Comparison of Airway Responses Induced in a Mouse Model by the Gas and Particulate Fractions of Gasoline Direct Injection Engine Exhaust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Murine Model of Allergic Airway Inflammation
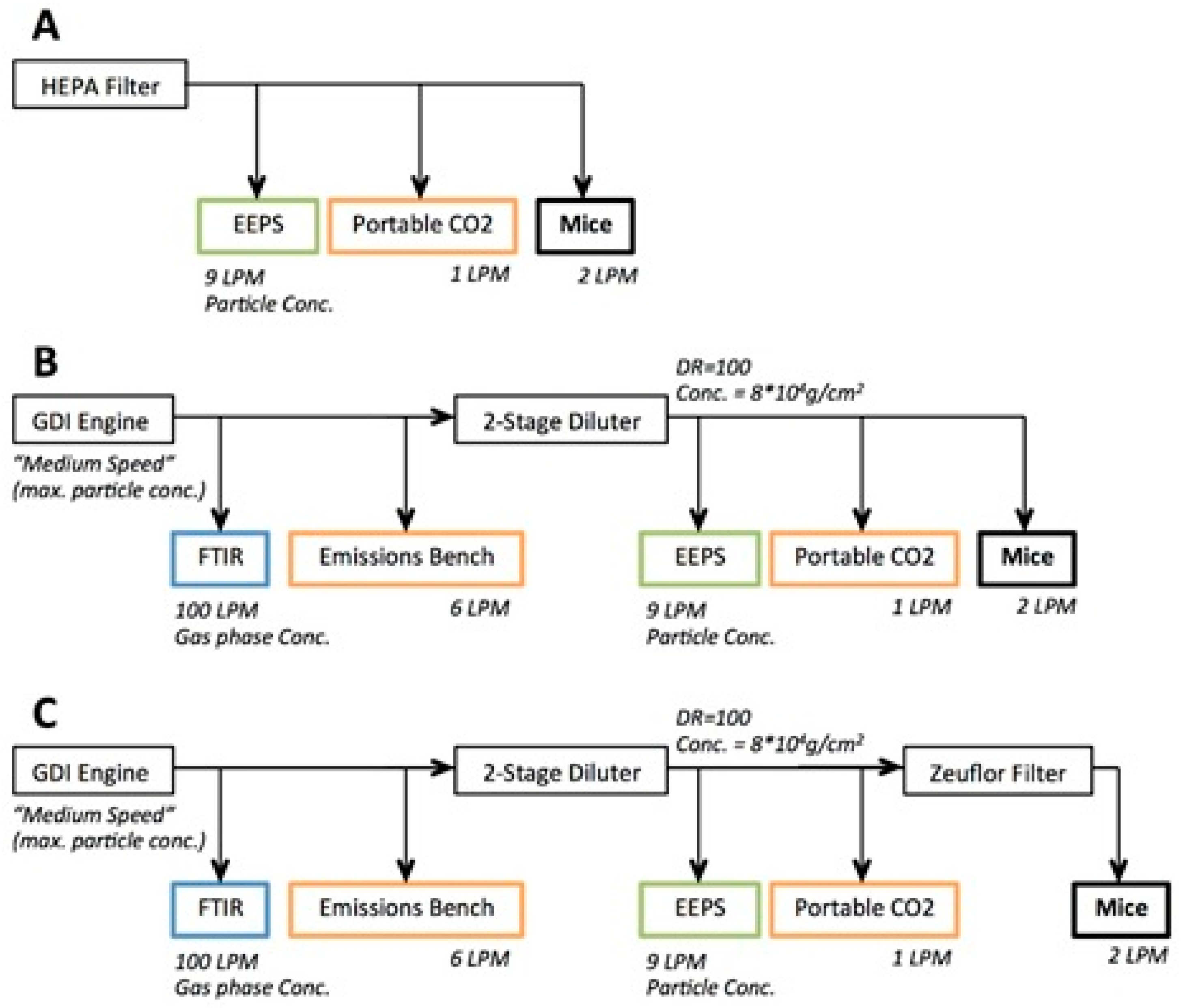
2.3. Engine Exhaust Characterisation
2.4. GDI Engine Exhaust Inhalation Exposure
2.5. Measurement of Airway Hyperresponsiveness and RNA Isolation from Murine Lungs
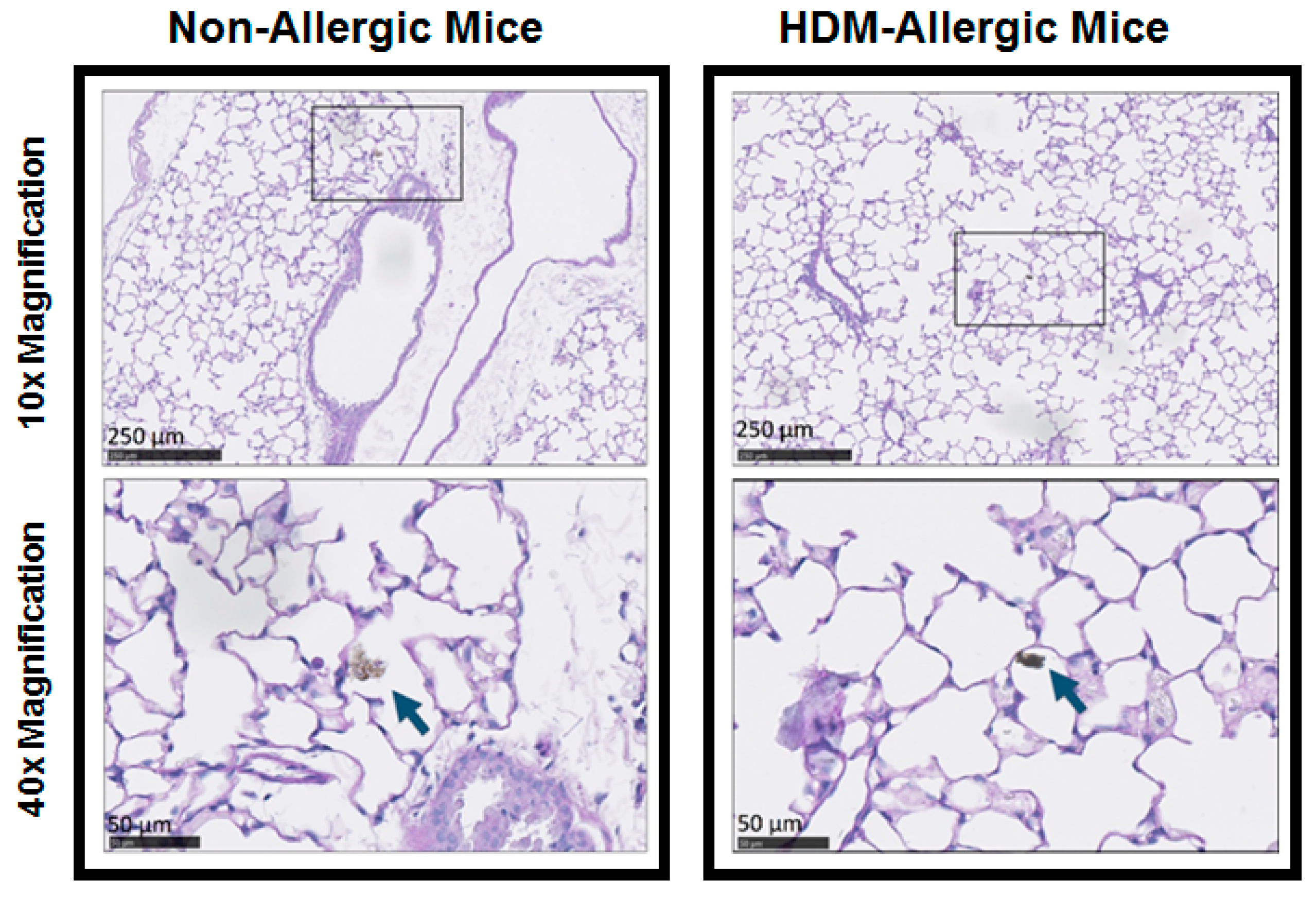
