Arsenic Concentration in the Surface Water of a Former Mining Area: The La Junta Creek, Baja California Sur, Mexico
Abstract
:1. Introduction
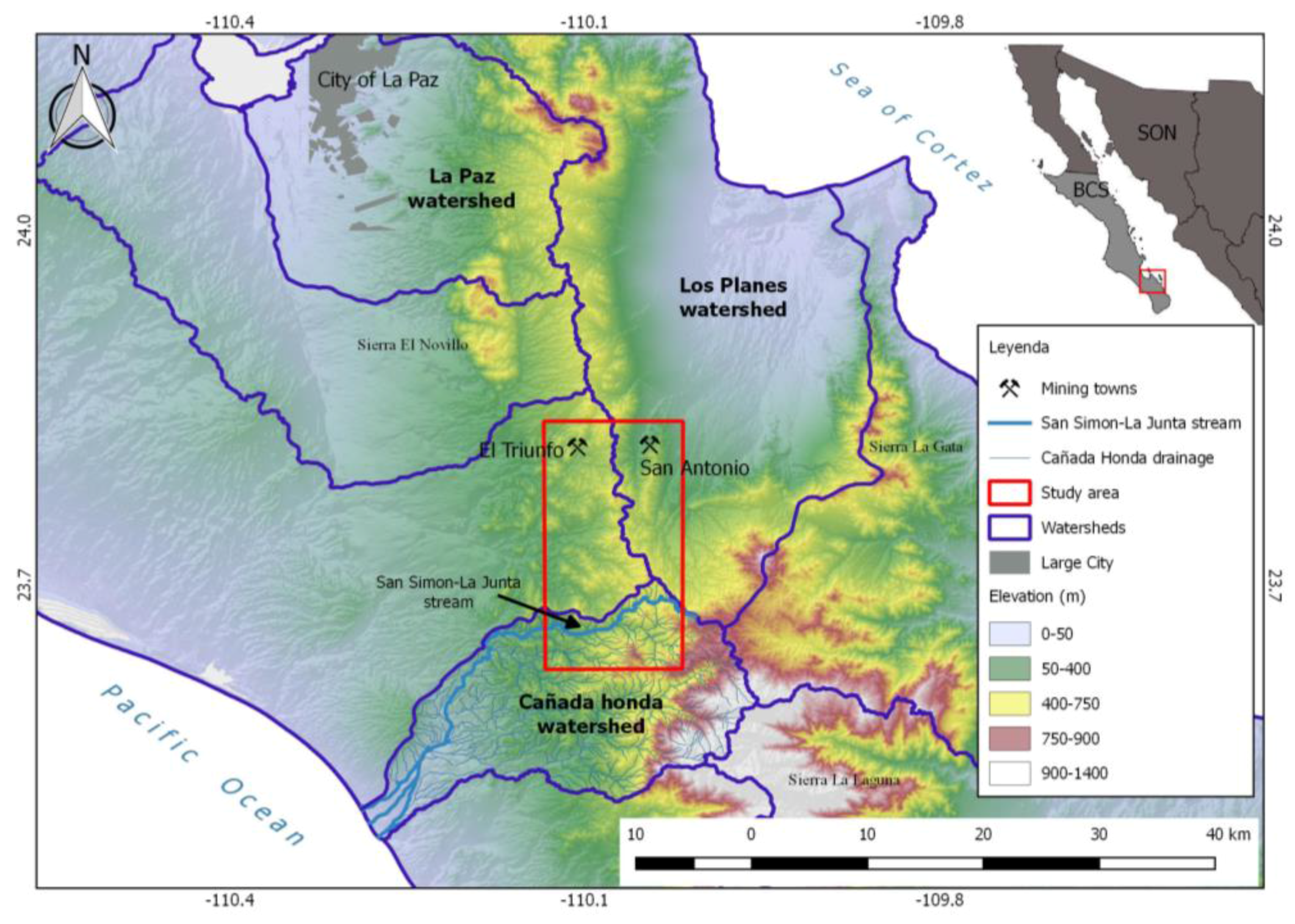
1.1. The Cañada Honda Watershed
1.2. The La Junta Sub-Basin
1.3. Purpose of the Study
2. Materials and Methods
2.1. Climate
2.2. Soil Types and Vegetation
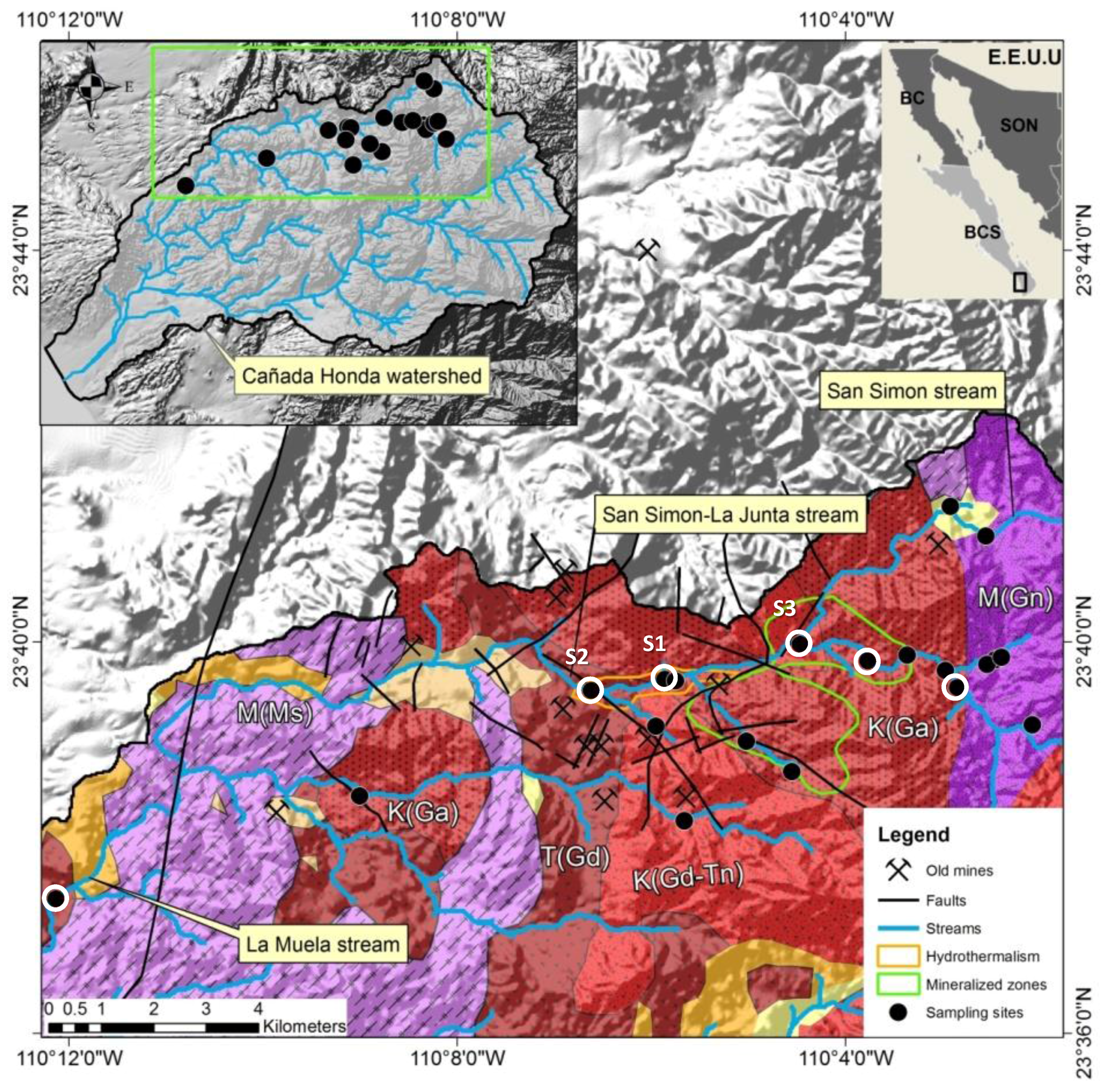
2.3. Geological Framework
2.4. Historical Mining Activity
2.5. Contamination with Arsenic and Byproducts in the Mining District
2.6. Contamination in the Cañada Honda Watershed
2.7. Analysis Revision
2.8. Statistical Analysis
