Characterization of a Microbial Consortium for the Bioremoval of Polycyclic Aromatic Hydrocarbons (PAHs) in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Materials
2.2. Enrichment and Selection for the Microbial Consortium Removal of Phenanthrene, Pyrene, and Naphthalene
2.3. Bioremoval Experiment
2.4. Sample Preparation and PAH Extraction
2.5. Instrumental Analysis
2.6. Microbial Consortium Identification
2.7. Data Analysis
3. Results and Discussion
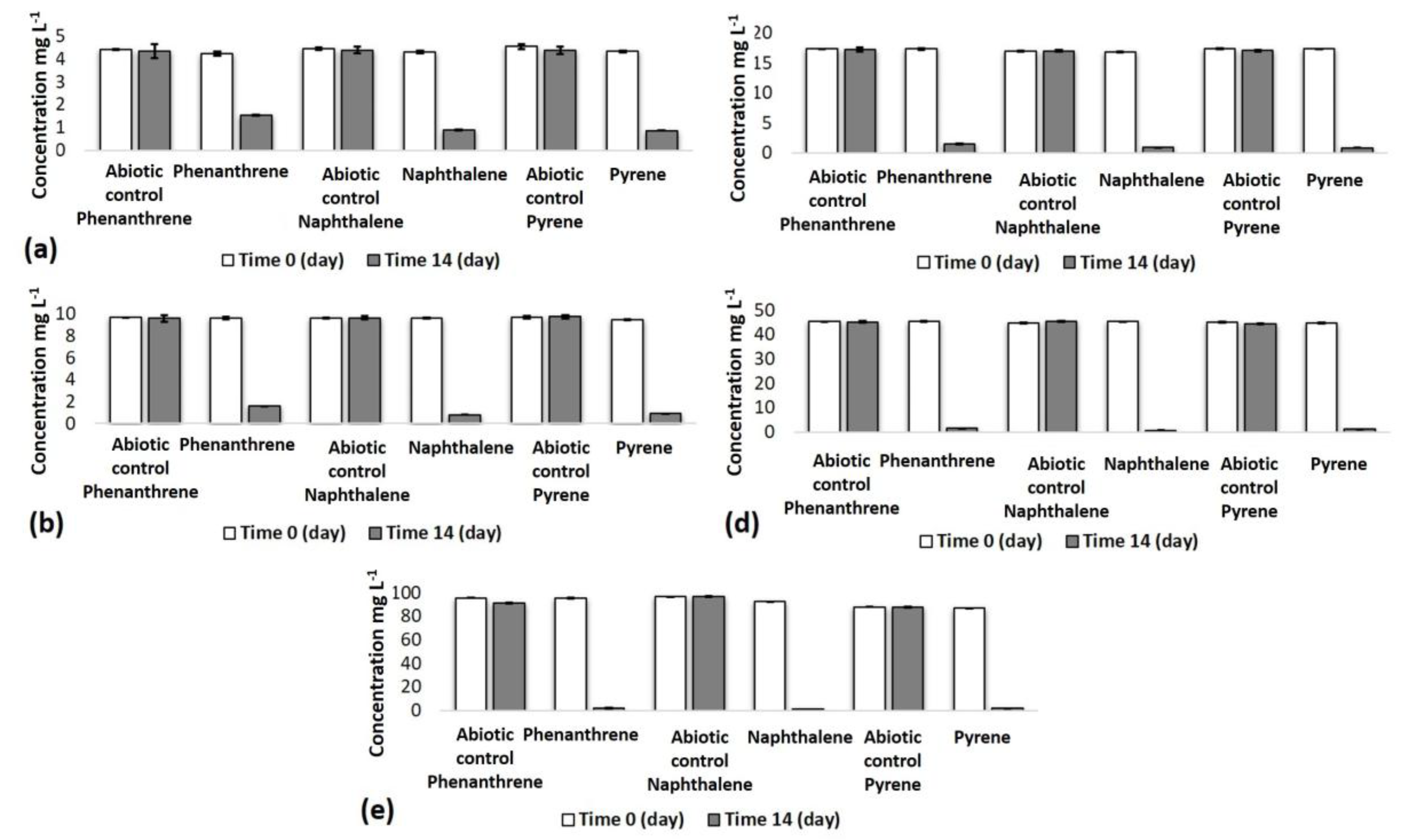
3.1. Removal of PAHs through the Use of Microorganisms from Ore Waste
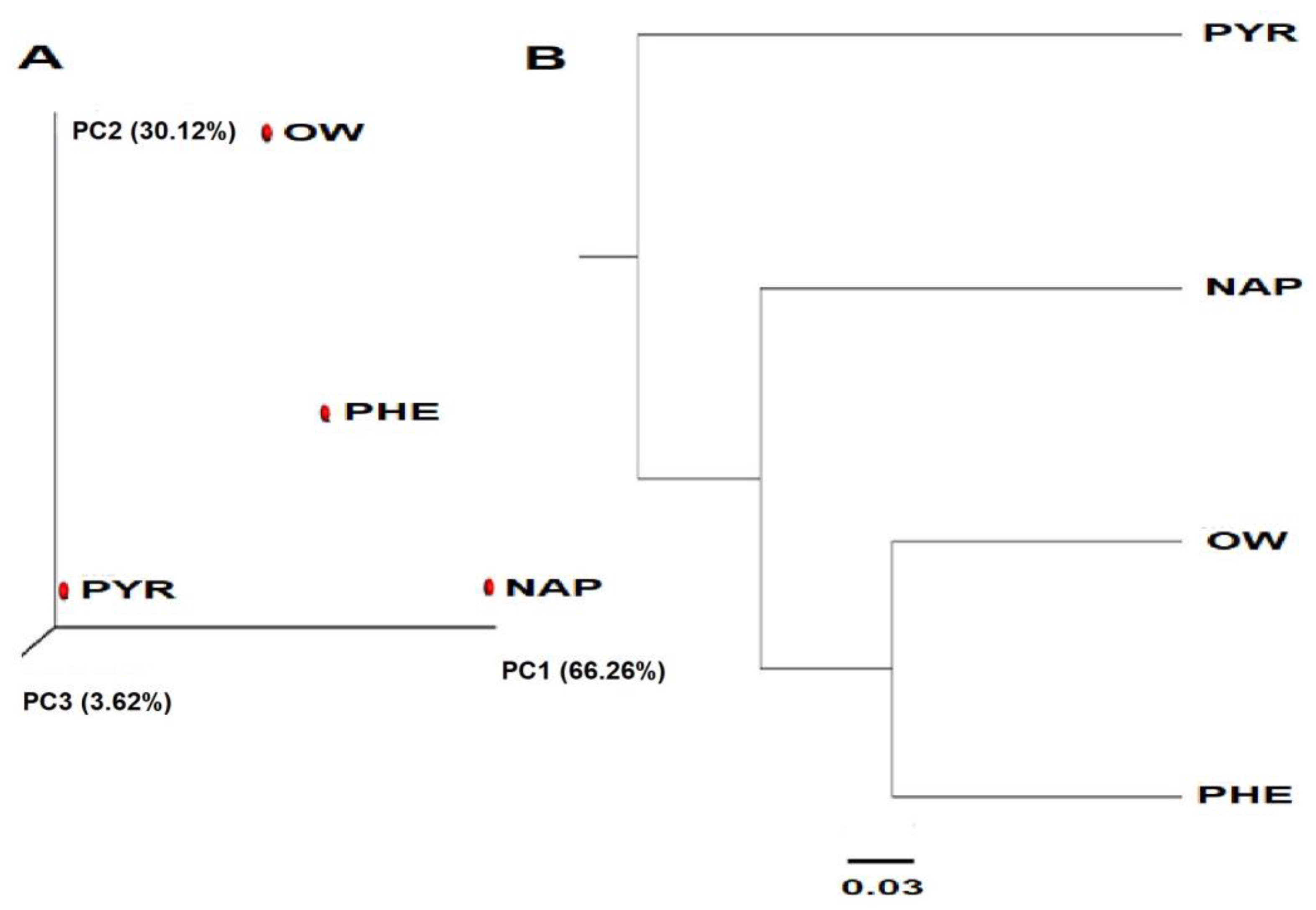
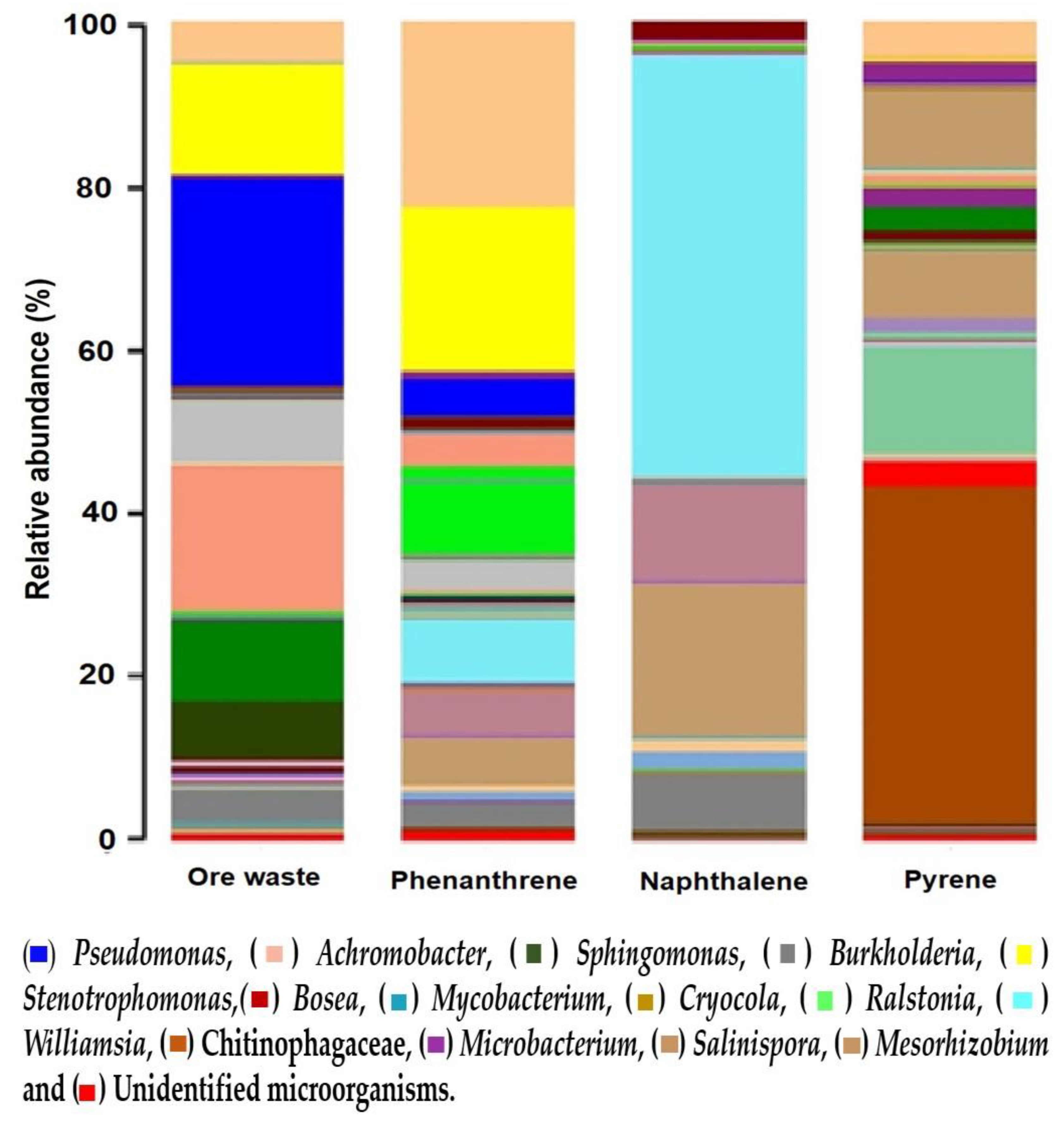
3.2. Sequencing Results and Diversity Index
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Liu, J.J.; Wang, X.C.; Fan, B. Characteristics of PAHs adsorption on inorganic particles and activated sludge in domestic wastewater treatment. Bioresour. Technol. 2011, 102, 5305–5311. [Google Scholar] [CrossRef] [PubMed]
- Patiño, S.; Díaz, Y.; Ordóñez, S. Water Micropollutants: Classification And Treatment Technologies. Avances en Ciencias e Ingeniería 2014, 5, 1–20. [Google Scholar]
