High Proportions of Multidrug-Resistant Acinetobacter spp. Isolates in a District in Western India: A Four-Year Antibiotic Susceptibility Study of Clinical Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Study Material
2.3. Laboratory Methods
2.4. Statistical Analyses
3. Results
3.1. Acinetobacter spp. Isolates
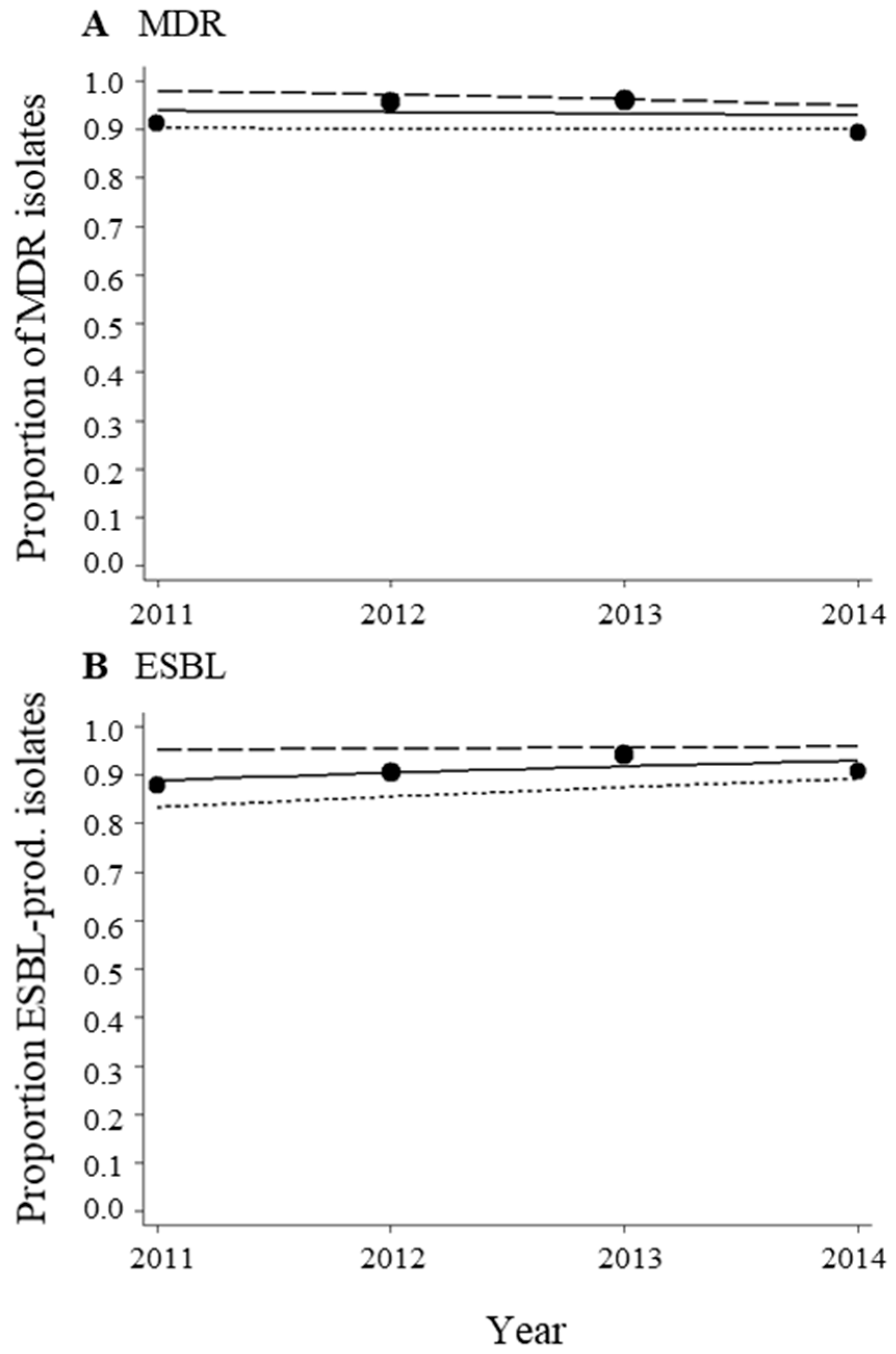
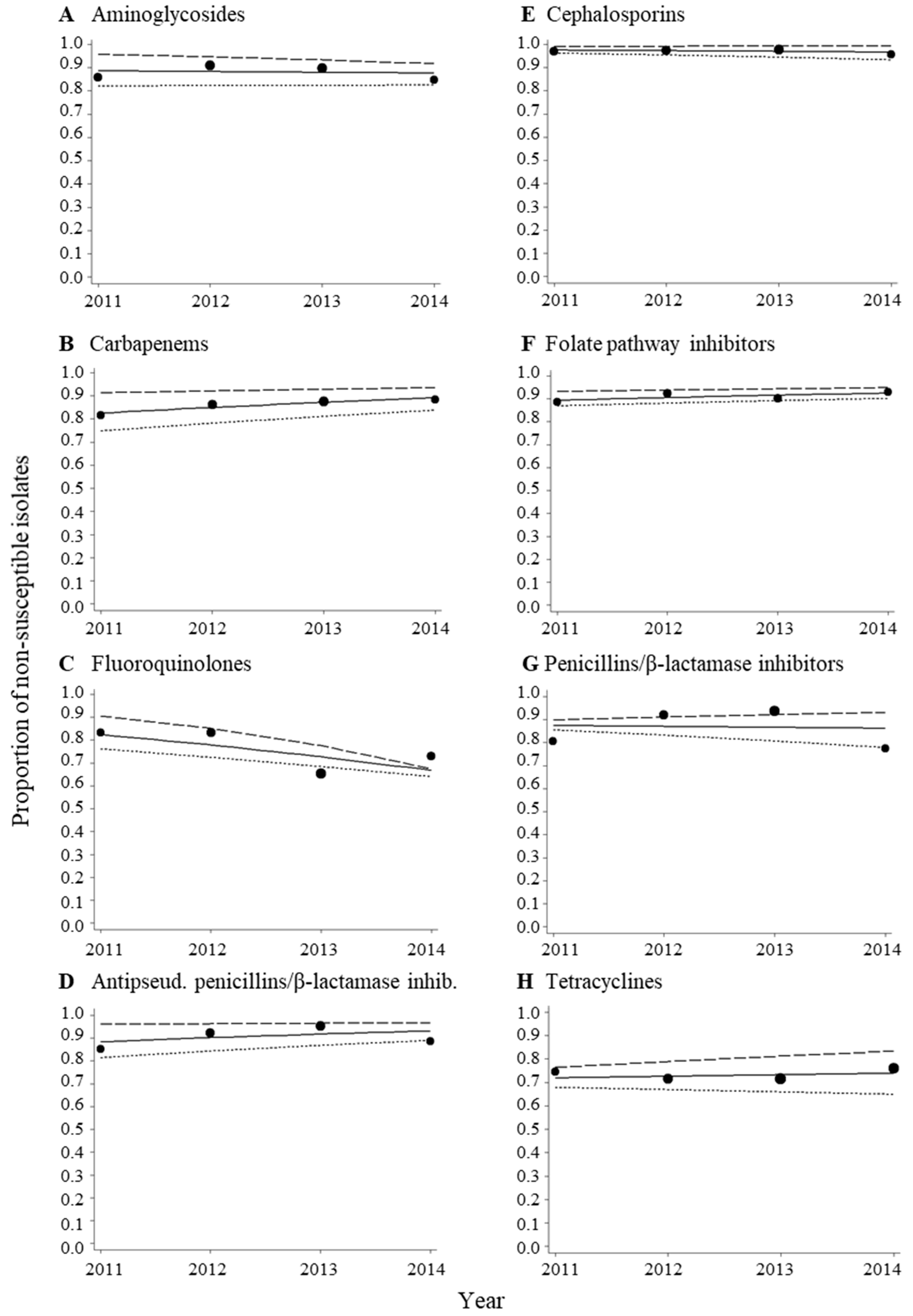
3.2. High Proportions of MDR Acinetobacter spp. Isolates during the Period from 2011–2014
3.3. No Change in Trends of MDR Acinetobacter spp. Isolates during the Period from 2011–2014
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Conflicts of Interest
Appendix A



| Multidrug Resistance/Antibacterial Category | 2011 | 2012 | 2013 | 2014 | ||||
|---|---|---|---|---|---|---|---|---|
| Multidrug resistance | % | n 1 | % | n 1 | % | n 1 | % | n 1 |
| MDR | 91.1 | 169 | 95.5 | 201 | 95.9 | 220 | 89.4 | 151 |
| ESBL | 87.9 | 157 | 90.5 | 190 | 94.0 | 201 | 90.9 | 142 |
| Antibacterial category | ||||||||
| Aminoglycosides | 85.8 | 169 | 91.0 | 199 | 89.6 | 211 | 84.7 | 137 |
| Carbapenems | 81.6 | 168 | 86.1 | 201 | 87.6 | 218 | 88.3 | 145 |
| Fluoroquinolones | 83.3 | 96 | 83.3 | 126 | 65.3 | 216 | 72.9 | 140 |
| Antipseudomonal penicillins/β-lactamase inhibitors | 85.1 | 161 | 92.3 | 195 | 95.3 | 212 | 88.7 | 142 |
| Cephalosporins | 97.0 | 168 | 97.4 | 194 | 97.7 | 219 | 95.7 | 141 |
| Folate pathway inhibitors | 88.7 | 106 | 92.3 | 117 | 90.2 | 132 | 93.1 | 101 |
| Penicillins/β-lactamase inhibitors | 80.7 | 155 | 91.9 | 173 | 93.7 | 205 | 77.5 | 142 |
