Occurrence and Composition of Microplastics in the Seabed Sediments of the Coral Communities in Proximity of a Metropolitan Area
Abstract
1. Introduction
2. Materials and Methods
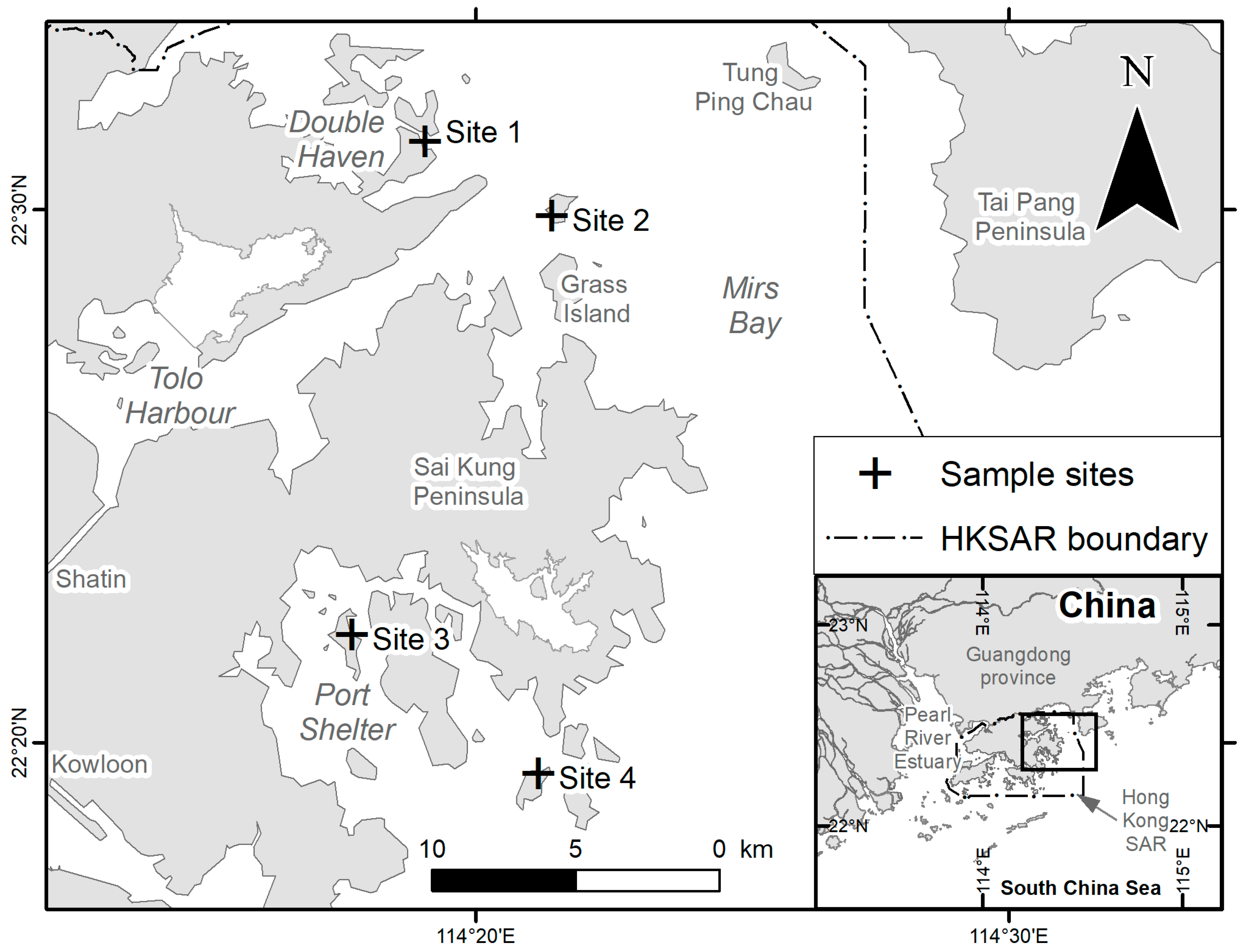
2.1. Study Area and the Coral Communities
2.2. Sample Collection
2.3. Extraction Procedures
2.4. Identification of Microplastics
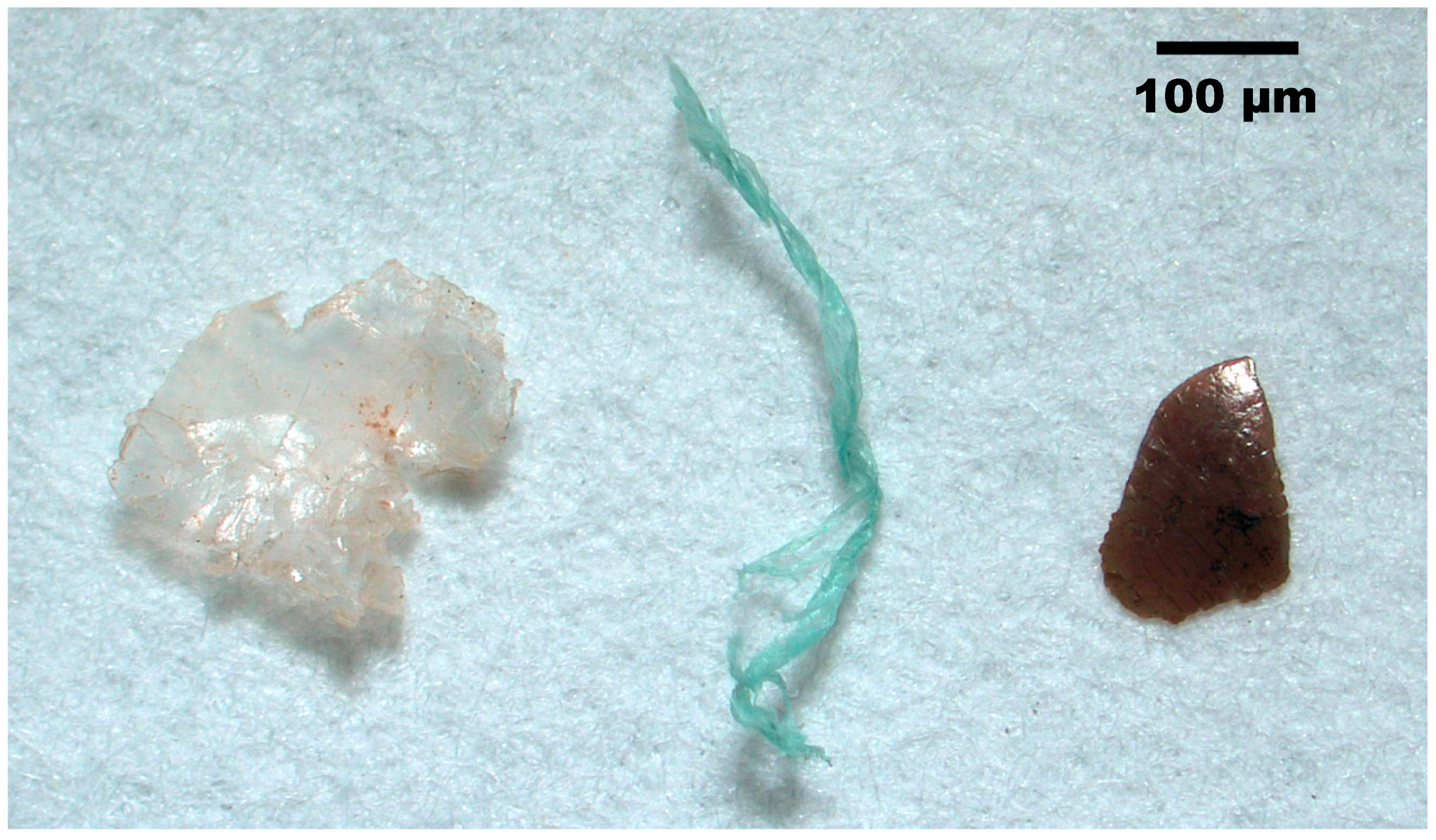
2.4.1. Microscope Examination
2.4.2. ATR-FTIR Analysis
2.5. Statistical Analysis
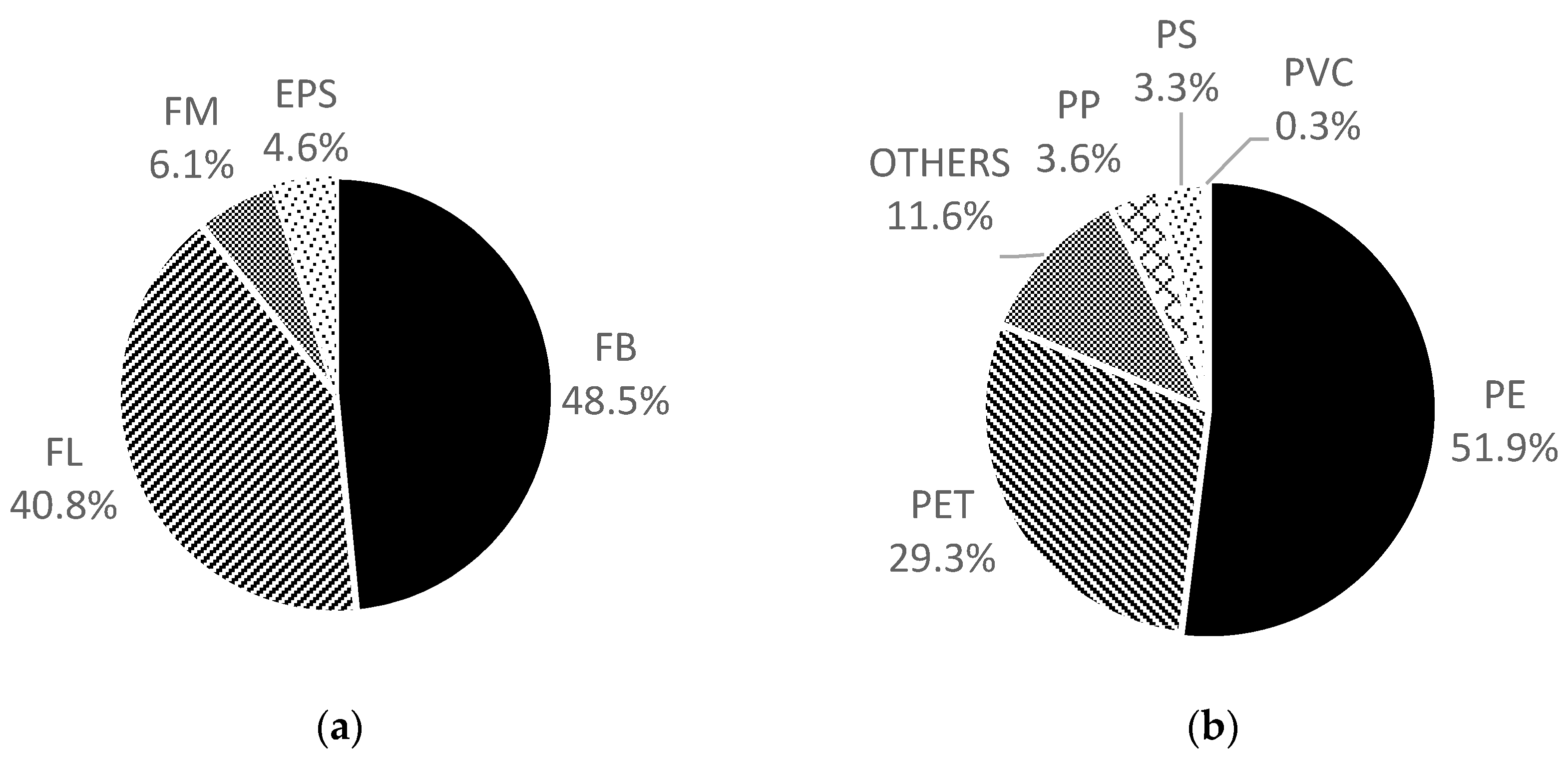
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Derraik, J.G.B. The pollution of the marine environment by plastic debris: A review. Mar. Pollut. Bull. 2002, 44, 842–852. [Google Scholar] [CrossRef]
- Lebreton, L.C.M.; van der Zwet, J.; Damsteeg, J.-W.; Slat, B.; Andrady, A.; Reisser, J. River plastic emissions to the world’s oceans. Nature 2017, 8, 15611. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Vanreusel, A.; Mees, J.; Janssen, C.R. Microplastic pollution in deep-sea sediments. Environ. Pollut. 2013, 182, 495–499. [Google Scholar] [CrossRef] [PubMed]
- PEMRG. Plastics—The Facts 2012: An Analysis of European Plastics Production, Demand and Recovery for 2011; Plastics Europe Market Research Group: Brussels, Belgium, 2012. [Google Scholar]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L. Microplastics in the marine environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.K.A. Biodiversity: Invasions by marine life on plastic debris. Nature 2002, 416, 808–809. [Google Scholar] [CrossRef] [PubMed]
