Poor Sleep Quality Is Associated with Higher Hemoglobin A1c in Pregnant Women: A Pilot Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Self-Rated Sleep Quality
2.3. Measurement of Sleep Fragmentation and Duration
2.4. Assessment of Depression Levels
2.5. Quantification of HbA1c and Total Cortisol
2.6. Oral Glucose Tolerance Test (OGTT) and Ponderal Index
2.7. Statistical Analyses
3. Results
3.1. Profiles of Study Cohort
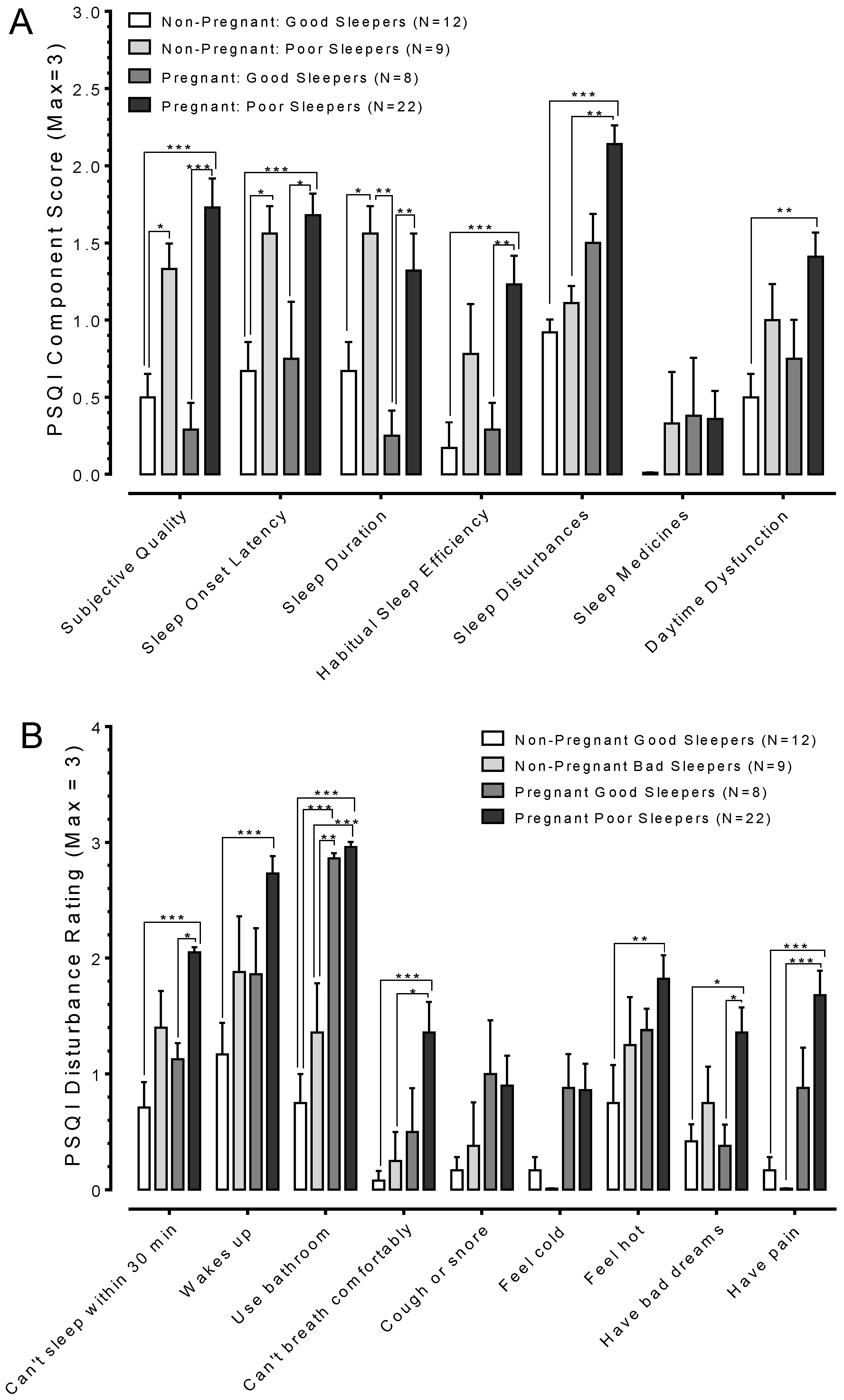
3.2. Pregnancy Changes Sleep Quality