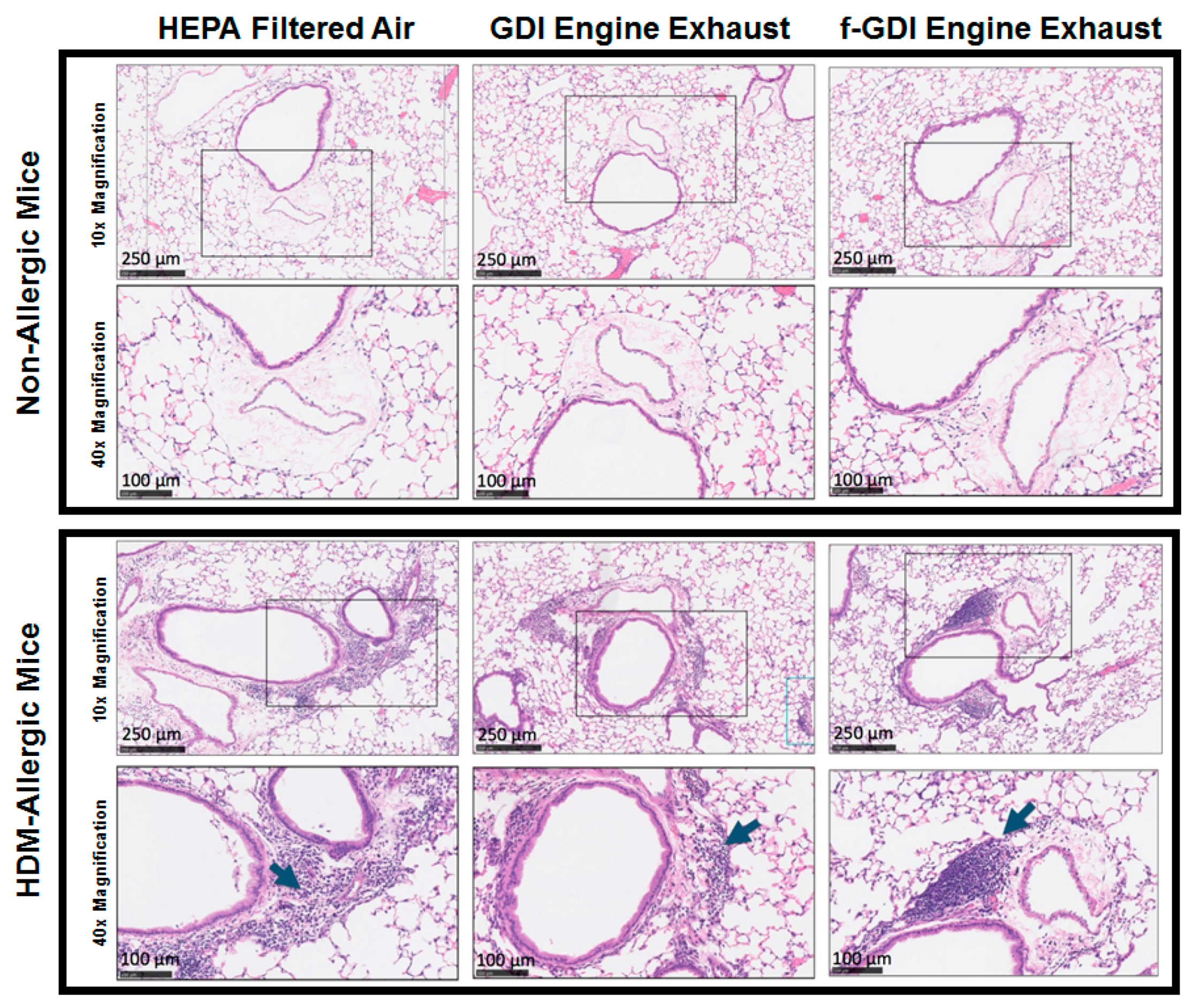
2.6. Histology Analysis
2.7. Assessment of Quantitative PCR in Airways
2.8. Statistical Analyses
3. Results
3.1. Characterisation of GDI Engine Exhaust Exposure Concentrations
3.2. Murine Model of Allergic Airways Inflammation
3.3. Single-Compartment Model of Respiratory Mechanics
3.4. Constant-Phase Model of Respiratory Mechanics
3.5. Histology of Lung Tissue
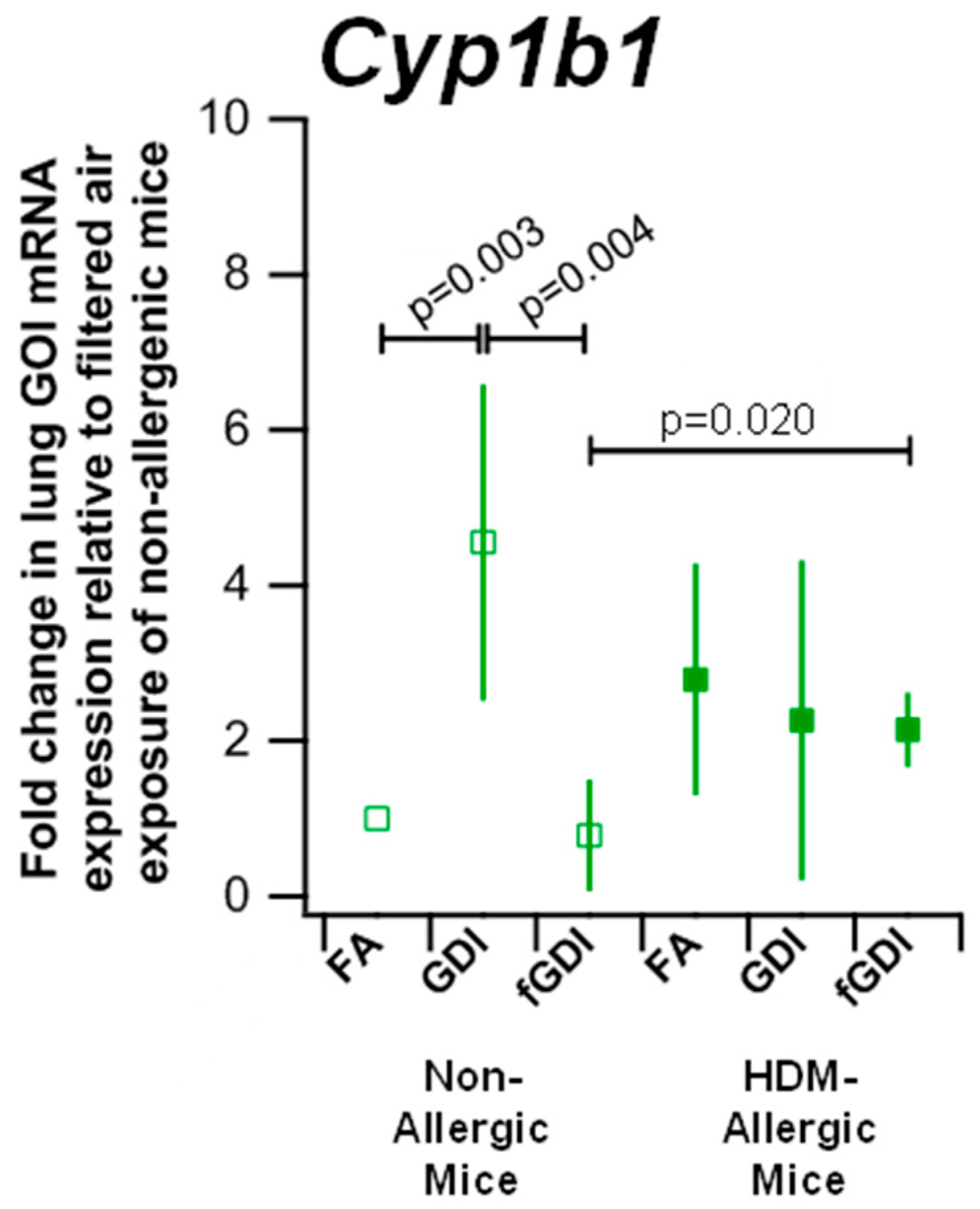
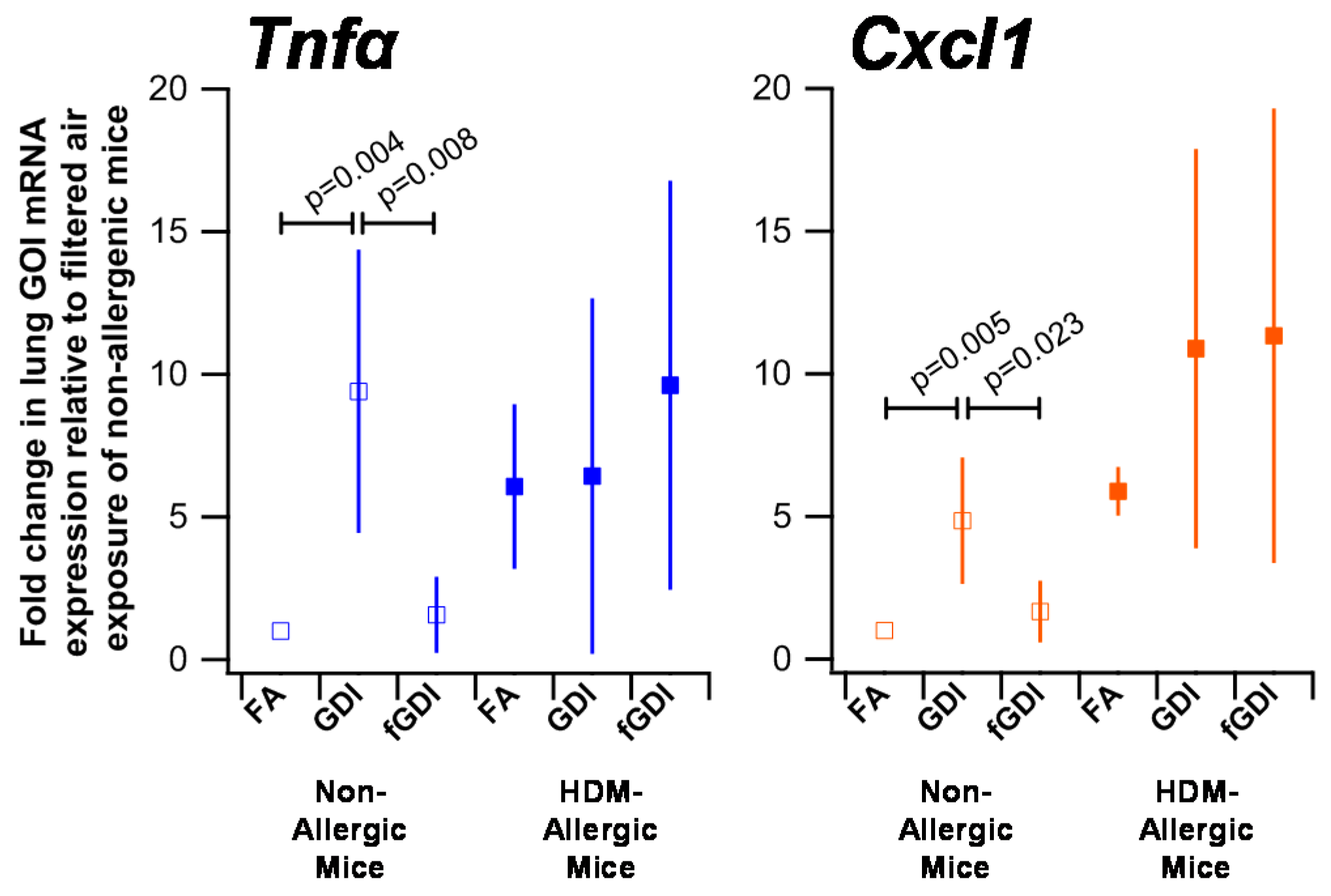