- Liu, Z.; Li, Q.; Wu, Q.; Kuo, D.T.; Chen, S.; Hu, X.; Deng, M.; Zhang, H.; Luo, M. Removal Efficiency and Risk Assessment of Polycyclic Aromatic Hydrocarbons in a Typical Municipal Wastewater Treatment Facility in Guangzhou, China. Int. J. Environ. Res. Public Health 2017, 14, 861. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhang, J.; Ma, Q.; Chen, Y. Human Health and Ecological Risk Assessment of 16 Polycyclic Aromatic Hydrocarbons in Drinking Source Water from a Large Mixed-Use Reservoir. Int. J. Environ. Res. Public Health 2015, 12, 13956–13969. [Google Scholar] [CrossRef] [PubMed]
- Kafilzadeh, F.; Shiva, A.H.; Malekpour, R. Determination of Polycyclic Aromatic Hydrocarbons (PAHs) in Water and Sediments of the Kor River, Iran. J. Sci. Res. 2011, 10, 1–7. [Google Scholar]
- Dutta, T.; Kwon, E.; Bhattacharya, S.S. Polycyclic aromatic hydrocarbons and volatile organic compounds in biochar and biochar-amended soil : A review. Bioenergy 2017, 9, 990–1004. [Google Scholar] [CrossRef]
- Lizardi, M.A.; Leal, R.M.; Ordaz, A.; Reyna, R. Airlift bioreactors for hydrocarbon water pollution remediation in a tourism development pole. Desalination Water Treat. 2013, 1–6. [Google Scholar] [CrossRef]
- Kadri, T.; Rouissi, T.; Brar, S.K.; Cledon, M.; Sarma, S.; Verma, M. Biodegradation of polycyclic aromatic hydrocarbons (PAHs) by fungal enzymes: A review. J. Environ. Sci. 2016, 51, 52–74. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, K.; Loh, K.C. Formulation of microbial cocktails for BTEX biodegradation. Biodegradation 2014, 26, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnol. Res. Int. 2011, 2011, 13. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, C.; Lv, C.; Lun, Z.; Zheng, C. Removal Capacities of Polycyclic Aromatic Hydrocarbons (PAHs) by a Newly Isolated Strain from Oilfield Produced Water. Int. J. Environ. Res. Public Health 2017, 14, 215. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.R.; Melchor, J.; Jiménez, C.; Hidalgo, J.; Lara, M.E.; Marsch, M.; Lara, H.; Dendooven, L.; Marsch, R.; Olivia, R.; et al. Phylogenetic and morphological identification of a photosynthetic microbial consortium of potential biotechnological interest. Hidrobiológica 2016, 26, 311–321. [Google Scholar]
- Echeverri, M.; Manjarrez, G.; Cabrera, G. Aislamiento de bacterias potencialmente degradadoras de petróleo en hábitats de ecosistemas costeros en la Bahía de Cartagena, Colombia. Cienc. Bioméd. 2011, 8, 76–86. (In Spanish) [Google Scholar]
- Pérez, B.; Mauricio, A.; Jimnez, T.; Tapia, A.; Santiesteban, A. Emulsification of Hydrocarbons Using Biosurfactant Producing Strains Isolated from Contaminated Soil in Puebla, Mexico. Biodegrad. Eng. Technol. 2013, 25–45. [Google Scholar] [CrossRef]