3.3. Pregnant Poor Sleepers Report Difficulties Falling Asleep at Bedtime
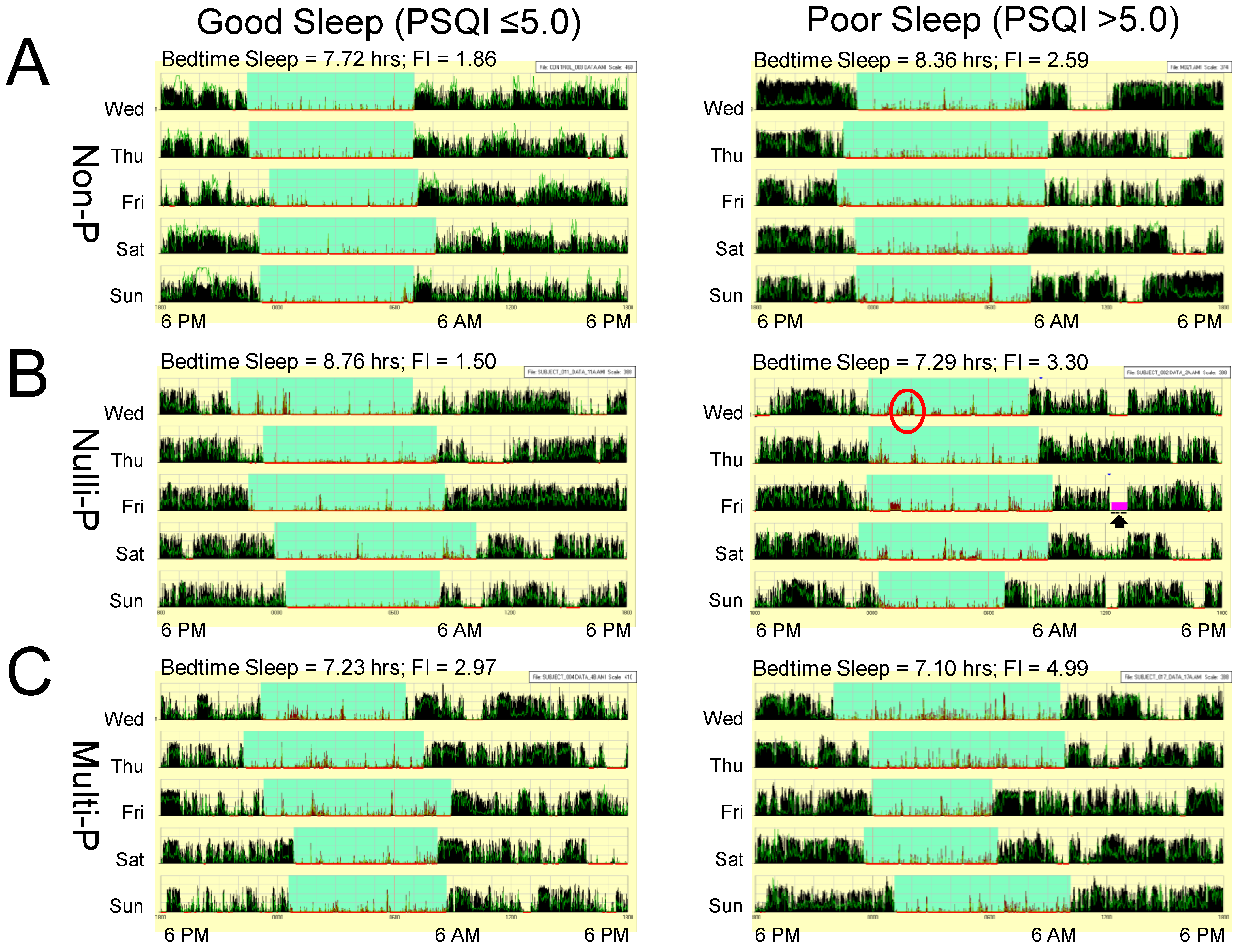
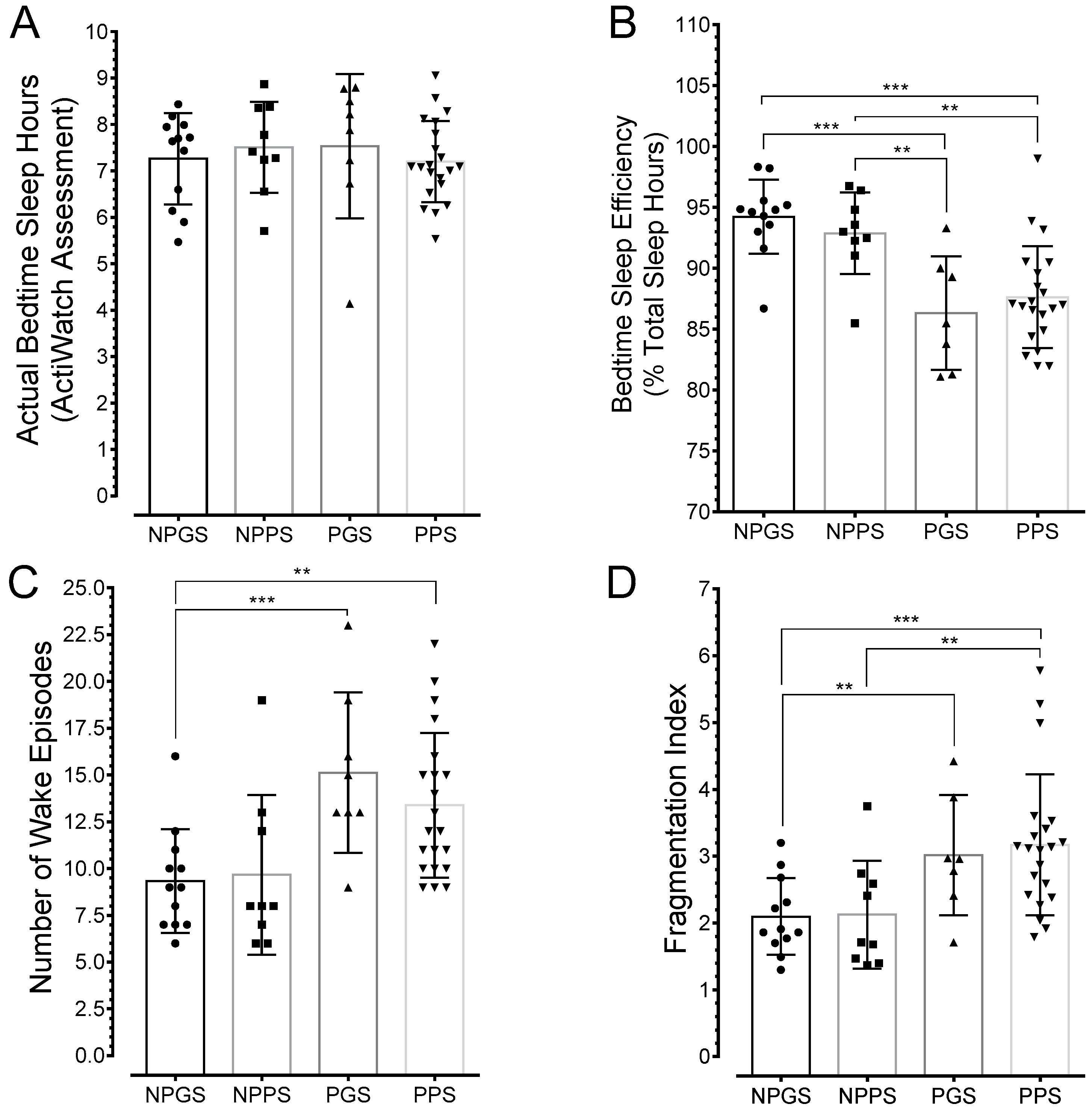
3.4. Sleep is Greatly Fragmented in Pregnant Poor Sleepers
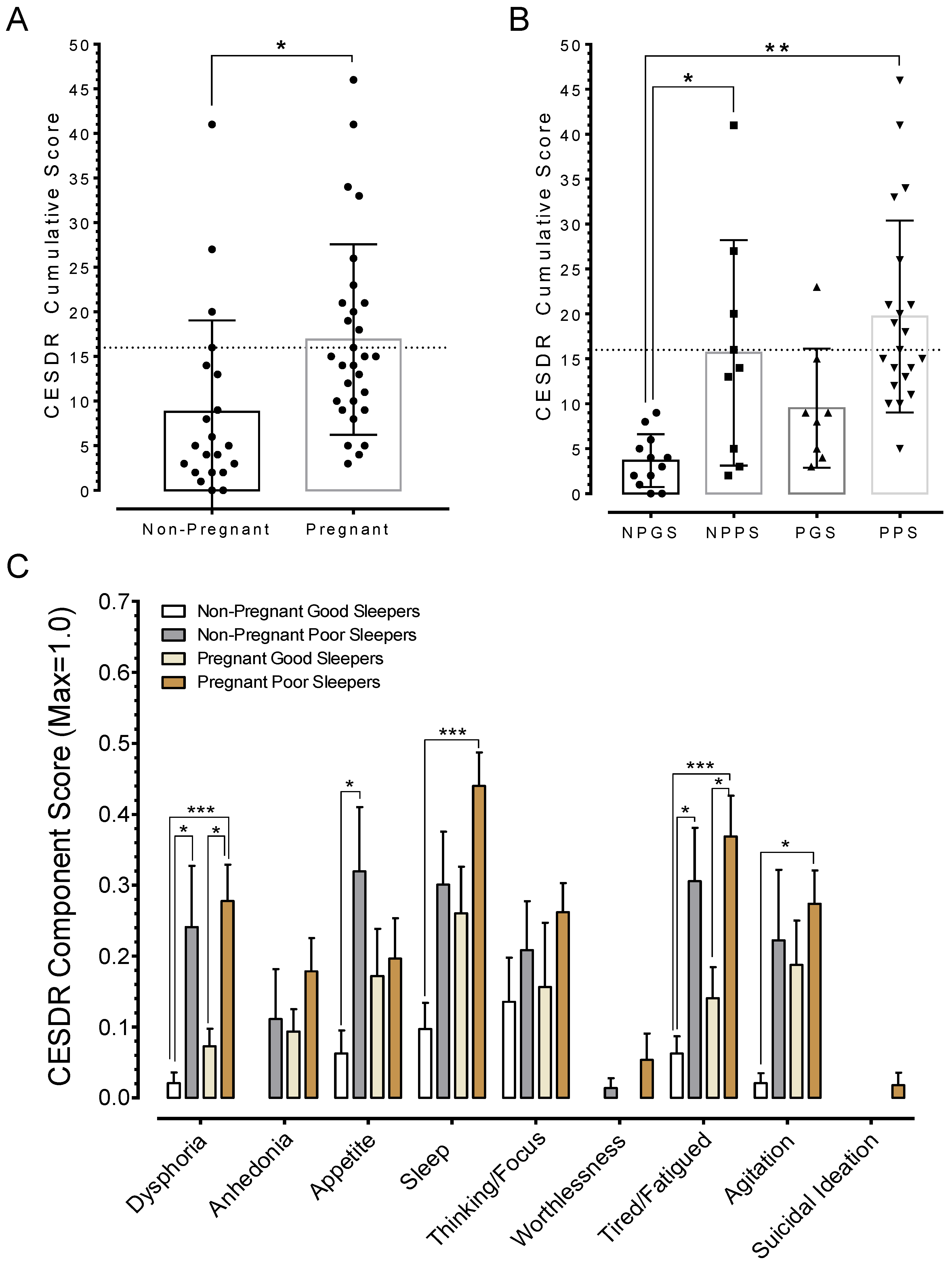