3.6. Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Akinbami, L.J.; Moorman, J.E.; Garbe, P.L.; Sondik, E.J. Status of childhood asthma in the United States, 1980–2007. Pediatrics 2009, 123, S131–S145. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, P.; Becker, A.; Brook, J.R.; Daley, D.; Mandhane, P.J.; Miller, G.E.; Turvey, S.E.; Sears, M.R. Epidemiology of asthma: Risk factors for development. Expert Rev. Clin. Immunol. 2009, 5, 77–95. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Tier 3 Vehicle Emission and Fuel Standards Program. 2014. Available online: https://www.theicct.org/sites/default/files/publications/ICCTupdate_Tier3_finalrule_mar2014.pdf (accessed on 22 February 2018).
- California Air Resources Board. Proposed Amendments to the LEV III Criteria Pollutant Requirements for Light and Medium-Duty Vehicles, the Hybrid Electric Vehicle Test Procedures, and the Heavy-Duty Otto-Cycle and Heavy-Duty Diesel Test Procedures. 2014. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32007R0715&from=en (accessed on 10 May 2017).
- European Parliament Council of the European Union. EUR-Lex—32007R0715—EN—EUR-Lex. 2007. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32007R0715&from=en (accessed on 15 February 2016).
- US EPA. Light-Duty Automotive Technology, Carbon Dioxide Emissions, and Fuel Economy Trends: 1975 through 2017. 2018. Available online: https://nepis.epa.gov/Exe/ZyPDF.cgi?Dockey=P100TGDW.pdf (accessed on 15 January 2018).
