A Geographical Information System Based Approach for Integrated Strategies of Tick Surveillance and Control in the Peri-Urban Natural Reserve of Monte Pellegrino (Palermo, Southern Italy)
Abstract
1. Introduction
2. Materials and Methods
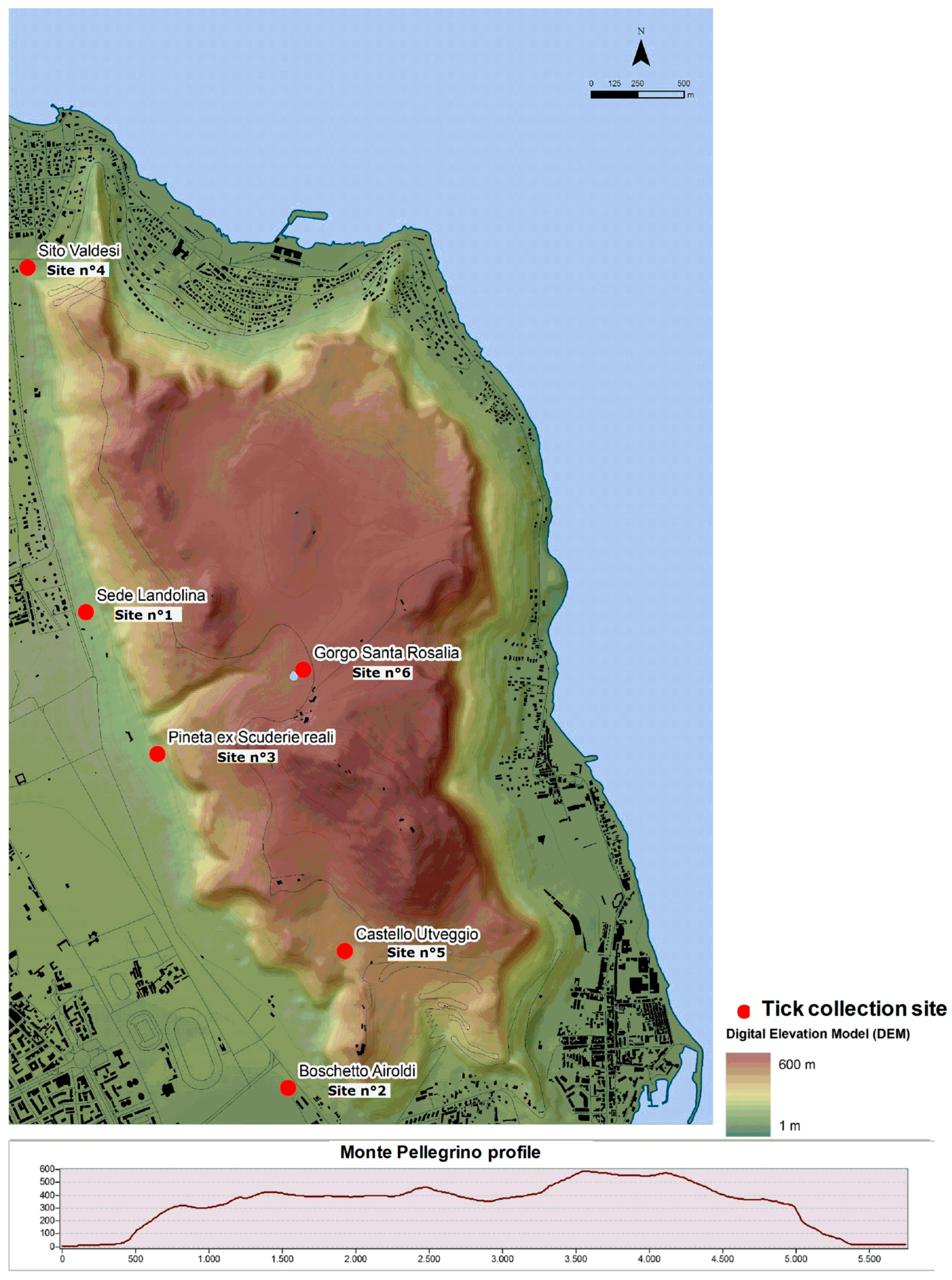
2.1. Collection Sites
2.2. Ticks Collection and Identification
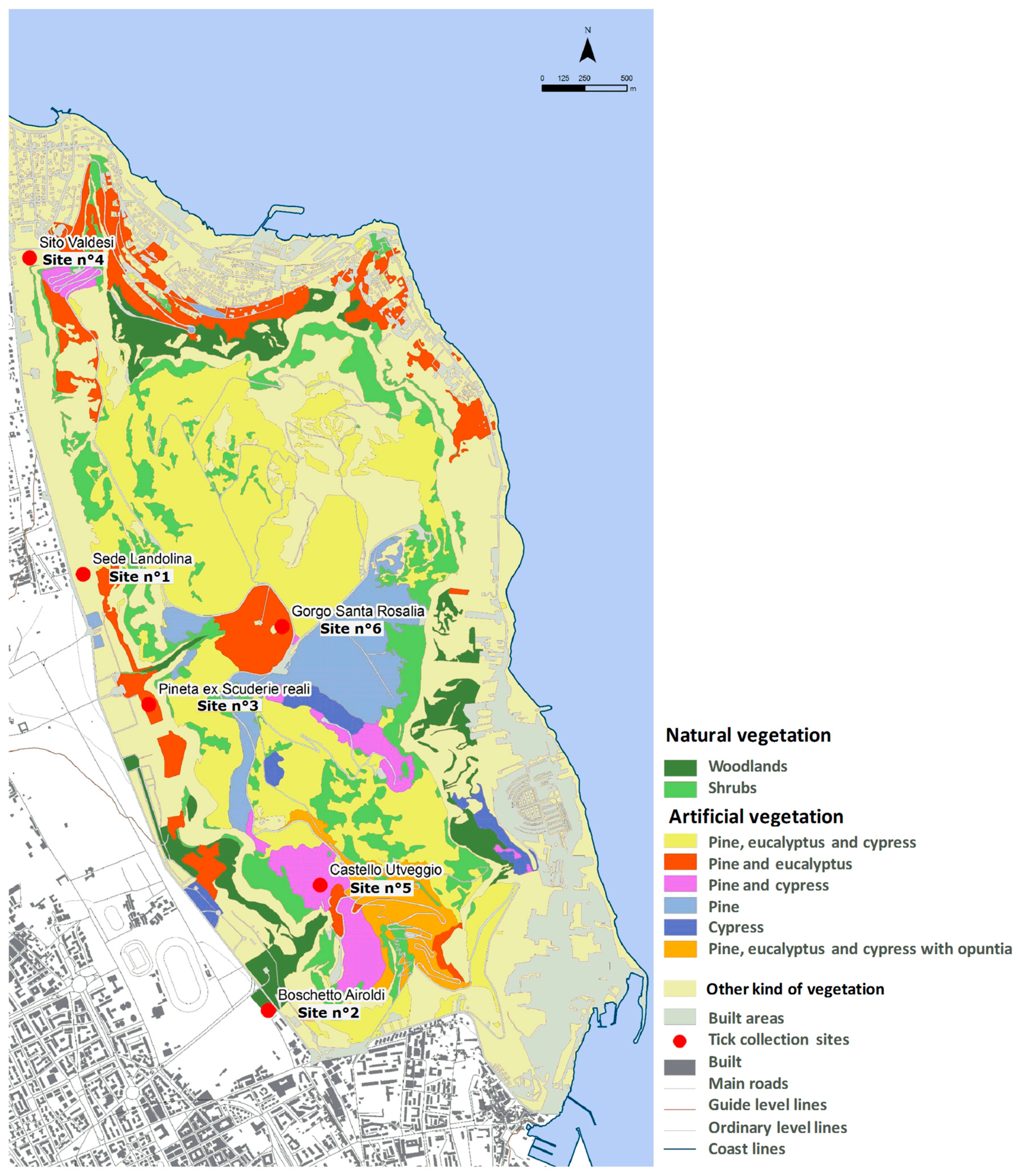
2.3. Ecological Analysis
2.4. Data Analysis
2.5. Processing of Maps with Proportionate Circles
3. Results
3.1. Ecological Analysis
3.2. Ticks Abundance
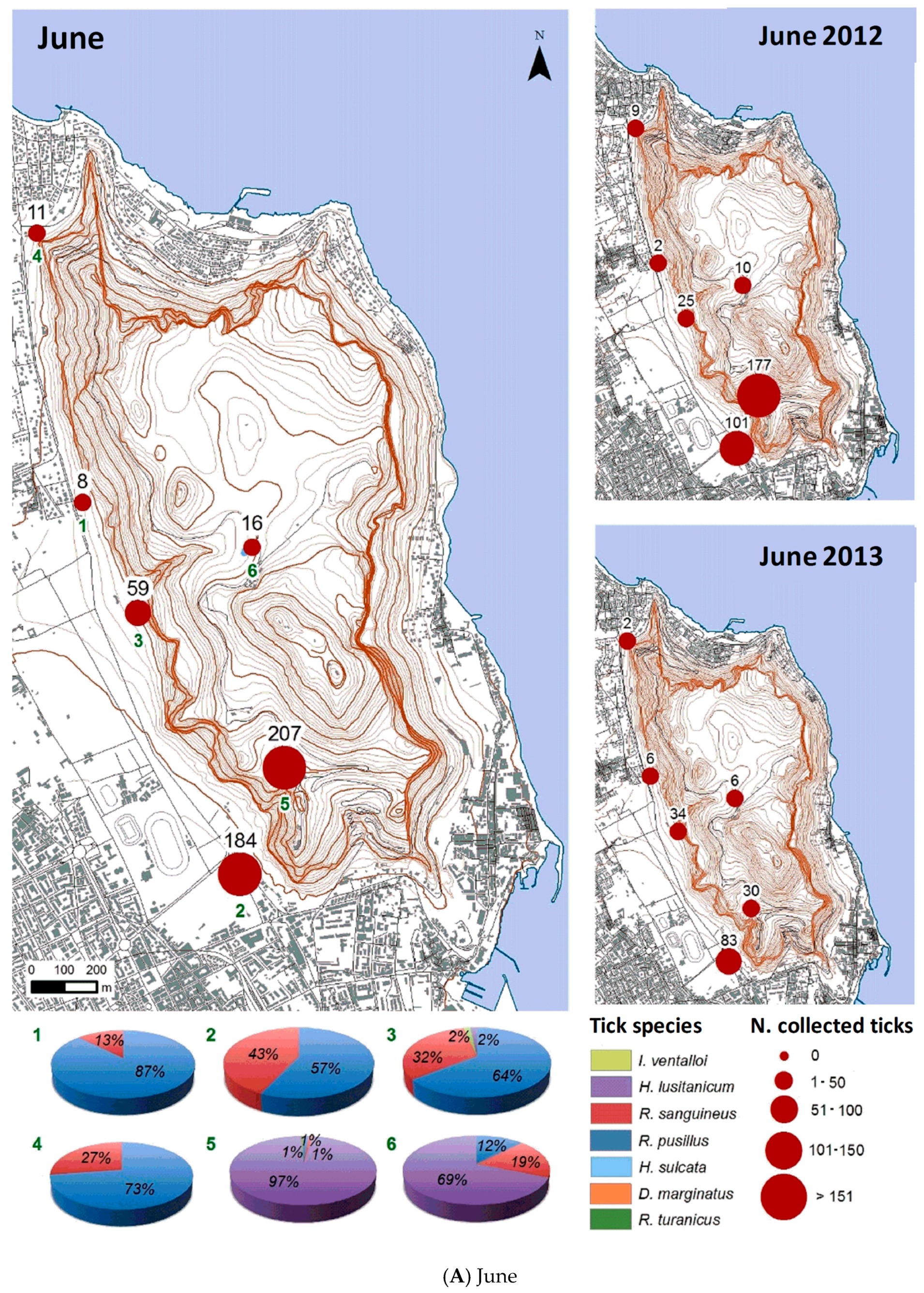
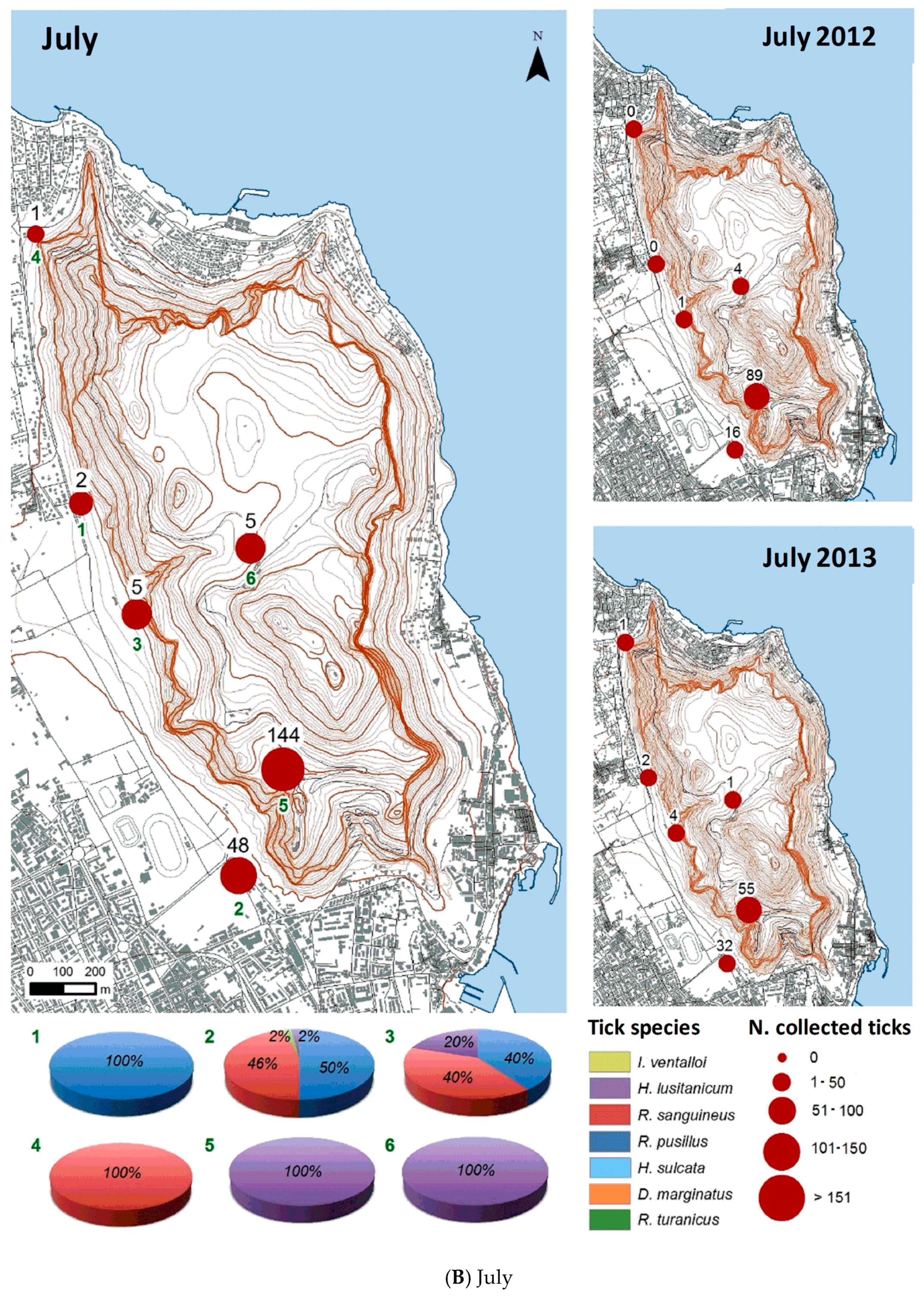
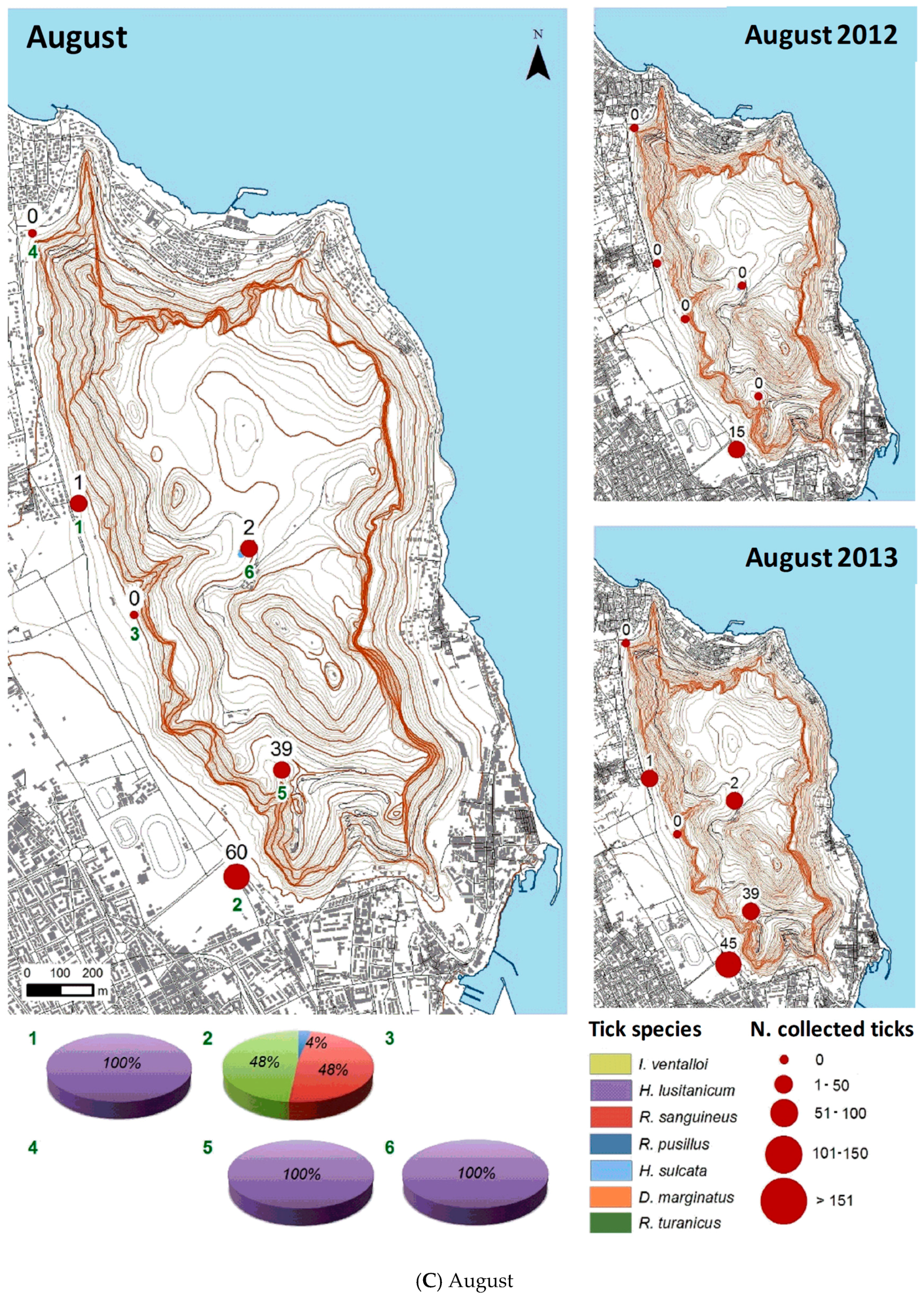
3.2.1. Monthly Trend
3.2.2. Spatial Distribution
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- De la Fuente, J.; Estrada-Peña, A.; Venzal, J.M.; Kocan, K.M.; Sonenshine, D.E. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front. Biosci. 2008, 13, 6938–6946. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Kopáček, P.; Lew-Tabor, A.; Maritz-Olivier, C. Strategies for new and improved vaccines against ticks and tick-borne diseases. Parasite Immunol. 2016, 38, 754–769. [Google Scholar] [CrossRef] [PubMed]
- Torina, A.; Moreno-Cid, J.A.; Blanda, V.; Fernández de Mera, I.G.; de la Lastra, J.M.; Scimeca, S.; Blanda, M.; Scariano, M.E.; Briganò, S.; Disclafani, R.; et al. Control of tick infestations and pathogen prevalence in cattle and sheep farms vaccinated with the recombinant Subolesin-Major Surface Protein 1a chimeric antigen. Parasites Vectors 2014, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J. Vaccines for vector control: Exciting possibilities for the future. Vet. J. 2012, 194, 139–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Estrada-Peña, A.; Ayllón, N.; De La Fuente, J. Impact of climate trends on tick-borne pathogen transmission. Front. Physiol. 2012, 3, 64. [Google Scholar] [CrossRef] [PubMed]
- Torina, A.; Alongi, A.; Naranjo, V.; Estrada-Peña, A.; Vicente, J.; Scimeca, S.; Marino, A.M.F.; Salina, F.; Caracappa, S.; De La Fuente, J. Prevalence and Genotypes of Anaplasma Species and Habitat Suitability for Ticks in a Mediterranean Ecosystem. Appl. Environ. Microbiol. 2008, 74, 7578–7584. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Rezza, C.; Ciervo, A. Le Rickettsiosi in Italia: Diagnosi e sorveglianza. Not. Ist. Super. Sanità 2015, 28, 3–8. [Google Scholar]
