Physiological Effects of Touching the Wood of Hinoki Cypress (Chamaecyparis obtusa) with the Soles of the Feet
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
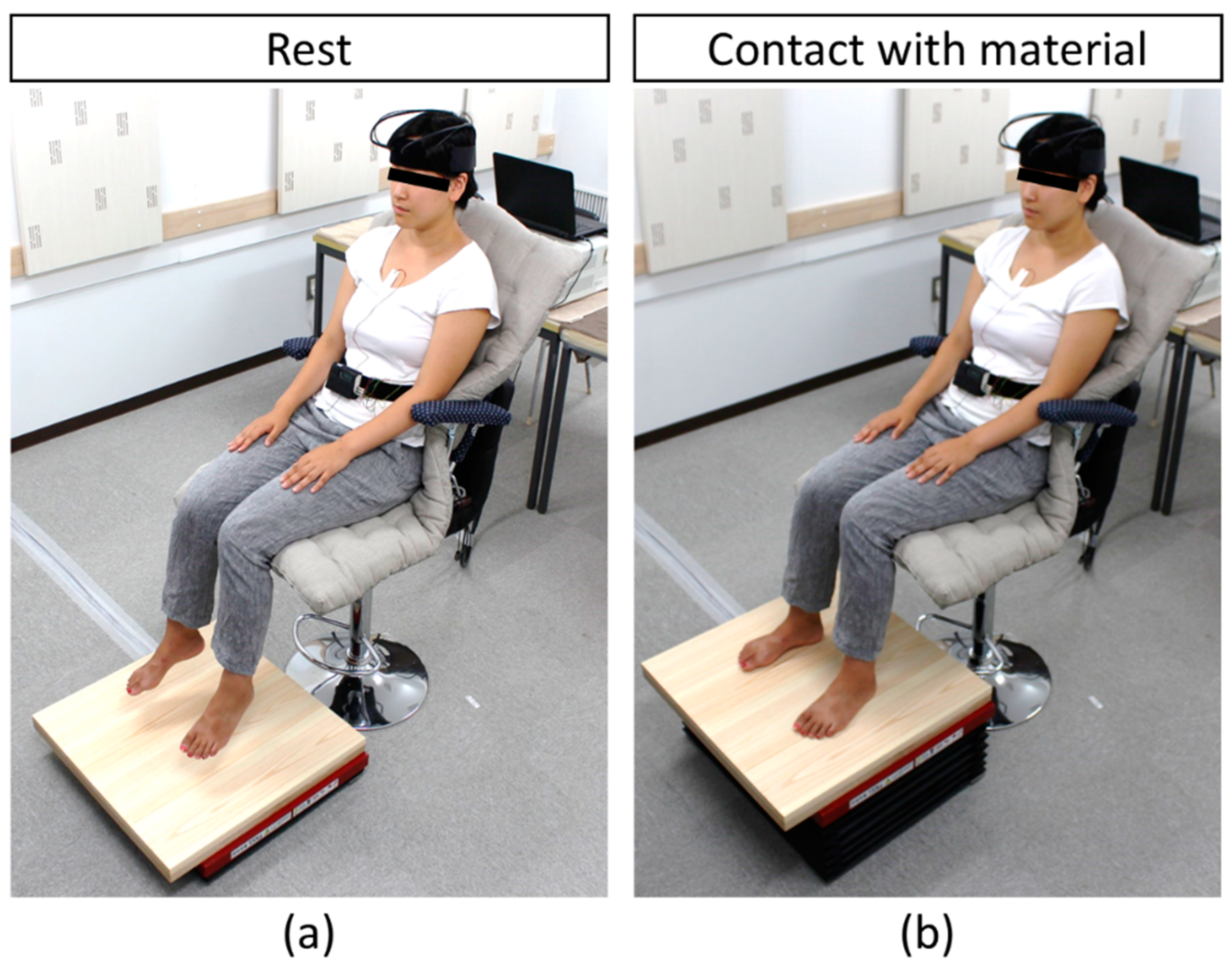
2.2. Study Protocol
2.3. Tactile Stimulation
2.4. Physiological Measurements
2.4.1. Near-Infrared Time-Resolved Spectroscopy (TRS)
2.4.2. Heart Rate Variability (HRV) and Heart Rate
2.5. Psychological Measurements
2.6. Statistical Analysis
3. Results
3.1. Physiological Effects
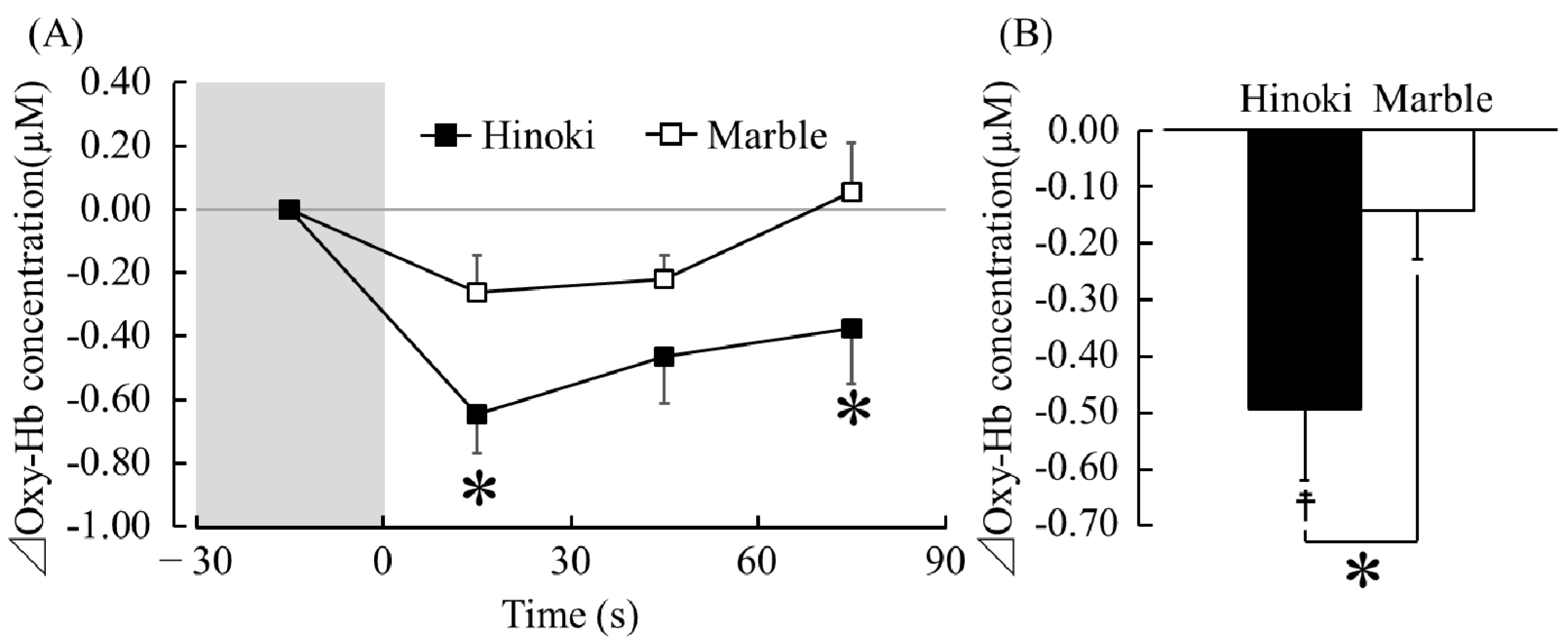
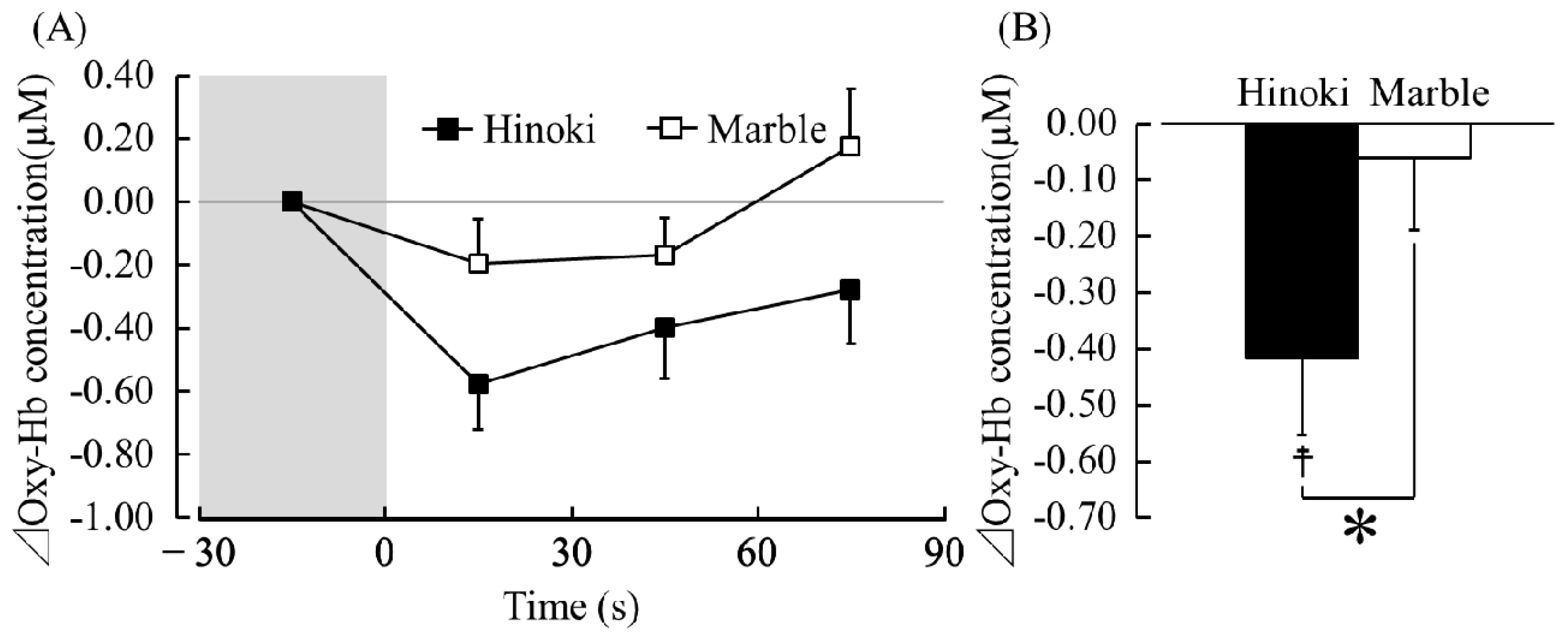
3.1.1. TRS
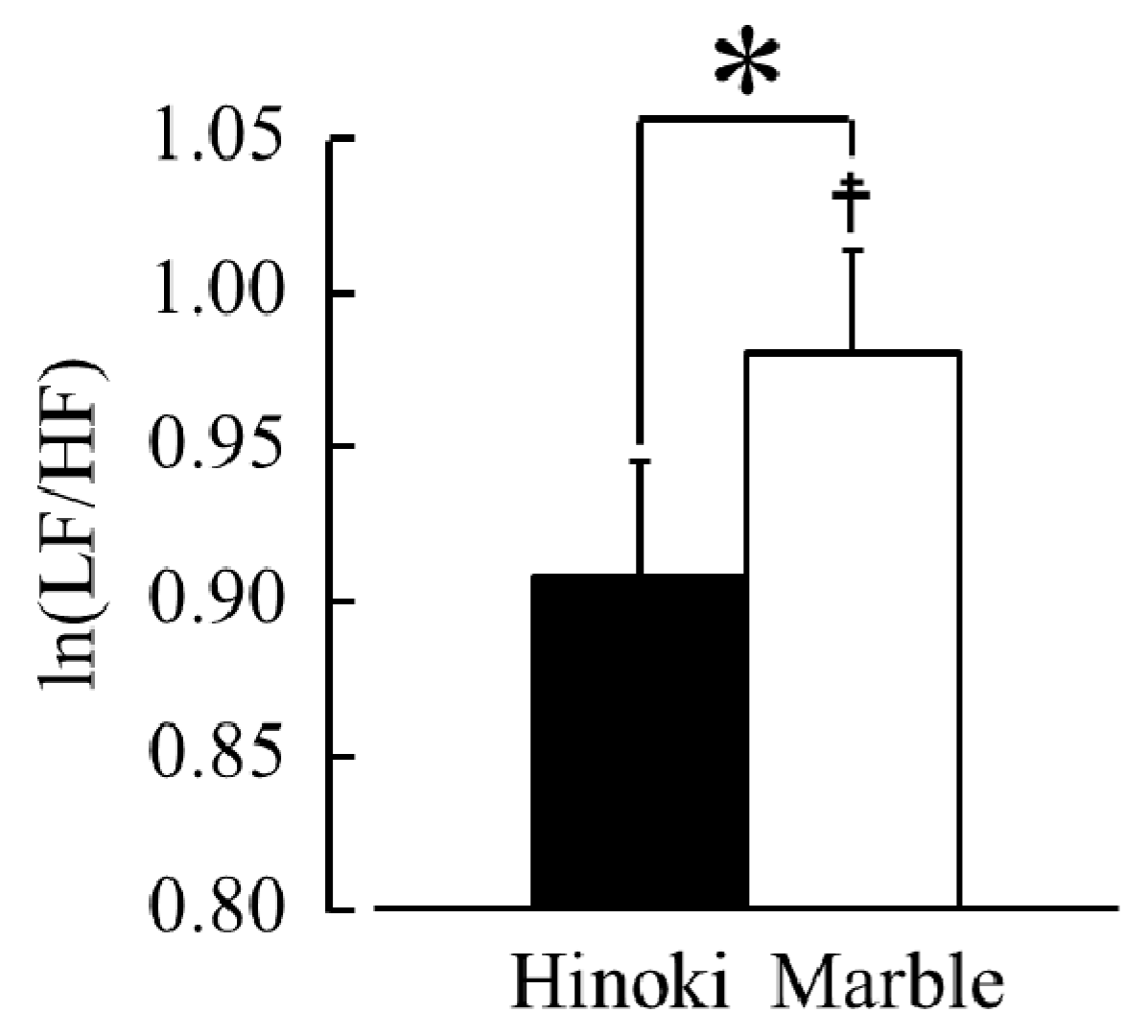
3.1.2. HRV and Heart Rate
3.2. Psychological Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations Population Fund. Trends in urbanization. In 2014 Revision of the World Urbanization Prospects; United Nations Population Fund: New York, NY, USA, 2014; pp. 7–12. ISBN 9789211231953. [Google Scholar]
- Lederbogen, F.; Kirsch, P.; Haddad, L.; Streit, F.; Tost, H.; Schuch, P.; Wüst, S.; Pruessner, J.C.; Rietschel, M.; Deuschle, M.; et al. City living and urban upbringing affect neural social stress processing in humans. Nature 2011, 474, 498. [Google Scholar] [CrossRef] [PubMed]
- Brod, C. Technostress: The Human Cost of the COMPUTER Revolution; Addison Wesley Press: Boston, MA, USA, 1984; p. 242. ISBN 9780201112115. [Google Scholar]
- Salanova, M.; Llorens, S.; Cifre, E. The dark side of technologies: Technostress among users of information and communication technologies. Int. J. Psychol. 2013, 48, 422–436. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Schmillen, R.; Sullivan, W.C. How to Waste a Break: Using Portable Electronic Devices Substantially Counteracts Attention Enhancement Effects of Green Spaces. Environ. Behav. 2018. [Google Scholar] [CrossRef]