- Gladstein, Ultrafine Particulate Matter and the Benefits of Reducing Particle Numbers in the U.S., a Report to the Manufacturers of Emission Controls Association (MECA). 2013. Available online: http://www.meca.org/resources/meca_ufp_white_paper_0713_final.pdf (accessed on 15 February 2016).
- Aakko, P.; Nylund, N.-O. Particle Emissions at Moderate and Cold Temperatures Using Different Fuels. SAE Tech. Paper 2003. [Google Scholar] [CrossRef]
- Karavalakis, G.; Short, D.; Vu, D.; Villela, M.; Asa-Awuku, A.; Durbin, T.D. Evaluating the regulated emissions, air toxics, ultrafine particles, and black carbon from SI-PFI and SI-DI vehicles operating on different ethanol and iso-butanol blends. Fuel 2014, 128, 410–421. [Google Scholar] [CrossRef]
- Zimmerman, N.; Wang, J.M.; Jeong, C.-H.; Ramos, M.; Hilker, N.; Healy, R.M.; Sabaliauskas, K.; Wallace, J.S.; Evans, G.J. Field Measurements of Gasoline Direct Injection Emission Factors: Spatial and Seasonal Variability. Environ. Sci. Technol. 2016, 50, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Maricq, M.M.; Xu, N.; Chase, R.E. Measuring Particulate Mass Emissions with the Electrical Low Pressure Impactor. Aerosol Sci. Technol. 2006, 40, 68–79. [Google Scholar] [CrossRef]
- Liang, B.; Ge, Y.; Tan, J.; Han, X.; Gao, L.; Hao, L.; Ye, W.; Dai, P. Comparison of PM emissions from a gasoline direct injected (GDI) vehicle and a port fuel injected (PFI) vehicle measured by electrical low pressure impactor (ELPI) with two fuels: Gasoline and M15 methanol gasoline. J. Aerosol Sci. 2013, 57, 22–31. [Google Scholar] [CrossRef]
- Maricq, M.M.; Szente, J.J.; Jahr, K. The Impact of Ethanol Fuel Blends on PM Emissions from a Light-Duty GDI Vehicle. Aerosol Sci. Technol. 2012, 46, 576–583. [Google Scholar] [CrossRef]
- Zielinska, B.; Sagebiel, J.; Arnott, W.P.; Rogers, C.F.; Kelly, K.E.; Wagner, D.A.; Lighty, J.S.; Sarofim, A.F.; Palmer, G. Phase and Size Distribution of Polycyclic Aromatic Hydrocarbons in Diesel and Gasoline Vehicle Emissions. Environ. Sci. Technol. 2004, 38, 2557–2567. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Fujii-Kuriyama, Y. Metabolic activation of polycyclic aromatic hydrocarbons to carcinogens by cytochromes P450 1A1 and 1B1. Cancer Sci. 2004, 95, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.J.; Malinow, M.R.; Willett, W.C.; Newcomer, L.M.; Upson, B.; Ullmann, D.; Tishler, P.V.; Hennekens, C.H. A prospective study of plasma homocyst(e)ine and risk of myocardial infarction in US physicians. JAMA 1992, 268, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Larcombe, A.N.; Phan, J.A.; Kicic, A.; Perks, K.L.; Mead-Hunter, R.; Mullins, B.J. Route of exposure alters inflammation and lung function responses to diesel exhaust. Inhal. Toxicol. 2014, 26, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Peden, D.B.; Dailey, L.; DeGraff, W.; Mitchell, J.B.; Lee, J.G.; Kaliner, M.A.; Hohman, R.J. Hydrogen peroxide effects on rat mast cell function. Am. J. Physiol. 1994, 267, L85–L93. [Google Scholar] [CrossRef] [PubMed]
- Cates, E.C.; Fattouh, R.; Wattie, J.; Inman, M.D.; Goncharova, S.; Coyle, A.J.; Gutierrez-Ramos, J.-C.; Jordana, M. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J. Immunol. 2004, 173, 6384–6392. [Google Scholar] [CrossRef] [PubMed]