- Simser, J.A.; Palmer, A.T.; Fingerle, V.; Wilske, B.; Kurtti, T.J.; Munderloh, U.G. Rickettsia monacensis sp. nov., a spotted fever group Rickettsia, from ticks (Ixodes ricinus) collected in a European city park. Appl. Environ. Microbiol. 2002, 68, 4559–4566. [Google Scholar] [CrossRef] [PubMed]
- Beati, L.; Raoult, D. Rickettsia massiliae sp. nov., a new spotted fever group Rickettsia. Int. J. Syst. Bacteriol. 1993, 43, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Sekeyova, Z.; Roux, V.; Xu, W.; Rehacek, J.; Raoult, D. Rickettsia slovaca sp. nov., a member of the spotted fever group Rickettsiae. Int. J. Syst. Bacteriol. 1998, 48, 1455–1462. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beati, L.; Peter, O.; Burgdorfer, W.; Aeschlimann, A.; Raoult, D. Confirmation that Rickettsia helvetica sp. nov. is a distinct species of the spotted fever group of Rickettsiae. Int. J. Syst. Bacteriol. 1993, 43, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Beati, L.; Meskini, M.; Thiers, B.; Raoult, D. Rickettsia aeschlimannii sp. nov., a new spotted fever group rickettsia associated with Hyalomma marginatum ticks. Int. J. Syst. Bacteriol. 1997, 47, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Blanda, V.; Torina, A.; La Russa, F.; D’Agostino, R.; Randazzo, K.; Scimeca, S.; Giudice, E.; Caracappa, S.; Cascio, A.; de la Fuente, J. A retrospective study of the characterization of Rickettsia species in ticks collected from humans. Ticks Tick Borne Dis. 2017, 8, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Otranto, D.; Dantas-Torres, F.; Giannelli, A.; Latrofa, M.S.; Cascio, A.; Cazzin, S.; Ravagnan, S.; Montarsi, F.; Zanzani, S.A.; Manfredi, M.T.; et al. Ticks infesting humans in Italy and associated pathogens. Parasites Vectors 2014, 14, 328. [Google Scholar] [CrossRef] [PubMed]
- Cascio, A.; Torina, A.; Valenzise, M.; Blanda, V.; Camarda, N.; Bombaci, S.; Iaria, C.; De Luca, F.; Wasniewska, M. Scalp eschar and neck lymphadenopathy caused by Rickettsia massiliae. Emerg. Infect. Dis. 2013, 19, 836–837. [Google Scholar] [CrossRef] [PubMed]
- Torina, A.; Fernández de Mera, I.; Alongi, A.; Mangold, A.J.; Blanda, V.; Scarlata, F.; Di Marco, V.; de la Fuente, J. Rickettsia conorii Indian Tick Typhus Strain and R. slovaca in Humans, Sicily. Emerg. Infect. Dis. 2012, 18, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Rikihisa, Y.; Isogai, E.; Takahashi, M.; Misumi, H.; Suto, C.; Shibata, S.; Zhang, C.; Tsuji, M. Ultrastructure and phylogenic analysis of ‘Candidatus Neoehrlichia mikurensis’ in the family Anaplasmataceae, isolated from wild rats and found in Ixodes ovatus ticks. Int. J. Syst. Evol. Microbiol. 2004, 54, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Torina, A.; Caracappa, S. Babesiosis in Italy: An overview. Parassitologia 2007, 49, 23–28. [Google Scholar] [PubMed]