- Fok, L.; Cheung, P.K. Hong kong at the pearl river estuary: A hotspot of microplastic pollution. Mar. Pollut. Bull. 2015, 99, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.K.; Fok, L. Characterisation of plastic microbeads in facial scrubs and their estimated emissions in mainland china. Water Res. 2017, 122, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Martí, E.; Martin, C.; Cózar, A.; Duarte, C.M. Low abundance of plastic fragments in the surface waters of the red sea. Front. Mar. Sci. 2017, 4, 333. [Google Scholar] [CrossRef]
- Hidalgo-Ruz, V.; Thiel, M. Distribution and abundance of small plastic debris on beaches in the se pacific (chile): A study supported by a citizen science project. Mar. Environ. Res. 2013, 87–88, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.-S.; Xu, X.; Wong, C.-Y.; Cheung, S.-G. Comparisons of microplastic pollution between mudflats and sandy beaches in hong kong. Environ. Pollut. 2018, 236, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Doyle, M.J.; Watson, W.; Bowlin, N.M.; Sheavly, S.B. Plastic particles in coastal pelagic ecosystems of the northeast pacific ocean. Mar. Environ. Res. 2011, 71, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Norén, F. Small Plastic Particles in Coastal Swedish Waters; KIMO Sweden: Göteborg, Sweden, 2007. [Google Scholar]
- Ng, S.L.; Zhang, Y.; Ng, K.H.; Wong, H.; Lee, J.W.Y. Living environment and quality of life in hong kong. Asian Geogr. 2018, 35, 35–51. [Google Scholar] [CrossRef]
