Activity of Antioxidant Enzymes and Their Association with Lipid Profile in Mexican People without Cardiovascular Disease: An Analysis of Interactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Volunteers
2.2. Lipid Profile and Classification of Dyslipidemia
2.3. Activity of Antioxidant Enzymes and FRAP Assays
2.3.1. SOD1 Activity
2.3.2. Catalase Activity
2.3.3. PON1 Activity
2.3.4. Ceruloplasmin Activity
2.3.5. FRAP Assay
2.4. Statistical Analysis
3. Results
3.1. General Characteristics of Participants
3.2. Linear Regression Models for the Association between Dyslipidemia and Antioxidant Enzymes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stahel, P.; Xiao, C.; Hegele, R.A.; Lewis, G.F. The atherogenic dyslipidemia complex and novel approaches to cardiovascular disease prevention in diabetes. Can. J. Cardiol. 2017, 34, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Nock, N.L.; Chandran Pillai, A.L. Dyslipidemia: Genetics and role in the metabolic syndrome. In Dyslipidemia-From Prevention to Treatment; INTECH Open Access Publisher: Croatia, Rijeka, 2012; pp. 15–40. ISBN 978-953-307-904-2. [Google Scholar]
- Choi, S.; Liu, X.; Pan, Z. Zinc deficiency and cellular oxidative stress: Prognostic implications in cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef] [PubMed]
- Betteridge, D.J. What is oxidative stress? Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Abbas, A.M.; Sakr, H.F. Simvastatin and vitamin E effects on cardiac and hepatic oxidative stress in rats fed on high fat diet. J. Physiol. Biochem. 2013, 69, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Suanarunsawat, T.; Devakul Na Ayutthaya, W.; Songsak, T.; Thirawarapan, S.; Poungshompoo, S. Antioxidant activity and lipid-lowering effect of essential oils extracted from Oocimum sanctum L. leaves in rats fed with a high cholesterol diet. J. Clin. Biochem. Nutr. 2009, 46, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Buyukhatıpoglu, H.; Sezen, Y.; Yildiz, A.; Guntekin, U.; Bas, M.; Polat, M.; Demirbag, R.; Taskin, A.; Celik, H.; Aksoy, N. Effects of statin use on total oxidant and antoxidant capacity and ceruloplasmin activity. Clin. Investig. Med. Med. Clin. Exp. 2010, 33, E313–E320. [Google Scholar] [CrossRef]
- Kalkan Ucar, S.; Coker, M.; Sözmen, E.; Goksen Simsek, D.; Darcan, S. An association among iron, copper, zinc, and selenium, and antioxidative status in dyslipidemic pediatric patients with glycogen storage disease types IA and III. J. Trace Elem. Med. Biol. 2010, 24, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Otunola, G.A.; Oloyede, O.B.; Oladiji, A.T.; Afolayan, A.J. Selected spices and their combination modulate hypercholesterolemia-induced oxidative stress in experimental rats. Biol. Res. 2014, 47, 5. [Google Scholar] [CrossRef]
