Antibiotic-Resistant Pathogenic Escherichia Coli Isolated from Rooftop Rainwater-Harvesting Tanks in the Eastern Cape, South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Sample Collection
2.2. Enumeration and Isolation of E. coli
2.3. Identification of Pathogenic Escherichia coli Strains Using Polymerase Chain Reaction (PCR)
DNA Extraction and Detection of Virulence Genes in E. coli Isolates
2.4. Screening for Antibiotic-Resistant E. coli
2.5. Data Analysis
3. Results
3.1. Concentration of E. coli in Harvested Rainwater (HRW)
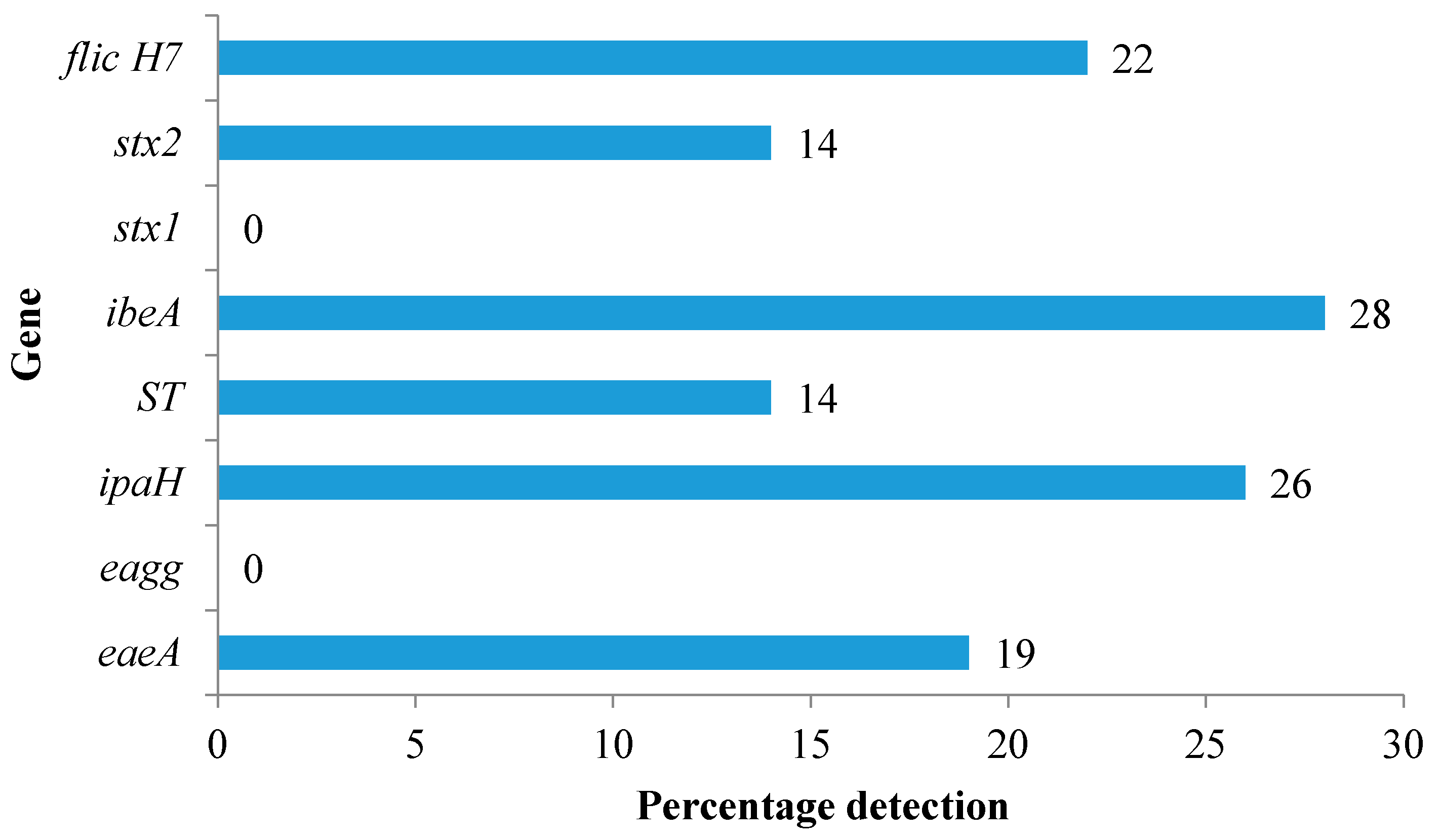
3.2. Identification of Virulence Genes among E. coli Isolates
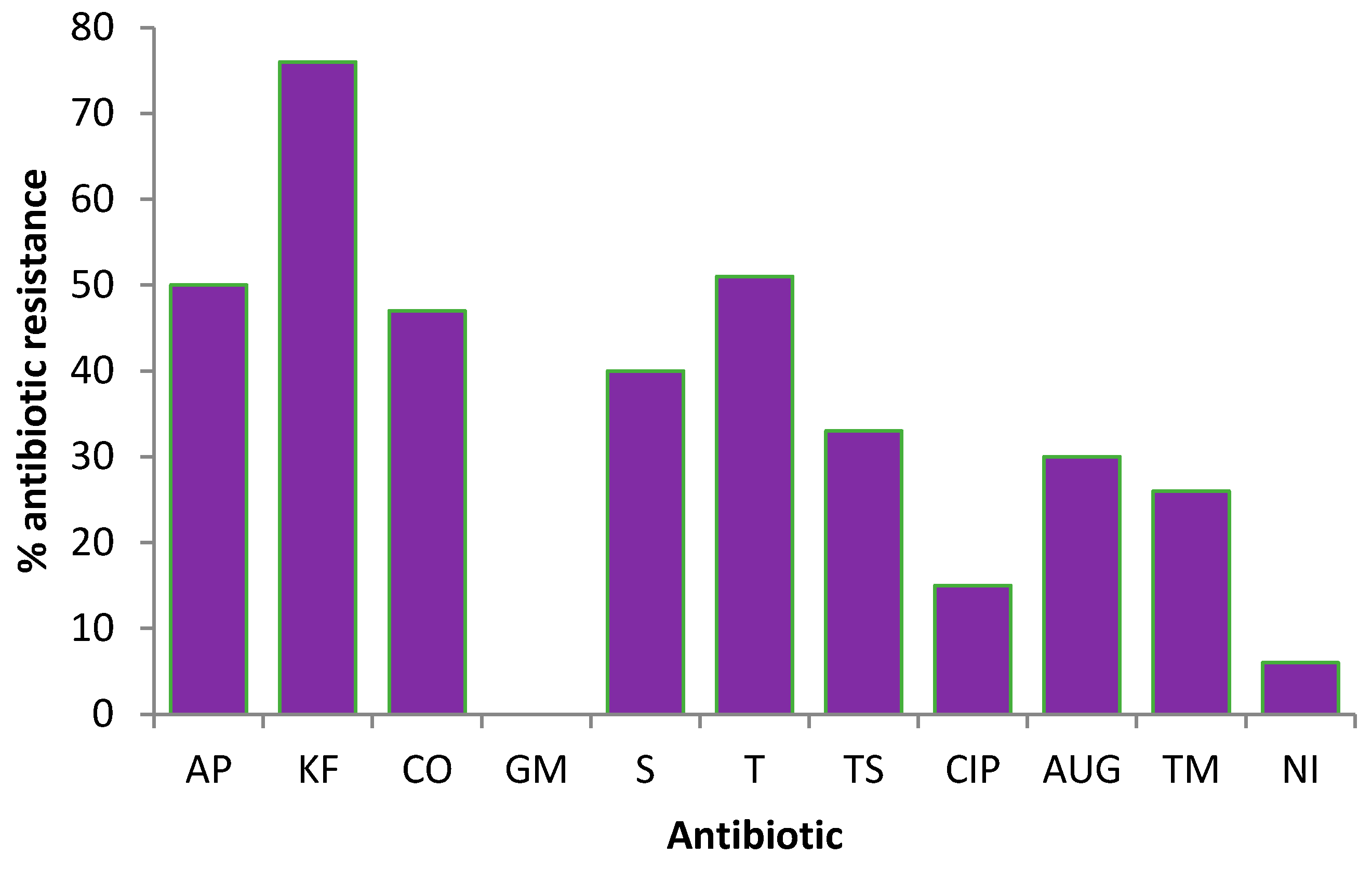
3.3. Antibiotic-Resistance Profiles of E. coli Isolated from the Harvested-Rainwater Samples
3.3.1. Overall Antibiotic Resistance Profiles of the E. coli
3.3.2. Prevalence of Multiple-Antibiotic Resistance
4. Discussion
4.1. Concentration of E. coli in Harvested Rainwater
4.2. Identification of Virulence Genes among E. coli Isolates
4.3. Detection of Antibiotic-Resistant E. coli in Harvested Rainwater
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ahmed, W.; Hodgers, L.; Masters, N.; Sidhu, J.P.S.; Katouli, M.; Toze, S. Occurrence of intestinal and extraintestinal virulence genes in Escherichia coli isolates from rainwater tanks in Southeast Queensland, Australia. Appl. Environ. Microbiol. 2011, 77, 7394–7400. [Google Scholar] [CrossRef] [PubMed]
- Masters, N.; Wiegand, A.; Ahmed, W.; Katouli, M. Escherichia coli virulence genes profile of surface waters as an indicator of water quality. Water Res. 2011, 45, 6321–6333. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Johnson, J.R. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli: ExPEC. J. Infect. Dis. 2000, 181, 1753–1754. [Google Scholar] [CrossRef] [PubMed]
- Canizalez-Roman, A.; Gonzalez-Nuñez, E.; Vidal, J.E.; Flores-Villaseñor, H.; León-Sicairos, N. Prevalence and antibiotic resistance profiles of diarrheagenic Escherichia coli strains isolated from food items in northwestern Mexico. Int. J. Food Microbiol. 2013, 164, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Aslani, M.M.; Bouzari, S. Escherichia coli: A brief review of diarrheagenic pathotypes and their role in diarrheal diseases in Iran. Iran. J. Microbiol. 2012, 4, 102–117. [Google Scholar] [PubMed]