- Murray, F.; Cowie, P.R. Plastic contamination in the decapod crustacean nephrops norvegicus (linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.; Lui, C.; Fok, L. Microplastic contamination of wild and captive flathead grey mullet (mugil cephalus). Int. J. Environ. Res. Public Health 2018, 15, 597. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, D.; Li, L.; Jabeen, K.; Shi, H. Microplastics in commercial bivalves from china. Environ. Pollut. 2015, 207, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-X.; Ma, L.-S.; Lin, L.; Ni, Z.-X.; Xu, X.-R.; Shi, H.-H.; Yan, Y.; Zheng, G.-M.; Rittschof, D. Microplastics in oysters saccostrea cucullata along the pearl river estuary, china. Environ. Pollut. 2018, 236, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global warming and recurrent mass bleaching of corals. Nature 2017, 543, 373. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, J.W.; Negri, A.P.; Uthicke, S.; Mueller, J.F. Chemical Pollution on Coral Reefs: Exposure and Ecological Effects. In Ecological Impacts of Toxic Chemicals; Bentham Science Publishers: Amsterdam, The Netherlands, 2011; pp. 187–211. [Google Scholar]
- Amid, C.; Olstedt, M.; Gunnarsson, J.S.; Le Lan, H.; Tran Thi Minh, H.; Van den Brink, P.J.; Hellström, M.; Tedengren, M. Additive effects of the herbicide glyphosate and elevated temperature on the branched coral acropora formosa in Nha Trang, Vietnam. Environ. Sci. Pollut. Res. 2018, 25, 13360–13372. [Google Scholar] [CrossRef] [PubMed]
- Shaw, C.M.; Brodie, J.; Mueller, J.F. Phytotoxicity induced in isolated zooxanthellae by herbicides extracted from great barrier reef flood waters. Mar. Pollut. Bull. 2012, 65, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Downs, C.A.; Kramarsky-Winter, E.; Segal, R.; Fauth, J.; Knutson, S.; Bronstein, O.; Ciner, F.R.; Jeger, R.; Lichtenfeld, Y.; Woodley, C.M.; et