Risk Factors for Abdominal Aortic Aneurysm in Population-Based Studies: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
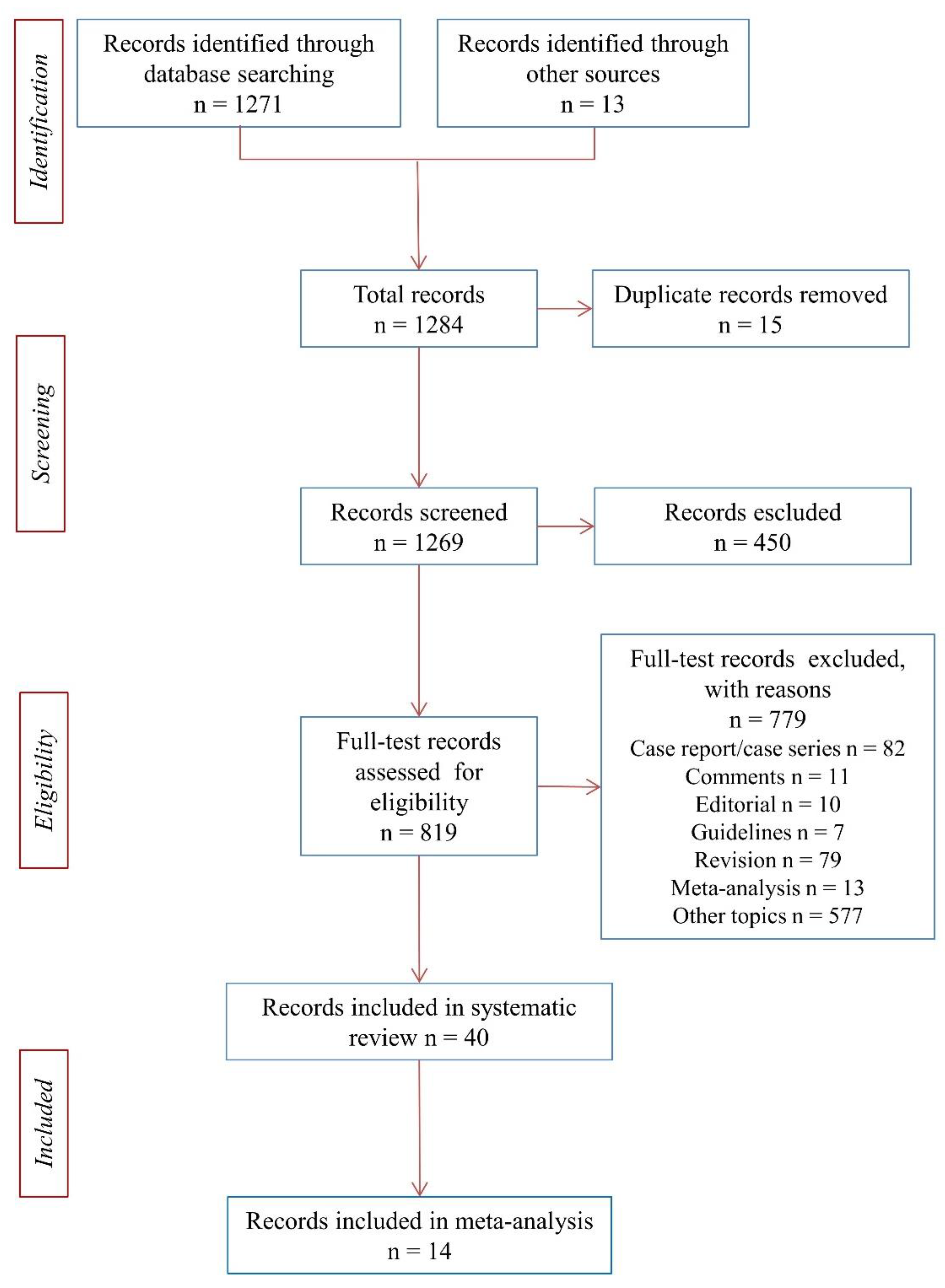
2.1. Search Method for Identification of Studies
2.2. Criteria for Selecting Studies
2.2.1. Participants
2.2.2. Outcome
2.2.3. Quality Assessment
2.3. Statistical Analysis
3. Results
3.1. Systematic Review of the Literature and Meta-Analysis
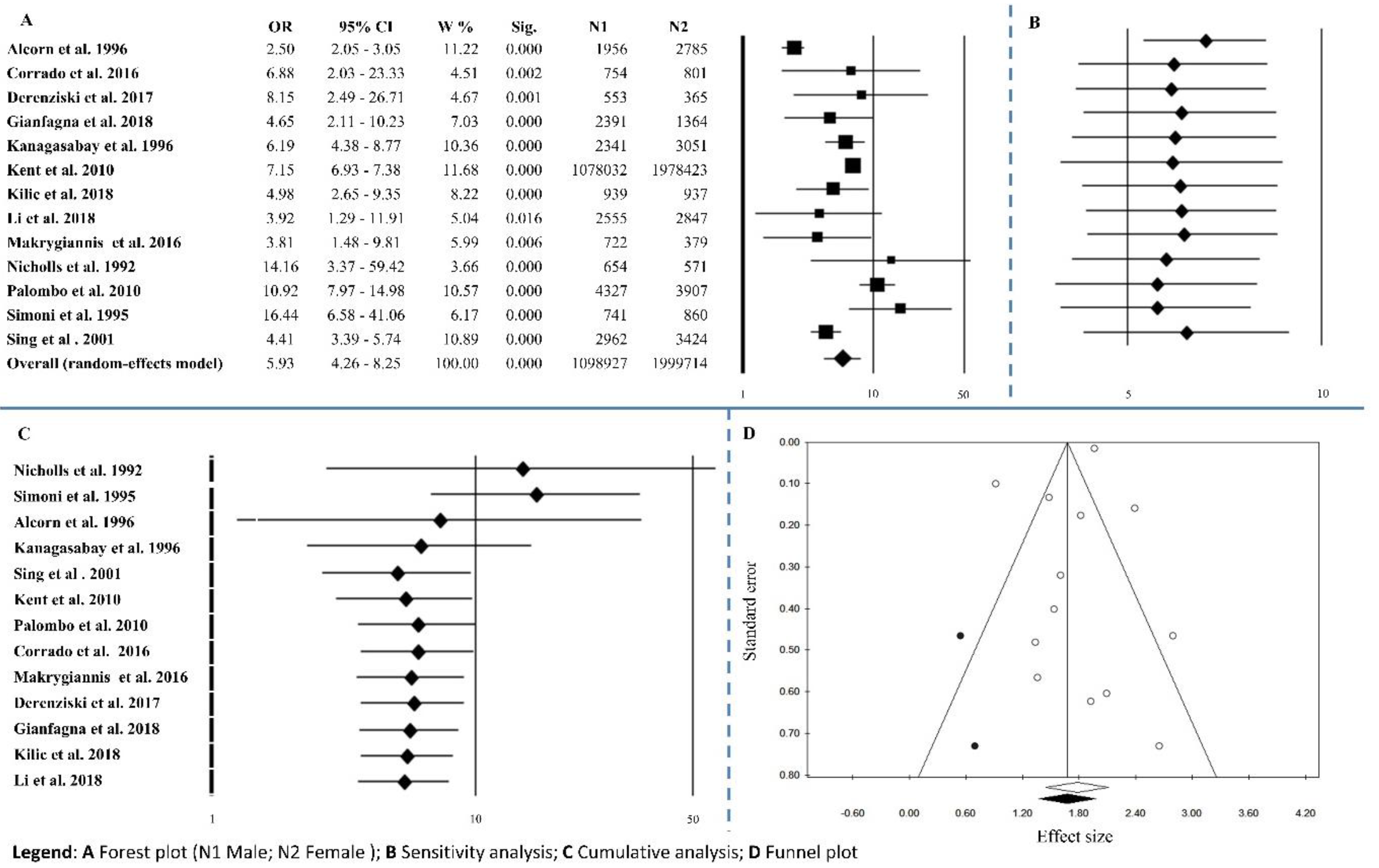
3.1.1. Gender
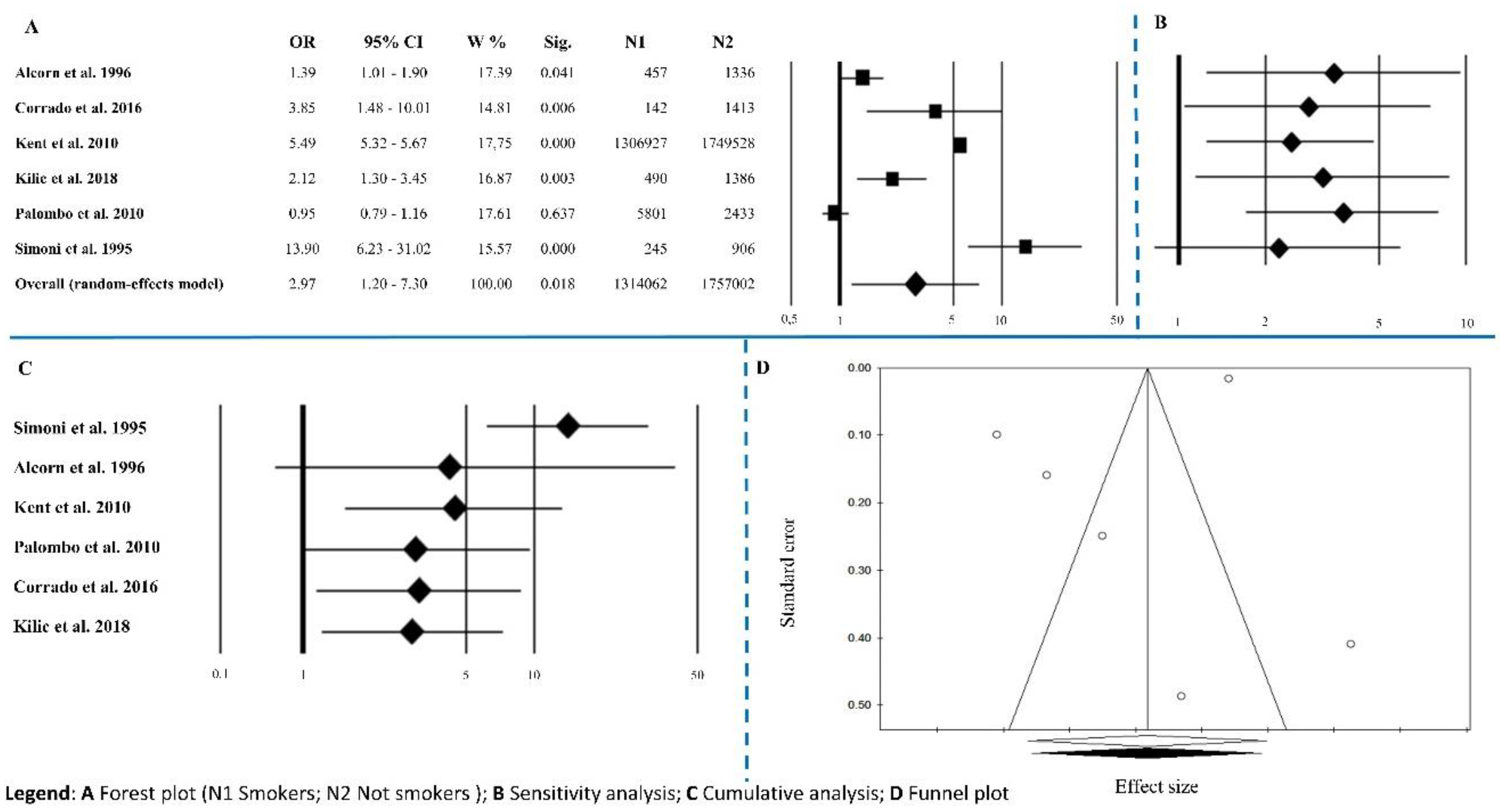
3.1.2. Smoking Habits
3.1.3. Hypertension
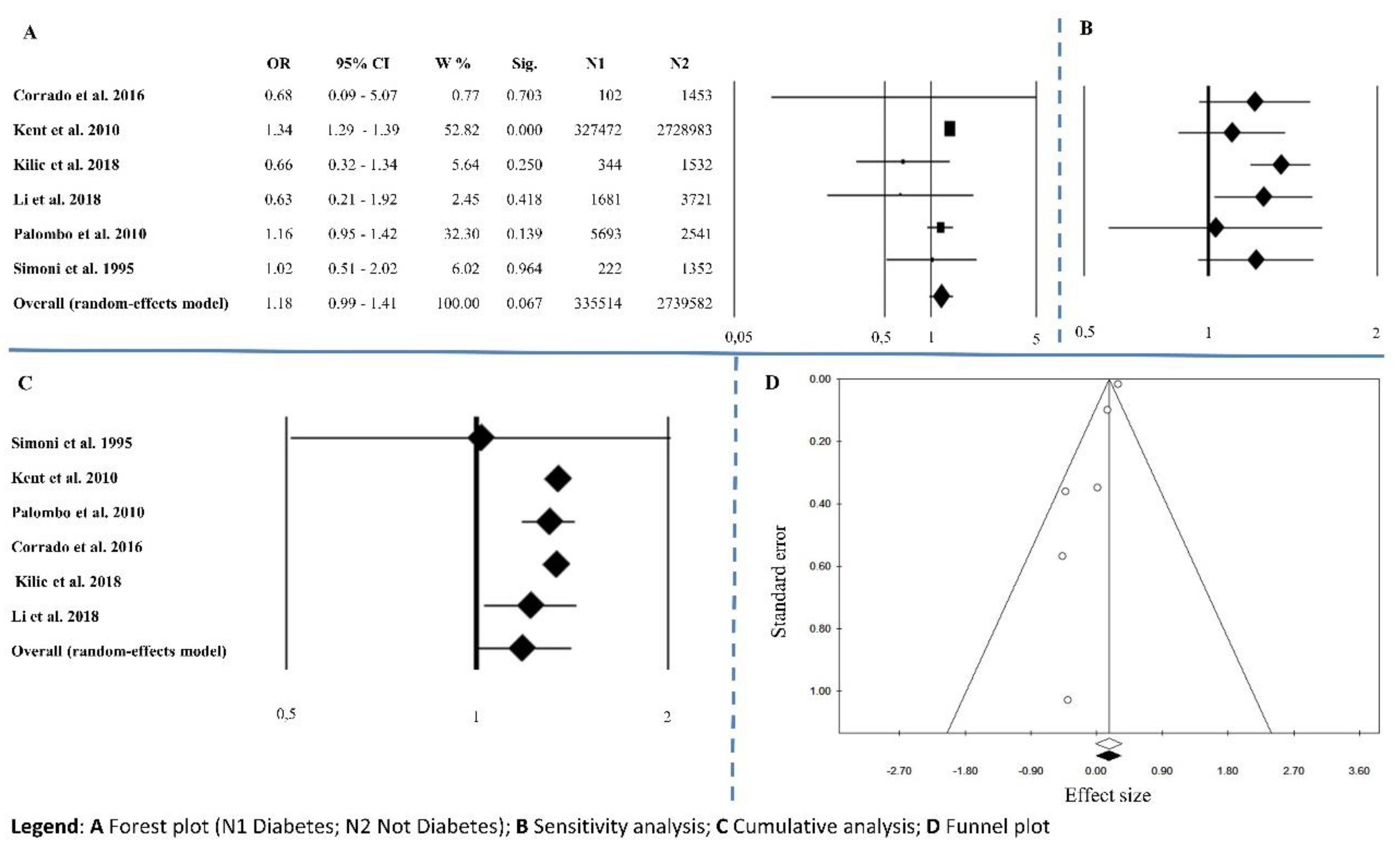
3.1.4. Diabetes Mellitus