- Servin, A.L. Pathogenesis of human diffusely adhering Escherichia coli expressing Afa/Dr adhesins (Afa/Dr DAEC): Current insights and future challenges. Clin. Microbiol. Rev. 2014, 27, 823–869. [Google Scholar] [CrossRef] [PubMed]
- Karmali, M.A.; Gannon, V.; Sargeant, J.M. Verocytotoxin-producing Escherichia coli (VTEC). Vet. Microbiol. 2010, 140, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Viazis, S.; Diez-Gonzalez, F. Enterohemorrhagic Escherichia coli. In The Twentieth Century’s Emerging Foodborne Pathogen: A Review, 1st ed.; Elsevier Inc.: New York, NY, USA, 2011; Volume 111. [Google Scholar]
- Anastasi, E.M.; Matthews, B.; Gundogdu, A.; Vollmerhausen, T.L.; Ramos, N.L.; Stratton, H.; Ahmed, W.; Katouli, M. Prevalence and persistence of Escherichia coli strains with uropathogenic virulence characteristics in sewage treatment plants. Appl. Environ. Microbiol. 2010, 76, 5882–5886. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.F.M.; Monteiro Neto, V.; Santos, B.R.d.C.; Costa, B.R.R.; Azevedo, A.; Serra, J.L.; Mendes, H.B.R.; Nascimento, A.R.; Mendes, M.B.P.; Kuppinger, O. Enterobacteria identification and detection of diarrheagenic Escherichia coli in a Port Complex. Braz. J. Microbiol. 2014, 45, 945–952. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abia, A.L.K.; Ubomba-Jaswa, E.; Momba, M.N.B. Occurrence of diarrhoeagenic Escherichia coli virulence genes in water and bed sediments of a river used by communities in Gauteng, South Africa. Environ. Sci. Pollut. Res. 2016, 23, 15665–15674. [Google Scholar] [CrossRef] [PubMed]
- Dobrowsky, P.H.; van Deventer, A.; De Kwaadsteniet, M.; Ndlovu, T.; Khan, S.; Cloete, T.E.; Khan, W. Prevalence of virulence genes associated with pathogenic Escherichia coli strains isolated from domestically harvested rainwater during low- and high-rainfall periods. Appl. Environ. Microbiol. 2014, 80, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.J.; van Vuuren, C.; Joubert, G. Antimicrobial resistance patterns in outpatient urinary tractinfections—The constant need to revise prescribing habits. S. Afr. Med. J. 2011, 101, 328–331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Habte, T.M.; Dube, S.; Ismail, N.; Hoosen, A.A. Hospital and community isolates of uropathogens at a tertiary hospital in South Africa. S. Afr. Med. J. 2009, 99, 584–587. [Google Scholar] [PubMed]