- Ebani, V.V.; Bertelloni, F.; Turchi, B.; Filogari, D.; Cerri, D. Molecular survey of tick-borne pathogens in Ixodid ticks collected from hunted wild animals in Tuscany, Italy. Asian Pac. J. Trop. Med. 2015, 8, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Torina, A.; Caracappa, S. Dog tick-borne diseases in Sicily. Parassitologia 2006, 48, 145–147. [Google Scholar] [PubMed]
- Di Luca, M.; Toma, L.; Bianchi, R.; Quarchioni, E.; Marini, L.; Mancini, F.; Ciervo, A.; Khoury, C. Seasonal dynamics of tick species in an urban park of Rome. Ticks Tick Borne Dis. 2013, 4, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Aureli, S.; Galuppi, R.; Ostanello, F.; Foley, J.E.; Bonoli, C.; Rejmanek, D.; Rocchi, G.; Orlandi, E.; Tampieri, M.P. Abundance of questing ticks and molecular evidence for pathogens in ticks in three parks of Emilia-Romagna region of Northern Italy. Ann. Agric. Environ. Med. 2015, 22, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Canale, A.; Mehlhorn, H. Tick repellents and acaricides of botanical origin: A green roadmap to control tick-borne diseases? Parasitol. Res. 2016, 115, 2545–2560. [Google Scholar] [CrossRef] [PubMed]
- McNair, C.M. Ectoparasites of medical and veterinary importance: Drug resistance and the need for alternative control methods. J. Pharm. Pharmacol. 2015, 67, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R. Repellence of essential oils and selected compounds against ticks—A systematic review. Acta Trop. 2018, 179, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Peña, A.; Venzal, J.M. A GIS framework for the assessment of tick impact on human health in a changing climate. Geospat. Health 2007, 1, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, G.; Iori, A.; Rinaldi, L.; Veneziano, V.; Genchi, C. Zecche Mappe Parassitologiche; Rolando Editore: Napoli, Italy, 2005; pp. 49–245. [Google Scholar]
- Rinaldi, L.; Musella, V.; Biggeri, A.; Cringoli, G. New insights into the application of geographical information systems and remote sensing in veterinary parasitology. Geospat. Health 2006, 1, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Blanda, V.; Blanda, M.; La Russa, F.; Scimeca, R.; Scimeca, S.; D’Agostino, R.; Auteri, M.; Torina, A. Geo-statistical analysis of Culicoides spp. distribution and abundance in Sicily. Parasites Vectors 2018. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, F.M.; Venturella, G. Lineamenti fisici e biogeografici del Promontorio di Monte Pellegrino (Palermo). Quad. Bot. Amb. Appl. 1993, 4, 7–11. [Google Scholar]