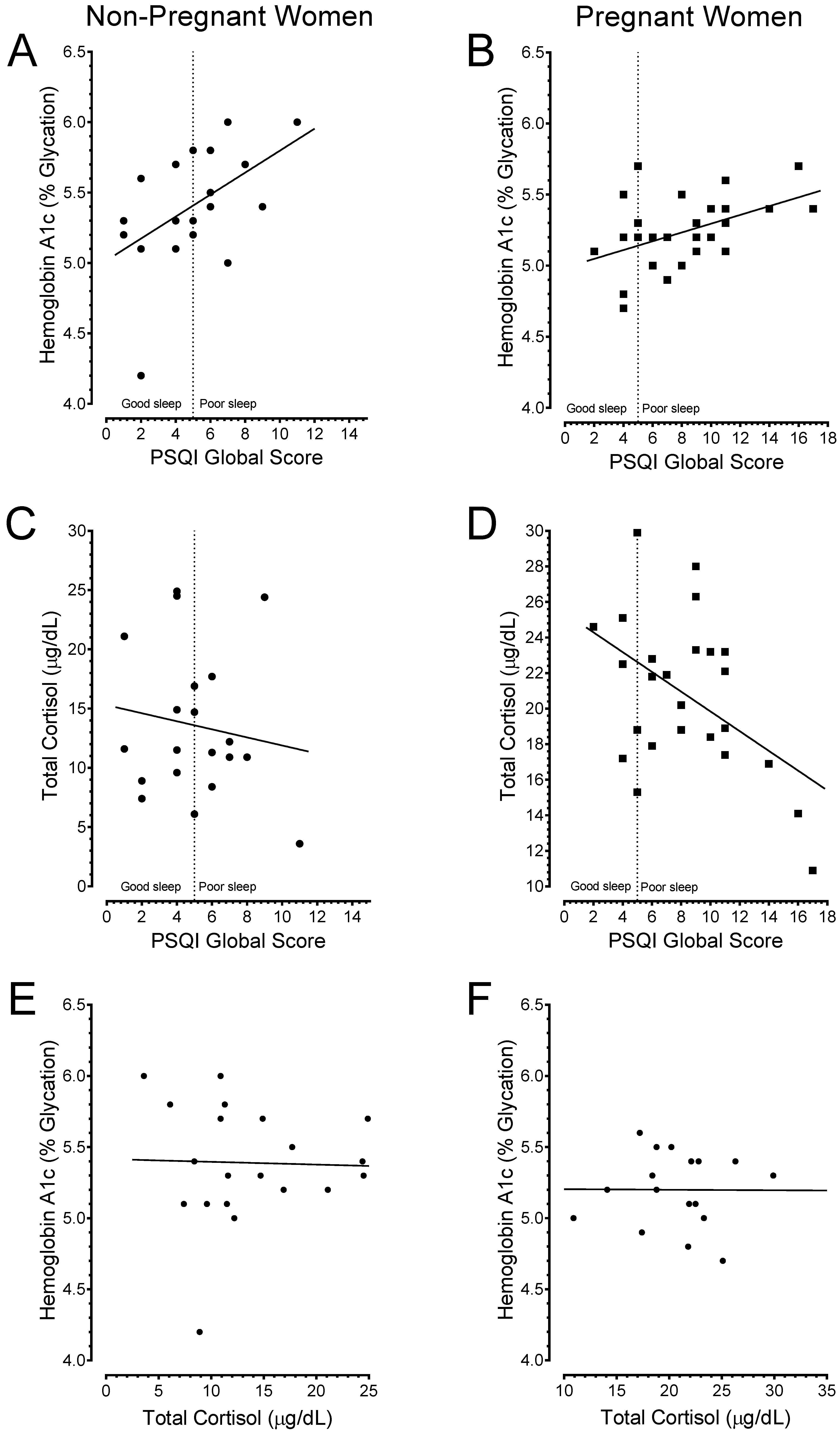
3.5. Poor Sleep Quality is Associated with Depression
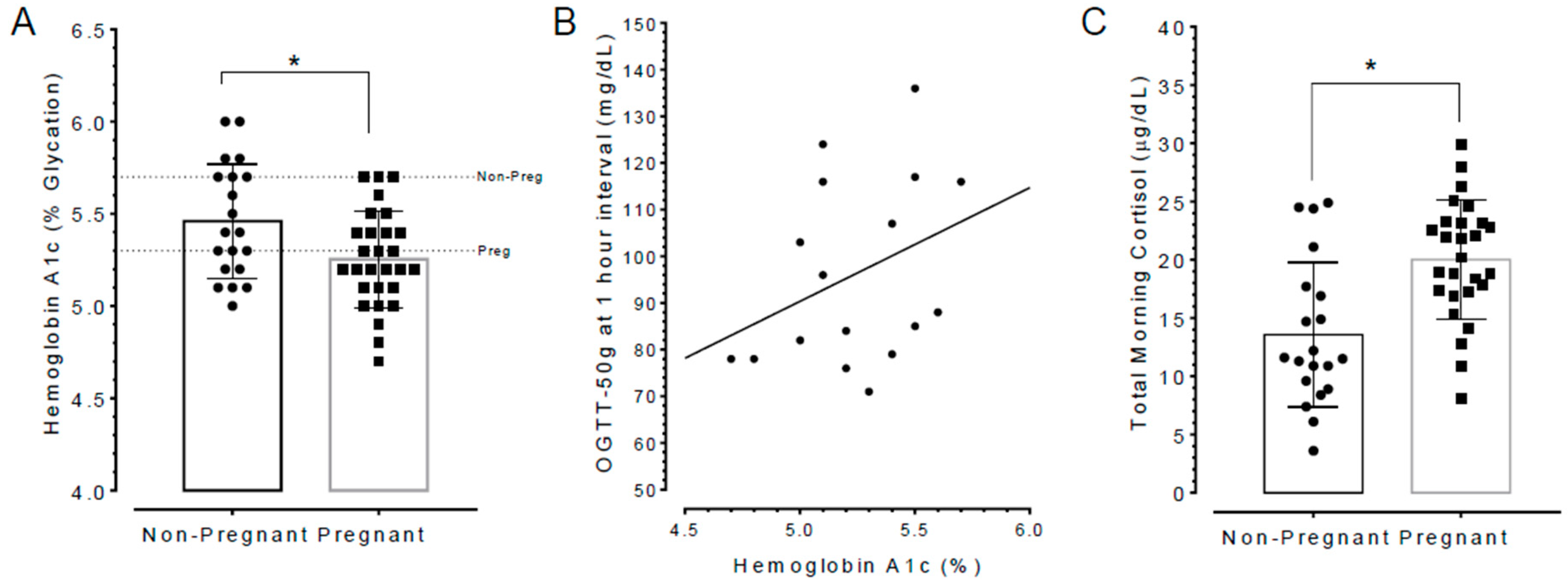
3.6. Poor Sleepers Exhibited Elevated HbA1c and Lowered Total Cortisol Levels than Good Sleepers
3.7. Poor Sleep Quality Is Associated with a Higher Incidence of Adverse Pregnancy Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Women and Sleep. Available online: www.sleepfoundation.org/article/sleep-topics/pregnancy-and-sleep (accessed on 15 August 2018).