- Song, C.; Ikei, H.; Miyazaki, Y. Physiological effects of nature therapy: A review of the research in Japan. Int. J. Environ. Res. Public Health 2016, 13, 781. [Google Scholar] [CrossRef] [PubMed]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effects of wood on humans: A review. J. Wood Sci. 2017, 63, 1–23. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effects of touching hinoki cypress (Chamaecyparis obtusa). J. Wood Sci. 2018, 64, 226–236. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effects of touching wood. Int. J. Environ. Res. Public Health 2017, 14, 801. [Google Scholar] [CrossRef] [PubMed]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effects of touching coated wood. Int. J. Environ. Res. Public Health 2017, 14, 773. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Miyazaki, Y.; Kobayashi, S. Time-series variations of blood pressure due to contact with wood. J. Wood Sci. 1998, 44, 495–497. [Google Scholar] [CrossRef]
- Sakuragawa, S.; Kaneko, T.; Miyazaki, Y. Effects of contact with wood on blood pressure and subjective evaluation. J. Wood Sci. 2008, 54, 107–113. [Google Scholar] [CrossRef]
- Berger, G.; Katz, H.; Petutschnigg, A.J. What consumers feel and prefer: Haptic perception of various wood flooring surface. For. Prod. J. 2006, 56, 42–47. [Google Scholar]
- ASTM C518–10. Standard Test Method for Steady-State Thermal Transmission Properties by Means of the Heat Flow Meter Apparatus; ASTM: West Conshohocken, PA, USA, 2003. [Google Scholar]
- ISO 8301. Thermal Insulation Determination of Steady-State Thermal Resistance and Related Properties Heat Flow Meter Apparatus; ISO: Geneva, Switzerland, 1991. [Google Scholar]