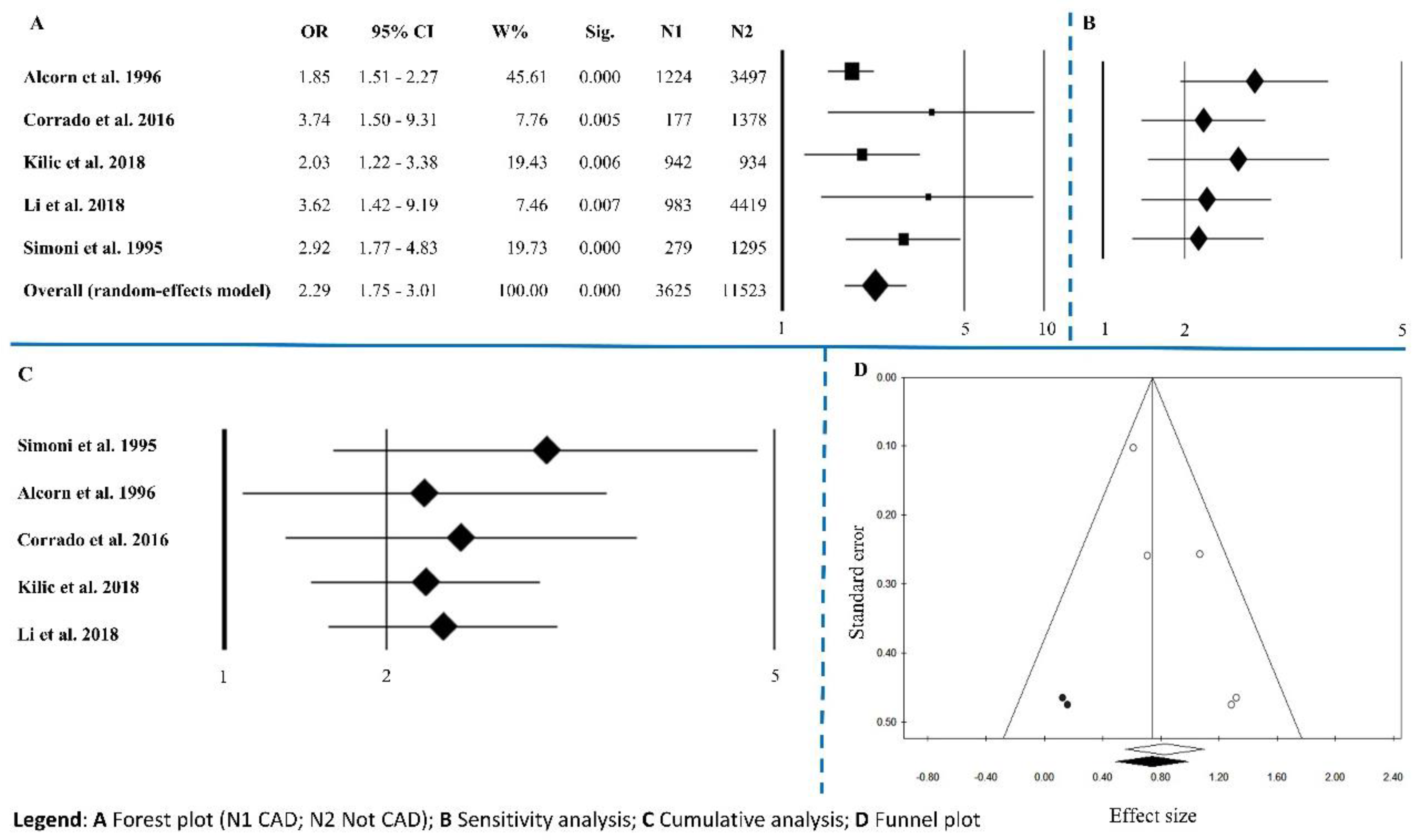
3.1.5. Coronary Artery Disease
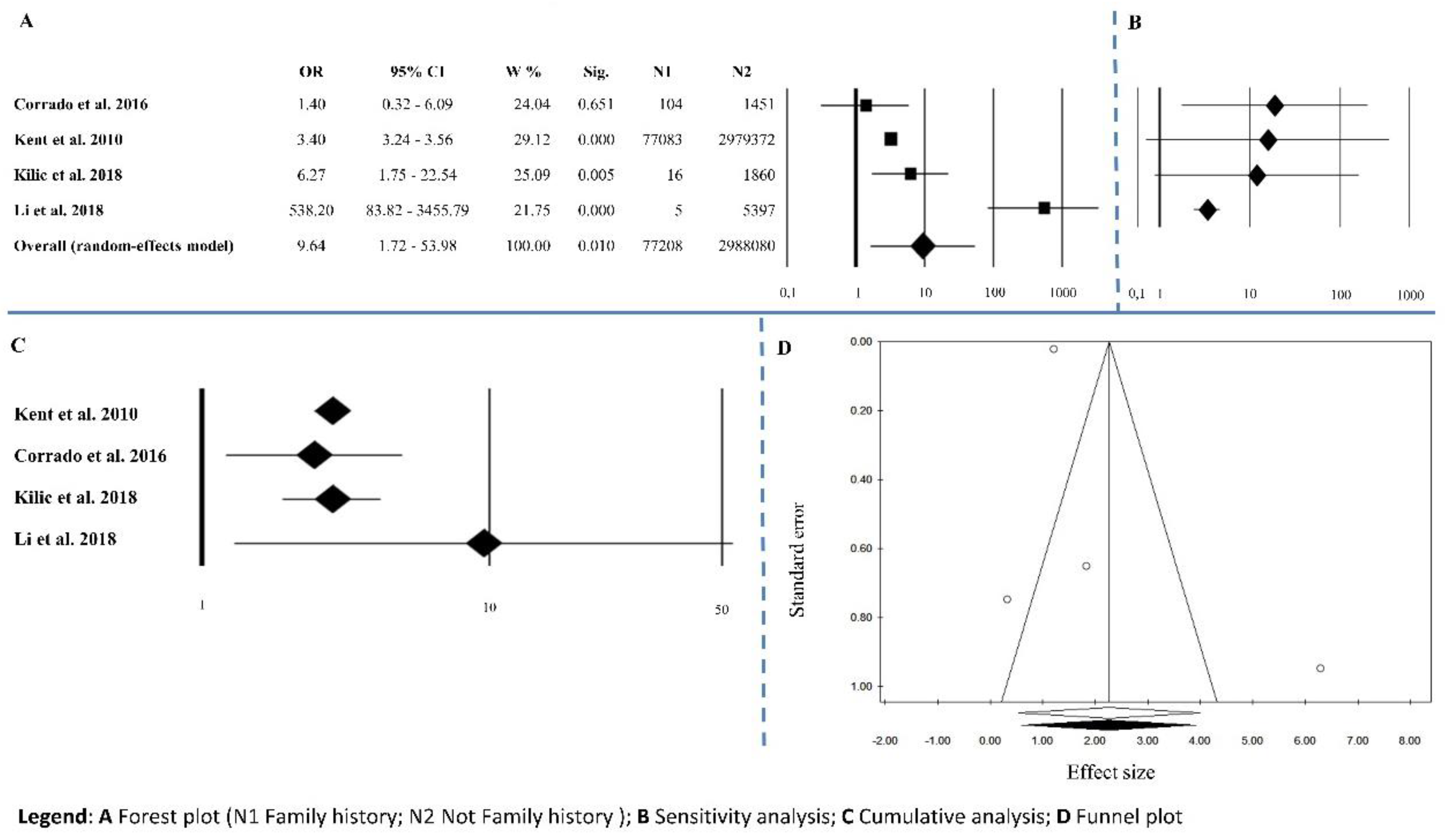
3.1.6. Family History of Abdominal Aortic Aneurysm
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moll, F.L.; Powell, J.T.; Fraedrich, G.; Verzini, F.; Haulon, S.; Waltham, M.; van Herwaarden, J.A.; Holt, P.J.; van Keulen, J.W.; Rantner, B.; et al. European Society for Vascular Surgery. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur. J. Vasc. Endovasc. Surg. 2011, 41 (Suppl. 1), S1–S58. [Google Scholar] [CrossRef] [PubMed]