- Raimondo, F.M.; Venturella, G.; Ilardi, E.V. Carta forestale del promontorio di Monte Pellegrino, (Palermo). Quad. Bot. Amb. Appl. 1993, 4, 145–152. [Google Scholar]
- Surano, N.; Gianguzzi, L.; Raimondo, F.M. Carta della vegetazione del promontorio di Monte Pellegrino (Palermo). Quad. Bot. Amb. Appl. 1993, 4, 139–144. [Google Scholar]
- Manilla, G. Fauna d’Italia Acari Ixodida; Edizioni Calderini: Bologna, Italy, 1998; pp. 42–242. [Google Scholar]
- Walker, J.B.; Keirans, J.E.; Horak, I.G. The Genus Rhipicephalus (Acari: Ixodidae): A Guide to the Brown Ticks of the World; Cambridge University Press: Cambridge, UK, 2000; pp. 40–583. [Google Scholar]
- Apanaskevich, D.A.; Santos-Silva, M.M.; Horak, I.G. The genus Hyalomma Koch, 1844. IV. Redescription of all parasitic stages of H. (Euhyalomma) lusitanicum Koch, 1844 and the adults of H. (E.) franchinii Tonelli Rondelli, 1932 (Acari: Ixodidae) with a first description of its immature stages. Folia Parasitol. 2008, 55, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Nava, S.; Estrada-Peña, A.; Petney, T.; Beati, L.; Labruna, M.B.; Szabó, M.P.; Venzal, J.M.; Mastropaolo, M.; Mangold, A.J.; Guglielmone, A.A. The taxonomic status of Rhipicephalus sanguineus (Latreille, 1806). Vet. Parasitol. 2015, 208, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Graci, G.; Pileri, P.; Sedazzari, M. Gis e Ambiente Guida All’uso di ArcGis per L’analisi del Territorio e la Valutazione Ambientale; Dario Flaccovio Editore: Palermo, Italy, 2008; pp. 1–268. [Google Scholar]
- Dantas-Torres, F. Climate change, biodiversity, ticks and tick-borne diseases: The butterfly effect. Int. J. Parasitol. Parasites Wildl. 2015, 4, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Torina, A.; Blanda, V.; Antoci, F.; Scimeca, S.; D’Agostino, R.; Scariano, E.; Piazza, A.; Galluzzo, P.; Giudice, E.; Caracappa, S. A Molecular survey of Anaplasma spp., Rickettsia spp., Ehrlichia canis and Babesia microti in foxes and fleas from Sicily. Transbound. Emerg. Dis. 2013, 60, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Rehse-Küpper, B.; Casals, J.; Rehse, E.; Ackermann, R. Eyach, an arthropod- borne virus related to Colorado tick fever virus in the Federal Republic of Germany. Acta Virol. 1976, 20, 339–342. [Google Scholar] [PubMed]