- Detels, R.; Gulliford, M.; Karim, Q.A.; Tan, C.C.; Press, O.U. Oxford Textbook of Global Public Health: The Practice of Public Health, 6th ed.; Detels, R., Beaglehole, R., Lansang, M., Gulliford, M., Eds.; Oxford University: New York, NY, USA, 2015; Volume 3. [Google Scholar]
- Xu, Y.; Guo, C.; Luo, Y.; Lv, J.; Zhang, Y.; Lin, H.; Wang, L.; Xu, J. Occurrence and distribution of antibiotics, antibiotic resistance genes in the urban rivers in Beijing, China. Environ. Pollut. 2016, 213, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Kinge, W.C.N.; Ateba, C.N.; Kawadza, D.T. Antibiotic resistance profiles of Escherichia coli isolated from different water sources in the Mmabatho locality, Northwest Province, South Africa. S. Afr. J. Sci. 2010, 106, 44–49. [Google Scholar]
- Adefisoye, M.A.; Okoh, A.I. Identification and antimicrobial resistance prevalence of pathogenic Escherichia coli strains from treated wastewater effluents in Eastern Cape, South Africa. Microbiologyopen 2016, 5, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Abia, A.L.K.; Schaefer, L.; Ubomba-Jaswa, E.; Le Roux, W. Abundance of pathogenic Escherichia coli virulence-associated genes in well and borehole water used for domestic purposes in a peri-urban community of South Africa. Int. J. Environ. Res. Public Health 2017, 14, 320. [Google Scholar] [CrossRef] [PubMed]
- Abia, A.L.K.; Ubomba-Jaswa, E.; Momba, M.N.B. High prevalence of multiple-antibiotic-resistant (MAR) Escherichia coli in river bed sediments of the Apies River, South Africa. Environ. Monit. Assess. 2015, 187, 652. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Tsen, H.Y. PCR primers designed from malic acid dehydrogenase gene and their use for detection of Escherichia coli in water and milk samples. Int. J. Food Microbiol. 2001, 64, 1–11. [Google Scholar] [CrossRef]