- Ohmae, E.; Ouchi, Y.; Oda, M.; Suzuki, T.; Nobesawa, S.; Kanno, T.; Yoshikawa, E.; Futatsubashi, M.; Ueda, Y.; Okada, H.; et al. Cerebral hemodynamics evaluation by near-infrared time-resolved spectroscopy: Correlation with simultaneous positron emission tomography measurements. Neuroimage 2006, 29, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Ohmae, E.; Oda, M.; Suzuki, T.; Yamashita, Y.; Kakihana, Y.; Matsunaga, A.; Kanmura, Y.; Tamura, M. Clinical evaluation of time-resolved spectroscopy by measuring cerebral hemodynamics during cardiopulmonary bypass surgery. J. Biomed. Opt. 2007, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, A.; Contini, D.; Pilled, A.; Caffini, M.; Re, R.; Zucchelli, L.; Spinelli, L. Time domain functional nirs imaging for human brain mapping. Neuroimage 2014, 85, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Seiyama, A.; Seki, J.; Tanabe, H.C.; Sase, I.; Takatsuki, A.; Miyauchi, S.; Eda, H.; Hayashi, S.; Imaruoka, T.; Iwakura, T.; et al. Circulatory basis of fMRI signals: Relationship between changes in the hemodynamic parameters and BOLD signal intensity. Neuroimage 2004, 21, 1204–1214. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Quaresima, V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012, 63, 921–935. [Google Scholar] [CrossRef] [PubMed]
- Boas, D.A.; Elwell, C.E.; Ferrari, M.; Taga, G. Twenty years of functional near-infrared spectroscopy: Introduction for the special issue. Neuroimage 2014, 1, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, Y.; Huang, J.; Kohri, S.; Iguchi, Y.; Naya, M.; Okamoto, T.; Ono, S. Recognition of human emotions from cerebral blood flow changes in the frontal region: A study with event-related near-infrared spectroscopy. J. Neuroimaging 2011, 21, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar]
- Kobayashi, H.; Ishibashi, K.; Noguchi, H. Heart rate variability; an index for monitoring and analyzing human autonomic activities. Appl. Hum. Sci. 1999, 18, 53–59. [Google Scholar] [CrossRef]