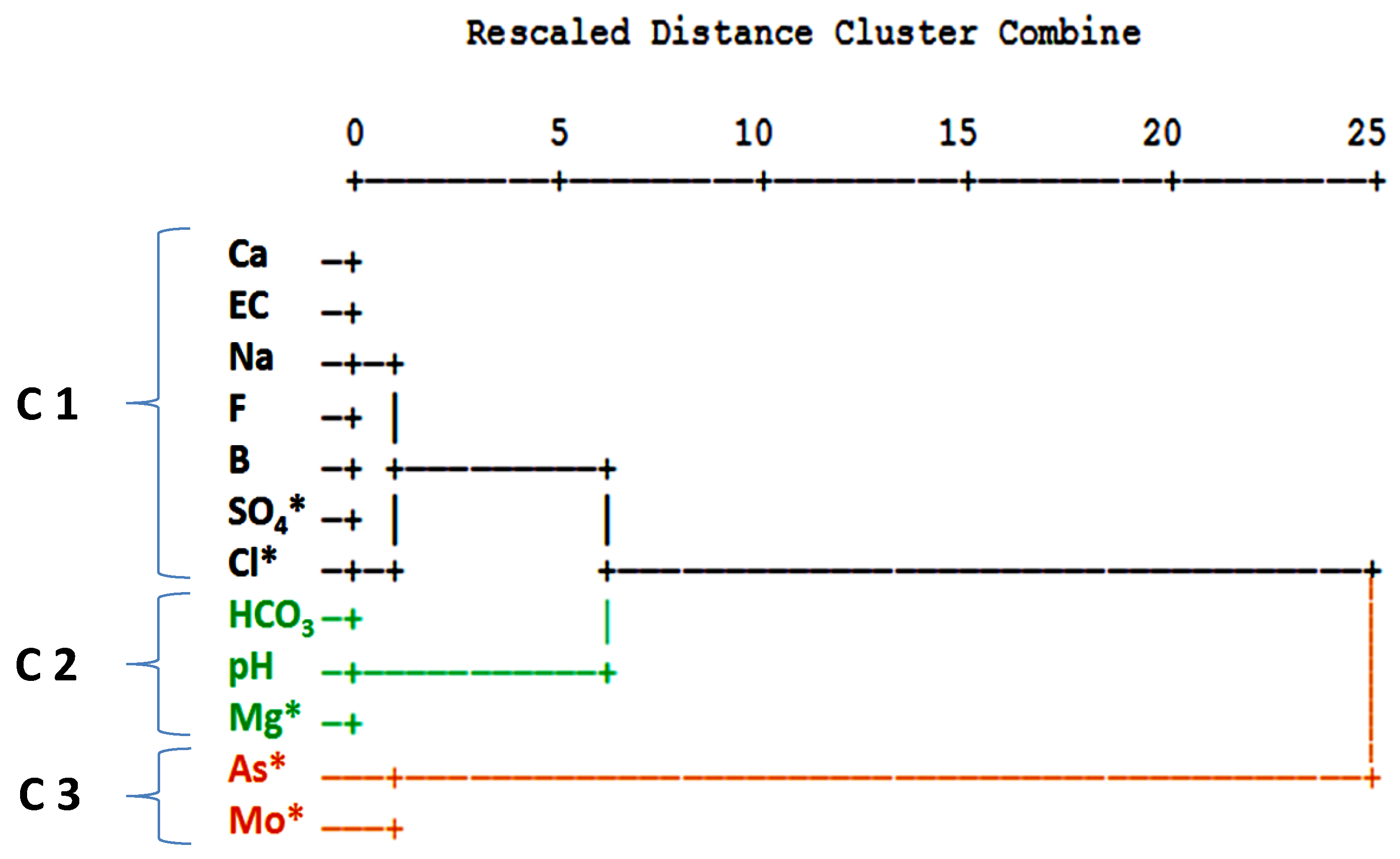
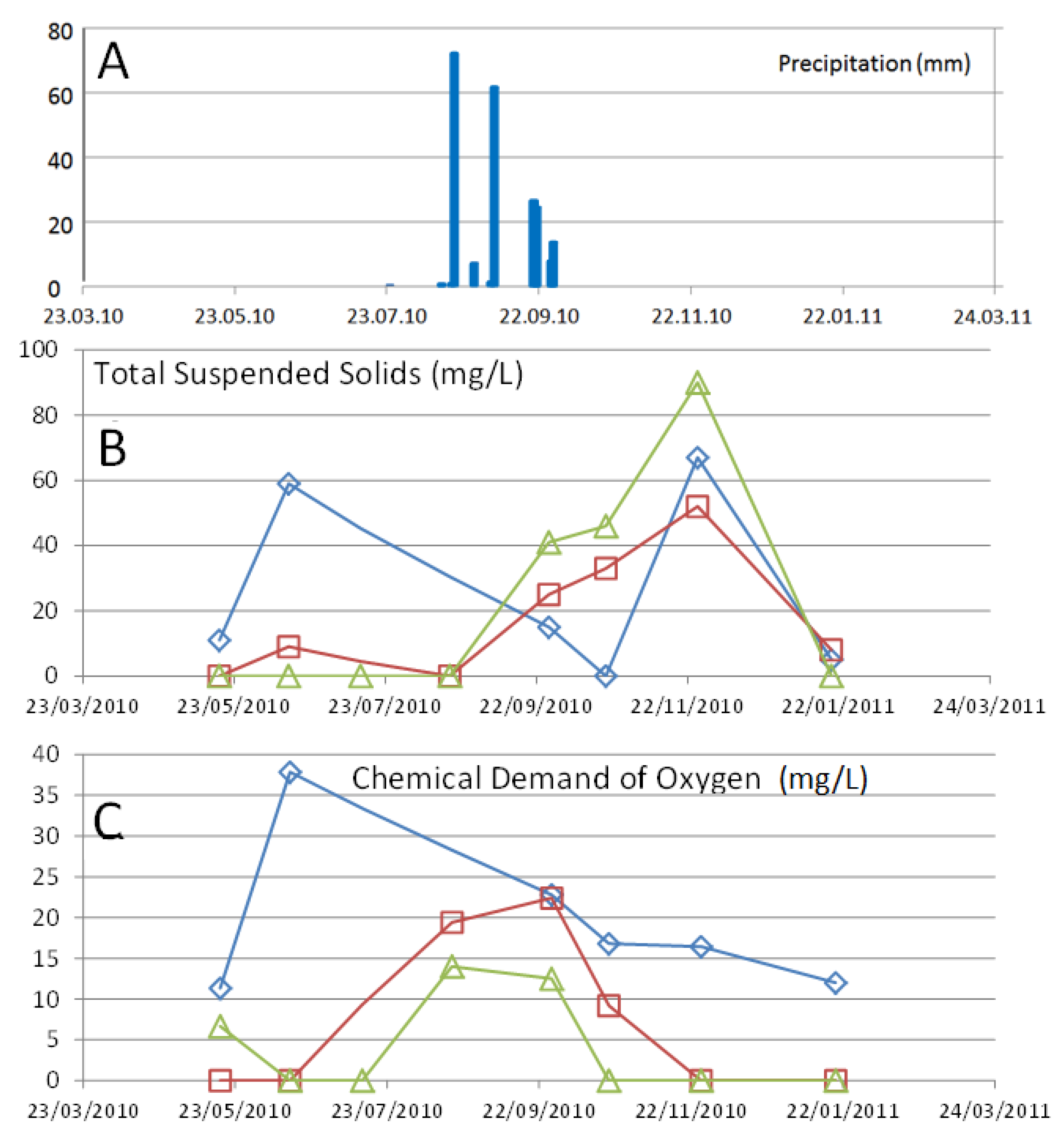
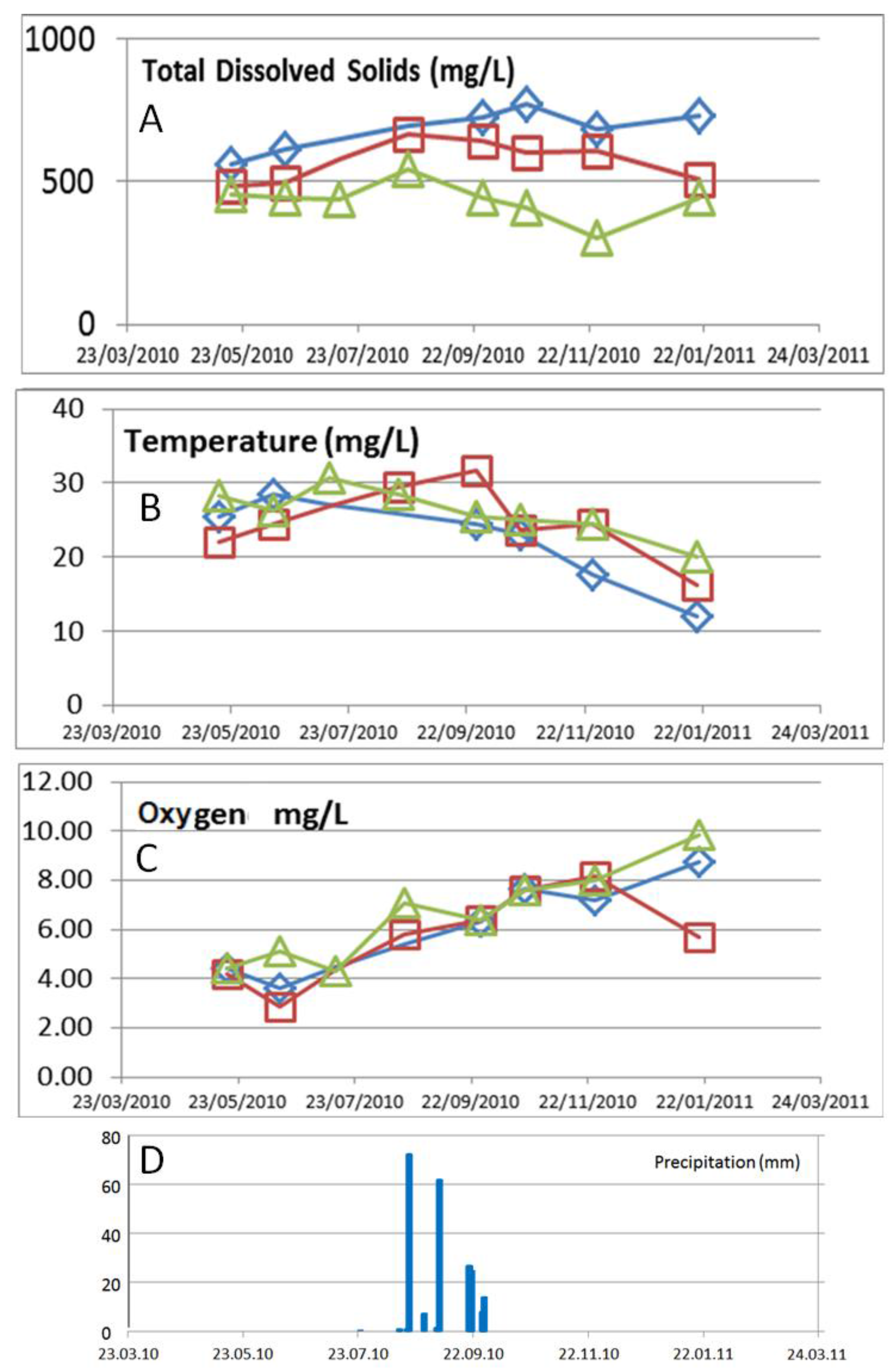
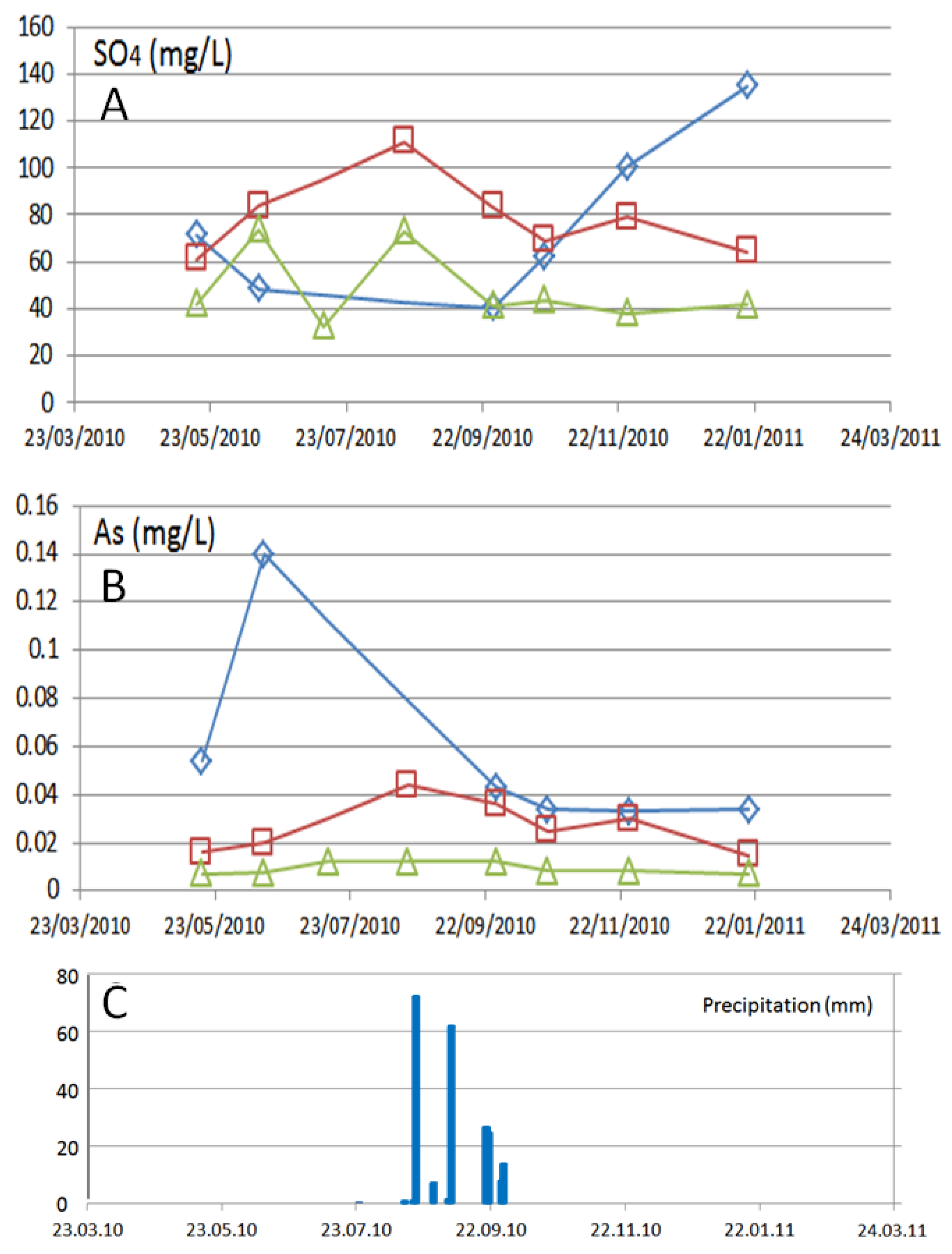
3. Results
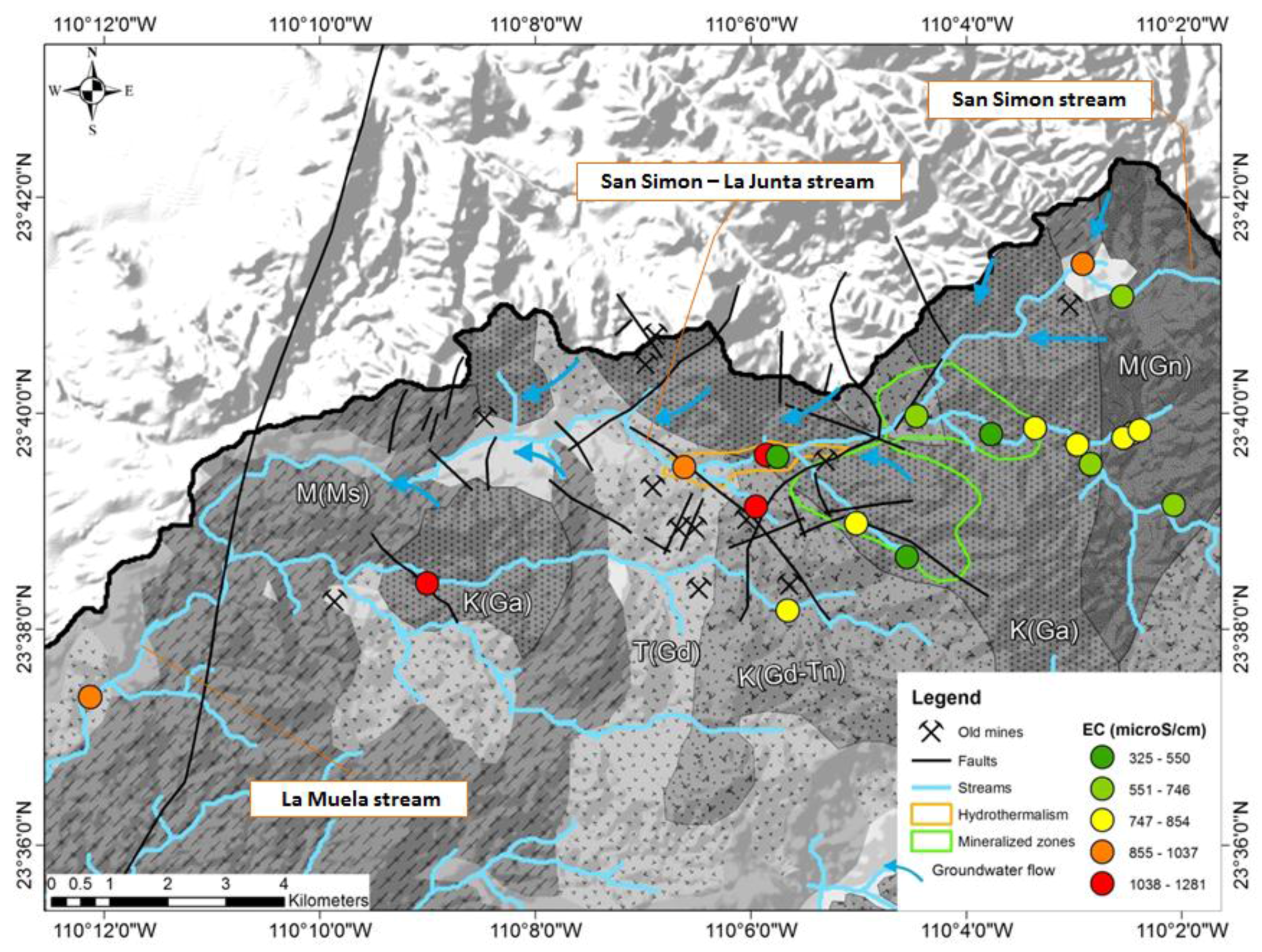
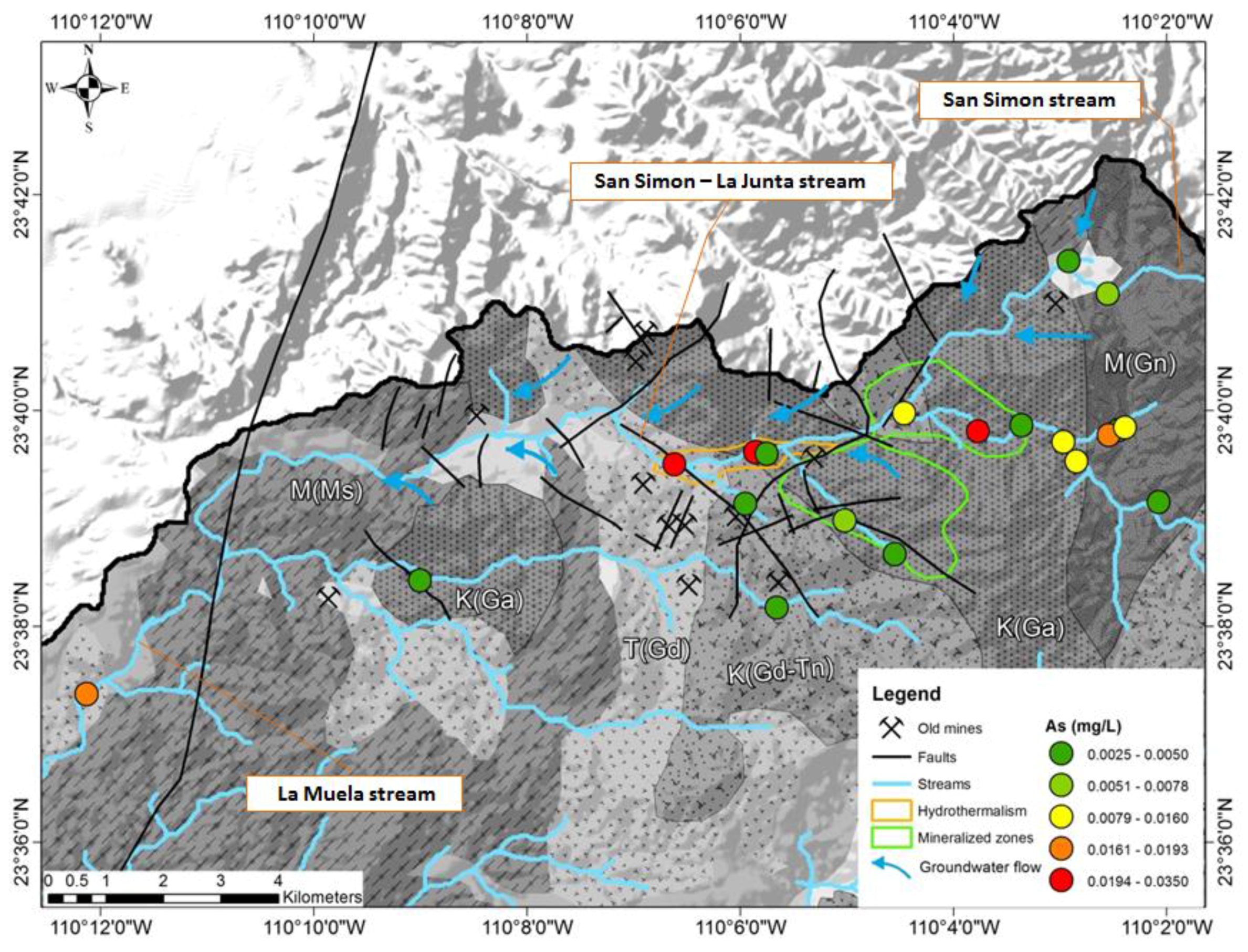
3.1. Statistical Analysis
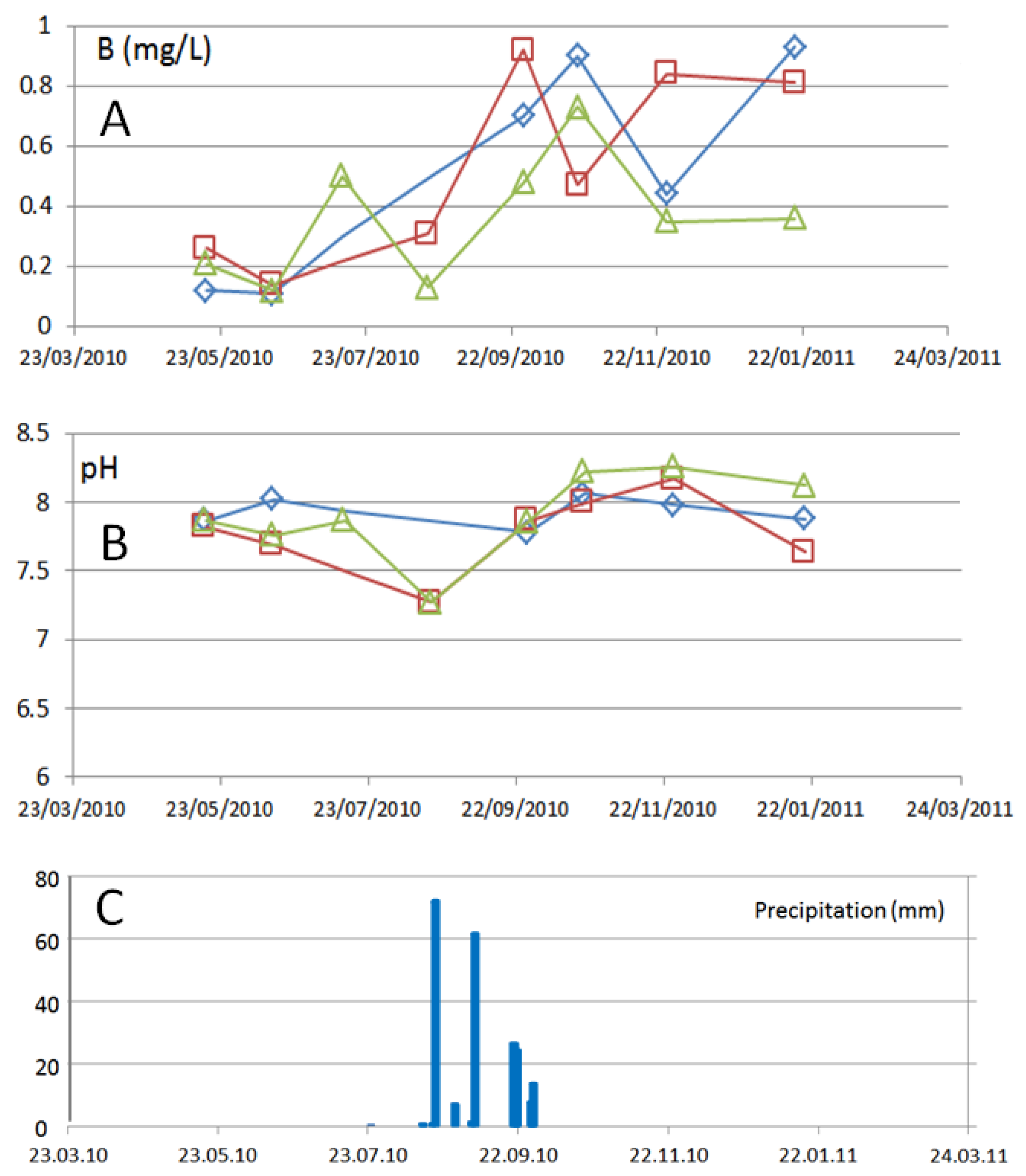
3.2. Temporal Variations
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cassassuce, F.; Olvera, J.; Loera-Pizarro, E. Estudio de la Calidad del Agua en 500 Pozos de Baja California Sur; Sociedad de Historia Natural Niparajá, A.C. en Colaboración con la CONAGUA: La Paz, Mexico, 2005; 27p. (In Spanish) [Google Scholar]