- Caine, L.; Nwodo, U.; Okoh, A.; Ndip, R.; Green, E. Occurrence of virulence genes associated with diarrheagenic Escherichia coli isolated from raw cow’s milk from two commercial dairy farms in the Eastern Cape Province, South Africa. Int. J. Environ. Res. Public Health 2014, 11, 11950–11963. [Google Scholar] [CrossRef] [PubMed]
- Titilawo, Y.; Obi, L.; Okoh, A. Occurrence of virulence gene signatures associated with diarrhoeagenic and non-diarrhoeagenic pathovars of Escherichia coli isolates from some selected rivers in South-Western Nigeria. BMC Microbiol. 2015, 15, 204. [Google Scholar] [CrossRef] [PubMed]
- Omar, K.B.; Barnard, T.G. Detection of diarrhoeagenic Escherichia coli in clinical and environmental water sources in South Africa using single-step 11-gene m-PCR. World J. Microbiol. Biotechnol. 2014, 30, 2663–2671. [Google Scholar] [CrossRef] [PubMed]
- Omar, K.B.; Potgieter, N.; Barnard, T.G. Development of a rapid screening method for the detection of pathogenic Escherichia coli using a combination of Colilert® Quanti-Trays/2000 and PCR. Water Sci. Technol. Water Supply 2010, 10, 7–13. [Google Scholar] [CrossRef]
- Stange, C.; Sidhu, J.P.S.; Tiehm, A.; Toze, S. Antibiotic resistance and virulence genes in coliform water isolates. Int. J. Hyg. Environ. Health 2016, 219, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Norusis, M. SPSS 16.0 Statistical Procedures Companion; Prentice Hall Press: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to indentify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [PubMed]
- Hachich, E.M.; Di Bari, M.; Christ, A.P.G.; Lamparelli, C.C.; Ramos, S.S.; Sato, M.I.Z. Comparison of thermotolerant coliforms and Escherichia coli densities in freshwater bodies. Braz. J. Microbiol. 2012, 43, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Spinks, J.; Phillips, S.; Robinson, P.; Van Buynder, P. Bushfires and tank rainwater quality: A cause for concern? J. Water Health 2006, 4, 21–28. [Google Scholar] [PubMed]
- Sazakli, E.; Alexopoulos, A.; Leotsinidis, M. Rainwater harvesting, quality assessment and utilization in Kefalonia Island, Greece. Water Res. 2007, 41, 2039–2047. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Sidhu, J.P.S.; Toze, S. An attempt to identify the likely sources of Escherichia coli harboring toxin genes in rainwater tanks. Environ. Sci. Technol. 2012, 46, 5193–5197. [Google Scholar] [CrossRef] [PubMed]