- Meerlo, P.; Sgoifo, A.; Suchecki, D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress and stress responsivity. Sleep Med. Rev. 2008, 12, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Nedeltcheva, A.V.; Kessler, L.; Imperial, J.; Penev, P.D. Exposure to recurrent sleep restriction in the setting of high caloric intake and physical inactivity results in increased insulin resistance and reduced glucose tolerance. J. Clin. Endocrinol. Metab. 2009, 94, 3242–3250. [Google Scholar] [CrossRef] [PubMed]
- Orzel-Gryglewska, J. Consequences of sleep deprivation. Int. J. Occup. Med. Environ. Health 2010, 23, 95–114. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Spiegel, K.; Tasali, E.; Leproult, R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008, 9, S23–S28. [Google Scholar] [CrossRef]
- Farrar, D. Hyperglycemia in pregnancy: Prevalence, impact, and management challenges. Int. J. Womens Health 2016, 8, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Metzger, B.E.; Lowe, L.P.; Dyer, A.R.; Trimble, E.R.; Chaovarindr, U.; Coustan, D.R.; Hadden, D.R.; McCance, D.R.; Hood, M.; McIntyre, H.D.; et al. Hyperglycemia and adverse pregnancy outcomes. N. Engl. J. Med. 2008, 358, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Guariguata, L.; Linnenkamp, U.; Beagley, J.; Whiting, D.R.; Cho, N.H. Global estimates of the prevalence of hyperglycaemia in pregnancy. Diabetes Res. Clin. Pract. 2014, 103, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, S.; Ji, J.; Ge, A.; Chen, C.; Zhu, Y.; Xie, N.; Wang, Y. Risk factors and management of gestational diabetes. Cell Biochem. Biophys. 2015, 71, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Kuenen, J.; Borg, R.; Zheng, H.; Schoenfeld, D.; Heine, R.J.; A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care 2008, 31, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Tyzbir, E.D.; Roman, N.M.; Amini, S.B.; Sims, E.A. Longitudinal changes in insulin release and insulin resistance in non-obese pregnant women. Am. J. Obstet. Gynecol. 1991, 165, 1667–1672. [Google Scholar] [CrossRef]
- Nadal, A.; Alonso-Magdalena, P.; Soriano, S.; Ropero, A.B.; Quesada, I. The role of oestrogens in the adaptation of islets to insulin resistance. J. Physiol. 2009, 587, 5031–5037. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, L.; Pettitt, D.J. Gestational diabetes mellitus. JAMA 2001, 286, 2516–2518. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Jung, I.; Kim, S.J. The association between maternal hyperglycemia and perinatal outcomes in gestational diabetes mellitus patients: A retrospective cohort study. Medicine 2016, 95, e4712. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Lee, C.N.; Wu, W.W.; Landis, C.A. Sleep hygiene and sleep quality of third-trimester pregnant women. Res. Nurs. Health 2016, 39, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, A.; Santeusanio, F.; Braaten, J.; Faloona, G.R.; Unger, R.H. Pancreatic alpha-cell function in trauma. JAMA 1974, 227, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Wilmore, D.W. Carbohydrate metabolism in trauma. Clin. Endocrinol. Metab. 1976, 5, 731–745. [Google Scholar] [CrossRef]
- Lager, I. The insulin-antagonistic effect of the counterregulatory hormones. J. Intern. Med. Suppl. 1991, 735, 41–47. [Google Scholar] [PubMed]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psych. Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Carpenter, J.S.; Andrykowski, M.A. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J. Psych. Res. 1998, 45, 5–13. [Google Scholar] [CrossRef]
- Baker, F.C.; Maloney, S.; Driver, H.S. A comparison of subjective estimates of sleep with objective polysomnographic data in healthy men and women. J. Psychosom. Res. 1999, 47, 335–341. [Google Scholar] [CrossRef]
- Backhaus, J.; Junghanns, K.; Broocks, A.; Riemann, D.; Hohagen, F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J. Psychosom. Res. 2002, 53, 737–740. [Google Scholar] [CrossRef]
- Skouteris, H.; Wertheim, E.H.; Germano, C.; Paxton, S.J.; Milgrom, J. Assessing sleep during pregnancy: A study across two time points examining the Pittsburgh Sleep Quality Index and associations with depressive symptoms. Womens Health Issues 2009, 19, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.J.; Kripke, D.F.; Gruen, W.; Mullaney, D.J.; Gillin, J.C. Automatic sleep/wake identification from wrist activity. Sleep 1992, 15, 461–469. [Google Scholar] [CrossRef] [PubMed]