- Latrofa, M.S.; Giannelli, A.; Persichetti, M.F.; Pennisi, M.G.; Solano-Gallego, L.; Brianti, E.; Parisi, A.; Wall, R.; Dantas-Torres, F.; Otranto, D. Ixodes ventalloi: Morphological and molecular support for species integrity. Parasitol. Res. 2017, 116, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Pardo, C.; Martínez-Grueiro, M.M. Some hydrolase activities from the tick Hyalomma lusitanicum koch, 1844 (Ixodoidea: Ixodida). Parasite 2008, 15, 589–593. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chastel, C.; Main, A.J.; Couatarmanach, A.; Le Lay, G.; Knudson, D.L.; Quillien, M.C.; Beaucournu, J.C. Isolation of Eyach virus (Reoviridae, Colorado tick fever group) from Ixodes ricinus and I. ventalloi ticks in France. Arch. Virol. 1984, 82, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.I.; Knight, E.M.; Courtois, G.; Williams, M.C.; Weinbren, M.P.; Kibukamusoke, J.W. Congo virus: A hitherto undescribed virus occurring in Africa. I. Human isolations—Clinical notes. East Afr. Med. J. 1967, 44, 86–92. [Google Scholar] [PubMed]
- Cajimat, M.N.B.; Rodriguez, S.E.; Schuster, I.U.E.; Swetnam, D.M.; Ksiazek, T.G.; Habela, M.A.; Negredo, A.I.; Estrada-Peña, A.; Barrett, A.D.T.; Bente, D.A. Characterization of Crimean-Congo Hemorrhagic Fever Virus in Hyalomma Tick from Spain, 2014. Vector Borne Zoonotic Dis. 2017, 17, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Bolaños-Rivero, M.; Carranza-Rodríguez, C.; Rodríguez, N.F.; Gutiérrez, C.; Pérez-Arellano, J.L. Detection of Coxiella burnetii DNA in Peridomestic and Wild Animals and Ticks in an Endemic Region (Canary Islands, Spain). Vector Borne Zoonotic Dis. 2017, 17, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Socolovschi, C.; Raoult, D. Deciphering the relationships between Rickettsia conorii conorii and Rhipicephalus sanguineus in the ecology and epidemiology of Mediterranean spotted fever. Ann. N. Y. Acad. Sci. 2009, 1166, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, W.L.; Paddock, C.D.; Demma, L.; Traeger, M.; Johnson, B.; Dickson, J.; McQuiston, J.; Swerdlow, D. Rocky Mountain spotted fever in Arizona: Documentation of heavy environmental infestations of Rhipicephalus sanguineus at an endemic site. Ann. N. Y. Acad. Sci. 2006, 1078, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Sainz, Á.; Roura, X.; Estrada-Peña, A.; Miró, G. A review of canine