- Bai, J.; Zheng, S.; Jiang, D.; Han, T.; Li, Y.; Zhang, Y.; Liu, W.; Cao, Y.; Hu, Y. Oxidative stress contributes to abnormal glucose metabolism and insulin sensitivity in two hyperlipidemia models. Int. J. Clin. Exp. Pathol. 2015, 8, 13193–13200. [Google Scholar]
- Mollazadeh, H.; Carbone, F.; Montecucco, F.; Pirro, M.; Sahebkar, A. Oxidative burden in familial hypercholesterolemia. J. Cell. Physiol. 2018, 233, 5716–5725. [Google Scholar] [CrossRef]
- Colín-Ramírez, E.; Rivera-Mancía, S.; Infante-Vázquez, O.; Cartas-Rosado, R.; Vargas-Barrón, J.; Madero, M.; Vallejo, M. Protocol for a prospective longitudinal study of risk factors for hypertension incidence in a Mexico City population: The Tlalpan 2020 cohort. BMJ Open 2017, 7, e016773. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A.; Phillips, E.; Gentzke, A.S.; Homa, D.M.; Babb, S.D.; King, B.A.; Neff, L.J. Current cigarette smoking among adults—United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 53–59. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Avila, M.; Romieu, I.; Parra, S.; Hernández-Avila, J.; Madrigal, H.; Willett, W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Pública México 1998, 40, 133–140. [Google Scholar] [CrossRef]
- International Society for Advancement of Kinanthropometry; Stewart, A.; Marfell-Jones, M.; Olds, T.; De Ridder, H. International Standards for Anthropometric Assessment; ISAK: Lower Hutt, New Zealand, 2011; ISBN 978-0-620-36207-8. [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) final report. Circulation 2002, 106, 3143–3421. [CrossRef]
- Oberley, L.W.; Spitz, D.R. Assay of superoxide dismutase activity in tumor tissue. Methods Enzymol. 1984, 105, 457–464. [Google Scholar] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Gan, K.N.; Smolen, A.; Eckerson, H.W.; La Du, B.N. Purification of human serum paraoxonase/arylesterase. Evidence for one esterase catalyzing both activities. Drug Metab. Dispos. Biol. Fate Chem. 1991, 19, 100–106. [Google Scholar]
- Arnaud, P.; Gianazza, E.; Miribel, L. Ceruloplasmin. Methods Enzymol. 1988, 163, 441–452. [Google Scholar]
- Tanabe, S.; Shioiri, T.; Murakami, K.; Imanari, T. A new method for assay of ferroxidase activity and its application to human and rabbit sera. Chem. Pharm. Bull. 1984, 32, 4029–4035. [Google Scholar] [CrossRef]