| Tetracyclines | 74.6 | 63 | 71.4 | 98 | 71.3 | 136 | 75.9 | 108 |
| Type of Patient/Specimen Type | 2011 | 2012 | 2013 | 2014 | ||||
|---|---|---|---|---|---|---|---|---|
| % | n 1 | % | n 1 | % | n 1 | % | n 1 | |
| Inpatients | 88.5 | 96 | 81.0 | 126 | 86.1 | 216 | 90.7 | 140 |
| Respiratory samples | 39.0 | 95 2 | 39.7 | 126 | 61.1 | 216 | 47.1 | 140 |
References
- Clark, N.M.; Zhanel, G.G.; Lynch, J.P. Emergence of antimicrobial resistance among Acinetobacter species: A global threat. Curr. Opin. Crit. Care 2016, 22, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Murray, G.L.; Peleg, A.Y. Acinetobacter baumannii: Evolution of antimicrobial resistance-treatment options. Semin. Respir. Crit. Care Med. 2015, 36, 85–98. [Google Scholar] [PubMed]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, G.J.; Domingues, S. Insights on the Horizontal Gene Transfer of Carbapenemase Determinants in the Opportunistic Pathogen Acinetobacter baumannii. Microorganisms 2016, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Amyes, S. OXA (beta)-lactamases in Acinetobacter: The story so far. J. Antimicrob. Chemother. 2006, 57, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Giske, C.G.; Monnet, D.L.; Cars, O.; Carmeli, Y. Clinical and economic impact of common multidrug-resistant gram-negative bacilli. Antimicrob. Agents Chemother. 2008, 52, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Poulikakos, P.; Tansarli, G.S.; Falagas, M.E. Combination antibiotic treatment versus monotherapy for multidrug-resistant, extensively drug-resistant, and pandrug-resistant Acinetobacter infections: A systematic review. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Chai, D.; Wang, R.; Liang, B.; Bai, N. Colistin resistance of Acinetobacter baumannii: Clinical reports, mechanisms and antimicrobial strategies. J. Antimicrob. Chemother. 2012, 67, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Gales, A.C.; Jones, R.N.; Sader, H.S. Global assessment of the antimicrobial activity of polymyxin B against 54 731 clinical isolates of Gram-negative bacilli: Report from the SENTRY antimicrobial surveillance programme (2001–2004). Clin. Microbiol. Infect. 2006, 12, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Bliziotis, I.A.; Siempos, I.I. Attributable mortality of Acinetobacter baumannii infections in critically ill patients: A systematic review of matched cohort and case-control studies. Crit. Care 2006, 10, R48. [Google Scholar] [CrossRef] [PubMed]
- Playford, E.G.; Craig, J.C.; Iredell, J.R. Carbapenem-resistant Acinetobacter baumannii in intensive care unit patients: Risk factors for acquisition, infection and their consequences. J. Hosp. Infect. 2007, 65, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.J.; Knipe, C.J.; Zieger, M.J.; Gabehart, K.M.; Goodman, J.E.; Volk, H.M.; Sood, R. Direct costs of multidrug-resistant Acinetobacter baumannii in the burn unit of a public teaching hospital. Am. J. Infect. Control 2004, 32, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- Bac-Test Laboratory Web Page. Available online: http://www.indiamart.com/bac-test-laboratory/ (accessed on 27 March 2017).
