Occurrence, Distribution, and Risk Assessment of Perfluoroalkyl Acids (PFAAs) in Muscle and Liver of Cattle in Xinjiang, China
Abstract
:1. Introduction
2. Materials and Methods
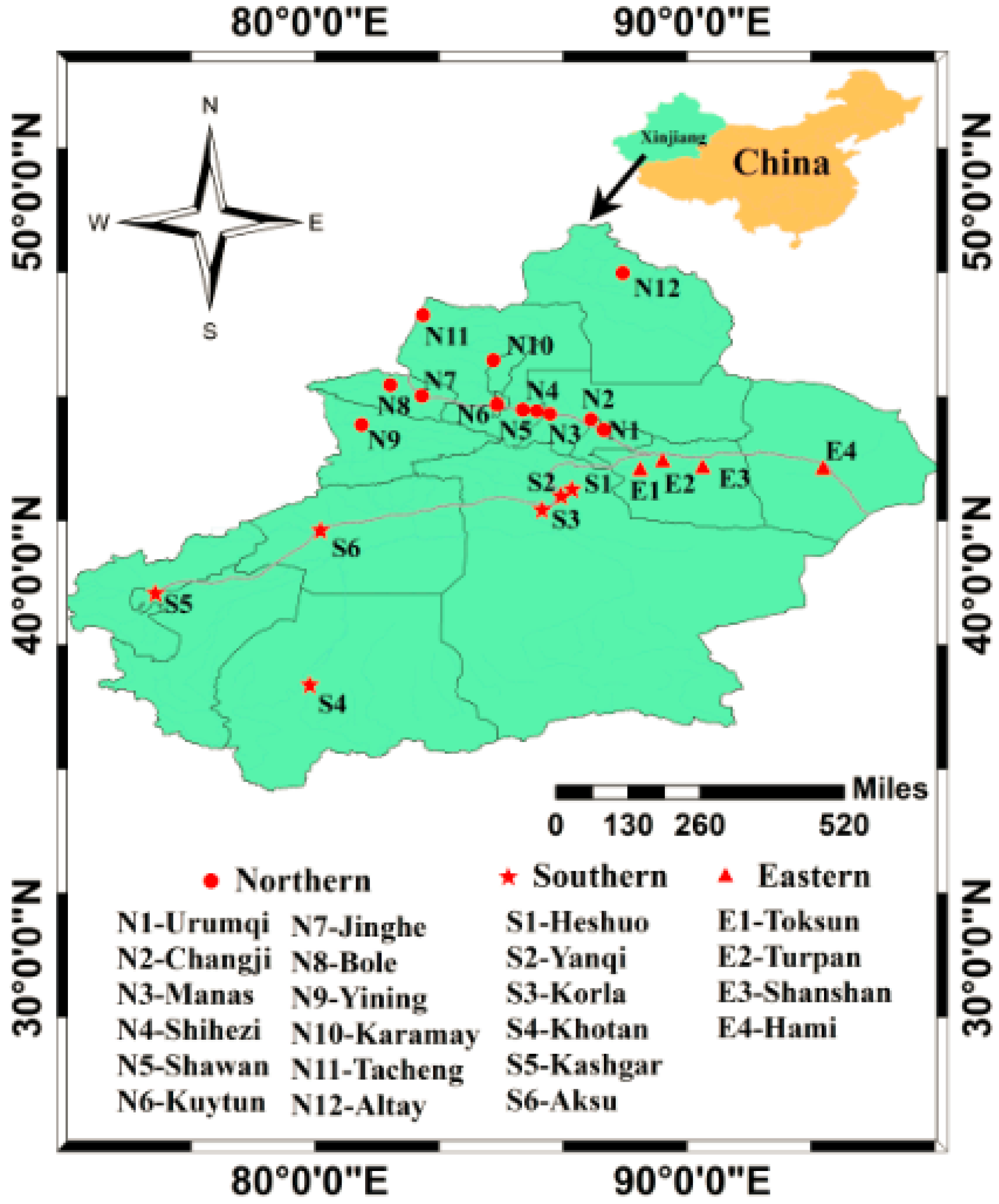
2.1. Sampling
2.2. Chemicals and Standards
2.3. Sample Preparation
2.4. Instrumental Conditions for HPLC-MS/MS
2.5. Quantification and Quality Control
2.6. Daily Intake Estimation and Risk Assessment
3. Results and Discussion
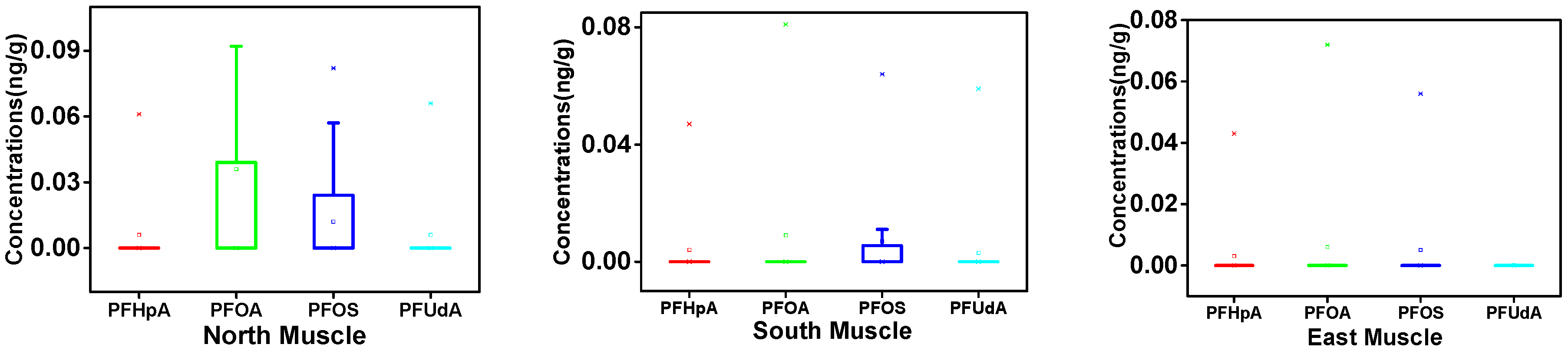
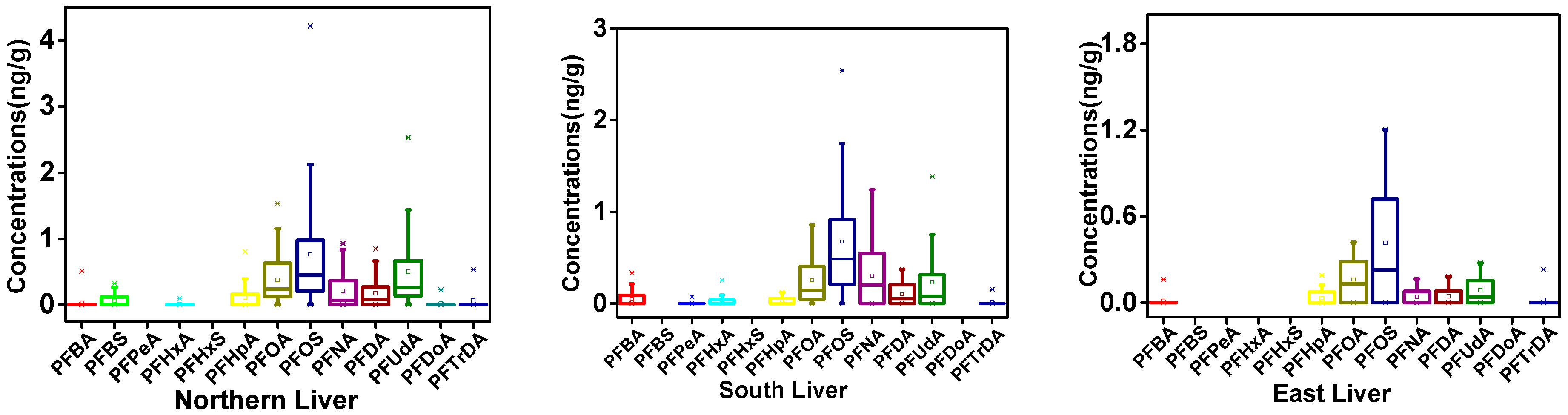
3.1. PFAA Concentrations across Beef Muscle and Beef Liver in Xinjiang
3.2. Regional Differences of PFAAs in Xinjiang
3.3. Comparison with Recent Studies of PFAAs in Animal Samples
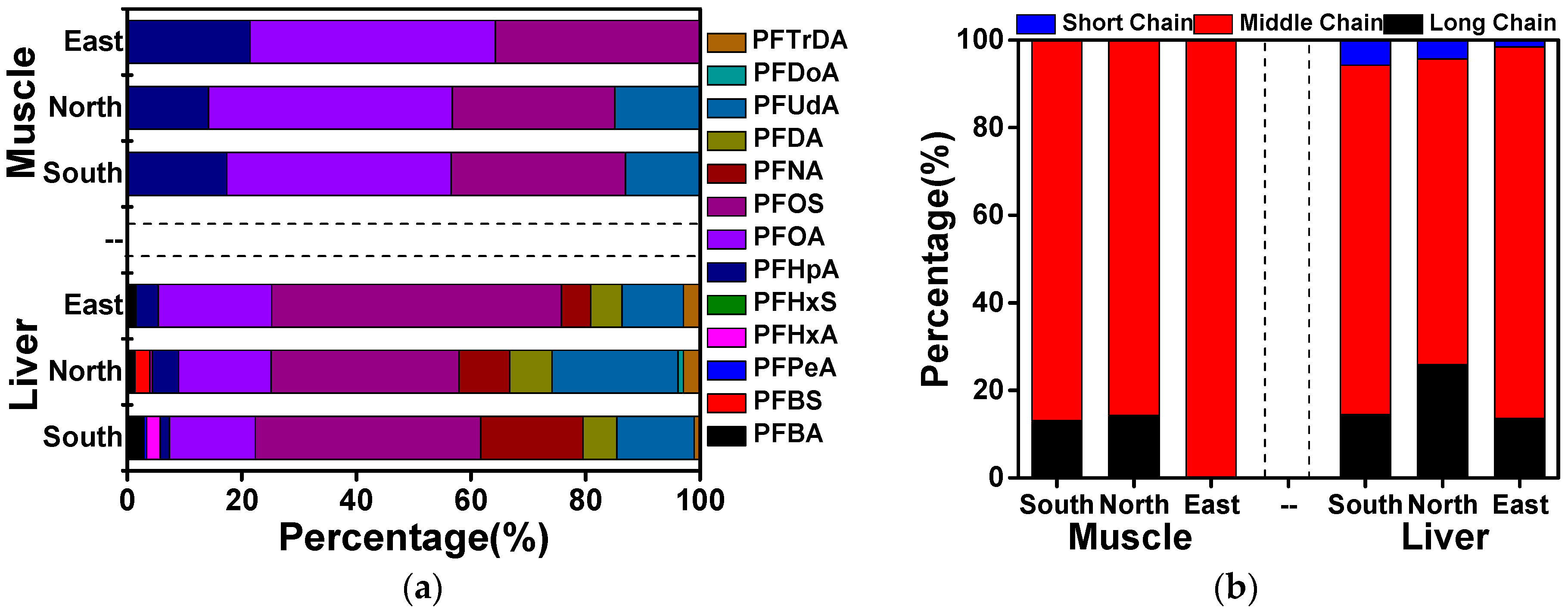
3.4. Source Analysis of PFAAs in Cattle of Xinjiang
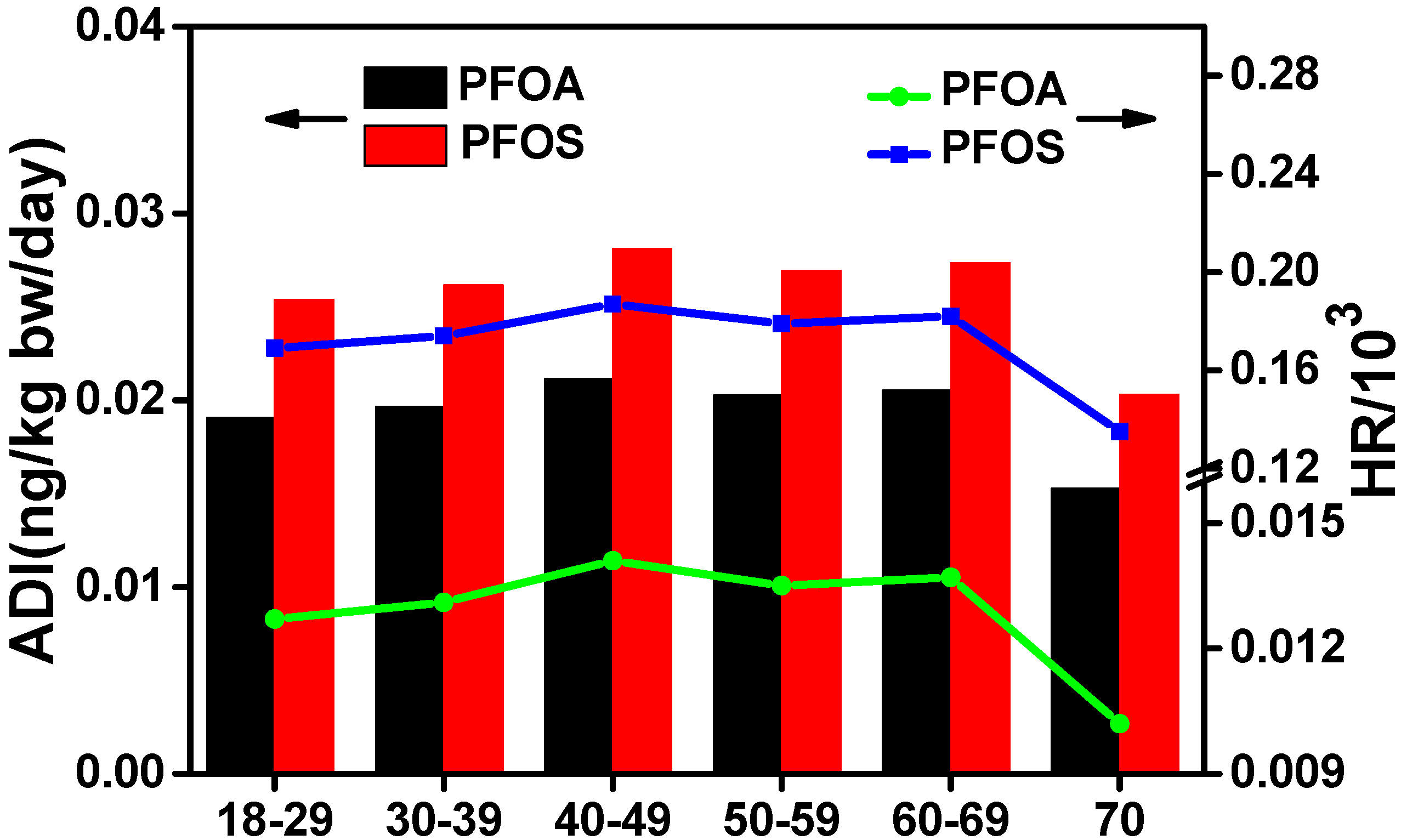
3.5. Dietary Intake and Health Risk Assessment in Xinjiang
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Habibullah-Al-Mamun, M.; Ahmed, M.K.; Raknuzzaman, M.; Islam, M.S.; Negishi, J.; Nakamichi, S.; Sekine, M.; Tokumura, M.; Masunaga, S. Occurrence and distribution of perfluoroalkyl acids (PFAAs) in surface water and sediment of a tropical coastal area (Bay of Bengal coast, Bangladesh). Sci. Total Environ. 