- Kanaya, N.; Hirata, N.; Kurosawa, S.; Nakayama, M.; Namiki, A. Differential effects of propofol and sevoflurane on heart rate variability. Anesthesiology 2003, 98, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Ohtomo, N.; Tanaka, Y.; Tanaka, G.; Yamakoshi, K.; Terachi, S.; Shimamoto, K.; Nakagawa, M.; Satoh, S.; Kuroda, S.; et al. New technique for time series analysis combining the maximum entropy method and non-linear least squares method: Its value in heart rate variability analysis. Med. Biol. Eng. Comput. 1997, 35, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Pagani, M.; Lombardi, F.; Guzzetti, S.; Rimoldi, O.; Furlan, R.; Pizzinelli, P.; Sandrone, G.; Malfatto, G.; Dell’Orto, S.; Piccaluga, E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympath o-vagal interaction in man and conscious dog. Circ. Res. 1986, 59, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Park, B.J.; Miyazaki, Y. Normative references of heart rate variability and salivary alpha-amylase in a healthy young male population. J. Physiol. Anthropol. 2012, 31, 9. [Google Scholar] [CrossRef] [PubMed]
- Osgood, C.E.; Suci, G.J.; Tannenbaum, P. The Measurement of Meaning; University of Illinois Press: Urbana, IL, USA, 1957. [Google Scholar]
- Victor, A.; Elsässer, A.; Hommel, G.; Blettner, M. Judging a plethora of p-values: How to contend with the problem of multiple testing. Part 10 of a series on evaluation of scientific publications. Dtsch. Arztebl. Int. 2010, 107, 50–56. [Google Scholar] [PubMed]
- Horiuchi, M.; Endo, J.; Takayama, N.; Murase, K.; Nishiyama, N.; Saito, H.; Fujiwara, A. Impact of Viewing vs. Not Viewing a Real Forest on Physiological and Psychological Responses in the Same Setting. Int. J. Environ. Res. Public Health 2014, 11, 10883–10901. [Google Scholar] [CrossRef] [PubMed]
- George, M.S.; Ketter, T.A.; Parekh, P.I.; Horwitz, B.; Herscovitch, P.; Post, R.M. Brain activity during transient sadness and happiness in healthy women. Am. J. Psychiatry 1995, 152, 341–351. [Google Scholar] [PubMed]