- Martínez-Hernández, R.; Montes, S.; Higuera-Calleja, J.; Yescas, P.; Boll, M.-C.; Diaz-Ruiz, A.; Rios, C. Plasma Ceruloplasmin Ferroxidase Activity Correlates with the Nigral Sonographic Area in Parkinson’s Disease Patients: A Pilot Study. Neurochem. Res. 2011, 36, 2111–2115. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-1-118-14692-7. [Google Scholar]
- Rozek, L.S.; Hatsukami, T.S.; Richter, R.J.; Ranchalis, J.; Nakayama, K.; McKinstry, L.A.; Gortner, D.A.; Boyko, E.; Schellenberg, G.D.; Furlong, C.E.; et al. The correlation of paraoxonase (PON1) activity with lipid and lipoprotein levels differs with vascular disease status. J. Lipid Res. 2005, 46, 1888–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowalska, K.; Socha, E.; Milnerowicz, H. Review: The role of paraoxonase in cardiovascular diseases. Ann. Clin. Lab. Sci. 2015, 45, 226–233. [Google Scholar] [PubMed]
- Camps, J.; Masrsillach, J.; Rull, A.; Alonso-Villaverde, C.; Joven, J. Interrelationships between paraoxonase-1 and monocyte chemoattractant protein-1 in the regulation of hepatic inflammation. In Paraoxonases in Inflammation, Infection, and Toxicology; Reddy, S.T., Ed.; Advances in Experimental Medicine and Biology; Humana Press: New York, NY, USA, 2010; Volume 660, pp. 5–18. ISBN 978-1-60761-349-7. [Google Scholar]
- Saha, N.; Roy, A.C.; Teo, S.H.; Tay, J.S.H.; Ratnam, S.S. Influence of serum paraoxonase polymorphism on serum lipids and apolipoproteins. Clin. Genet. 2008, 40, 277–282. [Google Scholar] [CrossRef]
- Kunutsor, S.K.; Bakker, S.J.L.; James, R.W.; Dullaart, R.P.F. Serum paraoxonase-1 activity and risk of incident cardiovascular disease: The PREVEND study and meta-analysis of prospective population studies. Atherosclerosis 2016, 245, 143–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, Z.-G.; Chen, H.-Z.; Yan, Y.; Li, H.; Liu, D.-P. The human paraoxonase gene cluster as a target in the treatment of atherosclerosis. Antioxid. Redox Signal. 2012, 16, 597–632. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.T.H.; Zhao, B.; Jose, P.O.; Azar, K.M.J.; Fortmann, S.P.; Palaniappan, L.P. Racial/ethnic differences in dyslipidemia patterns. Circulation 2014, 129, 570–579. [Google Scholar] [CrossRef]
- Dubick, M.; Barr, J.; Keen, C.; Atkins, J. Ceruloplasmin and hypoferremia: Studies in burn and non-burn trauma patients. Antioxidants 2015, 4, 153–169. [Google Scholar] [CrossRef]
- Wilson Tang, W.H.; Wu, Y.; Hartiala, J.; Fan, Y.; Stewart, A.F.R.; Roberts, R.; McPherson, R.; Fox, P.L.; Allayee, H.; Hazen, S.L. Clinical and genetic association of serum ceruloplasmin with cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 516–522. [Google Scholar] [CrossRef]