- Indian Census 2011. Available online: http://www.census2011.co.in/census/district/354-nashik.html (accessed on 27 March 2017).
- WHO. WHONET Software. Available online: http://www.whonet.org/software.html (accessed on 27 March 2017).
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; CLSI Documents M100-S21-M100-S24; CLSI: Wayne, PA, USA, 2014. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Tenth Edition: Approved Standard M07-A6; NCCLS: Wayne, PA, USA, 2015. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Alagesan, M.; Gopalakrishnan, R.; Panchatcharam, S.N.; Dorairajan, S.; Mandayam Ananth, T.; Venkatasubramanian, R. A decade of change in susceptibility patterns of Gram-negative blood culture isolates: A single center study. Germs 2015, 5, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.; Mojica, N.; Klein, E.Y.; Ashok, A.; Nerurkar, V.; Kumari, M.; Ramesh, U.; Dey, S.; Vadwai, V.; Das, B.R.; et al. Trends in antibiotic resistance among major bacterial pathogens isolated from blood cultures tested at a large private laboratory network in India, 2008–2014. Int. J. Infect. Dis. 2016, 50, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Jean, S.S.; Coombs, G.; Ling, T.; Balaji, V.; Rodrigues, C.; Mikamo, H.; Kim, M.J.; Rajasekaram, D.G.; Mendoza, M.; Tan, T.Y.; et al. Epidemiology and antimicrobial susceptibility profiles of pathogens causing urinary tract infections in the Asia-Pacific region: Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART), 2010–2013. Int. J. Antimicrob. Agents 2016, 47, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Lob, S.H.; Hoban, D.J.; Sahm, D.F.; Badal, R.E. Regional differences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int. J. Antimicrob. Agents 2016, 47, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, G.; Sheemar, S.; Chopra, S.; Kaur, J.; Chowdhary, D.; Makhija, S.K. Carbapenem resistance and phenotypic detection of carbapenemases in clinical isolates of Acinetobacter baumannii. Indian J. Med. Sci. 2011, 65, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Abbo, A.; Navon-Venezia, S.; Hammer-Muntz, O.; Krichali, T.; Siegman-Igra, Y.; Carmeli, Y. Multidrug-resistant Acinetobacter baumannii. Emerg. Infect. Dis. 2005, 11, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Kakati, B.; Khanduri, S.; Gupta, S. Emergence of Carbapenem Resistant Non-Fermenting Gram-Negative Bacilli Isolated in an ICU of a Tertiary Care Hospital. J. Clin. Diagn. Res. 2017, 11, DC04–DC07. [Google Scholar] [CrossRef] [PubMed]
- Khajuria, A.; Praharaj, A.K.; Kumar, M.; Grover, N. Molecular Characterization of Carbapenem Resistant Isolates of Acinetobacter baumannii in an Intensive Care Unit of a Tertiary Care Centre at Central India. J. Clin. Diagn. Res. 2014, 8, DC38–DC40. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjani, V.; Umadevi, S.; Srirangaraj, S.; Kali, A.; Seetha, K. Multi-drug resistant Acinetobacter species from various clinical samples in a tertiary care hospital from South India. Australas. Med. J. 2013, 6, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Rashid, A.; Aslam, B.; Waseem, M.; Shahid, M.; Akram, M.; Khurshid, M.; Rasool, M.H. Antimicrobial susceptibility of Acinetobacter clinical isolates and emerging antibiogram trends for nosocomial infection management. Rev. Soc. Bras. Med. Trop. 2016, 49, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Uwingabiye, J.; Frikh, M.; Lemnouer, A.; Bssaibis, F.; Belefquih, B.; Maleb, A.; Dahraoui, S.; Belyamani, L.; Bait, A.; Haimeur, C.; et al. Acinetobacter infections prevalence and frequency of the antibiotics resistance: Comparative study of intensive care units versus other hospital units. Pan Afr. Med. J. 2016, 23, 191. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Kollef, M.H.; Shorr, A.F. Secular trends in Acinetobacter baumannii resistance in respiratory and blood stream specimens in the United States, 2003 to 2012: A survey study. J. Hosp. Med. 2016, 11, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.R.; Song, J.H.; Kim, S.H.; Thamlikitkul, V.; Huang, S.G.; Wang, H.; So, T.M.; Yasin, R.M.; Hsueh, P.R.; Carlos, C.C.; et al. High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am. J. Respir. Crit. Care Med. 2011, 184, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Farrell, D.J.; Flamm, R.K.; Jones, R.N. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009–2011). Diagn. Microbiol. Infect. Dis. 2014, 78, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Wattal, C.; Oberoi, J.K.; Raveendran, R.; Datta, S.; Prasad, K.J. Trend analysis of antimicrobial consumption and development of resistance in non-fermenters in a tertiary care hospital in Delhi, India. J. Antimicrob. Chemother. 2011, 66, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Price, L.S.; Rosa, R.; Castro, J.G.; Laowansiri, P.; Latibeaudiere, R.; Namias, N.; Tarima, S. Evaluating the Impact of Antibiotic Exposures as Time-Dependent Variables on the Acquisition of Carbapenem-Resistant Acinetobacter baumannii. Crit. Care Med. 2016, 44, e949–e956. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.S.; Chu, C.M.; Tsang, K.Y.; Lo, F.H.; Lo, K.F.; Ho, P.L. Fulminant community-acquired Acinetobacter baumannii pneumonia as a distinct clinical syndrome. Chest 2006, 129, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.J.; Song, S.H.; Baik, S.H.; Lee, H.H.; Han, I.M.; Oh, D.H. A case of fulminant community-acquired Acinetobacter baumannii pneumonia in Korea. Korean J. Intern. Med. 2013, 28, 486–490. [Google Scholar] [CrossRef] [PubMed]
| Multidrug Resistance/Antibacterial Category | Inpatients | Outpatients | p 1 | ||
|---|---|---|---|---|---|
| Multidrug resistance | % | n 2 | % | n 2 | |
| MDR | 93.6 | 656 | 91.8 | 85 | 0.522 |
| ESBL | 91.3 | 611 | 88.6 | 79 | 0.427 |
| Antibacterial category | |||||
| Aminoglycosides | 89.3 | 634 | 79.3 | 82 | 0.008 |
| Carbapenems | 86.3 | 649 | 83.1 | 83 | 0.437 |
| Fluoroquinolones | 74.6 | 500 | 70.5 | 78 | 0.444 |
| Antipseudomonal penicillins/β-lactamase inhibitors | 91.6 | 631 | 84.8 | 79 | 0.048 |
| Cephalosporins | 97.2 | 638 | 96.4 | 84 | 0.701 |