al. Toxicopathological effects of the sunscreen uv filter, oxybenzone (benzophenone-3), on coral planulae and cultured primary cells and its environmental contamination in Hawaii and the U.S. Virgin Islands. Arch. Environ. Contam. Toxicol. 2016, 70, 265–288. [Google Scholar] [CrossRef] [PubMed]
- Koelmans, A.A.; Bakir, A.; Burton, G.A.; Janssen, C.R. Microplastic as a vector for chemicals in the aquatic environment: Critical review and model-supported reinterpretation of empirical studies. Environ. Sci. Technol. 2016, 50, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Mato, Y.; Isobe, T.; Takada, H.; Kanehiro, H.; Ohtake, C.; Kaminuma, T. Plastic resin pellets as a transport medium for toxic chemicals in the marine environment. Environ. Sci. Technol. 2001, 35, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, R. Microplastics are not important for the cycling and bioaccumulation of organic pollutants in the oceans—But should microplastics be considered pops themselves? Integr. Environ. Assess. Manag. 2017, 13, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Hall, N.M.; Berry, K.L.E.; Rintoul, L.; Hoogenboom, M.O. Microplastic ingestion by scleractinian corals. Mar. Biol. 2015, 162, 725–732. [Google Scholar] [CrossRef]
- Allen, A.S.; Seymour, A.C.; Rittschof, D. Chemoreception drives plastic consumption in a hard coral. Mar. Pollut. Bull. 2017, 124, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.; Schellenberg, J.; Schubert, P.; Wilke, T. Responses of reef building corals to microplastic exposure. Environ. Pollut. 