- Ikei, H.; Song, C.; Lee, J.; Miyazaki, Y. Comparison of the effects of olfactory stimulation by air-dried and high-temperature-dried wood chips of hinoki cypress (Chamaecyparis obtusa) on prefrontal cortex activity. J. Wood Sci. 2015, 61, 537–540. [Google Scholar] [CrossRef]
- Ikei, H.; Song, C.; Miyazaki, Y. Physiological effect of olfactory stimulation by hinoki cypress (Chamaecyparis obtusa) leaf oil. J. Physiol. Anthropol. 2015, 34, 44. [Google Scholar] [CrossRef] [PubMed]
- Ikei, H.; Song, C.; Miyazaki, Y. Effects of olfactory stimulation by α-pinene on autonomic nervous activity. J. Wood Sci. 2016, 62, 568–572. [Google Scholar] [CrossRef]
- Joung, D.; Song, C.; Ikei, H.; Okuda, T.; Igarashi, M.; Koizumi, H.; Park, B.J.; Yamaguchi, T.; Takagaki, M.; Miyazaki, Y. Physiological and psychological effects of olfactory stimulation with d-Limonene. Adv. Hortic. Sci. 2014, 28, 90–94. [Google Scholar]
- Brunet, M.; Guy, F.; Pilbeam, D.; Mackaye, H.T.; Likius, A.; Ahounta, D.; Beauvilain, A.; Blondel, C.; Bocherens, H.; Boisserie, J.R.; et al. A new hominid from the Upper Miocene of Chad, Central Africa. Nature 2002, 418, 141–151. [Google Scholar]
- Hansen, M.M.; Jones, R.; Tocchini, K. Shinrin-Yoku (Forest Bathing) and Nature Therapy: A State-of-the-Art Review. Int. J. Environ. Res. Public Health 2017, 14, 851. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y. Shinrin Yoku: The Art of Japanese Forest Bathing; Octopus Publishing Group: London, UK, 2018; p. 192. [Google Scholar]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Hirano, H.; Kagawa, T.; Sadoh, T.; Miyazaki, Y. Physiological effects of shinrin-yoku (taking in the atmosphere of the forest)—Using salivary cortisol and cerebral activity as indicators. J. Physiol. Anthropol. 