| Folate pathway inhibitors | 90.4 | 385 | 94.4 | 71 | 0.282 |
| Penicillins/β-lactamase inhibitors | 87.4 | 596 | 82.3 | 79 | 0.205 |
| Tetracyclines | 72.7 | 341 | 75.0 | 64 | 0.707 |
| Multidrug Resistance/Antibacterial Category | Respiratory 1 | Other 2 | p 3 | ||
|---|---|---|---|---|---|
| Multidrug resistance | % | n 4 | % | n 4 | |
| MDR | 96.5 | 375 | 90.1 | 364 | <0.001 |
| ESBL | 95.5 | 355 | 86.2 | 333 | <0.001 |
| Antibacterial category | |||||
| Aminoglycosides | 93.9 | 359 | 82.3 | 355 | <0.001 |
| Carbapenems | 92.4 | 370 | 79.2 | 360 | <0.001 |
| Fluoroquinolones | 78.3 | 285 | 69.9 | 292 | 0.022 |
| Antipseudomonal penicillins/β-lactamase inhibitors | 96.4 | 357 | 85.2 | 351 | <0.001 |
| Cephalosporins | 99.2 | 366 | 94.9 | 354 | 0.001 |
| Folate pathway inhibitors | 94.1 | 203 | 88.5 | 252 | 0.038 |
| Penicillins/β-lactamase inhibitors | 91.6 | 346 | 82.0 | 327 | <0.001 |
| Tetracyclines | 80.6 | 191 | 66.2 | 213 | 0.001 |
| Multidrug Resistance/Antibacterial Category | OR | 95% CI |
|---|---|---|
| Multidrug resistance | ||
| MDR | 0.95 | 0.72–1.25 |
| ESBL | 1.18 | 0.92–1.52 |
| Antibacterial category | ||
| Aminoglycosides | 0.97 | 0.78–1.20 |
| Carbapenems | 1.20 | 0.98–1.47 |
| Fluoroquinolones | 0.75 | 0.62–0.91 |
| Antipseudomonal penicillins/β-lactamase inhibitors | 1.22 | 0.95–1.56 |
| Cephalosporins | 0.90 | 0.60–1.37 |
| Folate pathway inhibitors | 1.14 | 0.84–1.53 |
| Penicillins/β-lactamase inhibitors | 0.97 | 0.78–1.20 |
| Tetracyclines | 1.03 | 0.83–1.28 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odsbu, I.; Khedkar, S.; Khedkar, U.; Nerkar, S.S.; Tamhankar, A.J.; Stålsby Lundborg, C. High Proportions of Multidrug-Resistant Acinetobacter spp. Isolates in a District in Western India: A Four-Year Antibiotic Susceptibility Study of Clinical Isolates. Int. J. Environ. Res. Public Health 2018, 15, 153. https://doi.org/10.3390/ijerph15010153
Odsbu I, Khedkar S, Khedkar U, Nerkar SS, Tamhankar AJ, Stålsby Lundborg C. High Proportions of Multidrug-Resistant Acinetobacter spp. Isolates in a District in Western India: A Four-Year Antibiotic Susceptibility Study of Clinical Isolates. International Journal of Environmental Research and Public Health. 2018; 15(1):153. https://doi.org/10.3390/ijerph15010153
Chicago/Turabian StyleOdsbu, Ingvild, Smita Khedkar, Uday Khedkar, Sandeep S. Nerkar, Ashok J. Tamhankar, and Cecilia Stålsby Lundborg. 2018. "High Proportions of Multidrug-Resistant Acinetobacter spp. Isolates in a District in Western India: A Four-Year Antibiotic Susceptibility Study of Clinical Isolates" International Journal of Environmental Research and Public Health 15, no. 1: 153. https://doi.org/10.3390/ijerph15010153
APA StyleOdsbu, I., Khedkar, S., Khedkar, U., Nerkar, S. S., Tamhankar, A. J., & Stålsby Lundborg, C. (2018). High Proportions of Multidrug-Resistant Acinetobacter spp. Isolates in a District in Western India: A Four-Year Antibiotic Susceptibility Study of Clinical Isolates. International Journal of Environmental Research and Public Health, 15(1), 153. https://doi.org/10.3390/ijerph15010153