2017, 237, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.K.; Cheung, L.T.O.; Fok, L. Seasonal variation in the abundance of marine plastic debris in the estuary of a subtropical macro-scale drainage basin in south china. Sci. Total Environ. 2016, 562, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Morton, B. Hong kong’s coral communities: Status, threats and management plans. Mar. Pollut. Bull. 1994, 29, 74–83. [Google Scholar] [CrossRef]
- Tam, T.W.; Ang, P.O. Repeated physical disturbances and the stability of sub-tropical coral communities in hong kong, china. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 1005–1024. [Google Scholar] [CrossRef]
- Morton, B.; Blackmore, G. Seasonal variations in the density of and corallivory by drupella rugosa and cronia margariticola (caenogastropoda: Muricidae) from the coastal waters of Hong Kong: ‘Plagues’ or ‘aggregations’? J. Mar. Biol. Assoc. UK 2009, 89, 147–159. [Google Scholar] [CrossRef]
- Qiu, J.W.; Lau, D.C.; Cheang, C.C.; Chow, W.K. Community-level destruction of hard corals by the sea urchin diadema setosum. Mar. Pollut. Bull. 2014, 85, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Duprey, N.N.; Yasuhara, M.; Baker, D.M. Reefs of tomorrow: Eutrophication reduces coral biodiversity in an urbanized seascape. Glob. Chang. Biol. 2016, 22, 3550–3565. [Google Scholar] [CrossRef] [PubMed]
- Masura, J.; Baker, J.; Foster, G.; Arthur, C.; Herring, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; National Oceanic and Atmospheric Administration: Silver Spring, MD, USA, 2015.