- Stather, P.W.; Sidloff, D.A.; Dattani, N.; Gokani, V.J.; Choke, E.; Sayers, R.D.; Bown, M.J. Meta-analysis and meta-regression analysis of biomarkers for abdominal aortic aneurysm. Br. J. Surg. 2014, 101, 1358–1372. [Google Scholar] [CrossRef] [PubMed]
- Krumholz, H.M.; Keenan, P.S.; Brush, J.E., Jr.; Bufalino, V.J.; Chernew, M.E.; Epstein, A.J.; Heidenreich, P.A.; Ho, V.; Masoudi, F.A.; Matchar, D.B.; et al. Standards for measures used for public reporting of efficiency in health care: A scientific statement from the American Heart Association Interdisciplinary Council on Quality of Care and Outcomes research and the American College of Cardiology Foundation. J. Am. Coll. Cardiol. 2008, 52, 1518–1526. [Google Scholar] [CrossRef] [PubMed]
- Makrygiannis, G.; Labalue, P.; Erpicum, M.; Schlitz, M.; Seidel, L.; El Hachemi, M.; Gangolf, M.; Albert, A.; Defraigne, J.O.; Lindholt, J.S.; et al. Extending abnominal aortic aneurysm detection to older age older age groups: Preliminary results from the Liege screening programme. Ann. Vasc. Surg. 2016, 36, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Institute for Health Metrics and Evaluation. Global Burden of Disease Study. Available online: http//www.healthmetricsandevaluation.org/gbd/visualizations/gbd-cause-patterns (accessed on 30 June 2018).