- Kennedy, D.J.; Fan, Y.; Wu, Y.; Pepoy, M.; Hazen, S.L.; Tang, W.H.W. Plasma ceruloplasmin, a regulator of nitric oxide activity, and incident cardiovascular risk in patients with CKD. Clin. J. Am. Soc. Nephrol. 2014, 9, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Rashtchizadeh, N.; Ettehad, S.; DiSilvestro, R.A.; Mahdavi, R. Antiatherogenic effects of zinc are associated with copper in iron-overloaded hypercholesterolemic rabbits. Nutr. Res. 2008, 28, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Kaviarasan, K.; Arjunan, M.M.; Pugalendi, K.V. Lipid profile, oxidant–antioxidant status and glycoprotein components in hyperlipidemic patients with/without diabetes. Clin. Chim. Acta 2005, 362, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Mansego, M.L.; Redon, J.; Martinez-Hervas, S.; Real, J.T.; Martinez, F.; Blesa, S.; Gonzalez-Albert, V.; Saez, G.T.; Carmena, R.; Chaves, F.J. Different impacts of cardiovascular risk factors on oxidative stress. Int. J. Mol. Sci. 2011, 12, 6146–6163. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Shimizu, T.; Shirasawa, T. CuZn-SOD deficiency causes ApoB degradation and induces hepatic lipid accumulation by impaired lipoprotein secretion in mice. J. Biol. Chem. 2006, 281, 31713–31719. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Guerrero, C.; Parra-Carriedo, A.; Ruiz-de-Santiago, D.; Galicia-Castillo, O.; Buenrostro-Jáuregui, M.; Díaz-Gutiérrez, C. Genetic polymorphisms of antioxidant enzymes CAT and SOD affect the outcome of clinical, biochemical, and anthropometric variables in people with obesity under a dietary intervention. Genes Nutr. 2018, 13, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khymenets, O.; Covas, M.I.; Farré, M.; Langohr, K.; Fitó, M.; de la Torre, R. Role of sex and time of blood sampling in SOD1 and SOD2 expression variability. Clin. Biochem. 2008, 41, 1348–1354. [Google Scholar] [CrossRef]
- Miri, R.; Saadati, H.; Ardi, P.; Firuzi, O. Alterations in oxidative stress biomarkers associated with mild hyperlipidemia and smoking. Food Chem. Toxicol. 2012, 50, 920–926. [Google Scholar] [CrossRef]
- Kosmidou, M.; Hatzitolios, A.I.; Molyva, D.; Raikos, N.; Savopoulos, C.; Daferera, N.; Kokkas, V.; Goulas, A. An association study between catalase -262C>T gene polymorphism, sodium-lithium countertrasport activity, insulin resistance, blood lipid parameters and their response to atorvastatin, in Greek dyslipidaemic patients and normolipidaemic controls. Free Radic. Res. 2009, 43, 385–389. [Google Scholar] [CrossRef]
- Peng, T.-C.; Wang, C.-C.; Kao, T.-W.; Chan, J.Y.-H.; Yang, Y.-H.; Chang, Y.-W.; Chen, W.-L. Relationship between hyperuricemia and lipid profiles in US Adults. BioMed Res. Int. 2015, 2015, 127596. [Google Scholar] [CrossRef]