- Clarke, K.R.; Gorley, R.N. Primer v7: User Manual/Tutorial; PRIMER-E: Plymouth, UK, 2015; p. 296. [Google Scholar]
- Imhof, H.K.; Sigl, R.; Brauer, E.; Feyl, S.; Giesemann, P.; Klink, S.; Leupolz, K.; Löder, M.G.J.; Löschel, L.A.; Missun, J.; et al. Spatial and temporal variation of macro-, meso- and microplastic abundance on a remote coral island of the maldives, indian ocean. Mar. Pollut. Bull. 2017, 116, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Tsang, Y.Y.; Mak, C.W.; Liebich, C.; Lam, S.W.; Sze, E.T.P.; Chan, K.M. Microplastic pollution in the marine waters and sediments of hong kong. Mar. Pollut. Bull. 2017, 115, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Claessens, M.; Meester, S.D.; Landuyt, L.V.; Clerck, K.D.; Janssen, C.R. Occurrence and distribution of microplastics in marine sediments along the belgian coast. Mar. Pollut. Bull. 2011, 62, 2199–2204. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic particles in sediments of lagoon of venice, italy: First observations on occurrence, spatial patterns and identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Qiu, Q.; Peng, J.; Yu, X.; Chen, F.; Wang, J.; Dong, F. Occurrence of microplastics in the coastal marine environment: First observation on sediment of china. Mar. Pollut. Bull. 2015, 98, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Dekiff, J.H.; Remy, D.; Klasmeier, J.; Fries, E. Occurrence and spatial distribution of microplastics in sediments from norderney. Environ. Pollut. 2014, 186, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Mathalon, A.; Hill, P. Microplastic fibers in the intertidal ecosystem surrounding halifax harbor, nova scotia. Mar. Pollut. Bull. 2014, 81, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Laglbauer, B.J.L.; Franco-Santos, R.M.; Andreu-Cazenave, M.; Brunelli, L.; Papadatou, M.; Palatinus, A.; Grego, M.; Deprez, T. Macrodebris and microplastics from beaches in slovenia. Mar. Pollut. Bull. 2014, 89, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.L.; Obbard, J.P. Prevalence of microplastics in singapore’s coastal marine environment. Mar. Pollut. Bull. 2006, 52, 761–767. [Google Scholar] [CrossRef] [PubMed]
- HKSAR News. Coastal Clean-Up Day Held at Sharp Island in Sai Kung (with Photos). Available online: http://www.info.gov.hk/gia/general/201509/19/P201509180537.htm (accessed on 12 October 2018).
- Pegram, J.E.; Andrady, A.L. Outdoor weathering of selected polymeric materials under marine exposure conditions. Polym. Degrad. Stab. 1989, 26, 333–345. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Li, D. Characterization of small plastic debris on tourism beaches around the south china sea. Reg. Stud. Mar. Sci. 2015, 1, 55–62. [Google Scholar] [CrossRef]
- Deng, L.; Chen, D.W.; Li, Y.; Luo, X.F.; Qiu, H.M. Organotin contamination in seawater and marine Animals along intertidal zone at dapeng bay and daya bay of Shenzhen, China. J. Trop. Oceanogr. 2010, 4, 112–117. (In Chinese) [Google Scholar]
- European Commission. Plastic Waste: Ecological and Human Health Impacts. Available online: http://ec.europa.eu/environment/integration/research/newsalert/pdf/IR1_en.pdf (accessed on 12 October 2018).
