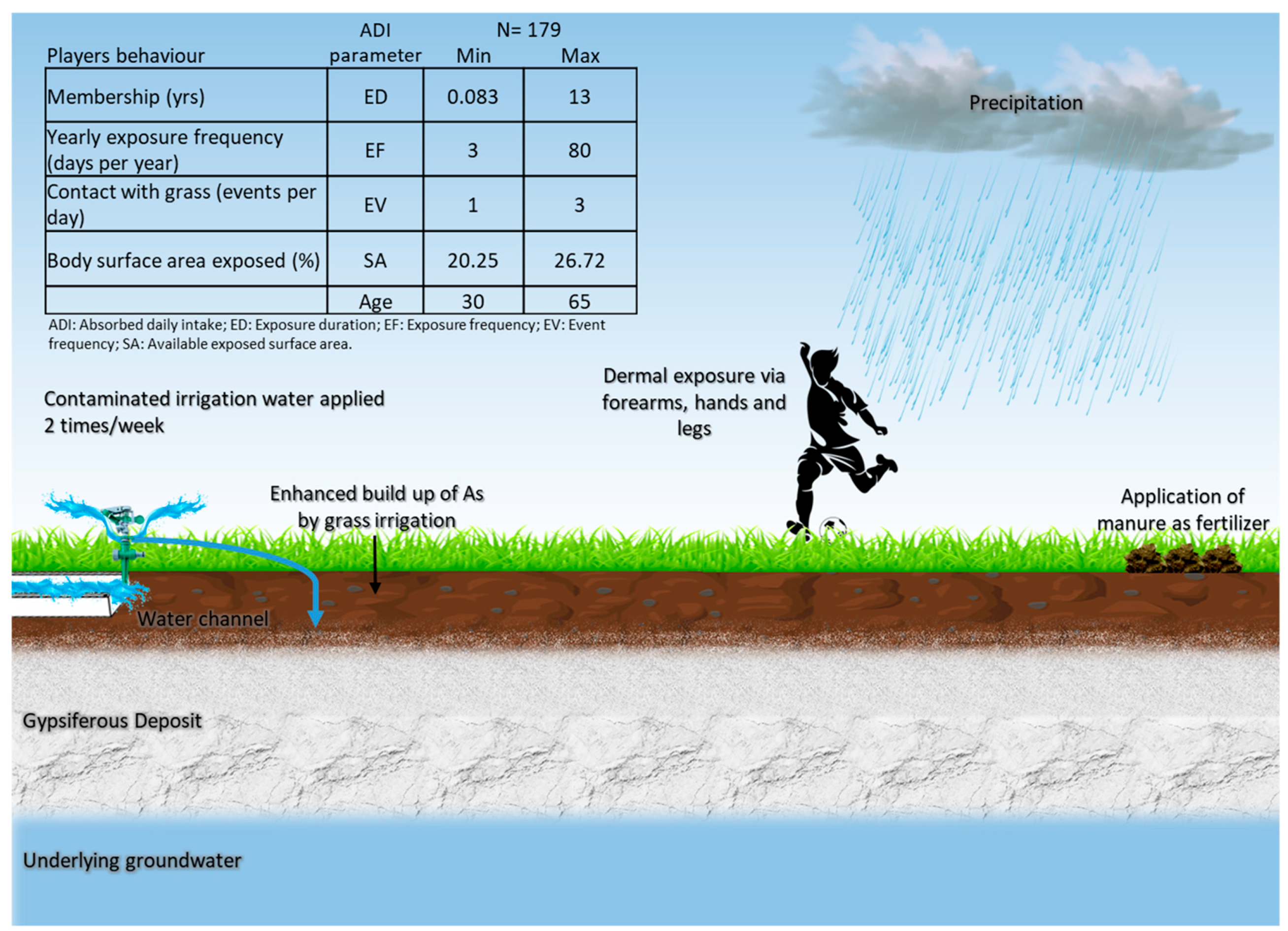
| Exposure Parameter | Description | Value | Source |
|---|---|---|---|
| Risk | The probability of an individual developing cancer over a lifetime | - | Site specific |
| ADI (mg kg−1-day) | Absorbed daily intake | 5 × 10−6 | Site specific |
| SF (mg kg−1-day)−1 | Dermal Slope Factor (based on a gastrointestinal absorption factor of 0.41) | 3.66 | RAIS (1998) |
| C (mg kg−1) | Concentration of As in soil | 13.14–591.31 | Site specific |
| AF (mg cm−2) | Resident soil adherence factor | 0.01–0.08 | USEPA (2004) |
| ABS | Absorption factor for As | 0.03 | USEPA (2004) |
| CF (kg mg−1) | Conversion factor | 10−6 | USEPA (2004) |
| SA (cm2) | Available exposed surface area | 3300–5700 | Site specific USEPA (2004) |
| EV (events day−1) | Event frequency | 1–3 | Site specific |
| EF (days yr−1) | Exposure frequency | 3–80 | Site specific |
| ED (yrs) | Exposure duration | 0.083–13 | Site specific |
| BW (kg) | Body weight | 74.8 | INEGI (2015) |
| AT (days yr−1) | Averaging lifetime for carcinogens | 25,550 | Agency for Toxic Substances and Disease Registry (2011) |
| Sample | T (°C) | pH | EC (µS/cm) | TDS (mg/L) | ORP (mV) | DO (mg/L) | Alkalinity (mgCaCO3/L) | As (mg/L) |
|---|---|---|---|---|---|---|---|---|
| Channel | 29.5 | 8.33 | 2567 | 1283 | 434.3 | 2.11 | 124 | 6.7 ± 1.6 |
| Irrigation faucet | 31.2 | 8.34 | 2601 | 1300 | 433.6 | 1.05 | 103 | 6.6 ± 0.2 |
| Sampling Areas | n (Number of Samples) | Mean (mg/kg) | Std Error (mg/kg) | Min (mg/kg) | Max (mg/kg) |