- Andriollo-Sanchez, M.; Hininger-Favier, I.; Meunier, N.; Venneria, E.; O’Connor, J.M.; Maiani, G.; Coudray, C.; Roussel, A.M. Age-related oxidative stress and antioxidant parameters in middle-aged and older European subjects: The ZENITH study. Eur. J. Clin. Nutr. 2005, 59, S58–S62. [Google Scholar] [CrossRef]
- Tóthová, L.; Ostatníková, D.; Šebeková, K.; Celec, P.; Hodosy, J. Sex differences of oxidative stress markers in young healthy subjects are marker-specific in plasma but not in saliva. Ann. Hum. Biol. 2013, 40, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Devore, E.E.; Kang, J.H.; Stampfer, M.J.; Grodstein, F. Total antioxidant capacity of diet in relation to cognitive function and decline. Am. J. Clin. Nutr. 2010, 92, 1157–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-F.; Hamada, M. Experiments: Planning, Analysis, and Parameter Design Optimization; Wiley Series in Probability and Statistics; Wiley: New York, NY, USA, 2000; ISBN 978-0-471-25511-6. [Google Scholar]
- Box, G.E.P.; Hunter, J.S.; Hunter, W.G. Statistics for Experimenters: Design, Innovation, and Discovery, 2nd ed.; Wiley Series in Probability and Statistics; Wiley-Interscience: Hoboken, NJ, USA, 2005; ISBN 978-0-471-71813-0. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Stat. Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- McDonald, J.H. Multiple comparisons. In Handbook of Biological Statistics; Sparky House Publishing: Baltimore, MD, USA, 2009; pp. 256–259. [Google Scholar]
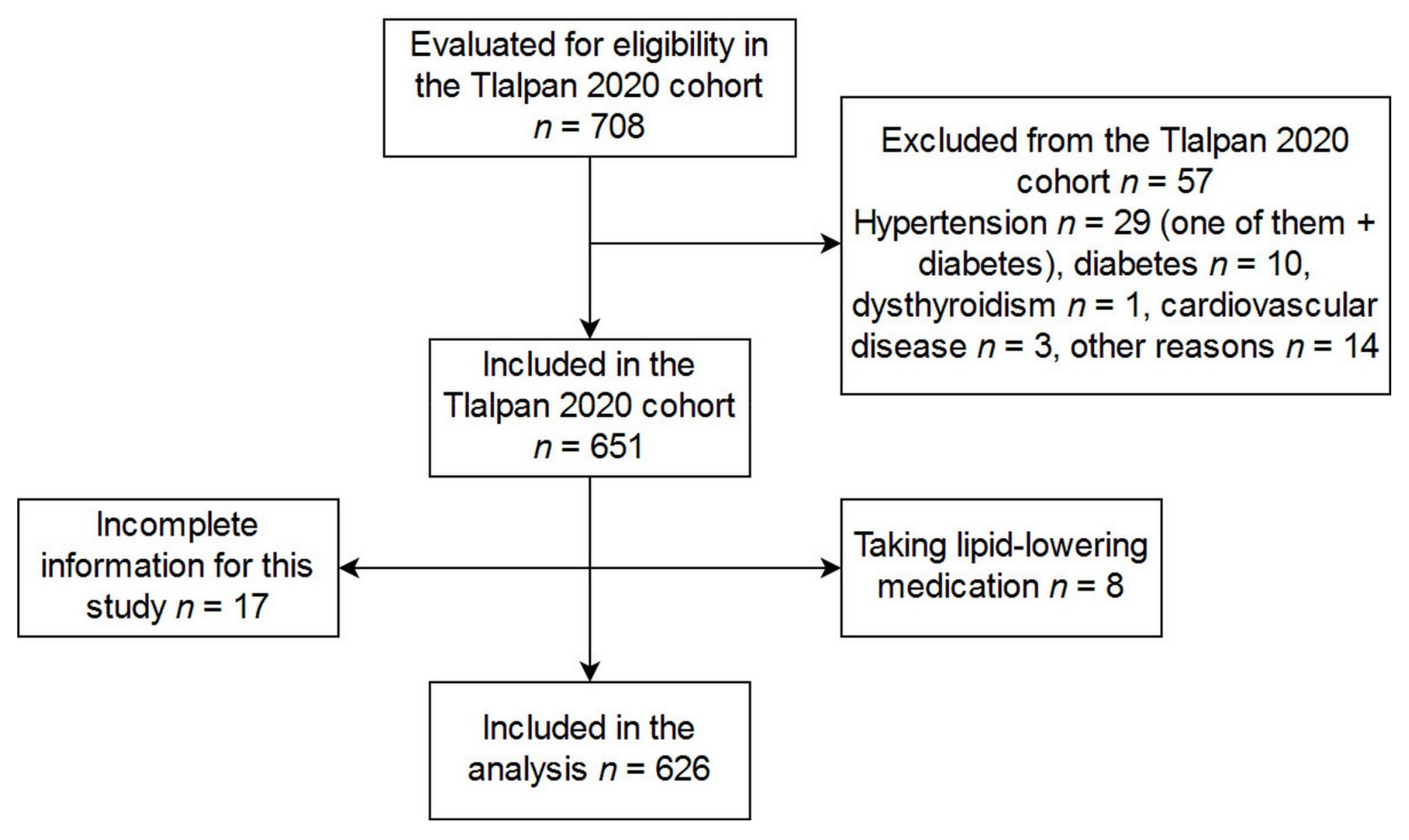
| Characteristic | All (n = 626) | Women (n = 395) | Men (n = 231) | p-Value | |
|---|---|---|---|---|---|
| Age (years) | 37.12 ± 8.97 | 37.67 ± 8.88 | 36.18 ± 9.06 | 0.046 | |
| Systolic blood pressure (mm Hg) | 106.66 ± 10.65 | 104.33 ± 10.29 | 110.64 ± 10.07 | <0.001 | |
| Diastolic blood pressure (mm Hg) | 71.23 ± 8.14 | 69.64 ± 7.89 | 73.94 ± 7.86 | <0.001 | |
| Weight (kg) | 71.13 ± 14.68 | 66.31 ± 12.81 | 79.36 ± 14.03 | <0.001 | |
| Body mass index (kg/m2) | 26.94 ± 4.56 | 26.83 ± 4.75 | 27.14 ± 4.25 | 0.411 | |
| Triglycerides (mmol/L) | 1.36 (0.96, 2.00) | 1.24 (0.89, 1.71) | 1.75 (1.15, 2.32) | <0.001 | |
| Total cholesterol (mmol/L) | 4.85 ± 0.92 | 4.82 ± 0.91 | 4.91 ± 0.95 | 0.225 | |
| LDL-cholesterol (mmol/L) | 3.14 ± 0.79 | 3.08 ± 0.79 | 3.24 ± 0.79 | 0.012 | |
| HDL-cholesterol (mmol/L) | 1.22 (1.03, 1.46) | 1.32 (1.11, 1.56) | 1.10 (0.93, 1.26) | <0.001 | |
| Caloric intake (kcal) | 2233.93 (1503.86, 2738.71) | 2063.82 (1455.35, 2511.08) | 2606.35 (2095.66, 3146.86) | <0.001 | |
| Tobacco smoking [n (%)] | Never | 395 (63.1) | 281 (71.1) | 114 (49.4) | <0.001 |