- Gutiérrez Caminero, L. Lead Isotopes as Tracers for Metal and Metalloid Sources in the San Antonio-El Triunfo Mining District, Baja California Sur. Master’s Thesis, CICESE, November 2013. Available online: https://cicese.repositorioinstitucional.mx/jspui/bitstream/1007/919/1/233611.pdf (accessed on 11 December 2017).
- Instituto Nacional de Estadística, Geografía e Informática, INEGI. Carta Topográfica 1:50,000, El Rosario F12B23. In stituto Nacional de Estadística, Geografía e Informática; INEGI City, México, 2002. Available online: http://internet.contenidos.inegi.org.mx/contenidos/Productos/prod_serv/contenidos/espanol/bvinegi/productos/geografia/imagen_cartografica/1_50_000/702825701604.pdf (accessed on 11 December 2017). (In Spanish)
- Vanderplank, S.; Wilder, B.T.; Ezcurra, E. Arroyo la Junta: Una Joya de Biodiversidad en la Reserva de la Biosfera Sierra la Laguna/a Biodiversity Jewel in the Sierra la Laguna Biosphere Reserve. Botanical Research Institute of Texas, Next Generation Sonoran Desert Researchers, and UC MEXUS, 2016. Available online: http://nextgensd.com/wp-content/uploads/2016/06/Los-Cardones_digital.pdf (accessed on 11 Decmber 2017). (In Spanish).