- De Souza, L.; Benedito-Silva, A.A.; Pires, M.L.; Poyares, D.; Tufik, S.; Calil, H.M. Further validation of actigraphy for sleep studies. Sleep 2003, 26, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Jean-Louis, G.; Kripke, D.F.; William, J.; Mason, W.J.; Jeffrey, A.; Elliott, J.A.; Shawn, D.; Youngstedt, S.D. Sleep estimation from wrist movement quantified by different actigraphic modalities. J. Neurosci. Methods 2001, 105, 185–191. [Google Scholar] [CrossRef]
- Sadeh, A.; Acebo, C. The role of actigraphy in sleep medicine. Sleep Med. Rev. 2002, 6, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Beebe, K.R.; Lee, K.A. Sleep disturbance in late pregnancy and early labor. J. Perinat. Neonatal Nurs. 2007, 21, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Rupp, T.L.; Balkin, T.J. Comparison of motion logger watch and actiwatch actigraphs to polysomnography for sleep/wake estimation in healthy young adults. Behav. Res. Methods 2011, 43, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, N.T.; Earleywine, M. Validation of the Center for Epidemiologic Studies Depression Scale—Revised (CESD-R): Pragmatic depression assessment in the general population. Psychiatry Res. 2011, 186, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; McGee, P.; Steffes, M.W.; Lachin, J.M.; DCCT/EDIC Research Group. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes 2014, 63, 282–290. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care 2014, 37, S14–S80. [Google Scholar] [CrossRef] [PubMed]
- International Association of Diabetes and Pregnancy Study Groups. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.J.; Berg, C.J.; Calle, E.E. The ponderal index in term newborn siblings. Am. J. Epidemiol. 1990, 132, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Dombrowski, M.P.; Berry, S.M.; Johnson, M.P.; Saleh, A.A.; Sokol, R.J. Birth weight-length ratios, ponderal indexes, placental weights and birth weight-placenta ratios in a large population. Arch. Pediatr. Adolesc. Med. 1994, 148, 508–512. [Google Scholar] [CrossRef] [PubMed]
- Tamim, H.; Beydoun, H.; Intani, M.; Khogali, M.; Chokr, I.; Yunis, K.A.; National Collaborative Perinatal Neonatal Network. Predicting neonatal outcomes: Birth weight, body mass index or ponderal index? J. Perinat. Med. 2004, 32, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Armangil, D.; Yurdakok, M.; Korkmaz, A.; Yigit, S.; Tekinalp, G. Ponderal index of a large-for-gestational age infants: Comparison between infants of diabetic and non-diabetic mothers. Turk. J. Pediatr. 2011, 53, 169–172. [Google Scholar] [PubMed]
- D’Agostino, R.; Pearson, E.S. Tests for departure from normality: Empirical results for the distributions of b2 and √b1. Biometrika 1973, 60, 613–622. [Google Scholar] [CrossRef]
- Carr, B.R.; Parker, C.R., Jr.; Madden, J.D.; MacDonald, P.C.; Porter, J.C. Maternal plasma adrenocorticotropin and cortisol relationships throughout human pregnancy. Am. J. Obstet. Gynecol. 1981, 139, 416–422. [Google Scholar] [CrossRef]
- Dorr, H.G.; Heller, A.; Versmold, H.T.; Sippell, W.G.; Herrmann, M.; Bidlingmaier, F.; Knorr, D. Longitudinal study of progestins, mineralcorticoids, and glucocorticoids throughout human pregnancy. J. Clin. Endocr. Metab. 1989, 68, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Hedderson, M.M.; Darbinian, J.A.; Ferrara, A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr. Perinat. Epidemiol. 2010, 24, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Beddoe, A.E.; Lee, K.A.; Weiss, S.J.; Kennedy, H.P.; Yang, C.P. Effects of mindful yoga on sleep in pregnant women: A pilot study. Biol. Res. Nurs. 2010, 11, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Gay, C.L. Sleep in late pregnancy predicts length of labor and type of delivery. Am. J. Obstet. Gynecol. 2004, 191, 2041–2046. [Google Scholar] [CrossRef] [PubMed]
- Enquobahrie, C.Q.; Frederick, I.O.; Abetew, D.; Williams, M.A. Glucose intolerance and gestational diabetes risk in relation to sleep duration and snoring during pregnancy: A pilot study. BMC Womens Health 2010, 10, 17. [Google Scholar] [CrossRef]