2007, 26, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Kagawa, T.; Miyazaki, Y. The restorative effects of viewing real forest landscapes: Based on a comparison with urban landscapes. Scand. J. For. Res. 2009, 24, 227–234. [Google Scholar] [CrossRef]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The physiological effects of shinrin-yoku (taking in the forest atmosphere or forest bathing): Evidence from field experiments in 24 forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu, Y.; Park, B.J.; Miyazaki, Y. Trends in research related to “shinrin-yoku” (taking in the forest atmosphere or forest bathing) in Japan. Environ. Health Prev. Med. 2010, 15, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, B.J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of forest bathing on physiological and psychological responses in young Japanese male subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Tsunetsugu, Y.; Lee, J.; Park, B.-J.; Tyrvainen, L.; Kagawa, T.; Miyazaki, Y. Physiological and psychological effects of viewing urban forest landscapes assessed by multiple measurements. Landsc. Urban Plan. 2013, 113, 90–93. [Google Scholar] [CrossRef]
- Lee, J.; Tsunetsugu, Y.; Takayama, N.; Park, B.J.; Li, Q.; Song, C.; Komatsu, M.; Ikei, H.; Tyrvainen, L.; Kagawa, T.; et al. Influence of forest therapy on cardiovascular relaxation in young adults. Evid. Based Complement. Alternat. Med. 2014, 2014, 834360. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Joung, D.; Ikei, H.; Igarashi, M.; Aga, M.; Park, B.J.; Miwa, M.; Takagaki, M.; Miyazaki, Y. Physiological and psychological effects of walking on young males in urban parks in winter. J. Physiol. Anthropol. 2013, 32, 18. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Ikei, H.; Igarashi, M.; Miwa, M.; Takagaki, M.; Miyazaki, Y. Physiological and psychological responses of young males during spring-time walks in urban parks. J. Physiol. Anthropol. 