- Norma Oficial Mexicana NOM-059-SEMARNAT-2001, Protección Ambiental-Especies Nativas de México de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones para su Inclusión, Exclusión o Cambio-Lista de Especies en en Riesgo. 2001. Available online: http://www.biodiversidad.gob.mx/pdf/NOM-059-ECOL-2001.pdf (accessed on 11 December 2017). (In Spanish)
- Servicio Meteorológico Nacional SMN. Base de Datos de Precipitaciones, Evaporación y Temperaturas Diarias de la Estación Climatológica 3049 San Antonio. Available online: http://smn.cna.gob.mx/tools/RESOURCES/Normales8110/NORMAL03049.TXT (accessed on 5 August 2017). (In Spanish)
- Wurl, J.; Martínez Gutiérrez, G. El efecto de ciclones tropicales sobre el clima en la cuenca de Santiago, Baja California Sur, México. In Proceedings of the III Simposio Internacional en Ingeniería y Ciencias para la Sustentabilidad Ambiental y Semana del Ambiente, Santiago de Compostela, Mexico, 5–6 June 2006. (In Spanish). [Google Scholar]
- Cuevas Limón, L.F. Estudio Geohidrológico-Geofísico Realizado en la Región del Proyecto Minero “Paredones Amarillos”, Cuenca del Arroyo San Simón La Muela; Municipio de La Paz, B.C.S.: La Paz, Mexico, 1996. (In Spanish) [Google Scholar]
- Arriaga, L.; León de la Luz, J.L. The Mexican tropical deciduous forest of Baja California Sur: A floristic and structural approach. Vegetatio 1989, 84, 45–52. [Google Scholar] [CrossRef]
- Aranda-Gómez, J.J.; Pérez-Venzor, J.A. Estratigrafía del Complejo Cristalino de la región de Todos Santos, Estado de Baja California Sur. Revista 1989, 8, 149–170. (In Spanish) [Google Scholar]
- Fletcher, J.M.; Kohn, B.P.; Foster, D.A.; Gleadow, A.J.W. Heterogeneous Neogene cooling and exhumation of the Los Cabos block, southern Baja California: Evidence from fission-track thermochronology. Geology 2000, 28, 107–110. [Google Scholar] [CrossRef]
- Koutz, F.R. 1994–1995 Annual Report: Surficial Geologic Work, Greater Paredones Project Internal Echo Bay Draft Report; La Paz Municipality: Baja California Sur, México, 1995; 33p. [Google Scholar]
- Bay, E. Exploration Mexico: Paredones Amarillos Project Level II Feasibility Study Board of Directors Briefing Document. 1996. (In Spanish) [Google Scholar]
- CAM (Corporación Ambiental de México S.A. de C.V.). Manifestación de Impacto Ambiental Modalidad Particular, Proyecto de Exploración Minera Paredones Amarillos, Municipio de La Paz B.C.S. In Paredones Amarillos S.A. de C.V.; La Paz B.C.S. Proyecto CAM 07023; 2007; 136p. Available online: http://sinat.semarnat.gob.mx/dgiraDocs/documentos/bcs/resumenes/2007/03BS2007M0013.pdf (accessed on 11 December 2017). (In Spanish)
- Carrillo-Chávez, A.; Drever, J.I.; Martínez, M. Arsenic content and groundwater geochemistry of the San Antonio-El Triunfo, Carrizal and Los Planes aquifers in Southernmost Baja California, Mexico. Environ. Geol. 2000, 39, 1295–1303. [Google Scholar] [CrossRef]
- Volke-Sepúlveda, T.; Solórzano-Ochoa, G.; Rosas-Domínguez, A.; Izumikawa, I.; Encarnación-Aguilar, G.; Velasco-Trejo, J.A.; Flores Martínez, S. Informe Final, Remediacion de Sitios Contaminados por Metales Provenientes de jales Mineros en los Distritos de El Triunfo-San Antonio y Santa Rosalía, Baja California Sur, Mexico. 2003; 37p. Available online: http://www2.ine.gob.mx/dgcenica/descargas/remediacion2003.pdf (accessed on 11 December 2014). (In Spanish)
- Carrillo, A. Environmental Geochemistry of the San Antonio-El Triunfo Mining Area, Baja California Peninsula, Mexico. Ph.D. Thesis, University of Wyoming, Laramie, WY, USA, May 1996. Available online: http://defiendelasierra.org/wp-content/uploads/2015/09/Carrillo-1996_Wyoming.pdf (accessed on 11 December 2017).
- Romero-Guadarrama, J.A. Geoquímica de As, Hg, Pb y Zn y Mineralogía en Sedimentos Superficiales de la Cuenca de Drenaje del Distrito Minero El Triunfo, BCS, México. Master’s Thesis, CICIMAR-IPN, Mexico, 2011. Available online: www.repositoriodigital.ipn.mx/bitstream/123456789/14758/1/Tesis-final.pdf (accessed on 11 December 2014). (In Spanish).
- Carrillo, A.; Drever, J. Environmental assessment of the potential for arsenic leaching into groundwater from mine wastes in Baja California Sur, México. Geofís. Int. 1998, 37, 35–39. [Google Scholar]
- SGM (Servicio Geologico Mexicano). Carta Geologica-Minera 1:250,000, El Rosario F12-B23. In Secretaria de Economia; La Paz, Baja California Sur, Mexico, 2000. Available online: http://mapserver.sgm.gob.mx/Cartas_Online/geologia/374_F12-B23_GM.pdf (accessed on 11 December 2017). (In Spanish)
- Posada-Ayala, I.H. Geoquímica Ambiental del Distrito Minero San Antonio, Sedimentos de los Streams de la Cuenca de San Juan de Los Planes y Plataforma Continental de Bahía La Ventana, BCS, México. Master’s Thesis, CICIMAR-IPN, 2011. Available online: http://tesis.ipn.mx/handle/123456789/19165 (accessed on 11 December 2014). (In Spanish).
- Sánchez-Martínez, M.A.; Marmolejo-Rodríguez, A.J.; Gómez Millán, R.; Sánchez-González, A.; Magallanes-Ordoñez, V.R.; Romero-Guadarrama, J.A. Sediment accumulation of Ag, Cu, and Ni through a semi-arid basin as a by-product of the El Triunfo gold mine, Baja California Sur Mexico. J. Iber. Geol. 2013, 39, 97–110. [Google Scholar] [CrossRef]
- Sobrino-Figueroa, A.S.; Becerra-Rueda, O.F.; Magañanes-Ordoñez, V.R.; Sánchez-González, A.; Marmolejo-Rodríguez, A.J. Toxicity in semiarid sediments influenced by tailings of an abandoned gold mine. Environ. Monit. Assess. 2015, 187, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Marmolejo-Rodríguez, A.J.; Sánchez-Martínez, M.A.; Romero-Guadarrama, J.A.; Sánchez-González, A.; Magallanes-Ordóñez, V.R. Migration of As, Hg, Pb, and Zn in arroyo sediments from a semiarid coastal system influenced by the abandoned gold mining district at El Triunfo, Baja California Sur, Mexico. J. Environ. Monit. 2011, 13, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Guidelines for Drinking-Water Quality. Health Criteria and Other Supporting Information, 4th ed.; WHO: Geneva, Switzerland, 2011; p. 564. [Google Scholar]
- Wurl, J.; Mendez-Rodriguez, L.; Acosta-Vargas, B. Arsenic content in groundwater from the southern part of the San Antonio-El Triunfo mining district, Baja California Sur, Mexico. J. Hydrol. 2014, 518, 447–459. [Google Scholar] [CrossRef]
- Wedepohl, K.H. The continental crust. Geochim. Cosmochim. Acta 1995, 59, 1217–1232. [Google Scholar] [CrossRef]
- American Public Health Association, APHA. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 1992. [Google Scholar]
- Norma Mexicana. NMX-AA-008-SCFI-2000. Que Establece la Metodología para la Determinación del pH en Aguas Residuales. Secretaría de Comercio y Fomento Industrial: México, 2000. Available online: https://agua.org.mx/wp-content/uploads/2011/01/nmx-aa-008-scfi-2000.pdf (accessed on 11 December 2017). (In Spanish).
- Norma Mexicana NMX-AA-093-SCFI-2000. Análisis de Agua. Determinación de la Conductividad Electrolítica. Método de Prueba. Secretaría de Comercio y Fomento Industrial CDU.631.879.27. Available online: http://lasa.ciga.unam.mx/monitoreo/images/biblioteca/46%20NMX-AA-093-SCFI-2000_Conductividad.pdf (accessed on 11 December 2017). (In Spanish).
- Norma Mexicana NMX-AA-036-SCFI-2001. Análisis de Agua, Determinación de acidez y Alcalinidad en Aguas Naturales, Residuales y Residuales Tratadas—Método de Prueba. 2001. Available online: https://www.gob.mx/cms/uploads/attachment/file/166776/NMX-AA-036-SCFI-2001.pdf (accessed on 11 December 2017). (In Spanish)
- Norma Mexicana NMX (2001a) Norma Mexicana NMX-AA-073-SCFI-2001. Análisis de agua-Determinación de Cloruros Totales en Aguas Naturales, Residuales, y Residuales Tratadas-Método de Prueba. Secretaría de Economía. 2001. Available online: https://agua.org.mx/biblioteca/nmx-aa-073-scfi-2001-analisis-de-agua-determinacion-de-cloruros-totales-en-aguas-naturales-residuales-y-residuales-tratadas-metodo-de-prueba/ (accessed on 11 December 2017). (In Spanish).