babesiosis: The European perspective. Parasites Vectors 2016, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P.E.; Zhu, Y.; Yu, X.; Raoult, D. Proposal to create subspecies of Rickettsia sibirica and an emended description of Rickettsia sibirica. Ann. N. Y. Acad. Sci. 2006, 1078, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Edouard, S.; Parola, P.; Socolovschi, C.; Davoust, B.; La Scola, B.; Raoult, D. Clustered Cases of Rickettsia sibirica mongolitimonae Infection, France. Emerg. Infect. Dis. 2013, 19, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Toledo, A.; Jado, I.; Olmeda, A.S.; Casado-Nistal, M.A.; Gil, H.; Escudero, R.; Anda, P. Detection of Coxiella burnetii in ticks collected from Central Spain. Vector Borne Zoonotic Dis. 2009, 9, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Parola, P.; Paddock, C.D.; Raoult, D. Tick-borne rickettsioses around the world: Emerging diseases challenging old concepts. Clin. Microbiol. 2005, 18, 719–756. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.; Ciccozzi, M.; Lo Presti, A.C.; Giovanetti, M.; Di Luca, M.; Toma, L.; Bianchi, R.; Khoury, C.; Rezza, G.; Ciervo, A. Characterization of spotted fever group Rickettsiae in ticks from a city park of Rome, Italy. Ann. Ist. Super. Sanita 2015, 51, 284–290. [Google Scholar] [PubMed]
- Mancini, F.; Di Luca, M.; Toma, L.; Vescio, F.; Bianchi, R.; Khoury, C.; Marini, L.; Rezza, G.; Ciervo, A. Prevalence of tick-borne pathogens in an urban park in Rome, Italy. Ann. Agric. Environ. Med. 2014, 21, 723–727. [Google Scholar] [CrossRef] [PubMed]


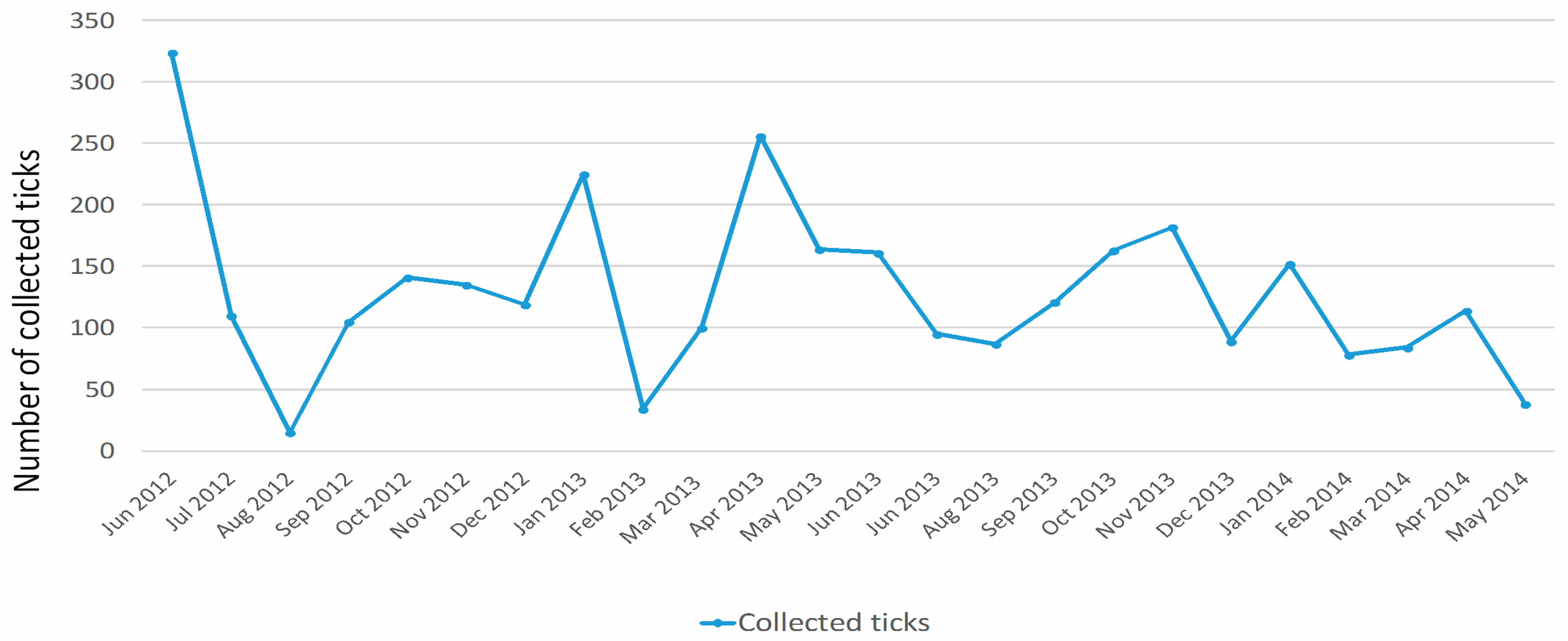
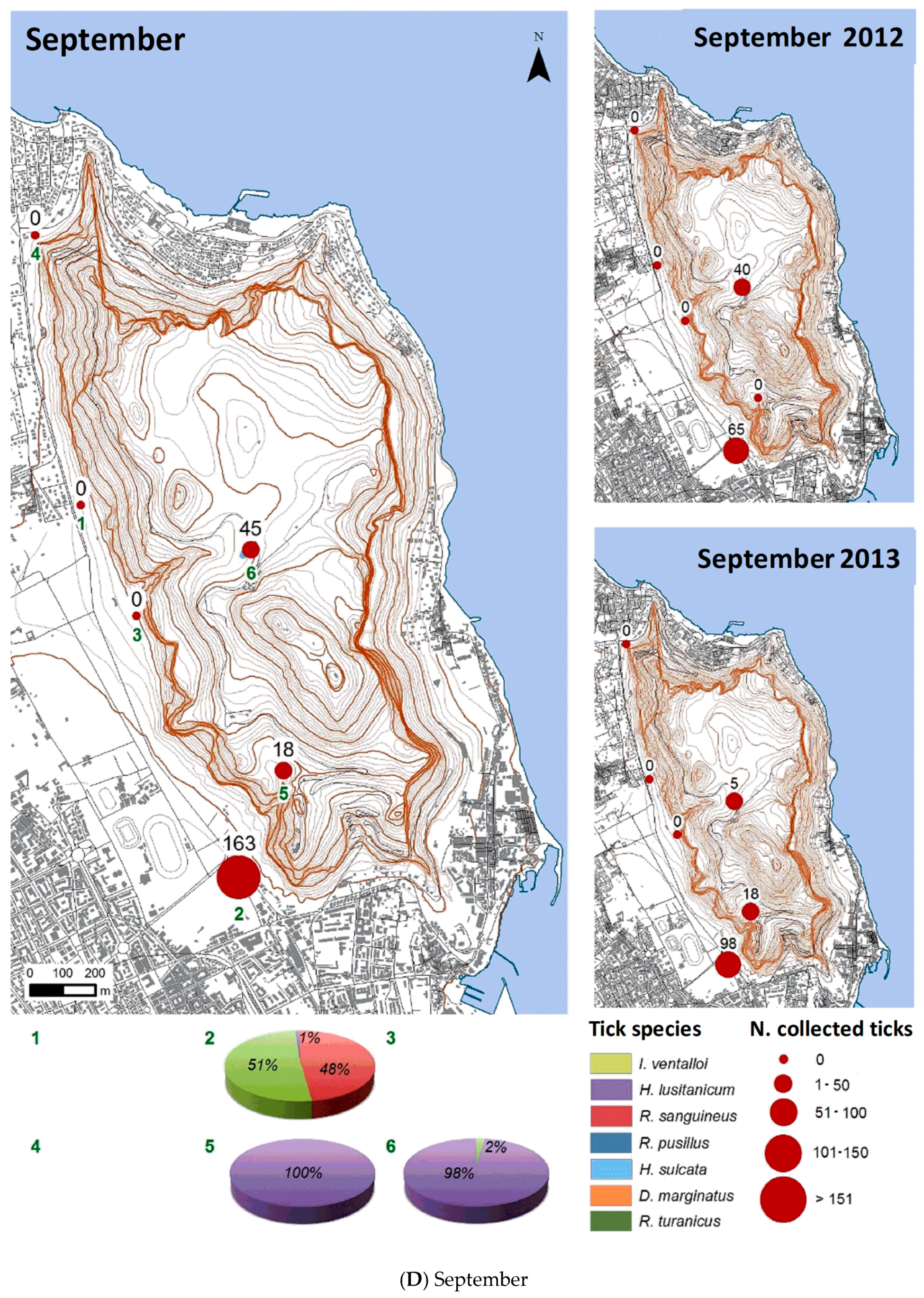
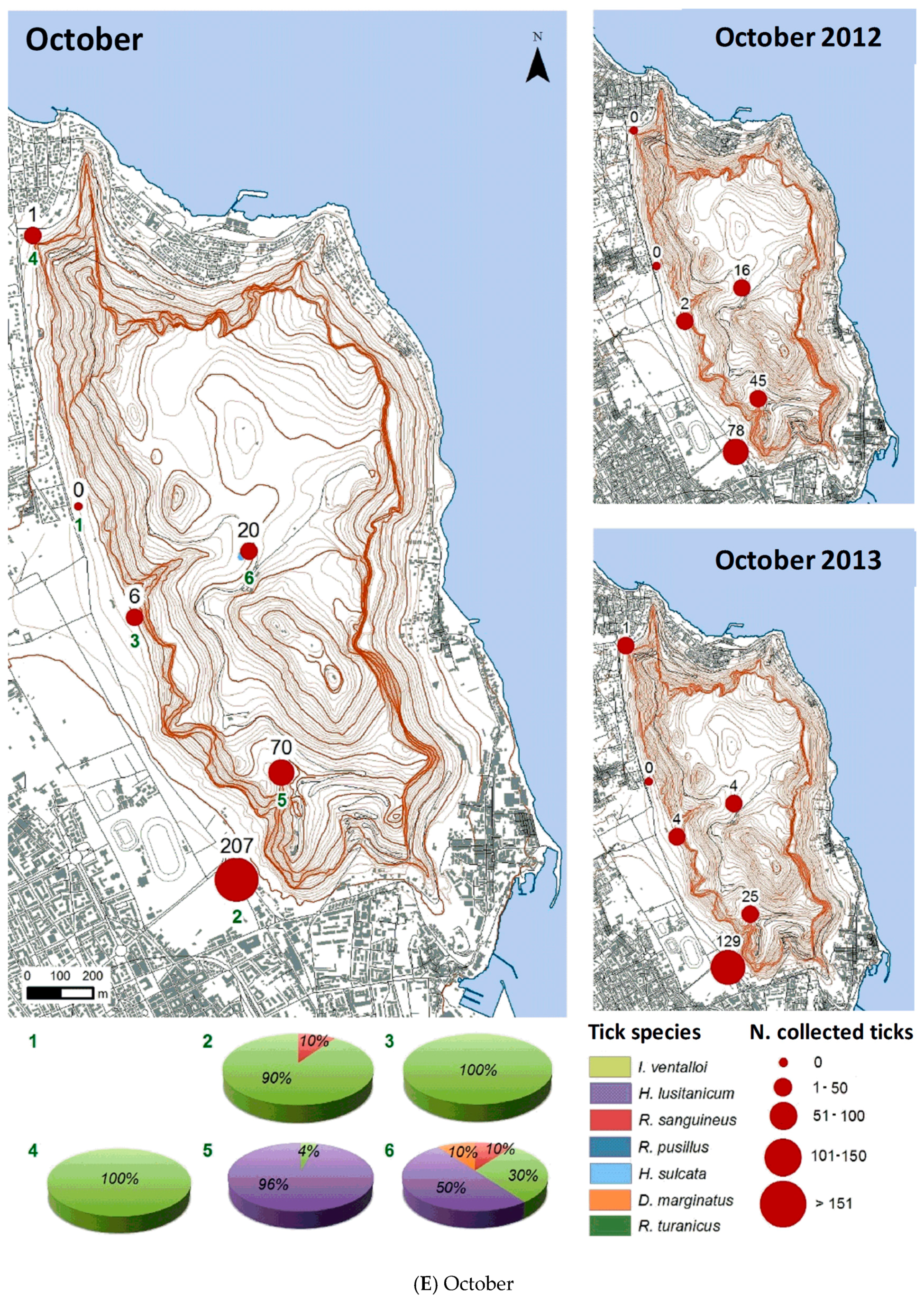
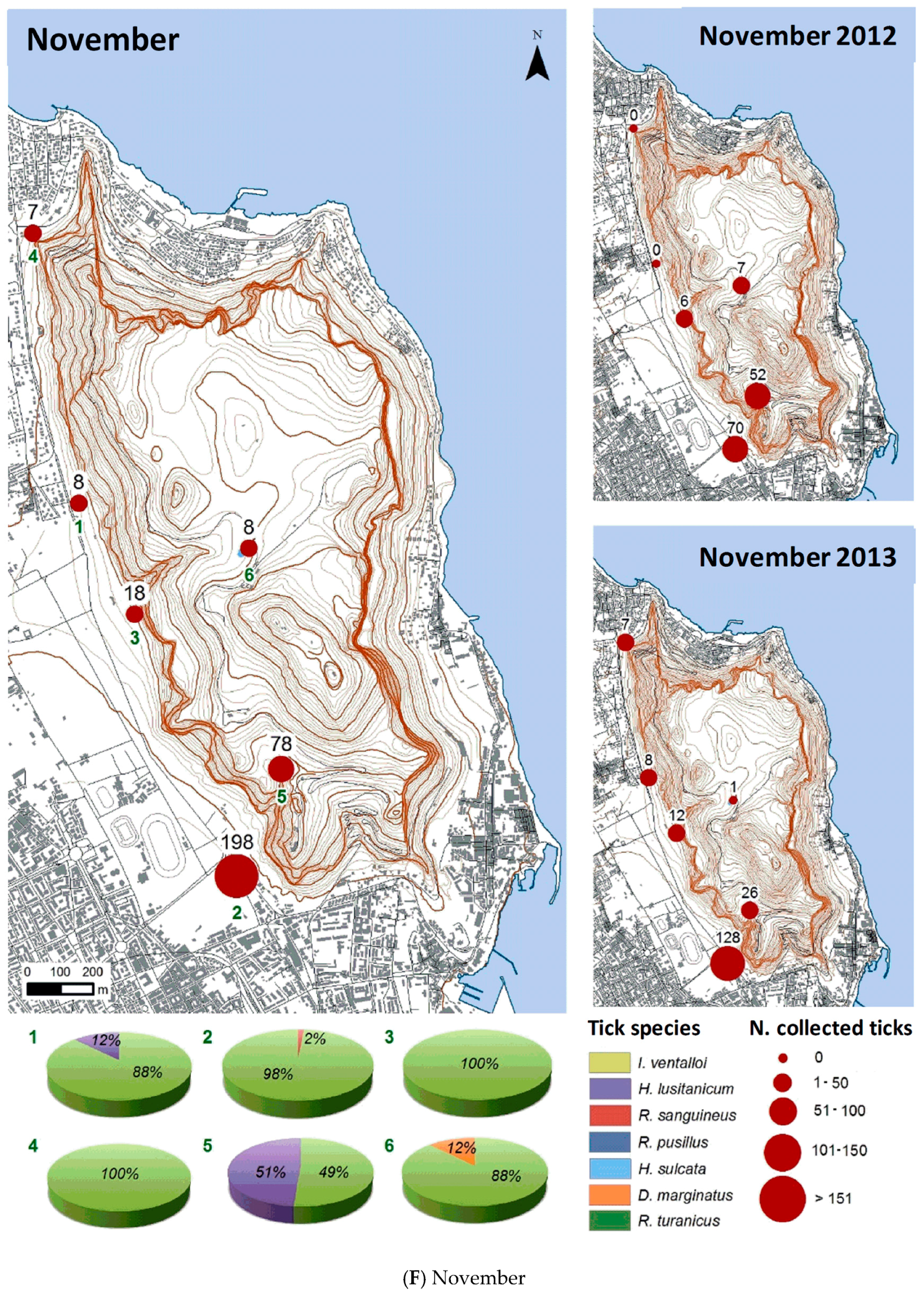
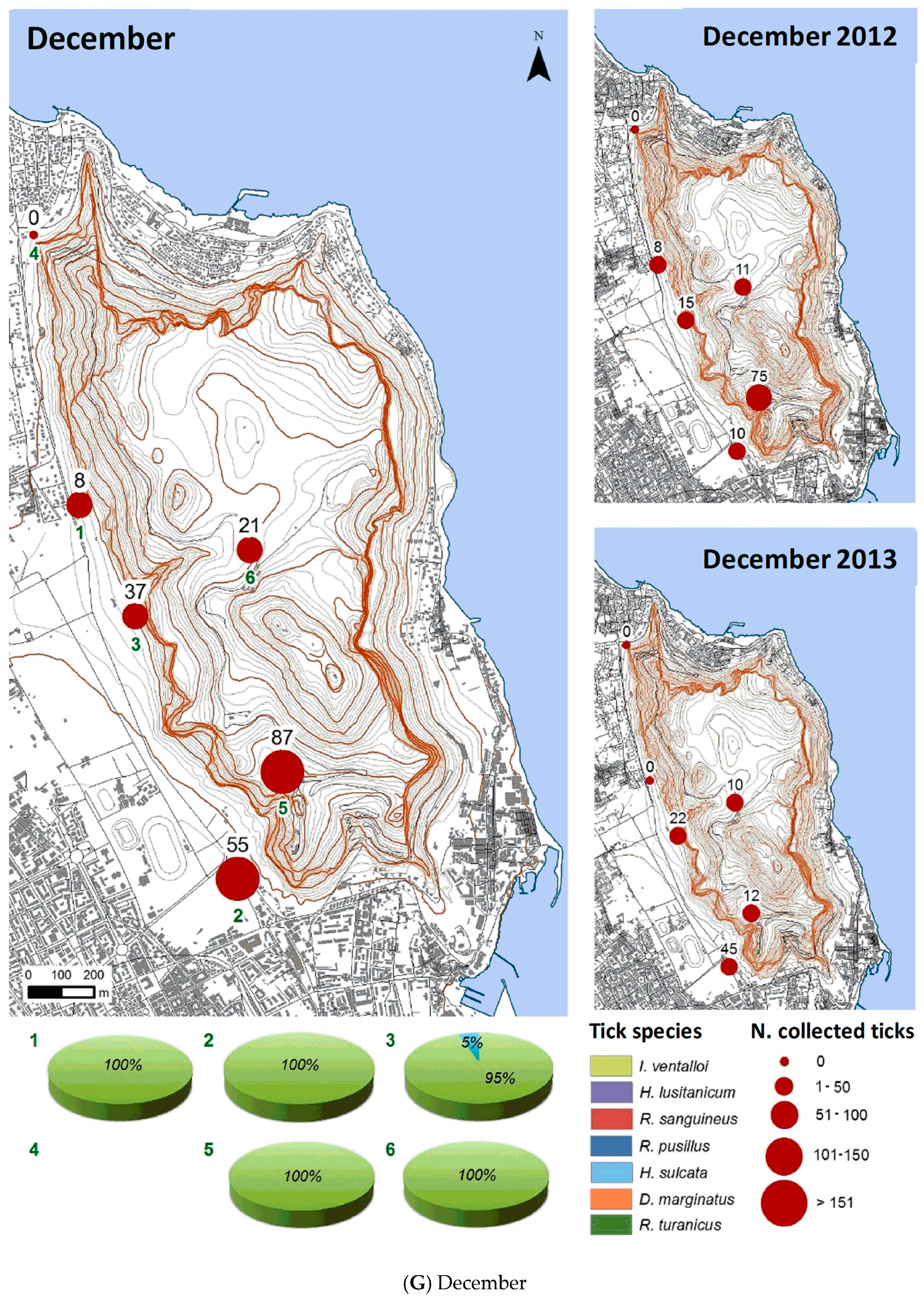
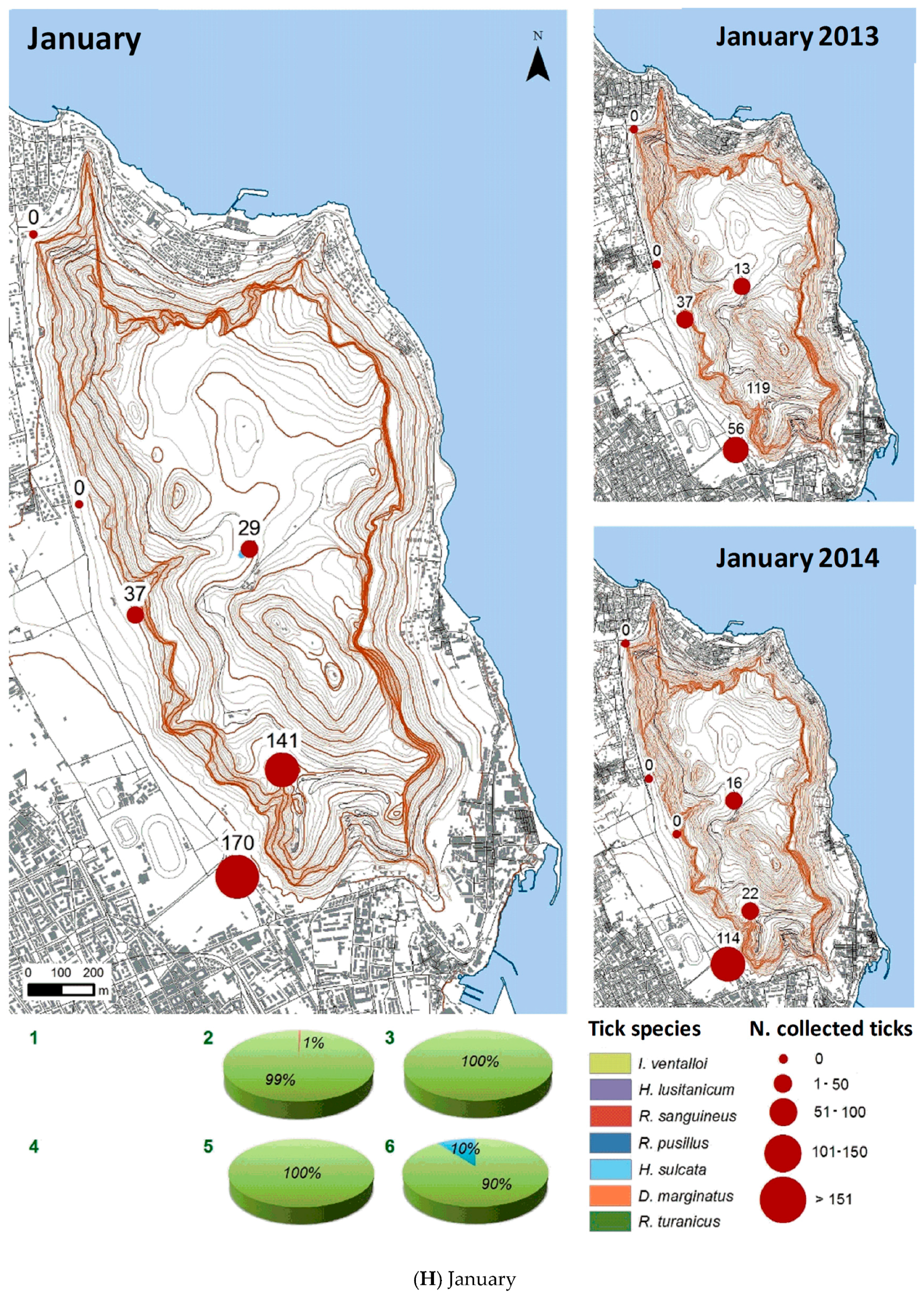
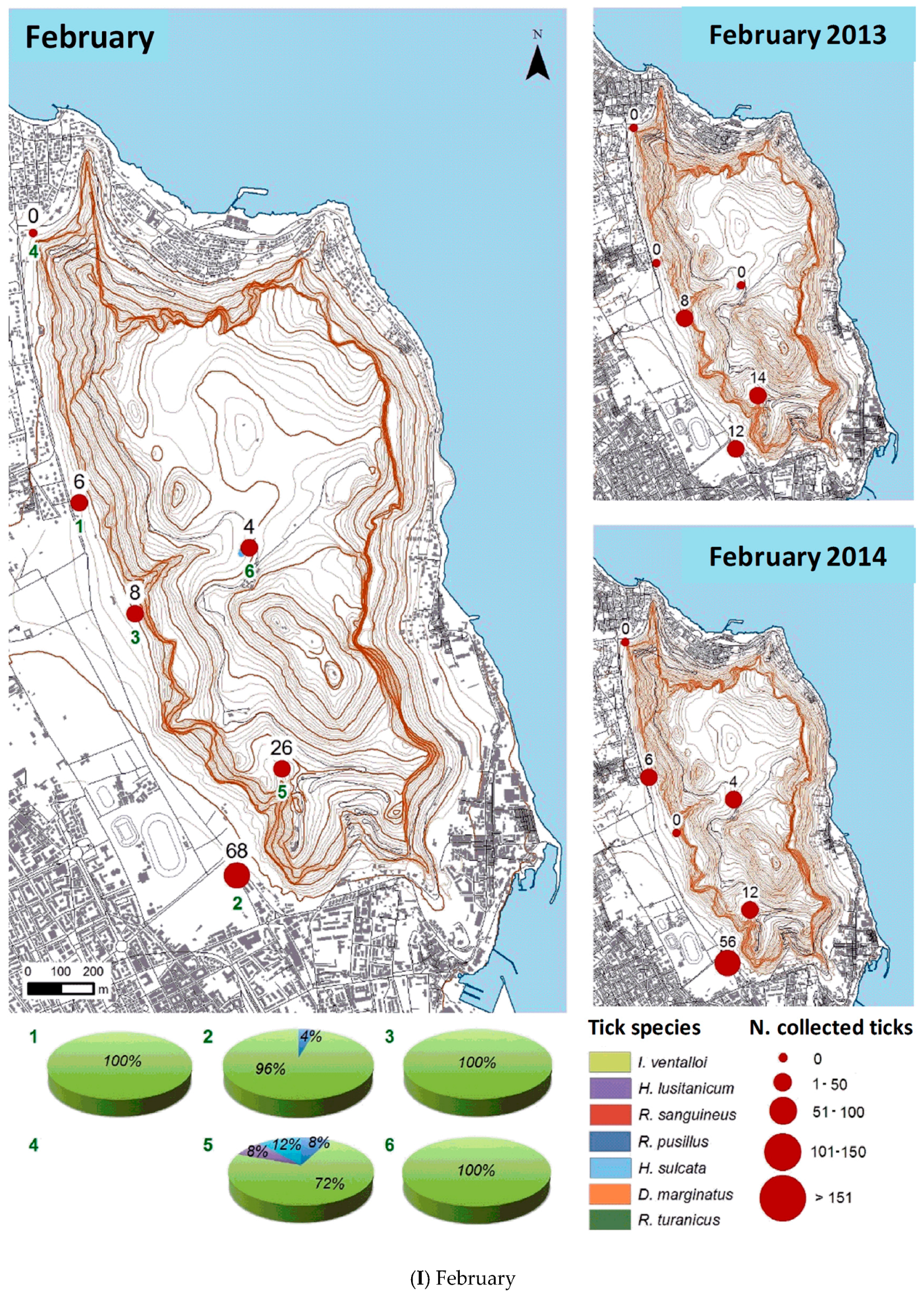

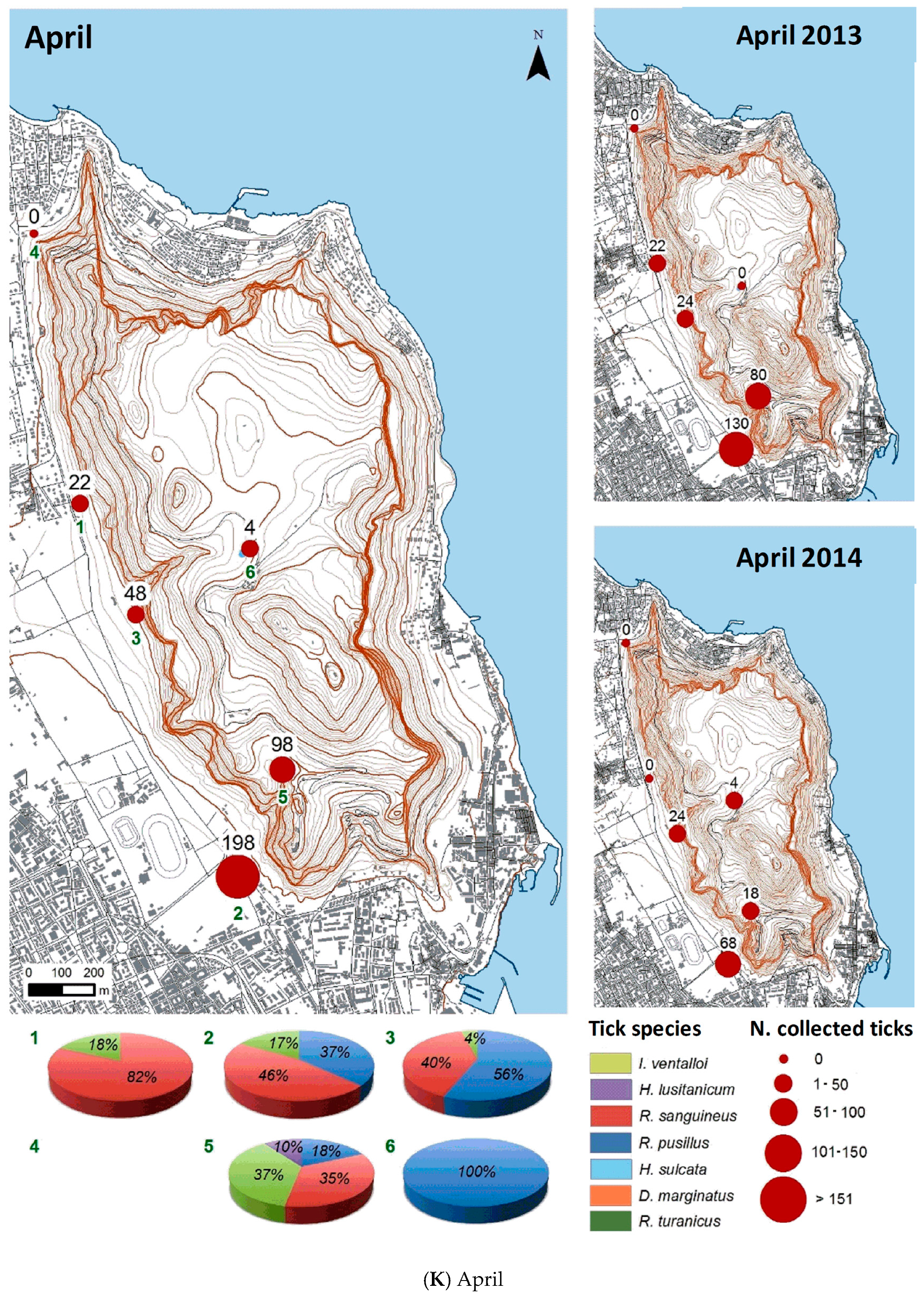
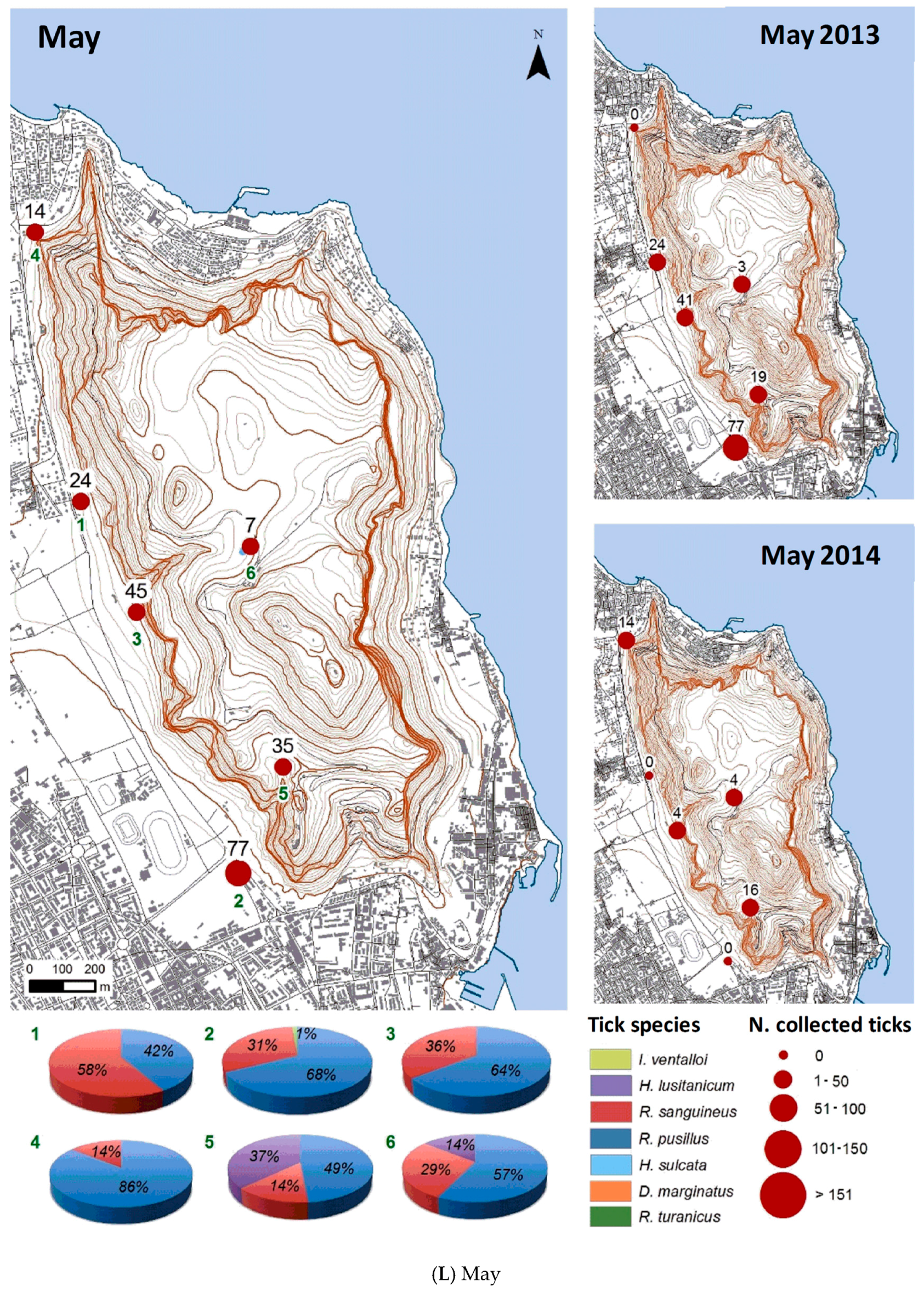
| Month | Total Tick Number | Ixodes ventalloi | Hyalomma lusitanicum | Rhipicephalus sanguineus | Rhipicephalus pusillus | Haemaphysalis sulcata | Dermacentor marginatus | Rhipicephalus turanicus |
|---|---|---|---|---|---|---|---|---|
| June 2012 | 324 | 0 | 181 | 76 | 67 | 0 | 0 | 0 |
| July 2012 | 110 | 0 | 94 | 10 | 6 | 0 | 0 | 0 |
| August 2012 | 15 | 6 | 0 | 9 | 0 | 0 | 0 | 0 |
| September 2012 | 105 | 39 | 39 | 27 | 0 | 0 | 0 | 0 |
| October 2012 | 141 | 69 | 52 | 18 | 0 | 0 | 2 | 0 |
| November 2012 | 135 | 97 | 34 | 3 | 0 | 0 | 1 | 0 |