- Kniemeyer, H.W.; Kessler, T.; Reber, P.U.; Ris, H.B.; Hakki, H.; Widmer, M.K. Treatment of ruptured abdominal aortic aneurysm, a permanent challenge or a waste of resources? Prediction of outcome using a multi-organ-dysfunction score. Eur. J. Vasc. Endovasc. Surg. 2000, 19, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.G.; Brown, L.C.; Sweeting, M.J.; Bown, M.J.; Kim, L.G.; Glover, M.J.; Buxton, M.; Powell, J. Systematic review and meta-analysis of the growth and rupture rates of small abdominal aortic aneurysms: Implications for surveillance intervals and their cost-effectiveness. Health Technol. Assess. 2013, 17, 1–118. [Google Scholar] [CrossRef] [PubMed]
- Durieux, R.; Van Damme, H.; Labropoulos, N.; Yazici, A.; Legrand, V.; Albert, A.; Defraigne, J.O.; Sakalihasan, N. High prevalence of abdominal aortic aneurysm in patients with three-vessel coronary artery disease. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Salcedo Jódar, L.; Alcázar Carmona, P.; Tenías Burillo, J.M.; García Tejada, R. Prevalence of abdominal aortic aneurysm in a rural population of 65–80 year-old males. Semergen 2014, 40, 425–430. [Google Scholar] [CrossRef]
- Salvador-González, B.; Martín-Baranera, M.; Borque-Ortega, Á.; Sáez-Sáez, R.M.; de Albert-Delas Vigo, M.; Carreño-García, E.; Tarín-Masriera, L.; Badia-Millán, P.; Martínez-Gil, M.; Torrabadella-Fàbrega, J. Prevalence of Abdominal Aortic Aneurysm in Men Aged 65-74 Years in a Metropolitan Area in North-East Spain. Eur. J. Vasc. Endovasc. Surg. 2016, 52, 75–81. [Google Scholar] [CrossRef]
- Dereziński, T.L.; Fórmankiewicz, B.; Migdalski, A.; Brazis, P.; Jakubowski, G.; Woda, Ł.; Jawień, A. The prevalence of abdominal aortic aneurysms in the rural/urban population in central Poland–Gniewkowo Aortic Study. Kardiol. Pol. 2017, 75, 705–710. [Google Scholar] [CrossRef]
- Kilic, S.; Saracoglu, E.; Cekici, Y. Clinical Efficacy of Transthoracic Echocardiography for Screening Abdominal Aortic Aneurysm in Turkish Patients. Acta Cardiol. Sin. 2018, 34, 137–143. [Google Scholar] [PubMed]
- Gianfagna, F.; Veronesi, G.; Tozzi, M.; Tarallo, A.; Borchini, R.; Ferrario, M.M.; Bertù, L.; Montonati, A.; Castelli, P.; Mara, L.; et al. Prevalence of abdominal aortic aneurysms in the general population and in subgroups at high cardiovascular risk in Italy. Results of the RoCAV population based study. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Bohlin, C.; Fröjd, A.; Wanhainen, M.; Björck, M. Change in smoking habits after been screened for abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 2014, 48, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, K.; Li, T.; Zhai, S. Primary results of abdominal aortic aneurysm screening in the at-risk residents in middle China. BMC Cardiovasc. Disord. 2018, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Belloch García, S.L. Abdominal aortic aneurysm. Prevalence and associated risk factors in a population of patients hospitalised in Internal Medicine. Rev. Clin. Esp. 2018, 218, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Itoga, N.K.; Rothenberg, K.A.; Suarez, P.; Ho, T.V.; Mell, M.W.; Xu, B.; Curtin, C.M.; Dalman, R.L. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J. Vasc. Surg. 2018. [Google Scholar] [CrossRef]
- Laroche, J.P.; Becker, F.; Baud, J.M.; Miserey, G.; Jaussent, A.; Picot, M.C.; Bura-Rivière, A.; Quéré, I. Ultrasound screening of abdominal aortic aneurysm: Lessons from Vesale 2013. J. Mal. Vasc. 2015, 40, 340–349. [Google Scholar] [CrossRef]
- Corrado, G.; Durante, A.; Genchi, V.; Trabattoni, L.; Beretta, S.; Rovelli, E.; Foglia-Manzillo, G.; Ferrari, G. Prevalence of previously undiagnosed abdominal aortic aneurysms in the area of Como: The ComoCuore “looking for AAA” ultrasonography screening. Int. J. Cardiovasc. Imaging 2016, 32, 1213–1217. [Google Scholar] [CrossRef]
- Saratzis, A.; Bown, M.J. The genetic basis for aortic aneurysmal disease. Heart 2014, 100, 916–922. [Google Scholar] [CrossRef]
- Toghill, B.J.; Saratzis, A.; Harrison, S.C.; Verissimo, A.R.; Mallon, E.B.; Bown, M.J. The potential role of DNA methylation in the pathogenesis of abdominal aortic aneurysm. Atherosclerosis 2015, 241, 121–129. [Google Scholar] [CrossRef]
- Ashton, H.A.; Gao, L.; Kim, L.G.; Druce, P.S.; Thompson, S.G.; Scott, R.A. Fifteen-year follow-up of a randomized clinical trial of ultrasonographic screening for abdominal aortic aneurysms. Br. J. Surg. 2007, 94, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Lindholt, J.S.; Sørensen, J.; Søgaard, R.; Henneberg, E.W. Long-term benefit and cost-effectiveness analysis of screening for abdominal aortic aneurysms from a randomized controlled trial. Br. J. Surg. 2010, 97, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Norman, P.E.; Jamrozik, K.; Lawrence-Brown, M.M.; Le, M.T.; Spencer, C.A.; Tuohy, R.J.; Parsons, R.W.; Dickinson, J.A. Population based randomized controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004, 329, 1259, Erratum in 2005, 330, 596. [Google Scholar] [CrossRef]
- Darwood, R.J.; Brooks, M.J. The impact of decreasing abdominal aortic aneurysm prevalence on a local aneurysm screening programme. Eur. J. Vasc. Endovasc. Surg. 2012, 44, 45–50. [Google Scholar] [CrossRef]
- Thompson, S.G.; Ashton, H.A.; Gao, L.; Scott, R.A. Multicentre Aneurysm Screening Study Group. Screening men for abdominal aortic aneurysm: 10 year mortality and cost effectiveness results from the randomized Multicentre Aneurysm Screening Study. BMJ 2009, 338, b2307. [Google Scholar] [CrossRef]
- Ali, M.U.; Fitzpatrick-Lewis, D.; Miller, J.; Warren, R.; Kenny, M.; Sherifali, D.; Raina, P. Screening for abdominal aortic aneurysm in asymptomatic adults. J. Vasc. Surg. 2016, 64, 1855–1868. [Google Scholar] [CrossRef]
- Stather, P.W.; Dattani, N.; Bown, M.J.; Earnshaw, J.J.; Lees, T.A. International variations in AAA screening. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 231–234. [Google Scholar] [CrossRef]