- Norma Mexicana NMX-AA-077-SCFI-2001. “Análisis de Aguas; Determinación de Fluoruros en Aguas Naturales, Residuales y Residuales Tratadas”. In Diario Oficial de la Nación; 2001; (Cancela a NMX-AA-077-1982). Available online: http://biblioteca.semarnat.gob.mx/janium/Documentos/Ciga/agenda/PPD1/DO86.pdf (accessed on 11 December 2017). (In Spanish)
- Norma Mexicana NMX-AA-074-SCFI-1981. Water Analysis. Determination of Sulfate Ion in Natural Waters, Wastewaters and Treated Waters. In Secretaría de Economía; 1981. Available online: https://www.gob.mx/cms/uploads/attachment/file/166149/nmx-aa-074-scfi-2014.pdf (accessed on 11 December 2017). (In Spanish)
- Edgell, K. USEPA Method Study 37 SW-846 Method 3050 acid Digestion of Sediments, Sludges, and Soils. 1989; US Environmental Protection Agency, Environmental Monitoring Systems Laboratory. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=2000TTR9.TXT (accessed on 11 December 2017).
- Norma Mexicana NMX-AA-051-SCFI-1981. Determinación de Metales, Método Espectrofotométrico de Absorción Atómica. 1982. Available online: https://www.gob.mx/cms/uploads/attachment/file/166785/NMX-AA-051-SCFI-2001.pdf (accessed on 11 December 2017). (In Spanish)
- NMX-AA-063-SCFI-2001, Análisis de Agua. Determinación de Boro en Aguas Naturales, Residuales y Residuales Tratadas. Método de Prueba. 2001. Available online: http://www.gob.mx/cms/uploads/attachment/file/166786/NMX-AA-063-SCFI-2001.pdf (accessed on 11 December 2017). (In Spanish)
- NMX-AA-058-SCFI-2001. Análisis de Aguas-Determinación de Cianuros Totales en Aguas na-Turales, Potables, Residuales y Residuales Trata-Das—Método de Prueba. 2001; pp. 1–23. Available online: https://www.gob.mx/cms/uploads/attachment/file/166784/NMX-AA-058-SCFI-2001.pdf (accessed on 11 December 2017). (In Spanish)
- DVWK-Regeln Zur Wasserwirtschaft H. Entnahme und Untersuchungsumfang von Grundwasserproben. In DK 556.32.001.5 Grundwasseruntersuchung, DK 543.3.053 Probenahme. DVWK-Regeln zur Wasserwirtschaft 128, 36; Deutscher Verband für Wasserwirtschaft und Kulturbau e. V.: Hamburg/Berlin, Germany, 1992. (In German)
- Carrillo, A.; Drever, J.I. Adsorption of arsenic by natural aquifer material in the San Antonio-El Triunfo mining area, Baja California. Environ. Geol. 1998, 35, 251–257. [Google Scholar] [CrossRef]
- Magdaleno Rico, C.A. Peligrosidad de Los Residuos Mineros Históricos del Distrito Minero San Antonio-El Triunfo en La Paz Baja California Sur y Evaluación de Generación de Drenaje Ácido a Través de Pruebas Estáticas, Tesis de Licenciatura, Universidad Nacional Autónoma de México UNAM, FACULTAD DE INGENIERÍA). Available online: www.ptolomeo.unam.mx:8080/xmlui/bitstream/handle/132.248.52.100/3026/Tesis.pdf (accessed on 10 February 2014). (In Spanish).
- Takeno, N. Atlas of Eh-pH Diagrams, Intercomparison of Thermodynamic Databases; File Report No. 419; National Institute of Advanced Industrial Science and Technology: Tokyo, Japan, 2005. [Google Scholar]
- NOM-127-SSA1-1994 Modificacion a la Norma Oficial Mexicana NOM-127-SSA1-1994, Salud Ambiental. Agua para uso y Consumo Humano. Lımites Permisibles de Calidad y Tratamientos a que debe Someterse el Agua Para su Potabilizacion. 2000; Mexico. Available online: http://www.salud.gob.mx/unidades/cdi/nom/m127ssa14.html (accessed on 11 December 2017). (In Spanish)
- Bale, C.W.; Chartrand, P.; Degterov, S.A.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melançon, J.; Pelton, A.D.; Petersen, S. FactSage Thermochemical Software and Databases. Calphad 2002, 26, 189. [Google Scholar] [CrossRef]