- Chidamba, L.; Korsten, L. Antibiotic resistance in Escherichia coli isolates from roof-harvested rainwater tanks and urban pigeon faeces as the likely source of contamination. Environ. Monit. Assess. 2015, 187, 405. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, W.; Hamilton, K.; Gyawali, P.; Toze, S.; Haas, C. Evidence of avian and possum fecal contamination in rainwater tanks as determined by microbial source tracking approaches. Appl. Environ. Microbiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.J.; Wannemuehler, Y.; Kariyawasam, S.; Johnson, J.R.; Logue, C.M.; Nolan, L.K. Prevalence of avian-pathogenic Escherichia coli strain O1 genomic islands among extraintestinal and commensal E. coli isolates. J. Bacteriol. 2012, 194, 2846–2853. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamilton, M.J.; Hadi, A.Z.; Griffith, J.F.; Ishii, S.; Sadowsky, M.J. Large scale analysis of virulence genes in Escherichia coli strains isolated from Avalon Bay, CA. Water Res. 2010, 44, 5463–5473. [Google Scholar] [CrossRef] [PubMed]
- Spinks, A.T.; Dunstan, R.H.; Coombes, P.; Kuczera, G. Thermal destruction analyses of water related pathogens at domestic hot water system temperatures. In Proceedings of the 28th International Hydrology and Water Resources Symposium, Wollongong, Australia, 10–13 November 2003. [Google Scholar]
- Dobrowsky, P.H.; Carstens, M.; De Villiers, J.; Cloete, T.E.; Khan, W. Efficiency of a closed-coupled solar pasteurization system in treating roof harvested rainwater. Sci. Total Environ. 2015, 536, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.T.; Nawaz, M.; Amin, M.N.; Han, M. Solar disinfection of Pseudomonas aeruginosa in harvested rainwater: A step towards potability of rainwater. PLoS ONE 2014, 9, e90743. [Google Scholar] [CrossRef] [PubMed]
- Essack, S.Y.; Schellack, N.; Pople, T.; van der Merwe, L.; Suleman, F.; Meyer, J.C.; Gous, A.G.S.; Benjamin, D. Part III. GARP: Antibiotic supply chain and management in human health. S. Afr. Med. J. 2011, 101, 562–566. [Google Scholar] [PubMed]
- Ruhe, J.J.; Menon, A. Tetracyclines as an oral treatment option for patients with community onset skin and soft tissue infections caused by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2007, 51, 3298–3303. [Google Scholar] [CrossRef] [PubMed]