2016, 571, 1089–1104. [Google Scholar] [CrossRef] [PubMed]
- Jantzen, C.E.; Annunziato, K.A.; Bugel, S.M.; Cooper, K.R. PFOS, PFNA, and PFOA sub-lethal exposure to embryonic zebrafish have different toxicity profiles in terms of morphometrics, behavior and gene expression. Aquat. Toxicol. 2016, 175, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Letcher, R.J.; Mcgoldrick, D.J.; Backus, S.M. A new fluorinated surfactant contaminant in biota: Perfluorobutane sulfonamide in several fish species. Environ. Sci. Technol. 2016, 50, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Pérez, F.; Llorca, M.; Köck-Schulmeyer, M.; Škrbić, B.; Oliveira, L.S.; Martinello, K.D.B.; Al-Dhabi, N.A.; Antić, I.; Farré, M.; Barceló, D. Assessment of perfluoroalkyl substances in food items at global scale. Environ. Res. 2014, 135, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.N.; Lu, J.J.; Liu, Z.L.; Li, S.M.; Wang, G.H.; Wang, X.L. Occurrence of perfluorooctanoic acid and perfluorooctane sulfonate in milk and yogurt and their risk assessment. Int. J. Environ. Res. Public Health 2016, 13, 1037. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Lu, Y.L.; Wang, P.; Wang, T.Y.; Liu, S.J.; Johnson, A.C.; Sweetman, A.J.; Baninla, Y. Pollution pathways and release estimation of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in central and eastern China. Sci. Total Environ. 2017, 580, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.W.; Qian, Z.M.; Vaughn, M.; Xian, H.; Elder, K.; Rodemich, E.; Bao, J.; Jin, Y.H.; Dong, G.H. Human serum levels of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in Uyghurs from Sinkiang-Uighur Autonomous Region, China: Background levels study. Environ. Sci. Pollut. Res. 2015, 22, 4736–4746. [Google Scholar] [CrossRef] [PubMed]
- Ashleymartin, J.; Dodds, L.; Arbuckle, T.E.; Morisset, A.S.; Fisher, M.; Bouchard, M.F.; Shapiro, G.D.; Ettinger, A.S.; Monnier, P.; Dallaire, R.; et al. Maternal and neonatal levels of perfluoroalkyl substances in relation to gestational weight gain. Int. J. Environ. Res. Public Health 2016, 13, 146. [Google Scholar] [CrossRef] [PubMed]