|---|---|---|---|---|---|
| Whole sport club | 39 | 119.4 | 109.5 | 13.1 | 591.3 |
| Irrigated Area | 23 | 138.1 | 82.9 | 13.5 | 353.3 |
| Non-Irrigated area | 16 | 92.5 | 137.9 | 13.6 | 591.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Villegas, N.; Hernández, A.; Meza-Figueroa, D.; Sen Gupta, B. Distribution of Arsenic and Risk Assessment of Activities on Soccer Pitches Irrigated with Arsenic-Contaminated Water. Int. J. Environ. Res. Public Health 2018, 15, 1060. https://doi.org/10.3390/ijerph15061060
Martínez-Villegas N, Hernández A, Meza-Figueroa D, Sen Gupta B. Distribution of Arsenic and Risk Assessment of Activities on Soccer Pitches Irrigated with Arsenic-Contaminated Water. International Journal of Environmental Research and Public Health. 2018; 15(6):1060. https://doi.org/10.3390/ijerph15061060
Chicago/Turabian StyleMartínez-Villegas, Nadia, Abraham Hernández, Diana Meza-Figueroa, and Bhaskar Sen Gupta. 2018. "Distribution of Arsenic and Risk Assessment of Activities on Soccer Pitches Irrigated with Arsenic-Contaminated Water" International Journal of Environmental Research and Public Health 15, no. 6: 1060. https://doi.org/10.3390/ijerph15061060
APA StyleMartínez-Villegas, N., Hernández, A., Meza-Figueroa, D., & Sen Gupta, B. (2018). Distribution of Arsenic and Risk Assessment of Activities on Soccer Pitches Irrigated with Arsenic-Contaminated Water. International Journal of Environmental Research and Public Health, 15(6), 1060. https://doi.org/10.3390/ijerph15061060