- Beceiro, A.; Tomás, M.; Bou, G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? Clin. Microbiol. Rev. 2013, 26, 185–230. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Bonomo, R.A. Extended-Spectrum beta-Lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef] [PubMed]
- Haenni, M.; Châtre, P.; Madec, J.Y. Emergence of Escherichia coli producing extended-spectrum AmpC β-lactamases (ESAC) in animals. Front. Microbiol. 2014, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
| Class | Antibiotic | Abbreviation | Concentration (µg) |
|---|---|---|---|
| β-Lactams | Ampicillin | AP | 10 |
| Cephalothin | KF | 5 | |
| Polypeptides | Colistin sulphate | CO | 25 |
| Aminoglycosides | Gentamicin | GM | 10 |
| Aminoglycosides | Streptomicin | S | 10 |
| Tetracyclines | Tetracycline | T | 25 |
| Folate pathway inhibitors | Cotrimoxazole | TS | 25 |
| Fluoroquinolones | Ciprofloxacin | CIP | 5 |
| Penicillin combination | Augmentin(amoxillin-clavulanate) | AUG | 30 |
| Sulfonamides | Trimethoprim | TM | 5 |
| Nitrofurans | Nitrofurantoin | NI | 300 |
| Tank ID | n | Minimum | Maximum | Mean ± Standard Deviation |
|---|---|---|---|---|
| T1 | 11 | 2.55 | 3.29 | 3.02 ± 0.21 |
| T2 | 11 | 1.95 | 3.11 | 2.62 ± 0.35 |
| T3 | 11 | 2.58 | 3.29 | 2.84 ± 0.25 |
| T4 | 11 | 1.64 | 2.89 | 2.52 ± 0.42 |
| T5 | 11 | 0.79 | 3.00 | 2.18 ± 0.82 |
| T6 | 11 | 2.53 | 3.04 | 2.88 ± 0.19 |
| T7 | 10 | 1.73 | 2.96 | 2.36 ± 0.37 |
| T8 | 10 | 1.78 | 2.41 | 2.09 ± 0.22 |
| T9 | 7 | 0.61 | 3.04 | 1.71 ± 0.82 |
| T10 | 10 | 0.3 | 3.19 | 1.57 ± 1.04 |
| T11 | 7 | 0.61 | 1.12 | 0.85 ± 0.26 |
| Tank Location | Tank ID | Number of E. coli Isolates Tested | EaeA (EPEC/EHEC) | Eagg (EAEC) | ipaH (EIEC) | ST (ETEC) | ibeA (NMEC) | Stx1 (EHEC) | Stx2 (EHEC) | flichH7 (EHEC) |
|---|---|---|---|---|---|---|---|---|---|---|
| Rhodes University | T1 | 11 | 6 (55%) | 0 | 4 (36%) | 0 | 4 (36%) | 0 | 2 (18%) | 4 (36%) |
| Rhodes University | T2 | 11 | 0 | 0 | 0 | 1 (9%) | 1 (9%) | 0 | 0 | 2 (18%) |
| Rhodes University | T3 | 11 | 1 (9%) | 0 | 0 | 0 | 3 (27%) | 0 | 0 | 1 (9%) |
| Rhodes University | T4 | 11 | 2 (18%) | 0 | 1 (9%) | 0 | 4 (36%) | 0 | 0 | 3 (27%) |