- Méndez-Rodríguez, L.; Zenteno-Savín, T.; Acosta-Vargas, B.; Wurl, J.; Imaz-Lamadrid, M. Differences in arsenic, molybdenum, barium, and other physicochemical relationships in groundwater between sites with and without mining activities. Nat. Sci. 2013, 5, 238–243. [Google Scholar] [CrossRef]
- Romero, F.M.; Armienta, M.A.; Villaseñor, G.; González, J.L. Mineralogical constraints on the mobility of arsenic in tailings from Zimapán, Hidalgo, Mexico. Int. J. Environ. Pollut. 2006, 26, 23–40. [Google Scholar] [CrossRef]
- Carrillo, A.; Huyck, H. A genetic model for the Los Uvares gold deposit, Baja California Sur, Mexico. Geofis. Int. 1997, 36, 111–119. [Google Scholar]
- Naranjo-Pulido, A.; Romero-Schmidt, H.; Mendez-Rodriguez, L.; Acosta-Vargas, B.; Ortega-Rubio, A. Soil arsenic contamination in the Cape Region, B.C.S. Mexico. J. Environ. Biol. 2002, 23, 347–352. [Google Scholar] [PubMed]
- Norma Oficial Mexicana Nom-053-Semarnat-1993 Que Establece El Procedimiento Para Llevar a Cabo la Prueba De Extracción Para Determinar Los Constituyentes Que Hacen a un Residuo Peligroso por Su Toxicidad al Ambiente. Available online: http://www.imss.gob.mx/sites/all/statics/profesionalesSalud/investigacionSalud/cbis/nom-053-semarnat-1993.pdf (accessed on 11 December 2017). (In Spanish)
- ONSITE Laboratories. Analysis to Determine the Characteristics of Corrosivity, Reactivity, Flammability and Toxicity to the Environment According to the Procedures and Sanctions in the Mexican Standard NOM-052-SEMARNAT-2005 Conducted on 10 Core Samples; ONSITE Laboratories: Bendigo, Australia, 2012; 100p. [Google Scholar]
- Feng, Z.; Xia, Y.; Tian, D.; Wu, K.; Schmitt, M.; Kwok, R.K.; Mumford, J.L. DNA damage in buccal epithelial cells from individuals chronically exposed to arsenic via drinking water in Inner Mongolia, China. Anticancer Res. 2001, 21, 51–58. [Google Scholar] [PubMed]
- Bell, F.G. Environmental Geology: Principles and Practice; Blackwell Science: London, UK, 1998; pp. 487–500. [Google Scholar]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behavior and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Cervantes, C.; Ji, G.; Ramírez, J.L.; Silver, S. Resistance to arsenic compounds in microorganisms. FEMS Microbiol. Rev. 1994, 15, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Burau, R.G. Environmental factors affecting rates of arsine evolution from and mineralization of arsenicals in soil. J. Environ. Qual. 1997, 26, 753–763. [Google Scholar] [CrossRef]
- Cheng, C.N.; Focht, D.D. Production of arsine and methylarsines in soil and culture. Appl. Environ. Microbiol. 1979, 38, 494–498. [Google Scholar] [PubMed]
- Smith, A.H.; Hopenhayn-Rich, C.; Bates, M.N.; Goeden, H.M.; Hertz-Picciotto, I.; Duggan, H.M.; Wood, R.; Kosnett, M.J.; Smith, M.T. Cancer risks from arsenic in drinking water. Environ. Health Perspect. 1992, 97, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Celik, I.; Gallicchio, L.; Boyd, K.; Lam, T.K.; Matanoski, G.; Tao, X.; Alberg, A.J. Arsenic in drinking water and lung cancer: A systematic review. Environ. Res. 2008, 108, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Colín-Torres, C.G.; Murillo-Jiménez, J.M.; Del Razo, L.M.; Sánchez-Peña, L.C.; Becerra-Rueda, O.F.; Marmolejo-Rodríguez, A.J. Urinary arsenic levels influenced by abandoned mine tailings in the Southernmost Baja California Peninsula, Mexico. Environ. Geochem. Health 2014, 36, 845–854. [Google Scholar] [CrossRef] [PubMed]
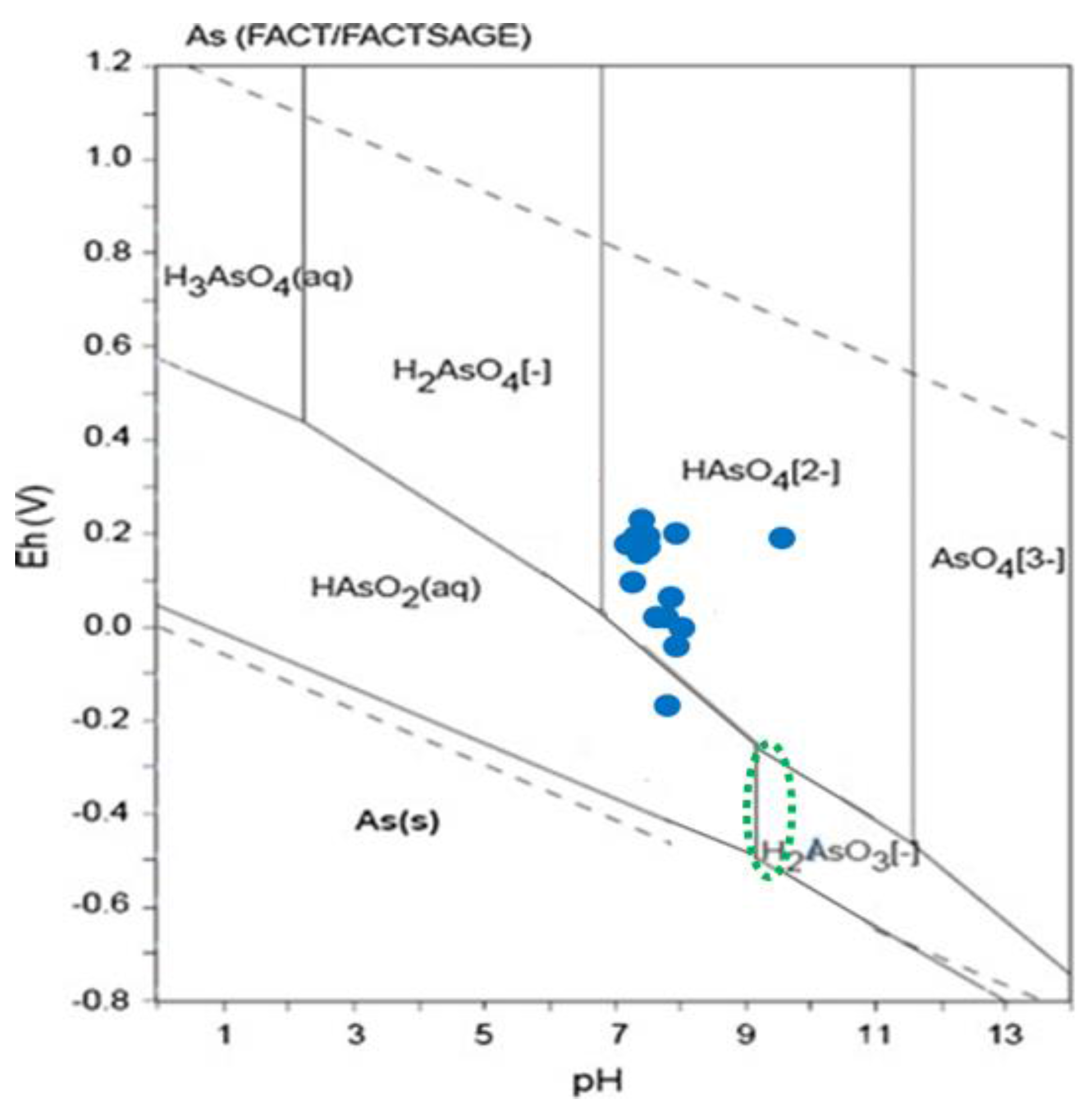
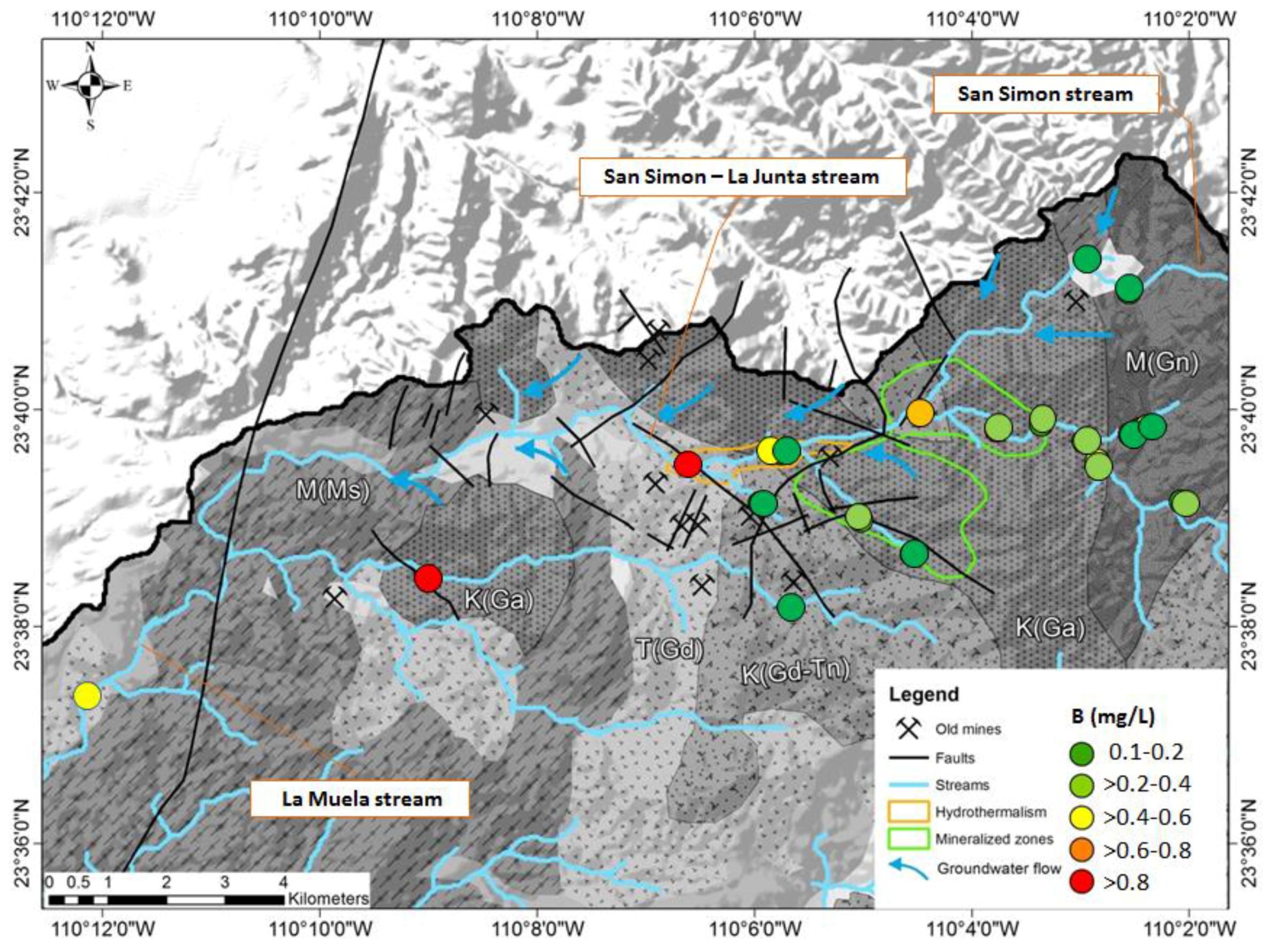
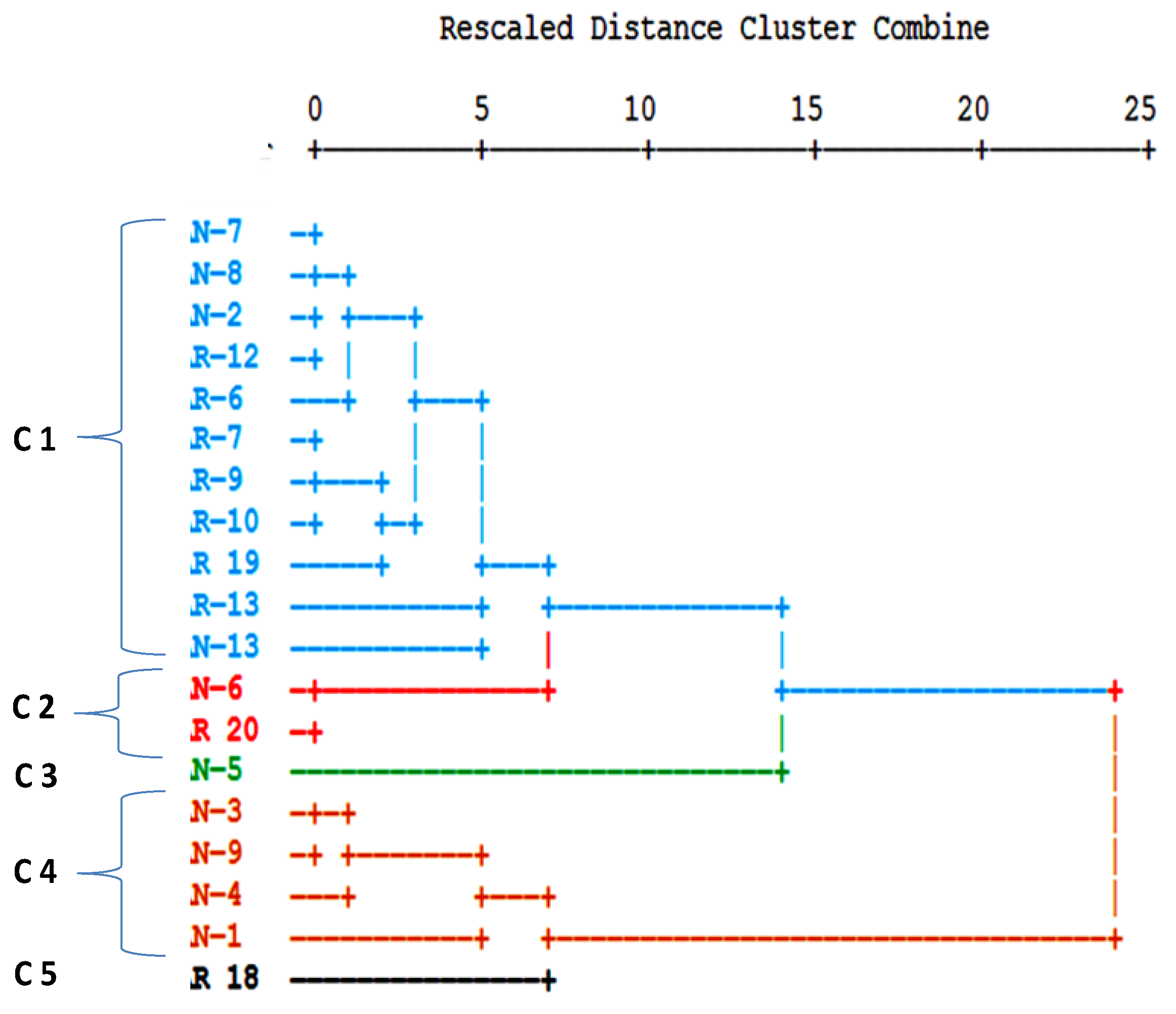