| December 2012 | 119 | 117 | 0 | 0 | 0 | 2 | 0 | 0 |
| January 2013 | 225 | 221 | 0 | 1 | 0 | 3 | 0 | 0 |
| February 2013 | 34 | 32 | 0 | 0 | 1 | 1 | 0 | 0 |
| March 2013 | 100 | 32 | 2 | 35 | 28 | 3 | 0 | 0 |
| April 2013 | 256 | 65 | 8 | 159 | 24 | 0 | 0 | 0 |
| May 2013 | 164 | 1 | 14 | 61 | 88 | 0 | 0 | 0 |
| June 2013 | 161 | 1 | 32 | 31 | 96 | 0 | 0 | 1 |
| July 2013 | 95 | 1 | 57 | 15 | 22 | 0 | 0 | 0 |
| August 2013 | 87 | 23 | 42 | 20 | 2 | 0 | 0 | 0 |
| September 2013 | 121 | 45 | 25 | 51 | 0 | 0 | 0 | 0 |
| October 2013 | 163 | 134 | 25 | 4 | 0 | 0 | 0 | 0 |
| November 2013 | 182 | 177 | 5 | 0 | 0 | 0 | 0 | 0 |
| December 2013 | 89 | 89 | 0 | 0 | 0 | 0 | 0 | 0 |
| January 2014 | 152 | 152 | 0 | 0 | 0 | 0 | 0 | 0 |
| February 2014 | 78 | 70 | 2 | 0 | 4 | 2 | 0 | 0 |
| March 2014 | 84 | 44 | 4 | 10 | 26 | 0 | 0 | 0 |
| April 2014 | 114 | 10 | 2 | 4 | 98 | 0 | 0 | 0 |
| May 2014 | 38 | 0 | 0 | 2 | 36 | 0 | 0 | 0 |
| Total | 3092 | 1425 | 618 | 536 | 498 | 11 | 3 | 1 |
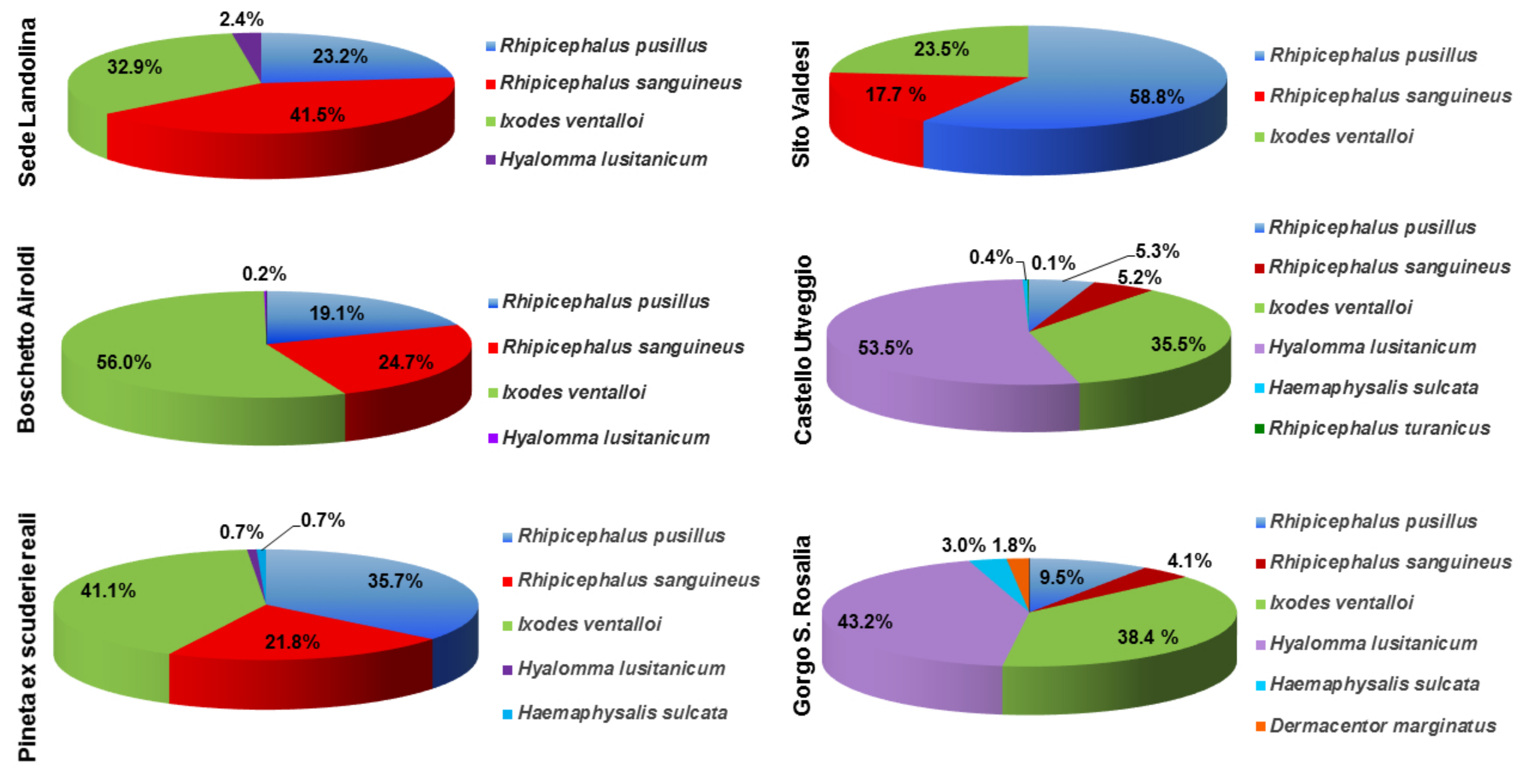
| Collection Site | Total | R. pusillus | R. sanguineus | I. ventalloi | H. lusitanicum | H. sulcata | D. marginatus | R. turanicus |
|---|---|---|---|---|---|---|---|---|
| Sede Landolina | 82 | 19 | 34 | 27 | 2 | 0 | 0 | 0 |
| Boschetto Airoldi | 1522 | 290 | 376 | 853 | 3 | 0 | 0 | 0 |
| Pineta Ex Scuderie Reali | 280 | 100 | 61 | 115 | 2 | 2 | 0 | 0 |
| Sito Valdesi | 34 | 20 | 6 | 8 | 0 | 0 | 0 | 0 |
| Castello Utveggio | 1005 | 53 | 52 | 357 | 538 | 4 | 0 | 1 |
| Gorgo S. Rosalia | 169 | 16 | 7 | 65 | 73 | 5 | 3 | 0 |
| 3092 | 498 | 536 | 1425 | 618 | 11 | 3 | 1 |
| Tick Species | Principal Habitat (GIS Forest Vegetation Map) | Pathogens Associated | Human Diseases | References |
|---|---|---|---|---|
| I. ventalloi | Natural vegetation (Woodland) | R. helvetica * R. monacensis * A. phagocytophilum * Bartonella clarridgeia * Eyach virus * | SFGR HGA CSD Eyach Virus Disease | [41,43] |
| H. lusitanicum | Artificial vegetation (Pine and Cypress) | CCHFV * (Simpson et al., 1967) [44] Coxiella burnetii * R. aeschlimannii * Theileria annulata Babesia spp. | CCHF SFGR Q fever | [13,42,45,46] |
| R. sanguineus | Natural vegetation (Woodland) | R. conorii * R. rickettsii * (Brumpt, 1922) Coxiella burnetii * Babesia canis (Piana & Galli-Valerio, 1895) Ehrlichia canis (Donatien and Lestoquard 1935) Anaplasma marginale | MSF RMSF Q fever | [47,48,49] |