- Bisht, S.; Pandey, P.; Bhargava, B.; Sharma, S.; Kumar, V.; Krishan, D. Bioremediation of polyaromatic hydrocarbons (PAHs) using rhizosphere technology. Braz. J. Microbiol. 2015, 46, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Ghazali, F.M.; Rahman, R.N.Z.A.; Salleh, A.B.; Basri, M. Biodegradation of hydrocarbons in soil by microbial consortium. Int. Biodeterior. Biodegrad. 2004, 54, 61–67. [Google Scholar] [CrossRef]
- Rosselló, R. El concepto de especie en Procariotas. Cientifica y Técnica de Ecología y Medio Ambiente 2005, 14, 11–16. (In Spanish) [Google Scholar]
- Salter, S.J.; Cox, M.J.; Turek, E.M.; Calus, S.T.; Cookson, W.O.; Moffatt, M.F.; Turner, P.; Parkhill, J.; Loman, N.J.; Walker, A.W. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 2014, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Staley, C.; Unno, T.; Gould, T.J.; Jarvis, B.; Phillips, J.; Cotner, J.B.; Sadowsky, M.J. Application of Illumina next-generation sequencing to characterize the bacterial community of the Upper Mississippi River. J. Appl. Microbiol. 2013, 115, 1147–1158. [Google Scholar] [CrossRef] [PubMed]
- NOM-021-RECNAT-2000. Segunda Sección, Diario Oficial de la Federación. 2000. Available online: http://www.ordenjuridico.gob.mx/Documentos/Federal/wo69255.pdf (accessed on 20 November 2017).