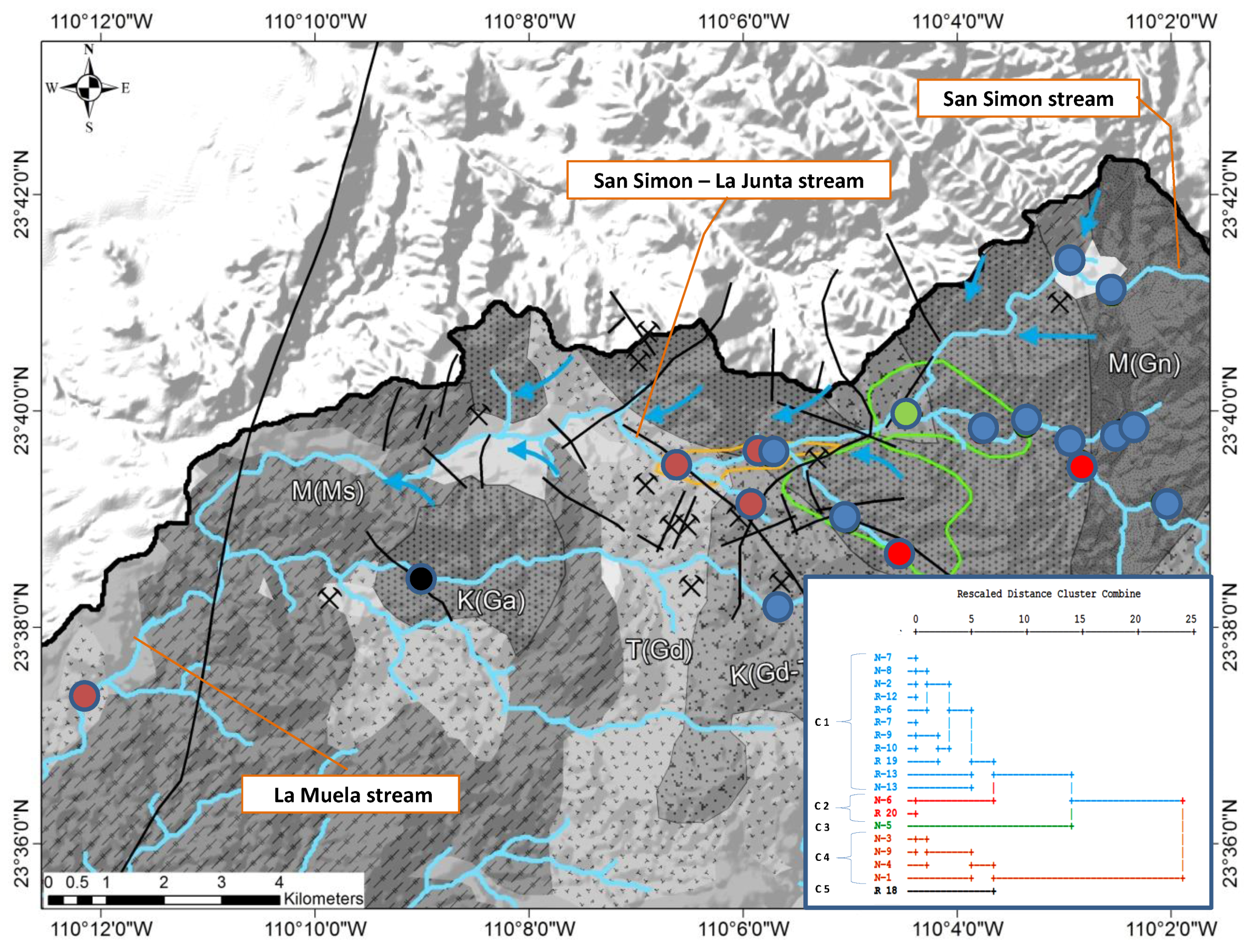
| No. | Name of the Gold Mine | Rock Type | Type of Ore Deposite | Mineralization Type | Metal Produced |
|---|---|---|---|---|---|
| 1 | El Saucito | Granite with diorite and granodiorite | Hydrothermal veins | Sulfurous | Au, Ag |
| 2 | Veta Arbol de Oro | Granodiorite | Hydrothermal veins | Sulfurous | Au |
| 3 | Bajo Veta Grande | Granodiorite | Hydrothermal veins | Sulfurous | Au, Ag |
| 4 | Veta Grande | Granodiorite | Hydrothermal veins | Sulfurous | Au, Ag |
| 5 | El Veladero | Granodiorite | Hydrothermal veins | Sulfurous | Au |
| 6 | Veta de Otto | Granite with diorite and granodiorite | Hydrothermal veins | Sulfurous | Au |
| 7 | Mr. Conn | Granite with diorite and granodiorite | Hydrothermal veins | Sulfurous | Au, Ag |
| 8 | Paredones Amarillos | Granite with diorite and granodiorite | Hydrothermal, disseminated gold deposit | Sulfurous | Au |
| 9 | Siempre Viva | Granodiorite and tonalite | Hydrothermal veins | Sulfurous | Au |
| 10 | Yerba de Manzo | Granodiorite and tonalite | Hydrothermal veins | Sulfurous | Au, Ag, Pb |
| 11 | La Zorra | Granodiorite and tonalite | Hydrothermal veins | Sulfurous | Au, Ag |
| 12 | La Encantada | Granite with diorite and granodiorite | Hydrothermal veins | Sulfurous | Au, Ag, Pb |
| 13 | Adonde | Granodiorite and tonalite | Hydrothermal veins | Sulfurous | Au, Ag, Pb |
| Parameter | Methods | Reference |
|---|---|---|
| The pH value | NMX-AA-008-SCFI-2000 | [29] |
| Electrolytic conductivity | NMX-AA-093-SCFI-2000 | [30] |
| Total alkalinity | NMX-AA-036-SCFI-2001 | [31] |
| Chlorides | NMX-AA-073-SCFI-2001 | [32] |
| Fluorides | NMXAA-077-SCFI-2001 | [33] |
| Sulfates | NMX-AA-074-1981 | [34] |
| Ca, Mg, Na, Sr, K, Fe, Ag, Al, As, Ba, Cd, Co, Cr, Cu, Li, Mn, Mo, Ni, Pb, Sb, Zn, Be, Se, Sn, Bi, Tl | ICP-MS according to USEPA Method 6020A [35]. The samples were measured twice, with a filtrated sample and without filtration so that dissolved concentration and total concentration were obtained. | |
| Mercury | Cold vapor atomic absorption spectroscopy, according to the Mexican Norm NMX-AA-051-SCFI-2001 [36]. | |
| Cyanide and boron | Spectrophotometric techniques according to the Mexican Norms NMX-AA-063-SCFI-2001 [37] and NMX-AA-058-SCFI-2001 at 540 nm and 578 nm [38] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wurl, J.; Imaz Lamadrid, M.; Mendez-Rodriguez, L.; Acosta Vargas, B. Arsenic Concentration in the Surface Water of a Former Mining Area: The La Junta Creek, Baja California Sur, Mexico. Int. J. Environ. Res. Public Health 2018, 15, 437. https://doi.org/10.3390/ijerph15030437
Wurl J, Imaz Lamadrid M, Mendez-Rodriguez L, Acosta Vargas B. Arsenic Concentration in the Surface Water of a Former Mining Area: The La Junta Creek, Baja California Sur, Mexico. International Journal of Environmental Research and Public Health. 2018; 15(3):437. https://doi.org/10.3390/ijerph15030437
Chicago/Turabian StyleWurl, Jobst, Miguel Imaz Lamadrid, Lía Mendez-Rodriguez, and Baudilio Acosta Vargas. 2018. "Arsenic Concentration in the Surface Water of a Former Mining Area: The La Junta Creek, Baja California Sur, Mexico" International Journal of Environmental Research and Public Health 15, no. 3: 437. https://doi.org/10.3390/ijerph15030437
APA StyleWurl, J., Imaz Lamadrid, M., Mendez-Rodriguez, L., & Acosta Vargas, B. (2018). Arsenic Concentration in the Surface Water of a Former Mining Area: The La Junta Creek, Baja California Sur, Mexico. International Journal of Environmental Research and Public Health, 15(3), 437. https://doi.org/10.3390/ijerph15030437