- Sadeh, A.; Sharkey, K.M.; Carskadon, M.A. Activity-based sleep-wake identification: An empirical test of methodological issues. Sleep 1994, 17, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Morin, A.K.; Jarvis, C.I.; Lynch, A.M. Therapeutic options for sleep-maintenance and sleep-onset insomnia. Pharm. 2007, 27, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Van Liempt, S.; Vermetten, E.; Lentjes, E.; Arends, J.; Westenberg, H. Decreased nocturnal growth hormone secretion and sleep fragmentation in combat-related posttraumatic stress disorder; potential predictors of impaired memory consolidation. Psychoneuroendocrinology 2011, 36, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Herring, S.J.; Nelson, D.B.; Pien, G.W.; Homko, C.; Goetzl, L.M.; Davey, A.; Forster, G.D. Objectively-measured sleep duration and hyperglycemia in pregnancy. Sleep Med. 2014, 15, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Schmid, S.M.; Hallschmid, M.; Schultes, B. The metabolic burden of sleep loss. Lancet Diabetes Endocr. 2015, 3, 52–62. [Google Scholar] [CrossRef]
- Lenz, H.J.; Raedler, A.; Greten, H.; Brown, M.R. CRF initiates biological actions within the brain that are observed in response to stress. Am. J. Physiol. 1987, 21, 34–39. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.; Smith, R. Corticotropin-releasing hormone and human parturition. Reproduction 2001, 121, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Chernow, B.; Alexander, H.R.; Smallridge, R.C.; Thompson, W.R.; Cook, D.; Beardsley, D.; Fink, M.P.; Lake, C.R.; Fletcher, J.R. Hormonal responses to graded surgical stress. Arch. Intern. Med. 1987, 147, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Dorn, L.D.; Lucke, J.F.; Loucks, T.L.; Berga, S.L. Salivary cortisol reflects serum cortisol: Analysis of circadian profiles. Ann. Clin. Biochem. 2007, 44, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Krieger, D.T. Rhythms of ACTH and corticosteroid secretion in health and disease and their experimental modification. J. Steroid Biochem. 1975, 6, 758–791. [Google Scholar] [CrossRef]
- Nielsen, G.L.; Moller, M.; Sorensen, H.T. HbA1c in early diabetic pregnancy and pregnancy outcomes: A Danish population-based cohort study of 573 pregnancies of women with type 1 diabetes. Diabetes Care 2006, 29, 2612–2616. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.C.; Moore, M.P.; Gullam, J.E.; Mohamed, K.; Rowan, J. An early pregnancy HbA1c ≥ 5.9% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care 2014, 37, 2953–2959. [Google Scholar] [CrossRef] [PubMed]
- Mañé, L.; Flores-Le Roux, J.A.; Benaiges, D.; Rodríguez, M.; Marcelo, I.; Chillarón, J.J.; Pedro-Botet, J.; Llauradó, G.; Gortazar, L.; Carreras, R.; et al. Role of first-trimester HbA1c as a predictor of adverse obstetric outcomes in a multiethnic cohort. J. Clin. Endocr. Metab. 2017, 102, 390–397. [Google Scholar] [PubMed]
- Sweeting, A.N.; Ross, G.P.; Hyett, J.; Molyneaux, L.; Tan, K.; Constantino, M.; Harding, A.J.; Wong, J. Baseline HbA1c to identify high-risk gestational diabetes: Utility in early vs standard gestational diabetes. J. Clin. Endocr. Metab. 2017, 102, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Reutrakul, S.; Anothaisintawee, T.; Herring, S.J.; Balserak, B.I.; Marc, I.; Thakkinstian, A. Short sleep duration and hyperglycemia in pregnancy: Aggregate and individual patient data meta-analysis. Sleep Med. Rev. 2018, 40, 31–42. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chirwa, S.; Nwabuisi, C.R.; Ladson, G.M.; Korley, L.; Whitty, J.E.; Atkinson, R.; Clark, J.T. Poor Sleep Quality Is Associated with Higher Hemoglobin A1c in Pregnant Women: A Pilot Observational Study. Int. J. Environ. Res. Public Health 2018, 15, 2287. https://doi.org/10.3390/ijerph15102287
Chirwa S, Nwabuisi CR, Ladson GM, Korley L, Whitty JE, Atkinson R, Clark JT. Poor Sleep Quality Is Associated with Higher Hemoglobin A1c in Pregnant Women: A Pilot Observational Study. International Journal of Environmental Research and Public Health. 2018; 15(10):2287. https://doi.org/10.3390/ijerph15102287
Chicago/Turabian StyleChirwa, Sanika, Chioma R. Nwabuisi, Gwinnett M. Ladson, Linda Korley, Janice E. Whitty, Robin Atkinson, and John T. Clark. 2018. "Poor Sleep Quality Is Associated with Higher Hemoglobin A1c in Pregnant Women: A Pilot Observational Study" International Journal of Environmental Research and Public Health 15, no. 10: 2287. https://doi.org/10.3390/ijerph15102287
APA StyleChirwa, S., Nwabuisi, C. R., Ladson, G. M., Korley, L., Whitty, J. E., Atkinson, R., & Clark, J. T. (2018). Poor Sleep Quality Is Associated with Higher Hemoglobin A1c in Pregnant Women: A Pilot Observational Study. International Journal of Environmental Research and Public Health, 15(10), 2287. https://doi.org/10.3390/ijerph15102287