- Ballesteros, R.; Lyons, L.L.; Herna, J.J. Determination of PAHs in diesel particulate matter using thermal extraction and solid phase micro-extraction. Atmos. Environ. 2009, 43, 655–662. [Google Scholar] [CrossRef]
- Janbandhu, A.; Fulekar, M.H. Biodegradation of phenanthrene using adapted microbial consortium isolated from petrochemical contaminated environment. J. Hazard. Mater. 2011, 187, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Doong, R.; Chang, S.; Sun, Y. Solid-phase microextraction for determining the distribution of sixteen US Environmental Protection Agency polycyclic aromatic hydrocarbons in water samples. J. Chromatogr. A 2000, 879, 177–188. [Google Scholar] [CrossRef]
- Menezes, A.; Neves, F.; Afonso, P.; Pereira, D.P. Development, validation and application of a method based on DI-SPME and GC–MS for determination of pesticides of different chemical groups in surface and groundwater samples. Microchem. J. 2010, 96, 139–145. [Google Scholar] [CrossRef]
- Xu, H.; Ding, Z.; Lv, L.; Song, D.; Feng, Y. A novel dispersive liquid—liquid microextraction based on solidification of floating organic droplet method for determination of polycyclic aromatic hydrocarbons in aqueous samples. Anal. Chim. Acta 2009, 636, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Doong, R.; Chang, S.; Sun, Y. Solid-phase microextraction and headspace solid-phase microextraction for the determination of high molecular-weight polycyclic aromatic hydrocarbons in water and soil samples. J. Chromatogr. Sci. 2000, 38, 528–534. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pawliszyn, J. Solid Phase Microextraction; Plenum: New York, NY, USA, 2001. [Google Scholar]
- Dias, A.N.; Sim, V.; Merib, J.; Carasek, E. Cork as a new (green) coating for solid-phase microextraction: Determination of polycyclic aromatic hydrocarbons in water samples by gas chromatography-mass spectrometry. Anal. Chim. Acta 2013, 772, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, C.S.; Winston, F. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for trausforma. Gene 1987, 51, 267–272. [Google Scholar] [CrossRef]
- Macrogen. MiSeq Raw Data. Rep. of Korea. 2017. Available online: http://dna.macrogen.com (accessed on 20 December 2017).
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- QIIME2. Illumina Overview Tutorial (an IPython Notebook): Open Reference OTU Picking and Core Diversity Analyses. 2015. Available online: http://qiime.org/tutorials/illumina_overview_tutorial.html (accessed on 24 October 2017).
- Vázquez, Y.; Pirrung, M.; Gonzalez, A.; Knight, R. EMPeror : A tool for visualizing high-throughput microbial community data. GigaScience 2013, 2, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Sanches, S.; Martins, M.; Silva, A.F.; Galinha, C.F.; Santos, M.A.; Pereira, I.A.; Teresa, M.; Crespo, B. Bioremoval of priority polycyclic aromatic hydrocarbons by a microbial community with high sorption ability. Environ. Sci. Pollut. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Bautista, L.F.; Sanz, R.; Molina, M.C.; Gonza, N. Effect of different non-ionic surfactants on the biodegradation of PAHs by diverse aerobic bacteria. Int. Biodeterior. Biodegrad. 2009, 63, 913–922. [Google Scholar] [CrossRef]
- Huijie, L.; Caiyun, Y.; Yun, T.; Guanghui, L.; Tianling, Z. Using population dynamics analysis by DGGE to design the bacterial consortium isolated from mangrove sediments for biodegradation of PAHs. Int. Biodeterior. Biodegrad. 2011, 65, 269–275. [Google Scholar] [CrossRef]