- Southam, D.S.; Ellis, R.; Wattie, J.; Inman, M.D. Components of airway hyperresponsiveness and their associations with inflammation and remodeling in mice. J. Allergy Clin. Immunol. 2007, 119, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Wakahara, K.; Tanaka, H.; Takahashi, G.; Tamari, M.; Nasu, R.; Toyohara, T.; Takano, H.; Saito, S.; Inagaki, N.; Shimokata, K.; et al. Repeated instillations of Dermatophagoides farinae into the airways can induce Th2-dependent airway hyperresponsiveness, eosinophilia and remodeling in mice: Effect of intratracheal treatment of fluticasone propionate. Eur. J. Pharmacol. 2008, 578, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Penton, P.C.; Wang, X.; Amatullah, H.; Cooper, J.; Godri, K.; North, M.L.; Khanna, N.; Scott, J.A.; Chow, C.-W. Spleen tyrosine kinase inhibition attenuates airway hyperresponsiveness and pollution-induced enhanced airway response in a chronic mouse model of asthma. J. Allergy Clin. Immunol. 2013, 131, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, N.; Rais, K.; Jeong, C.-H.; Pant, P.; Delgado-Saborit, J.M.; Wallace, J.S.; Evans, G.J.; Brook, J. R.; Godri Pollitt, K.J. Carbonaceous aerosol sampling of gasoline direct injection engine exhaust with an integrated organic gas and particle sampler. Sci. Total Environ. 2018, Submitted. [Google Scholar]
- Delgado-Saborit, J.M.; Aquilina, N.; Baker, S.; Harrad, S.; Meddings, C.; Harrison, R.M. Determination of atmospheric particulate-phase polycyclic aromatic hydrocarbons from low volume air samples. Anal. Methods 2010, 2, 231. [Google Scholar] [CrossRef]
- Shah, M.; Gburcik, V.; Reilly, P.; Sankey, R.A.; Emery, R.J.; Clarkin, C.E.; Pitsillides, A.A. Local origins impart conserved bone type-related differences in human osteoblast behaviour. Eur. Cell. Mater. 2015, 29, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Tian, F.; Frankenberger, B.; de Angelis, M.H.; Stoeger, T. Selection and evaluation of stable housekeeping genes for gene expression normalization in carbon nanoparticle-induced acute pulmonary inflammation in mice. Biochem. Biophys. Res. Commun. 2010, 399, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Kouadjo, K.E.; Nishida, Y.; Cadrin-Girard, J.F.; Yoshioka, M.; St-Amand, J. Housekeeping and tissue-specific genes in mouse tissues. BMC Genomics 2007, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- North, M.L.; Amatullah, H.; Khanna, N.; Urch, B.; Grasemann, H.; Silverman, F.; Scott, J.A. Augmentation of arginase 1 expression by exposure to air pollution exacerbates the airways hyperresponsiveness in murine models of asthma. Respir. Res. 2011, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Plant, P.J.; North, M.L.; Ward, A.; Ward, M.; Khanna, N.; Correa, J.; Scott, J.A.; Batt, J. Hypertrophic Airway Smooth Muscle Mass Correlates with Increased Airway Responsiveness in a Murine Model of Asthma. Am. J. Respir. Cell Mol. Biol. 2012, 46, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Sabaliauskas, K.; Jeong, C.-H.; Yao, X.; Jun, Y.-S.; Jadidian, P.; Evans, G.J. Five-year roadside measurements of ultrafine particles in a major Canadian city. Atmos. Environ. 2012, 49, 245–256. [Google Scholar] [CrossRef]
- Maikawa, C.L.; Zimmerman, N.; Rais, K.; Shah, M.; Hawley, B.; Pant, P.; Jeong, C.-H.; Delgado-Saborit, J.M.; Volckens, J.; Evans, G.; et al. Murine precision-cut lung slices exhibit acute responses following exposure to gasoline direct injection engine emissions. Sci. Total Environ. 