2014, 33, 8. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Ikei, H.; Igarashi, M.; Takagaki, M.; Miyazaki, Y. Physiological and psychological effects of a walk in urban parks in fall. Int. J. Environ. Res. Public Health 2015, 12, 14216–14228. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Igarashi, M.; Ikei, H.; Miyazaki, Y. Physiological effects of viewing fresh red roses. Complement. Ther. Med. 2017, 35, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Ikei, H.; Komatsu, M.; Song, C.; Himoro, E.; Miyazaki, Y. The physiological and psychological relaxing effects of viewing rose flowers in office workers. J. Physiol. Anthropol. 2014, 33, 6. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Yamamoto, T.; Lee, J.; Song, C.; Ikei, H.; Miyazaki, Y. Effects of stimulation by three-dimensional natural images on prefrontal cortex and autonomic nerve activity: A comparison with stimulation using two-dimensional images. Cogn. Process. 2014, 15, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Ikei, H.; Song, C.; Miyazaki, Y. Effects of olfactory stimulation with rose and orange oil on prefrontal cortex activity. Complement. Ther. Med. 2014, 22, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Song, C.; Ikei, H.; Ohira, T.; Miyazaki, Y. Effect of olfactory stimulation by fresh rose flowers on autonomic nervous activity. J. Altern. Complement. Med. 2014, 20, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Aga, M.; Ikei, H.; Namekawa, T.; Miyazaki, T. Physiological and psychological effects on high school students of viewing real and artificial pansies. Int. J. Environ. Res. Public Health 2015, 12, 2521–2531. [Google Scholar] [CrossRef] [PubMed]