- Ferradji, F.; Mnif, S.; Badis, A.; Rebbani, S.; Fodil, D.; Eddouaouda, K.; Sayadi, S. Naphthalene and crude oil degradation by biosurfactant producing Streptomyces spp. isolated from Mitidja plain soil (North of Algeria). Int. Biodeterior. Biodegrad. 2014, 86, 300–308. [Google Scholar] [CrossRef]
- Herrera, M.C.; Chaves, J. Validación de un método de análisis para la determinación de hidrocarburos aromáticos policíclicos por cromatografía líquida de alta eficiencia en partículas PM10 Y PM2.5. Tecnol. Marcha 2012, 25. (In Spanish) [Google Scholar] [CrossRef]
- Hong, C.; Si, Y.; Xing, Y.; Li, Y. Illumina MiSeq sequencing investigation on the contrasting soil bacterial community structures in different iron mining areas. Environ. Sci. Pollut. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hwang, H.S.; Kim, W.; Kim, H.; Kim, H.; Hwang, H.S.; Kim, W. Metagenomic analysis of the marine coastal invertebrates of South Korea as assessed by Ilumina MiSeq. Anim. Cells Syst. 2017, 21, 1–8. [Google Scholar] [CrossRef]
- Sawulski, P.; Clipson, N.; Doyle, E. Effects of polycyclic aromatic hydrocarbons on microbial community structure and PAH ring hydroxylating dioxygenase gene abundance in soil. Biodegradation 2014, 25, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gang, T.; Zou, F.; Fung, N.; Tam, Y. Different bacterial groups for biodegradation of three- and four-ring PAHs isolated from a Hong Kong mangrove sediment. J. Hazard. Mater. 2008, 152, 1179–1185. [Google Scholar] [CrossRef]
- Tikilili, P.V.; Nkhalambayausi, E.M. Characterization and biodegradation of polycyclic aromatic hydrocarbons in radioactive wastewater. J. Hazard. Mater. 2011, 192, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Cydzik, A.; Zielin, M. Bacterial communities in full-scale wastewater treatment systems. World J. Microbiol. Biotechnol. 2016, 32, 66. [Google Scholar] [CrossRef] [PubMed]
- Benavides, J.; De Mesa, L.; Quintero, G.; Liliana, A.; Vizcaíno, G.; Jaimes, D.C. Bioremediación de suelos contaminados con hidrocarburos derivados del petróleo. Nova 2006, 4, 82–90. (In Spanish) [Google Scholar] [CrossRef]
- Haritash, A.K.; Kaushik, C.P. Biodegradation aspects of Polycyclic Aromatic Hydrocarbons (PAHs): A review. J. Hazard. Mater. 2009, 169, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Soler, G.; Guerrero, A.; Alonso, M.D.; Cuesta, J.L. Biodegradation potential and molecular detection of the cathecol 1, 2-dioxygenase gene of actinobacteria isolated from wastewater treatment plants in Spain. Microbes Appl. Res. 2018, 44–48. [Google Scholar] [CrossRef]
- Lim, J.H.; Baek, S.; Lee, S.; Lee, S. Ferruginibacter lapsinanis sp. nov., novel members of the family ‘Chitinophagaceae’ in the phylum Bacteroidetes, isolated from freshwater sediment. Int. J. Syst. Evolut. Microbiol. 2009, 59, 2394–2399. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Lin, X.; Zhang, J.; Zeng, J. Soil Biology & Biochemistry Dissipation of polycyclic aromatic hydrocarbons (PAHs) in soil microcosms amended with mushroom cultivation substrate. Soil Biol. Biochemi. 2012, 47, 191–197. [Google Scholar] [CrossRef]
- Gao, S.; Seo, J.; Wang, J.; Keum, Y.; Li, J.; Li, Q.X. International Biodeterioration & Biodegradation Multiple degradation pathways of phenanthrene by Stenotrophomonas maltophilia C6. Int. Biodeterior. Biodegrad. 2013, 79, 98–104. [Google Scholar] [CrossRef]