- Available online: https://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/abdominal-aortic-aneurysm-screening (accessed on 31 May 2017).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef]
- Vazquez, C.; Sakalihasan, N.; D’Harcour, J.B.; Limet, R. Routine ultrasound screening for abdominal aortic aneurysm among 65- and 75-year-old men in a city of 200,000 inhabitants. Ann. Vasc. Surg. 1998, 12, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Kvist, T.V.; Lindholt, J.S.; Rasmussen, L.M.; Søgaard, R.; Lambrechtsen, J.; Steffensen, F.H.; Frost, L.; Olsen, M.H.; Mickley, H.; Hallas, J.; et al. The DanCavas Pilot Study of Multifaceted Screening for Subclinical Cardiovascular Disease in Men and Women Aged 65-74 Years. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Palombo, D.; Lucertini, G.; Pane, B.; Mazzei, R.; Spinella, G.; Brasesco, P.C. District-based abdominal aortic aneurysm screening in population aged 65 years and older. J. Cardiovasc. Surg. 2010, 51, 777–782. [Google Scholar]
- Simoni, G.; Pastorino, C.; Perrone, R.; Ardia, A.; Gianrossi, R.; De Cian, F.; Cittadini, G., Jr.; Baiardi, A.; Bachi, V. Screening for abdominal aortic aneurysms and associated risk factors in a general population. Eur. J. Vasc. Endovasc. Surg. 1995, 10, 207–210. [Google Scholar] [CrossRef]
- Jawien, A.; Formankiewicz, B.; Derezinski, T.; Migdalski, A.; Brazis, P.; Woda, L. Abdominal aortic aneurysm screening program in Poland. Gefasschirurgie 2014, 19, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Sisó-Almirall, A.; Kostov, B.; Navarro González, M.; Cararach Salami, D.; Pérez Jiménez, A.; Gilabert Solé, R.; Saumell, C.B.; Bach, L.D.; Martí, M.V.; González-de Paz, L.; et al. Abdominal aortic aneurysm screening program using hand-held ultrasound in primary healthcare. PLoS ONE 2017, 12, e0176877. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Martín, J.M.; Fernández-Morána, M.C.; Alonso-Álvareza, M.I.; García-Gimenob, M.; Fernández-Samosa, R.; Vaquero-Morilloa, F. The prevalence of abdominal aortic aneurysms in a high risk population. Angiologia 2007, 59, 305–315. [Google Scholar] [CrossRef]
- Stackelberg, O.; Wolk, A.; Eliasson, K.; Hellberg, A.; Bersztel, A.; Larsson, S.C.; Orsini, N.; Wanhainen, A.; Björck, M. Lifestyle and Risk of Screening-Detected Abdominal Aortic Aneurysm in Men. J. Am. Heart Assoc. 2017, 6, e004725. [Google Scholar] [CrossRef]
- Wanhainen, A.; Hultgren, R.; Linné, A.; Holst, J.; Gottsäter, A.; Langenskiöld, M.; Smidfelt, K.; Björck, M.; Svensjö, S.; Swedish Aneurysm Screening Study Group (SASS); et al. Outcome of the Swedish Nationwide Abdominal Aortic Aneurysm Screening Program. Swedish Aneurysm Screening Study Group (SASS). Circulation 2016, 134, 1141–1148. [Google Scholar] [CrossRef]
- Hager, J.; Länne, T.; Carlsson, P.; Lundgren, F. Lower prevalence than expected when screening 70-year-old men for abdominal aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 453–459. [Google Scholar] [CrossRef]
- Svensjö, S.; Björck, M.; Wanhainen, A. Current prevalence of abdominal aortic aneurysm in 70-year-old women. Br. J. Surg. 2013, 100, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Svensjö, S.; Björck, M.; Gürtelschmid, M.; Djavani Gidlund, K.; Hellberg, A.; Wanhainen, A. Low prevalence of abdominal aortic aneurysm among 65-year-old Swedish men indicates a change in the epidemiology of the disease. Circulation 2011, 124, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Kanagasabay, R.; Gajraj, H.; Pointon, L.; Scott, R.A. Co-morbidity in patients with abdominal aortic aneurysm. J. Med. Screen. 1996, 3, 208–210. [Google Scholar] [CrossRef]
- Smith, F.C.; Grimshaw, G.M.; Paterson, I.S.; Shearman, C.P.; Hamer, J.D. Ultrasonographic screening for abdominal aortic aneurysm in an urban community. Br. J. Surg. 1993, 80, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Grimshaw, G.M.; Thompson, J.M.; Hamer, J.D. Prevalence of abdominal aortic aneurysm associated with hypertension in an urban population. J. Med. Screen. 1994, 1, 226–228. [Google Scholar] [CrossRef]
- Singh, K.; Bønaa, K.H.; Jacobsen, B.K.; Bjørk, L.; Solberg, S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: The Tromsø Study. Am. J. Epidemiol. 2001, 154, 236–244. [Google Scholar] [CrossRef]
- Krohn, C.D.; Kullmann, G.; Kvernebo, K.; Rosén, L.; Kroese, A. Ultrasonographic screening for abdominal aortic aneurysm. Eur. J. Surg. 1992, 158, 527–530. [Google Scholar]
- Engelberger, S.; Rosso, R.; Sarti, M.; Del Grande, F.; Canevascini, R.; van den Berg, J.C.; Prouse, G.; Giovannacci, L. Ultrasound screening for abdominal aortic aneurysms. Swiss Med. Wkly. 2017, 147, w14412. [Google Scholar] [PubMed]
- Nicholls, E.A.; Norman, P.E.; Lawrence-Brown, M.M.; Goodman, M.A.; Pedersen, B. Screening for abdominal aortic aneurysms in Western Australia. Aust. N. Z. J. Surg. 1992, 62, 858–861. [Google Scholar] [CrossRef]
- Takei, H.; Ishikawa, S.; Otaki, A.; Sakata, K.; Aizaki, M.; Sato, Y.; Suzuki, M.; Ishikita, T.; Iino, Y.; Yokoe, T.; et al. Screening for abdominal aortic aneurysm and occlusive peripheral vascular disease in Japanese residents. Surg. Today 1995, 25, 608–611. [Google Scholar] [CrossRef]
- Al-Zahrani, H.A.; Rawas, M.; Maimani, A.; Gasab, M.; Aba al Khail, B.A. Screening for abdominal aortic aneurysm in the Jeddah area, western Saudi Arabia. Cardiovasc. Surg. 1996, 4, 87–92. [Google Scholar] [CrossRef]