| Former | 105 (16.8) | 47 (11.9) | 58 (25.1) | ||
| Current | 126 (20.1) | 67 (17.0) | 59 (25.5) | ||
| Alcohol use [n (%)] | Yes | 450 (71.9) | 273 (69.1) | 177 (76.6) | 0.044 |
| No | 176 (28.1) | 122 (30.9) | 54 (23.4) | ||
| Physical activity [n (%)] | Low | 64 (10.2) | 38 (9.6) | 26 (11.3) | 0.003 |
| Medium | 263 (42.0) | 186 (47.1) | 77 (33.3) | ||
| High | 299 (47.8) | 171 (43.3) | 128 (55.4) | ||
| Combination of alterations of lipid profile [n (%)] | Without dyslipidemia | 197 (31.5) | 132 (33.4) | 65 (28.1) | <0.001 |
| Only ↑TG | 24 (3.8) | 6 (1.5) | 18 (7.8) | ||
| Only ↑LDLC | 75 (12.0) | 55 (13.9) | 20 (8.7) | ||
| Only ↓HDLC | 101 (16.1) | 81 (20.5) | 20 (8.7) | ||
| ↑TG↑LDLC | 58 (9.3) | 17 (4.3) | 41 (17.7) | ||
| ↑TG↓HDLC | 82 (13.1) | 44 (11.1) | 38 (16.5) | ||
| ↑LDLC↓HDLC | 28 (4.5) | 25 (6.3) | 3 (1.3) | ||
| ↑TG↑LDLC↓HDLC | 61 (9.7) | 35 (8.9) | 26 (11.3) | ||
| Parameter | Without Dyslipidemia | ↑TG | ↑LDLC | ↓HDLC | ↑TG ↑LDLC | ↑TG ↓HDLC | ↑LDLC ↓HDLC | ↑TG ↑LDLC ↓HDLC |
|---|---|---|---|---|---|---|---|---|
| Paraoxonase-1 activity (U/mL) | 278.9 ± 131.0 | 321.6 ± 102.7 | 266.9 ± 142.8 | 257.4 ± 127.9 | 298.9 ± 165.1 | 243.7 ± 117.6 | 228.4 ± 122.3 | 309.6 ± 145.6 |
| Ceruloplasmin activity (U/mL) | 2.28 ± 0.31 | 2.37 ± 0.41 | 2.35 ± 0.36 | 2.26 ± 0.26 | 2.24 ± 0.34 | 2.30 ± 0.36 | 2.34 ± 0.32 | 2.28 ± 0.31 |
| Superoxide dismutase-1 activity (U/mg) | 11.1 ± 2.8 | 11.2 ± 2.9 | 11.3 ± 3.0 | 11.0 ± 2.7 | 10.5 ± 2.7 | 11.0 ± 3.2 | 11.9 ± 3.3 | 10.8 ± 2.4 |
| Catalase activity (κ/mg) | 0.34 ± 0.18 | 0.34 ± 0.15 | 0.33 ± 0.20 | 0.32 ± 0.18 | 0.35 ± 0.16 | 0.33 ± 0.18 | 0.34 ± 0.19 | 0.31 ± 0.15 |
| FRAP (micromoles of Trolox equivalents) | 953.1 ± 352.0 a | 1048.6 ± 383.9 | 964.2 ± 332.3 b | 964.2 ± 328.9 c | 1125.3 ± 364.6 | 1162.9 ± 526.1 a,b,c | 1021.9 ± 315.0 | 1069.4 ± 380.1 |
| Parameter | Term in the Equation | Unadjusted β Coefficient (95% CI) | Unadjusted Standardized β Coefficient | p-Value | Adjusted β Coefficient (95% CI) | Adjusted Standardized β Coefficient | p-Value |
|---|---|---|---|---|---|---|---|
| Paraoxonase-1 activity | ↑TG | 23.23 (−1.03, 47.50) | 0.082 | 0.061 | 22.59 (−3.33, 48.50) | 0.080 | 0.087 |
| ↑LDLC | 5.17 (−18.05, 28.39) | 0.018 | 0.662 | 5.79 (−17.97, 29.54) | 0.020 | 0.478 | |
| ↓HDLC | −27.12 (−49.75, −4.50) | −0.099 | 0.019 | −29.31 (−52.48, −6.14) | −0.107 | 0.013 | |
| Superoxide dismutase-1 activity | ↑TG | −0.38 (−0.89, 0.13) | −0.065 | 0.143 | −0.11 (−0.65, 0.44) | −0.018 | 0.705 |
| ↑LDLC | 0.06 (−0.43, 0.55) | 0.010 | 0.818 | 0.01 (−0.51, 0.50) | −0.001 | 0.983 | |
| ↓HDLC | 0.11 (−0.37, 0.58) | 0.019 | 0.662 | −0.05 (−0.54, 0.43) | −0.010 | 0.829 | |