- Galgani, F.; Fleet, D.; Van Franeker, J.; Katsanevakis, S.; Maes, T.; Mouat, J.; Oosterbaan, L.; Poitou, I.; Hanke, G.; Thompson, R.; et al. Marine Strategy Framework Directive Task Group 10 Report: Marine Litter; European Commission Joint Research Centre Luxembourg: Luxembourg, 2010. [Google Scholar]
| Site | No. of Samples | Mean | Median | SD | CV% | MAD |
|---|---|---|---|---|---|---|
| 1 | 6 | 185.0 | 194.5 | 38.3 | 20.7 | 25.0 |
| 2 | 6 | 171.7 | 168.5 | 57.6 | 33.6 | 51.0 |
| 3 | 6 | 223.0 | 221.0 | 51.4 | 23.1 | 38.5 |
| 4 | 6 | 198.5 | 198.5 | 47.9 | 24.1 | 42.0 |
| All | 24 | 194.5 | 196.0 | 49.9 | 25.7 | 39.5 |
| Region | Site | Habitat | Particle Size (mm) | Concentration (items/kg) | Reference |
|---|---|---|---|---|---|
| Hong Kong, China | Double Haven, Tolo Harbour & Port Shelter | Coastal sea with fringing reef | 0.3–5 | Range: 95–298 Mean: 185 | This study |
| Hong Kong, China | Deep Bay, Tolo Harbour, Tsing Yi & Victoria Harbour | Coastal sea | 0.01–5 | Range 44–458 Mean: 158 | [40] |
| Belgium | Nieuwpoort, Oostende and Zeebrugge | Coastal sea | 0.038–1 | Maximum: 390 Mean: 166.7 ± 92.1 | [41] |
| Italy | Lagoon of Venice | Lagoon | <1 | Range: 672–2175 Mean: 1445 | [42] |
| Hong Kong, China | Local coastal shores | Beach | 0.25–5 | Range: 0.58–2116 Mean: 161 | [12] |
| China | Shapawan, Haikou, Wanning, Sanya and Beihai | Beach | <5 | Range: 5014–8714 Mean: 6923 | [43] |
| Germany | The Island of Norderney | Beach | <1 | Range: 1–4 Mean: 1.8 | [44] |
| Canada | Halifax Harbour | Beach | <5 | Range: 2000–8000 (fibre) | [45] |
| Yugoslavia | Along the Slovenian coast | Beach | 0.25–5 | Maximum: 444.4 Mean: 177.8 | [46] |
| Singapore | Local coastal shores | Beach | >0.0016 | Maximum: 10.7 Mean: 2.3 | [47] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheang, C.C.; Ma, Y.; Fok, L. Occurrence and Composition of Microplastics in the Seabed Sediments of the Coral Communities in Proximity of a Metropolitan Area. Int. J. Environ. Res. Public Health 2018, 15, 2270. https://doi.org/10.3390/ijerph15102270
Cheang CC, Ma Y, Fok L. Occurrence and Composition of Microplastics in the Seabed Sediments of the Coral Communities in Proximity of a Metropolitan Area. International Journal of Environmental Research and Public Health. 2018; 15(10):2270. https://doi.org/10.3390/ijerph15102270
Chicago/Turabian StyleCheang, Chi Chiu, Yue Ma, and Lincoln Fok. 2018. "Occurrence and Composition of Microplastics in the Seabed Sediments of the Coral Communities in Proximity of a Metropolitan Area" International Journal of Environmental Research and Public Health 15, no. 10: 2270. https://doi.org/10.3390/ijerph15102270
APA StyleCheang, C. C., Ma, Y., & Fok, L. (2018). Occurrence and Composition of Microplastics in the Seabed Sediments of the Coral Communities in Proximity of a Metropolitan Area. International Journal of Environmental Research and Public Health, 15(10), 2270. https://doi.org/10.3390/ijerph15102270