| Rhodes University | T5 | 11 | 1 (9%) | 0 | 0 | 0 | 4 (36%) | 0 | 0 | 2 (18%) |
| Rhodes University | T6 | 11 | 1 (9%) | 0 | 2 (18%) | 2 (18%) | 8 (72%) | 0 | 0 | 3 (27%) |
| Kenton-on-sea | T7 | 10 | 2 (20%) | 0 | 0 | 0 | 2 (20%) | 0 | 0 | 2 (20%) |
| Kenton-on-sea | T8 | 10 | 0 | 0 | 4 (40%) | 0 | 2 (20%) | 0 | 1(10%) | 2 (20%) |
| Grahamstown west | T9 | 7 | 1 (14%) | 0 | 0 | 0 | 1 (13%) | 0 | 0 | 0 |
| Grahamstown west | T11 | 7 | 0 | 0 | 0 | 0 | 1 (13%) | 0 | 0 | 0 |
| Tank ID | n | % Resistance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AP | KF | CO | GM | S | T | TS | CIP | AUG | TM | NI | ||
| T1 | 11 | 72 | 91 | 63 | 0 | 45 | 72 | 39 | 27 | 27 | 63 | 9 |
| T2 | 11 | 72 | 81 | 36 | 0 | 27 | 45 | 45 | 9 | 54 | 45 | 18 |
| T3 | 11 | 27 | 36 | 27 | 0 | 45 | 18 | 27 | 18 | 9 | 18 | 0 |
| T4 | 11 | 36 | 90 | 54 | 0 | 36 | 36 | 27 | 0 | 9 | 27 | 0 |
| T5 | 11 | 36 | 100 | 54 | 0 | 0 | 54 | 36 | 27 | 18 | 45 | 0 |
| T6 | 11 | 45 | 100 | 45 | 0 | 18 | 36 | 36 | 0 | 9 | 27 | 0 |
| T7 | 10 | 30 | 30 | 20 | 0 | 100 | 20 | 30 | 20 | 30 | 30 | 0 |
| T8 | 10 | 30 | 100 | 10 | 0 | 20 | 10 | 0 | 0 | 40 | 0 | 0 |
| T9 | 7 | 75 | 87 | 75 | 0 | 62 | 100 | 87 | 0 | 75 | 50 | 0 |
| T11 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 |
| T1 | T2 | ||
|---|---|---|---|
| MAR Phenotype | Number of Isolates | MAR Phenotype | Number of Isolates |
| AP-KF-CO-T | 1 | KF-T-NI | 1 |
| AP-KF-CO-T-TM | 1 | AP-KF-AUG | 1 |
| AP-KF-CO-S-T-TS-TM | 1 | AP-KF-NI | 1 |
| AP-KF-CO-S-T-TS-CIP-AUG-TM | 1 | AP-KF-S-T-TM | 1 |
| AP-KF-CO-S-T-TS-CIP-TM | 1 | AP-KF-CO-T-AUG | 1 |
| KF-CO-S-T-TS-AUG-TM | 1 | AP-KF-CO-S-T-TS-TM | 1 |
| AP-KF-CO-S-TS-CIP-TM | 1 | AP-KF-CO-S-T-TS-CIP-AUG-TM | 1 |
| AP-KF-CO-S-T-TS-AUG-TM | 1 | AP-KF-CO-T-TS-CIP-AUG-TM | 1 |
| AP-KF-CO-S-T-TS-AUG-TM | 1 | ||
| T3 | T4 | ||
| MAR Phenotype | Number of Isolates | MAR Phenotype | Number of Isolates |
| AP-KF-CO-S-TS-CIP-TM | 1 | KF-ST-AUG | 1 |
| AP-KF-CO-S-T-TS-TM | 1 | KF-T-NI | 1 |
| AP-KF-CO-S-T-TS-CIP-AUG-TM | 1 | AP-KF-CO-S-T-TS-TM | 2 |
| AP-KF-CO-S-T-TS-CIP-TM | 1 | ||
| AP-KF-CO-S-T-TS-CIP-AUG-TM | 2 | ||
| T5 | T6 | ||
| MAR Phenotype | Number of Isolates | MAR Phenotype | Number of Isolates |