| Log catalase activity | ↑TG | 0.02 (−0.03, 0.06) | 0.035 | 0.428 | −0.01 (−0.05, 0.04) | −0.010 | 0.833 |
| ↑LDLC | 0.00 (−0.04, 0.04) | 0.002 | 0.964 | 0.00 (−0.04, 0.04) | −0.002 | 0.964 | |
| ↓HDLC | −0.03 (−0.07, 0.01) | −0.070 | 0.100 | −0.03 (−0.07, 0.01) | −0.061 | 0.162 | |
| Ceruloplasmin activity | ↑TG | −0.01 (−0.07, 0.05) | −0.019 | 0.664 | −0.02 (−0.09, 0.04) | −0.033 | 0.486 |
| ↑LDLC | 0.02 (−0.04, 0.07) | 0.024 | 0.574 | 0.01 (−0.04, 0.07) | 0.021 | 0.631 | |
| ↓HDLC | −0.01 (−0.07, 0.04) | −0.017 | 0.696 | −0.01 (−0.06, 0.05) | −0.008 | 0.860 | |
| Log ferric-reducing ability of plasma | ↑TG | 0.06 (0.03, 0.08) | 0.175 | <0.001 | 0.03 (0.00, 0.06) | 0.089 | 0.041 |
| ↑LDLC | 0.01 (−0.02, 0.03) | 0.023 | 0.575 | 0.02 (−0.01, 0.05) | 0.060 | 0.131 | |
| ↓HDLC | 0.01 (−0.02, 0.04) | 0.029 | 0.490 | 0.03 (0.01, 0.06) | 0.096 | 0.018 |
| Parameter | Term in the Equation | Unadjusted β Coefficient (95% CI) | Unadjusted Standardized β Coefficient | p-Value | Adjusted β Coefficient (95% CI) | Adjusted Standardized β Coefficient | p-Value |
|---|---|---|---|---|---|---|---|
| Paraoxonase-1 activity | ↑TG | 42.28 (−14.62, 99.17) | 0.150 | 0.145 | 36.61 (−21.70, 94.93) | 0.130 | 0.218 |
| ↑LDLC | −12.41 (−48.12, 23.30) | −0.044 | 0.495 | −10.66 (−47.13, 25.81) | −0.038 | 0.566 | |
| ↓HDLC | −21.44 (−53.64, 10.77) | −0.079 | 0.192 | 23.77 (−56.17, 8.63) | −0.087 | 0.150 | |
| ↑TG × ↑LDLC | −10.28 (−83.46, 62.89) | −0.030 | 0.783 | −5.33 (−78.85, 68.19) | −0.015 | 0.887 | |
| ↑TG × ↓HDLC | −56.42 (−125.46, 12.63) | −0.175 | 0.109 | −48.46 (−117.87, 20.96) | −0.151 | 0.171 | |
| ↑LDLC × ↓HDLC | −17.08 (−83.66, 49.51) | −0.044 | 0.615 | −17.26 (−83.91, 49.39) | −0.045 | 0.611 | |
| ↑TG × ↑LDLC × ↓HDLC | 105.81 (3.37, 208.24) | 0.232 | 0.043 | 94.32 (−8.39, 197.03) | 0.207 | 0.072 | |
| Ceruloplasmin activity | ↑TG | 0.09 (−0.044, 0.23) | 0.137 | 0.186 | 0.09 (−0.05, 0.23) | 0.130 | 0.225 |
| ↑LDLC | 0.07 (−0.016, 0.16) | 0.104 | 0.111 | 0.07 (−0.02, 0.16) | 0.109 | 0.103 | |
| ↓HDLC | −0.03 (−0.10, 0.05) | −0.038 | 0.527 | −0.02 (−0.10, 0.06) | −0.029 | 0.632 | |
| ↑TG × ↑LDLC | −0.21 (−0.38, −0.03) | −0.254 | 0.020 | −0.22 (−0.39, −0.04) | −0.265 | 0.016 | |
| ↑TG × ↓HDLC | −0.05 (−0.22, 0.11) | −0.069 | 0.530 | −0.05 (−0.22, 0.12) | −0.068 | 0.542 | |
| ↑LDLC × ↓HDLC | 0.016 (−0.14, 0.18) | 0.081 | 0.848 | 0.016 (−0.15, 0.18) | 0.017 | 0.849 | |
| ↑TG × ↑LDLC × ↓HDLC | 0.10 (−0.15, 0.34) | 0.088 | 0.448 | 0.093 (−0.15, 0.34) | 0.126 | 0.459 |