- Chun, K.C.; Schmidt, A.S.; Bains, S.; Nguyen, A.T.; Samadzadeh, K.M.; Wilson, M.D.; Peters, J.H.; Lee, E.S. Surveillance outcomes of small abdominal aortic aneurysms identified from a large screening program. J. Vasc. Surg. 2016, 63, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kent, K.C.; Zwolak, R.M.; Egorova, N.N.; Riles, T.S.; Manganaro, A.; Moskowitz, A.J.; Gelijns, A.C.; Greco, G. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J. Vasc. Surg. 2010, 52, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Johnson, G.R.; Wilson, S.E.; Chute, E.P.; Hye, R.J.; Makaroun, M.S.; Barone, G.W.; Bandyk, D.; Moneta, G.L.; Makhoul, R.G. The aneurysm detection and management study screening program: Validation cohort and final results. Aneurysm Detection and Management Veterans Affairs Cooperative Study Investigators. Arch. Intern. Med. 2000, 160, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Alcorn, H.G.; Wolfson, S.K., Jr.; Sutton-Tyrrell, K.; Kuller, L.H.; O’Leary, D. Risk factors for abdominal aortic aneurysms in older adults enrolled in The Cardiovascular Health Study. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Williams, C.; Sweeting, M.J.; Turton, G.; Parkin, D.; Cooper, D.; Rodd, C.; Thompson, S.G.; Earnshaw, J.J.; Gloucestershire and Swindon Abdominal Aortic Aneurysm Screening Programme. Lessons learned about prevalence and growth rates of abdominal aortic aneurysms from a 25-year ultrasound population screening programme. Br. J. Surg. 2018, 105, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Barba, Á.; Vega de Céniga, M.; Estallo, L.; de la Fuente, N.; Viviens, B.; Izagirre, M. Prevalence of abdominal aortic aneurysm is still high in certain areas of southern Europe. Ann. Vasc. Surg. 2013, 27, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Makrygiannis, G.; Mourmoura, E.; Spanos, K.; Roussas, N.; Kuivaniemi, H.; Sakalihasan, N.; Tsezou, A.; Giannoukas, A. Risk Factor Assessment in a Greek Cohort of Patients with Large Abdominal Aortic Aneurysms. Angiology 2018. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.; Søgaard, R.; Frost, L.; Høgh, A.; Lindholt, J. Effectiveness of Screening Postmenopausal Women for Cardiovascular Diseases: A Population Based, Prospective Parallel Cohort Study. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Howard, D.P.J.; Banerjee, A.; Fairhead, J.F.; Handa, A.; Silver, L.E.; Rothwell, P.M. On behalf of the Oxford Vascular Study Population-Based study of incidence of Acute Abdominal Aortic Aneurysm with projected impact of screnning strategy. J. Am. Heart Assoc. 2015, 4, e001926. [Google Scholar] [CrossRef]
- Johansson, M.; Zahl, P.H.; Siersma, V.; Jørgensen, K.J.; Marklund, B.; Brodersen, J. Benefits and harms of screening men for abdominal aortic aneurysm in Sweden: A registry-based cohort study. Lancet 2018, 391, 2441–2447. [Google Scholar] [CrossRef]
- Cornuz, J.; Sidoti Pinto, C.; Tevaearai, H.; Egger, M. Risk factors for asymptomatic abdominal aortic aneurysm: Systematic review and meta-analysis of population-based screening studies. Eur. J. Public Health 2004, 14, 343–349. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials test. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Altobelli, E.; Rapacchietta, L.; Angeletti, P.M.; Barbante, L.; Profeta, F.V.; Faganno, R. Breast cancer screening programmes across the WHO European Region: Differences among Countries based on national income level. Int. J. Environ. Res. Public Health 2017, 14, 452. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; D’Aloisio, F.; Angeletti, P.M. Colorectal cancer screening in countries of European Council outside of the EU-28. World J. Gastroenterol. 2016, 22, 4946–4957. [Google Scholar] [CrossRef]
- Altobelli, E.; Lattanzi, A. Cervical carcinoma in the European Union: An update on disease burden screening program state of activation, and coverage as of March 2014. Int. J. Gynecol. Cancer 2015, 25, 474–483. [Google Scholar] [CrossRef]
- Altobelli, E.; Lattanzi, A.; Paduano, R.; Varassi, G.; di Orio, F. Colorectal cancer prevention in Europe: Burden of disease and status of screening programs. Prev. Med. 2014, 62, 132–141. [Google Scholar] [CrossRef]
- Altobelli, E.; Lattanzi, A. Brest cancer in European Union: An update of screening programmes as of March 2014 (review). Int. J. Oncol. 2014, 45, 1785–1792. [Google Scholar] [CrossRef]
- Sildoff, D.; Stather, P.; Dattani, N.; Bown, M.; Thompson, J.; Sayers, R.; Choke, E. Aneurysm global epidemiology study: Public health measures can further abdominal aortic aneurysm mortality. Circulation 2014, 129, 747–753. [Google Scholar]
- Kim, L.G.; Scott, R.A.P.; Asthon, H.A.; Thompson, S.G. For the Multicentre Aneurysm Screening Study Group A sustained mortality benefit from screening for Abdominal Aortic Aneurysm. Ann. Intern. Med. 2007, 146, 699–706. [Google Scholar] [CrossRef]
- Scott, R.A.P.; Wilson, N.M.; Asthon, H.A.; Kay, D.N. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br. J. Surg. 1995, 82, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Forsdahl, S.H.; Singh, K.; Solberg, S.; Jacobsen, B.K. Risk factors for Abdominal Aortic Aneurism a 7-year prospective study: The Tromsø study 1994–2001. Circulation 2009, 119, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.G.; Ashton, H.A.; Gao, L.; Buxton, M.J.; Scott, R.A.P. On behalf of the Multicentre Aneurysm Screening Study (MASS) Group Final follow-up of Multicentre Aneurysm Screening Study (MASS) randomized trial od abdominal aortic aneurysm screening. Br. J. Surg. 2012, 99, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- De Rango, P.; Farchioni, L.; Fiorucci, B.; Lenti, M. Diabetes and abdominal aortic aneurysms. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 243–261. [Google Scholar] [CrossRef] [PubMed]
- De Vos, L.C.; Noordzij, M.J.; Mulder, D.J.; Smit, A.J.; Lutgers, H.L.; Dullaart, R.P.; Kamphuisen, P.W.; Zeebregts, C.J.; Lefrandt, J.D. Skin autofluorescence as a measure of advanced glycation end products deposition is elevated in peripheral artery disease. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Del Negro, V.; Angeletti, P.M.; Latella, G. Low-FODMAP Diet Improves Irritable Bowel Syndrome Symptoms: A Meta-Analysis. Nutrients 2017, 9, 940. [Google Scholar] [CrossRef] [PubMed]

| Country a Reference, Year | Region | Age-Group | Level of Participation (%) or Screened People | AAA Detection Rate (%) | Program Start | Included in Meta-Analysis |
|---|---|---|---|---|---|---|
| Population-Based | ||||||
| Italy | ||||||
| Gianfagna, 2018 [13] | Varese, Lombardia | M 50–75 F 60–75 | M 65.3 F 61.3 T 63.8 | M 1.3 F 0.3 T 0.9 | 2013 | Yes |
| Palombo, 2010 [35] | Genoa, Liguria | M, F 65–92 | M 61.6 F 48.8 T 54.3 | M 10.8 F 1.1 T 6.2 | 2007–2009 | Yes |