| AP-KF-CO-S-T-TS-CIP-TM | 1 | AP-KF-AUG | 1 |
| AP-KF-CO-T-TS-TM | 1 | KF-CO-S-T-TS-AUG-TM | 1 |
| AP-KF-CO-S-T-TS-CIP-AUG-TM | 1 | AP-KF-CO-S-TS-TM | 1 |
| KF-CO-T-TS-AUG-TM | 1 | AP-KF-CO-T-TS-TM-NI | 1 |
| AP-KF-CO-S-T-TS-CIP-AUG-TM | 1 | AP-KF-CO-S-T-TS-CIP-AUG-TM | 2 |
| T7 | T8 | ||
| MAR Phenotype | Number of Isolates | MAR Phenotype | Number of Isolates |
| AP-KF-CO-S-T-TS-CIP-AUG-TM | 1 | AP-KF-T | 1 |
| AP-KF-CO-S-TS-AUG-TM | 1 | KF-CO-S-TS | 1 |
| AP-KF-CO-S-T-CIP-AUG-TM | 1 | AP-KF-CO-S-T-TS-CIP-AUG-TM | 2 |
| AP-KF-CO-S-T-TS-AUG-TM | 1 | ||
| T9 | T11 | ||
| MAR Phenotype | Number of Isolates | MAR Phenotype | Number of Isolates |
| AP-KF-CO-S-T-TS-CIP-AUG-TM | 1 | AP-KF-CO-S-T-TS-AUG-TM | 2 |
| AP-KF-CO-S-T-TS-AUG-TM | 2 | AP-KF-CO-S-T-TS-TM | 1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malema, M.S.; Abia, A.L.K.; Tandlich, R.; Zuma, B.; Mwenge Kahinda, J.-M.; Ubomba-Jaswa, E. Antibiotic-Resistant Pathogenic Escherichia Coli Isolated from Rooftop Rainwater-Harvesting Tanks in the Eastern Cape, South Africa. Int. J. Environ. Res. Public Health 2018, 15, 892. https://doi.org/10.3390/ijerph15050892
Malema MS, Abia ALK, Tandlich R, Zuma B, Mwenge Kahinda J-M, Ubomba-Jaswa E. Antibiotic-Resistant Pathogenic Escherichia Coli Isolated from Rooftop Rainwater-Harvesting Tanks in the Eastern Cape, South Africa. International Journal of Environmental Research and Public Health. 2018; 15(5):892. https://doi.org/10.3390/ijerph15050892
Chicago/Turabian StyleMalema, Mokaba Shirley, Akebe Luther King Abia, Roman Tandlich, Bonga Zuma, Jean-Marc Mwenge Kahinda, and Eunice Ubomba-Jaswa. 2018. "Antibiotic-Resistant Pathogenic Escherichia Coli Isolated from Rooftop Rainwater-Harvesting Tanks in the Eastern Cape, South Africa" International Journal of Environmental Research and Public Health 15, no. 5: 892. https://doi.org/10.3390/ijerph15050892
APA StyleMalema, M. S., Abia, A. L. K., Tandlich, R., Zuma, B., Mwenge Kahinda, J.-M., & Ubomba-Jaswa, E. (2018). Antibiotic-Resistant Pathogenic Escherichia Coli Isolated from Rooftop Rainwater-Harvesting Tanks in the Eastern Cape, South Africa. International Journal of Environmental Research and Public Health, 15(5), 892. https://doi.org/10.3390/ijerph15050892