| Double Interaction Tested | Fixed Condition | Unadjusted β Coefficient (CI 95%) | Unadjusted Standardized β Coefficient | p-Value | Adjusted β Coefficient (CI 95%) | Adjusted Standardized β Coefficient | p-Value |
|---|---|---|---|---|---|---|---|
| ↑TG × ↓HDLC | Normal LDLC | −56.42 (−121.38, 8.55) | −0.179 | 0.089 | −52.79 (−118.15, 12.57) | −0.167 | 0.113 |
| ↑LDLC | 49.39 (−34.28, 133.06) | 0.148 | 0.246 | 52.14 (−31.17, 135.45) | 0.156 | 0.219 | |
| ↑LDLC × ↓HDLC | Normal TG | −17.08 (−72.63, 48.48) | −0.033 | 0.609 | −16.96 (−82.95, 49.04) | −0.033 | 0.614 |
| ↑TG | 88.73 (8.34, 169.12) | 0.281 | 0.031 | 74.55 (−7.30, 156.40) | 0.236 | 0.074 | |
| ↑TG × ↑LDLC | Normal HDLC | −10.28 (−85.71, 65.15) | −0.028 | 0.789 | −3.29 (−79.19, 72.61) | −0.009 | 0.932 |
| ↓HDLC | 95.53 (26.50, 164.55) | 0.305 | 0.007 | 91.59 (21.18, 162.00) | 0.293 | 0.011 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Mancía, S.; Jiménez-Osorio, A.S.; Medina-Campos, O.N.; Colín-Ramírez, E.; Vallejo, M.; Alcántara-Gaspar, A.; Cartas-Rosado, R.; Vargas-Barrón, J.; Pedraza-Chaverri, J. Activity of Antioxidant Enzymes and Their Association with Lipid Profile in Mexican People without Cardiovascular Disease: An Analysis of Interactions. Int. J. Environ. Res. Public Health 2018, 15, 2687. https://doi.org/10.3390/ijerph15122687
Rivera-Mancía S, Jiménez-Osorio AS, Medina-Campos ON, Colín-Ramírez E, Vallejo M, Alcántara-Gaspar A, Cartas-Rosado R, Vargas-Barrón J, Pedraza-Chaverri J. Activity of Antioxidant Enzymes and Their Association with Lipid Profile in Mexican People without Cardiovascular Disease: An Analysis of Interactions. International Journal of Environmental Research and Public Health. 2018; 15(12):2687. https://doi.org/10.3390/ijerph15122687
Chicago/Turabian StyleRivera-Mancía, Susana, Angélica Saraí Jiménez-Osorio, Omar Noel Medina-Campos, Eloísa Colín-Ramírez, Maite Vallejo, Ariadna Alcántara-Gaspar, Raúl Cartas-Rosado, Jesús Vargas-Barrón, and José Pedraza-Chaverri. 2018. "Activity of Antioxidant Enzymes and Their Association with Lipid Profile in Mexican People without Cardiovascular Disease: An Analysis of Interactions" International Journal of Environmental Research and Public Health 15, no. 12: 2687. https://doi.org/10.3390/ijerph15122687
APA StyleRivera-Mancía, S., Jiménez-Osorio, A. S., Medina-Campos, O. N., Colín-Ramírez, E., Vallejo, M., Alcántara-Gaspar, A., Cartas-Rosado, R., Vargas-Barrón, J., & Pedraza-Chaverri, J. (2018). Activity of Antioxidant Enzymes and Their Association with Lipid Profile in Mexican People without Cardiovascular Disease: An Analysis of Interactions. International Journal of Environmental Research and Public Health, 15(12), 2687. https://doi.org/10.3390/ijerph15122687