- Mangwani, N.; Shukla, S.K.; Kumari, S.; Rao, T.S.; Das, S. Characterization of Stenotrophomonas acidaminiphila NCW-702 biofilm for implication in the degradation of polycyclic aromatic hydrocarbons. J. Appl. Microbiol. 2014, 117, 1012–1024. [Google Scholar] [CrossRef] [PubMed]

| Sample | Diversity Indexes | |||
|---|---|---|---|---|
| Reads | Chao | Shannon | Simpson | |
| OW | 390,118 | 37,123 | 7.37 | 0.9576 |
| PHE | 365,294 | 39,895 | 7.71 | 0.9508 |
| NAP | 390,788 | 32,348 | 6.55 | 0.9426 |
| PYR | 376,710 | 36,657 | 6.83 | 0.9249 |
| Microorganisms | Phylum |
|---|---|
| Archaea | Crenarchaeota |
| Bacteria | Thermi |
| Acidobacteria | |
| Actinobacteria | |
| Armatimonadetes | |
| Bacteroidetes | |
| Chloroflexi | |
| Cyanobacteria | |
| Fibrobacteres | |
| Firmicutes | |
| Fusobacteria | |
| Gemmatimonadetes | |
| Planctomycetes | |
| Proteobacteria | |
| Synergistetes | |
| Tenericutes | |
| Verrucomicrobia |
| Phylum/Sample | Ore Waste | Phenanthrene | Naphthalene | Pyrene |
|---|---|---|---|---|
| Proteobacteria | 92.6 | 72.7 | 4.3 | 53.5 |
| Actinobacteria | 5.5 | 25.8 | 95 | 0.4 |
| Bacteroidetes | 0.5 | 0.01 | 0.2 | 44.6 |
| Unidentified | 1.1 | 1.4 | 0.4 | 1.2 |
| Genus/Sample | Ore Waste | Phenantrene | Naphthalene | Pyrene |
|---|---|---|---|---|
| Pseudomonas | 25.3 * | 4.7 | 0.2 | 0.01 |
| Achromobacter | 18.1 * | 0.5 | 0.01 | 1.1 |
| Sphingomonas | 9.8 * | 0.4 | 0.01 | 2.7 |
| Burkholderia | 7.4 * | 3.8 | 0.1 | 0.1 |
| Stenotrophomonas | 5.5 * | 23.0 * | 0.1 | 4.6 * |
| Ralstonia | 0.1 | 9.0 * | 0.4 * | 0.0 |
| Williamsia | 0.01 | 8.1 * | 51.2 * | 0.1 |
| Cryocola | 0.2 | 5.9 * | 18.9 * | 0.01 |
| Microbacterium | 0.1 | 5.7 * | 11.8 * | 0.01 |
| Serratia | 0.2 | 1.0 | 2.6 * | 0.01 |
| Chitinophagaceae | 0.01 | 0.01 | 0.01 | 40.9 * |
| Salinispora | 0.01 | 0.01 | 0.01 | 9.8 * |
| Mesorhizobium | 0.1 | 0.6 | 0.01 | 8.2 * |
| Chitinophaga | 0.01 | 0.01 | 0.01 | 3.1 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Enríquez, E.G.; Zavala-Díaz de la Serna, F.J.; Peralta-Pérez, M.D.R.; Ballinas-Casarrubias, L.; Salmerón, I.; Rubio-Arias, H.; Rocha-Gutiérrez, B.A. Characterization of a Microbial Consortium for the Bioremoval of Polycyclic Aromatic Hydrocarbons (PAHs) in Water. Int. J. Environ. Res. Public Health 2018, 15, 975. https://doi.org/10.3390/ijerph15050975
Blanco-Enríquez EG, Zavala-Díaz de la Serna FJ, Peralta-Pérez MDR, Ballinas-Casarrubias L, Salmerón I, Rubio-Arias H, Rocha-Gutiérrez BA. Characterization of a Microbial Consortium for the Bioremoval of Polycyclic Aromatic Hydrocarbons (PAHs) in Water. International Journal of Environmental Research and Public Health. 2018; 15(5):975. https://doi.org/10.3390/ijerph15050975
Chicago/Turabian StyleBlanco-Enríquez, Esmeralda G., Francisco Javier Zavala-Díaz de la Serna, María Del Rosario Peralta-Pérez, Lourdes Ballinas-Casarrubias, Iván Salmerón, Héctor Rubio-Arias, and Beatriz A. Rocha-Gutiérrez. 2018. "Characterization of a Microbial Consortium for the Bioremoval of Polycyclic Aromatic Hydrocarbons (PAHs) in Water" International Journal of Environmental Research and Public Health 15, no. 5: 975. https://doi.org/10.3390/ijerph15050975
APA StyleBlanco-Enríquez, E. G., Zavala-Díaz de la Serna, F. J., Peralta-Pérez, M. D. R., Ballinas-Casarrubias, L., Salmerón, I., Rubio-Arias, H., & Rocha-Gutiérrez, B. A. (2018). Characterization of a Microbial Consortium for the Bioremoval of Polycyclic Aromatic Hydrocarbons (PAHs) in Water. International Journal of Environmental Research and Public Health, 15(5), 975. https://doi.org/10.3390/ijerph15050975