| Simoni, 1995 [36] | Genoa, Liguria | M, F 65–75 | M 58.5 | M 8.8 F 0.6 T 4.4 | 1991–1994 | Yes |
| Belgium | ||||||
| Makrygiannis, 2016 [4] | Chaudfontaine, Liege, Wallonia | M 65–85 F 74–85 | M 39.5 F 31.7 T 36.0 | M 4.8 F 1.3 T 3.6 | 2014 | |
| Vazquez, 1998 [33] | Liege, Wallonia | M 75–65 | T 41.0 | T 4.5 | 1995 | |
| China | ||||||
| Kun Li, 2018 [15] | Zhengzhou City, Middle China | M, F <55 M, F 55–75 M, F >75 | M 2555 F 2847 T 5402 | M 0.55 F 0.14 T 0.33 | 2014–2015 | Yes |
| Denmark | ||||||
| Dahl, 2018 [61] | Viborg, Central Denmark | F (Born 1936, 1941, 1946, 1951) | F 107,491 | NR | 2011–2013 | |
| Kvist, 2016 [34] | Northen part of Funen and City of Odense | T 65–74 | M 64.9 F 63.0 | M 12.4 F 1.1 | 2014–2015 | |
| Poland | ||||||
| Dereńzíski, 2017 [11] | Gniewkowo, Central Poland | M >60 F >65 | M 61.0 | M 6.3 F 0.82 T 4.12 | 2009–2012 | yes |
| Janwien, 2014 [37] | Kuyavia-Pomeranian | M >60 | M 1556 | M 6.0 | 2009–2011 | |
| Spain | ||||||
| Sisó-Almirall, 2017 [38] | Barcelona, Catalonia | M 60–65 | M 74.9 | M 1.5 | 2013 | |
| Salcedo Jódar, 2014 [9] | Ciudad Real, Castilla La Mancia | M 65–80 | M 93.5 | M 3.3 | 2012 | |
| Salvador-González, 2016 [10] | Barcelona, Catalonia | M 65–74 | M 66.9 | M 2.3 | 2007 | |
| Barba, 2013 [59] | Asturias | M (born in 1943) | M 70.8 | M 4.7 | 2013 | |
| Sweden | ||||||
| Johansson, 2018 [63] | Uppsala, Dalarna, Södermanland, Västra Götaland | M >65 | M 25,265 | NR | 2006–2009 | |
| Stackelberg, 2017 [40] | Vastmanland, Orebro | M 65–75 | M 49.0 | M 1.2 | 2007–2009 | |
| Wanhainen, 2016 [41] | All Nation except Halland Country | M 65–75 | M 84.0 | M 1.5 | 2006–2014 | |
| Hager, 2013 [42] | Őstergötland | M >70 | M 84.0 | M 3.0 | 2008–2010 | |
| Svensjö, 2013 [43] | Uppsala and Darlana | F >70 | M 74.2 | F 0.4 | 2007–2009 | |
| Svensjö, 2011 [44] | Uppsala, Darlana, Sörmland, Gävleborg | M >65 | M 85.0 | M 1.7 | 2006–2010 | |
| United Kingdom | ||||||
| Oliver-Williams, 2018 [58] | Gloucestershire, England | M 65 | M 80.7 | M 1.9 | 1990–2015 | |
| Kanagasabay, 1996 [45] | London, England | M, F 65–80 | NR | M 7.6 F 1.3 | 1995 | Yes |
| Smith, 1993 [46] | Birmingham, England | M 65–75 | M 76.3 | T 8.4 | 1981–1999 | |
| Grismhaw, 1994 [47] | Birmingham, England | M, F 60–75 | M 76.1 | M 7.2 | 1989–1991 | |
| Norway | ||||||
| Singh, 2001 [48] | Tromsø | M, F 25–84 | 25–44 62.0 45–54 81.0 55–64 83.0 65–74 79.0 75–84 58.0 | M 9.7 F 2.2 T 4.7 | 1994–1995 | Yes |
| Japan | ||||||
| Takei, 1995 * [52] | Ueno, Central Japan | M, F 60–79 | M 69.0 | M 3.9 F 5.0 T 4.6 | 1992 | |
| United States | ||||||
| Alcorn, 1996 [57] | Pittsburgh cohort | M, F >65 | T 656 | T 2.9 | 1990–1992 | Yes |
| Not Population-Based | ||||||
| Australia | ||||||
| Nicholls, 1992 [51] | Perth | M, F 60–80 | T 1225 | M 4.7 F 0.35 T 2.64 | 1991 | Yes |
| Italy | ||||||
| Corrado, 2016 [19] | Como, Lombardia | M, F 60–85 | T 1555 | M 2.5 F 0.4 T 1.4 | 2010–2013 | Yes |
| France | ||||||
| Laroche, 2015 [18] | All Nation (metropolitan and overseas departement “Operation Vésale”) | M 50–75 F 60–75 | T 6691 | M 3.1 F 0.3 T 1.7 | 2013 | |
| Greece | ||||||
| Makrygiannis, 2018 [60] | Larissa, Central Greece | NR | NR | NR | 2010–2013 | Yes |
| Spain | ||||||
| Belloch García, 2018 [16] | La Ribera, Spain | T >50 | T 241 | T 2.9 | 2016–2017 | |
| Ortega-Martín, 2007 [39] | León | M 65–75 | M 66.0 | M 4.2 | 2000–2001 | |
| Norway | ||||||
| Krohn, 1992 * [49] | Oslo | M, F 60–89 | T 500 ** | NR | 1991 | |
| Switzerland | ||||||
| Engelberger, 2017 [50] | Lugano, Ticino | M 65–80 | M 68.2 | M 4.1 | 2013 | |
| Saudi Arabia | ||||||
| Al-Zahrani, 1996 [53] | Jeddah, Western Saudi Arabia | M, F 60–80 | NR | T 2.0 | 1991–1992 | Yes |
| Turkey | ||||||
| Kilic, 2018 [12] | Turkey | T ≥ 65 | T 1948 | T 3.7 | 2016–2017 | Yes |
| United States | ||||||
| Chun, 2016 [54] | North Carolina (Veterans Affair Health care system) | M 65–75 | T 9571 | T 7.1 | 2007–2011 | |
| Kent, 2010 [55] | All Nation | M, F <85 | T 3,056,455 | M 1.7 F 0.2 T 0.7 | 2003–2008 | Yes |
| Lederle, 2000 [56] | 15 Department of veterans affair | M, F 50–79 | NR | T 1.4 | 1994–1997 | |
| Risk Factors | Pooled Analysis | Heterogeneity | Publication Bias | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k = n. of Studies | ES (OR) | 95% CI | p-Value | Q | p-Value | I2 | Egger p-Value | Begg and Mazumdar p-Value | |
| Gender | 13 | 5.93 | 4.26–8.25 | <0.0001 | 132.89 | <0.0001 | 90.97 | 0.339 | 0.542 |
| Smoking habits | 6 | 2.97 | 1.20–7.30 | 0.018 | 390.71 | <0.0001 | 98.72 | 0.229 | 0.573 |
| Hypertension | 8 | 1.55 | 1.02–2.34 | 0.039 | 112.34 | <0.0001 | 93.77 | 0.127 | 0.322 |
| Diabetes mellitus | 6 | 1.18 | 0.99–1.41 | 0.067 | 8.45 | 0.133 | 40.85 | 0.008 | 0.851 |
| Coronary Artery Disease (CAD) | 5 | 2.29 | 1.75–3.01 | <0.0001 | 5.98 | 0.200 | 33.15 | 0.032 | 0.624 |
| Family history of AAA | 4 | 9.64 | 1.72–53.98 | 0.01 | 30.77 | <0.0001 | 90.25 | 0.467 | 0.174 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altobelli, E.; Rapacchietta, L.; Profeta, V.F.; Fagnano, R. Risk Factors for Abdominal Aortic Aneurysm in Population-Based Studies: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2018, 15, 2805. https://doi.org/10.3390/ijerph15122805
Altobelli E, Rapacchietta L, Profeta VF, Fagnano R. Risk Factors for Abdominal Aortic Aneurysm in Population-Based Studies: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2018; 15(12):2805. https://doi.org/10.3390/ijerph15122805
Chicago/Turabian StyleAltobelli, Emma, Leonardo Rapacchietta, Valerio F. Profeta, and Roberto Fagnano. 2018. "Risk Factors for Abdominal Aortic Aneurysm in Population-Based Studies: A Systematic Review and Meta-Analysis" International Journal of Environmental Research and Public Health 15, no. 12: 2805. https://doi.org/10.3390/ijerph15122805
APA StyleAltobelli, E., Rapacchietta, L., Profeta, V. F., & Fagnano, R. (2018). Risk Factors for Abdominal Aortic Aneurysm in Population-Based Studies: A Systematic Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 15(12), 2805. https://doi.org/10.3390/ijerph15122805