- Ikei, H.; Song, C.; Igarashi, M.; Namekawa, T.; Miyazaki, Y. Physiological and psychological relaxing effects of visual stimulation with foliage plants in high school students. Adv. Hortic. Sci. 2014, 28, 111–116. [Google Scholar]
- Park, S.A.; Song, C.; Choi, J.Y.; Son, K.C.; Miyazaki, Y. Foliage plants cause physiological and psychological relaxation-as evidenced by measurements of prefrontal cortex activity and profile of mood states. HortScience 2016, 51, 1308–1312. [Google Scholar] [CrossRef]

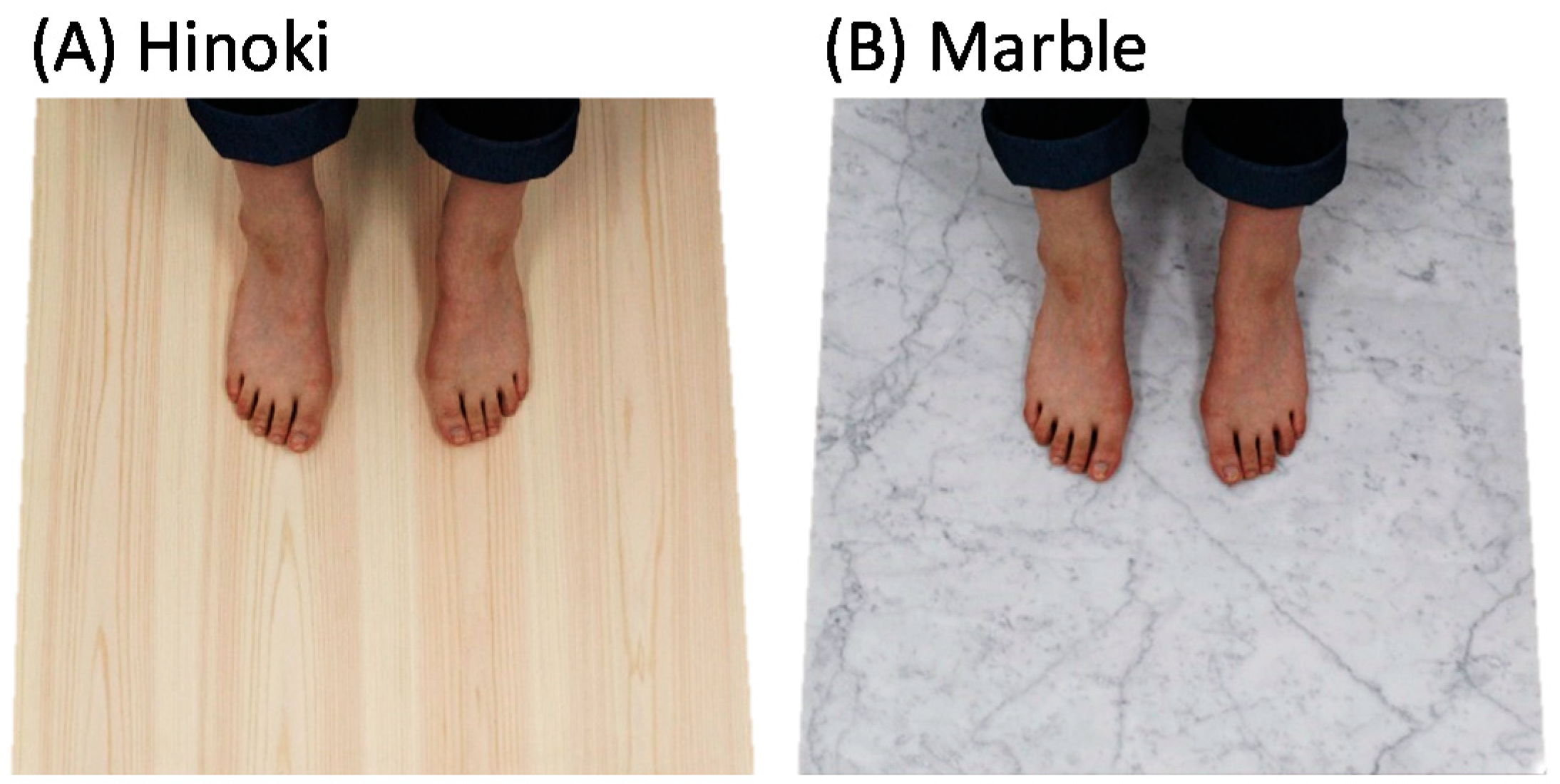


| Material 1 | h (mm) | λ [W/(m K)] 2 | ρ (g/cm3) 3 | Ra (µm) 4 | Condition |
|---|---|---|---|---|---|
| Hinoki | 20 (+JCP 25) | 0.121 | 0.52 | 35.03 | Brushing |
| Marble | 20 (+JCP 25) | 1.947 | 2.49 | 0.16 | Buffing |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikei, H.; Song, C.; Miyazaki, Y. Physiological Effects of Touching the Wood of Hinoki Cypress (Chamaecyparis obtusa) with the Soles of the Feet. Int. J. Environ. Res. Public Health 2018, 15, 2135. https://doi.org/10.3390/ijerph15102135
Ikei H, Song C, Miyazaki Y. Physiological Effects of Touching the Wood of Hinoki Cypress (Chamaecyparis obtusa) with the Soles of the Feet. International Journal of Environmental Research and Public Health. 2018; 15(10):2135. https://doi.org/10.3390/ijerph15102135
Chicago/Turabian StyleIkei, Harumi, Chorong Song, and Yoshifumi Miyazaki. 2018. "Physiological Effects of Touching the Wood of Hinoki Cypress (Chamaecyparis obtusa) with the Soles of the Feet" International Journal of Environmental Research and Public Health 15, no. 10: 2135. https://doi.org/10.3390/ijerph15102135
APA StyleIkei, H., Song, C., & Miyazaki, Y. (2018). Physiological Effects of Touching the Wood of Hinoki Cypress (Chamaecyparis obtusa) with the Soles of the Feet. International Journal of Environmental Research and Public Health, 15(10), 2135. https://doi.org/10.3390/ijerph15102135