| R. pusillus | Natural vegetation (Woodland) | R. sibirica mongolitimonae * (Fournier et al., 2006) [50] Coxiella burnetiid * | Lymphangitis-associated rickettsiosis Q fever | [51,52] |
| Hae. sulcata | Artificial vegetation (Pine and cypress/eucalyptus) | Anaplasma ovis (Lestoquard, 1924) Theileria annulata | - | [33] |
| D. marginatus | Artificial vegetation (Pine and eucalyptus) | R. sibirica * (Zdrodovskii, 1948) R. slovaca * R. aeschlimannii * Babesia canis | Siberian tick typhus SFGR SENLAT | [53] |
| R. turanicus | Artificial vegetation (Pine and cypress) | R. monacensis * R. massiliae * R. conorii * R. aeschlimannii * Babesia spp. | MSF SFGR | [54] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torina, A.; Blanda, V.; Blanda, M.; Auteri, M.; La Russa, F.; Scimeca, S.; D’Agostino, R.; Disclafani, R.; Villari, S.; Currò, V.; et al. A Geographical Information System Based Approach for Integrated Strategies of Tick Surveillance and Control in the Peri-Urban Natural Reserve of Monte Pellegrino (Palermo, Southern Italy). Int. J. Environ. Res. Public Health 2018, 15, 404. https://doi.org/10.3390/ijerph15030404
Torina A, Blanda V, Blanda M, Auteri M, La Russa F, Scimeca S, D’Agostino R, Disclafani R, Villari S, Currò V, et al. A Geographical Information System Based Approach for Integrated Strategies of Tick Surveillance and Control in the Peri-Urban Natural Reserve of Monte Pellegrino (Palermo, Southern Italy). International Journal of Environmental Research and Public Health. 2018; 15(3):404. https://doi.org/10.3390/ijerph15030404
Chicago/Turabian StyleTorina, Alessandra, Valeria Blanda, Marcellocalogero Blanda, Michelangelo Auteri, Francesco La Russa, Salvatore Scimeca, Rosalia D’Agostino, Rosaria Disclafani, Sara Villari, Vittoria Currò, and et al. 2018. "A Geographical Information System Based Approach for Integrated Strategies of Tick Surveillance and Control in the Peri-Urban Natural Reserve of Monte Pellegrino (Palermo, Southern Italy)" International Journal of Environmental Research and Public Health 15, no. 3: 404. https://doi.org/10.3390/ijerph15030404
APA StyleTorina, A., Blanda, V., Blanda, M., Auteri, M., La Russa, F., Scimeca, S., D’Agostino, R., Disclafani, R., Villari, S., Currò, V., & Caracappa, S. (2018). A Geographical Information System Based Approach for Integrated Strategies of Tick Surveillance and Control in the Peri-Urban Natural Reserve of Monte Pellegrino (Palermo, Southern Italy). International Journal of Environmental Research and Public Health, 15(3), 404. https://doi.org/10.3390/ijerph15030404