2016, 568, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Chew, A.D.; Hirota, J.A.; Ellis, R.; Wattie, J.; Inman, M.D.; Janssen, L.J. Effects of allergen on airway narrowing dynamics as assessed by lung-slice technique. Eur. Respir. J. 2008, 31, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Buters, J.T.; Sakai, S.; Richter, T.; Pineau, T.; Alexander, D.L.; Savas, U.; Doehmer, J.; Ward, J.M.; Jefcoate, C.R.; Gonzalez, F.J. Cytochrome P450 CYP1B1 determines susceptibility to 7, 12-dimethylbenz[a]anthracene-induced lymphomas. Proc. Natl. Acad. Sci. USA 1999, 96, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Al-Attas, O.S.; Al-Daghri, N.M.; Mohammed, A.A.; De Rosas, E.; Ibrahim, S.; Vinodson, B.; Ansari, M.G.; El-Din, K.I.A. Induction of CYP1A1, CYP1A2, CYP1B1, increased oxidative stress and inflammation in the lung and liver tissues of rats exposed to incense smoke. Mol. Cell. Biochem. 2014, 391, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Cheruyiot, N.K.; Lee, W.-J.; Mwangi, J.K.; Wang, L.-C.; Lin, N.-H.; Lin, Y.-C.; Cao, J.; Zhang, R.; Chang-Chien, G.-P. An Overview: Polycyclic Aromatic Hydrocarbon Emissions from the Stationary and Mobile Sources and in the Ambient Air. Aerosol Air Qual. Res. 2015, 15, 2730–2762. [Google Scholar] [CrossRef]
- Westphal, G.A.; Krahl, J.; Munack, A.; Ruschel, Y.; Schröder, O.; Hallier, E.; Brüning, T.; Bünger, J. Mutagenicity of Diesel Engine Exhaust Is Eliminated in the Gas Phase by an Oxidation Catalyst but Only Slightly Reduced in the Particle Phase. Environ. Sci. Technol. 2012, 46, 6417–6424. [Google Scholar] [CrossRef] [PubMed]
- Miyabara, Y.; Ichinose, T.; Takano, H.; Lim, H.B.; Sagai, M.; Sagai, M. Effects of diesel exhaust on allergic airway inflammation in mice. J. Allergy Clin. Immunol. 1998, 102, 805–812. [Google Scholar] [CrossRef]
- Ontario Ministry of Labour. Current Occupational Exposure Limits for Ontario Workplaces Required under Regulation 833. 2015. Available online: https://www.labour.gov.on.ca/english/hs/pubs/oel_table.php (accessed on 1 March 2018).
| Title | Exposure Group | Non-Allergic Mice | HDM-Allergic Mice |
|---|---|---|---|
| Total Airway Resistance Max | FA | 6.34 ± 2.34 | 10.15 ± 3.50 |
| GDI | 7.19 ± 4.26 | 14.78 ± 2.32 | |
| fGDI | 7.63 ± 2.48 | 14.49 ± 2.52 | |
| Central Tissue Max | FA | 2.01 ± 0.41 | 2.51 ± 1.08 |
| GDI | 1.83 ± 0.64 | 3.99 ± 1.11 | |
| fGDI | 2.28 ± 0.73 | 2.55 ± 0.76 | |
| Peripheral Tissue Max | FA | 31.05 ± 15.93 | 48.84 ± 16.05 |
| GDI | 29.81 ± 15.22 | 95.56 ± 30.90 | |
| fGDI | 36.45 ± 12.41 | 78.94 ± 33.64 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maikawa, C.L.; Zimmerman, N.; Ramos, M.; Shah, M.; Wallace, J.S.; Pollitt, K.J.G. Comparison of Airway Responses Induced in a Mouse Model by the Gas and Particulate Fractions of Gasoline Direct Injection Engine Exhaust. Int. J. Environ. Res. Public Health 2018, 15, 429. https://doi.org/10.3390/ijerph15030429
Maikawa CL, Zimmerman N, Ramos M, Shah M, Wallace JS, Pollitt KJG. Comparison of Airway Responses Induced in a Mouse Model by the Gas and Particulate Fractions of Gasoline Direct Injection Engine Exhaust. International Journal of Environmental Research and Public Health. 2018; 15(3):429. https://doi.org/10.3390/ijerph15030429
Chicago/Turabian StyleMaikawa, Caitlin L., Naomi Zimmerman, Manuel Ramos, Mittal Shah, James S. Wallace, and Krystal J. Godri Pollitt. 2018. "Comparison of Airway Responses Induced in a Mouse Model by the Gas and Particulate Fractions of Gasoline Direct Injection Engine Exhaust" International Journal of Environmental Research and Public Health 15, no. 3: 429. https://doi.org/10.3390/ijerph15030429
APA StyleMaikawa, C. L., Zimmerman, N., Ramos, M., Shah, M., Wallace, J. S., & Pollitt, K. J. G. (2018). Comparison of Airway Responses Induced in a Mouse Model by the Gas and Particulate Fractions of Gasoline Direct Injection Engine Exhaust. International Journal of Environmental Research and Public Health, 15(3), 429. https://doi.org/10.3390/ijerph15030429