- Cariou, R.; Veyrand, B.; Yamada, A.; Berrebi, A.; Zalko, D.; Durand, S.; Pollono, C.; Marchand, P.; Leblanc, J.C.; Antignac, J.P. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ. Int. 2015, 84, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.B. Estimation of the total concentration of perfluoroalkyl acids (PFAA) in human serum: Data from NHANES 2005-2012. Chemosphere 2015, 134, 387–394. [Google Scholar] [CrossRef] [PubMed]
- De, F.E.; Abballe, A.; Albano, F.L.; Battista, T.; Carraro, V.; Conversano, M.; Franchini, S.; Giambanco, L.; Iacovella, N.; Ingelido, A.M. Current exposure of Italian women of reproductive age to PFOS and PFOA: A human biomonitoring study. Chemosphere 2015, 137, 1–8. [Google Scholar]
- Porpora, M.G.; Lucchini, R.; Abballe, A.; Ingelido, A.M.; Valentini, S.; Fuggetta, E.; Cardi, V.; Ticino, A.; Marra, V.; Fulgenzi, A.R. Placental transfer of persistent organic pollutants: A preliminary study on mother-newborn pairs. Int. J. Environ. Res. Public Health 2013, 10, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Noorlander, C.W.; Leeuwen, S.P.J.V.; Biesebeek, J.D.T.; Mengelers, M.J.B.; Zeilmaker, M.J. Levels of perfluorinated compounds in food and dietary intake of PFOS and PFOA in The Netherlands. J. Agric. Food Chem. 2011, 59, 7496–7505. [Google Scholar] [CrossRef] [PubMed]
- D’Hollander, W.; de Voogt, P.; De Coen, W.; Bervoets, L. Perfluorinated substances in human food and other sources of human exposure. Rev. Environ. Contam. Toxicol. 2010, 208, 179–215. [Google Scholar] [PubMed]
- Vestergren, R.; Berger, U.; Glynn, A.; Cousins, I.T. Dietary exposure to perfluoroalkyl acids for the Swedish population in 1999, 2005 and 2010. Environ. Int. 2012, 49, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L. Health risks of dietary exposure to perfluorinated compounds. Environ. Int. 2012, 40, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Su, H.Q.; Shi, Y.J.; Lu, Y.L.; Wang, P.; Zhang, M.; Sweetman, A.; Jones, K.; Johnson, A. Home produced eggs: An important pathway of human exposure to perfluorobutanoic acid (PFBA) and perfluorooctanoic acid (PFOA) around a fluorochemical industrial park in China. Environ. Int. 2017, 101, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Hlouskova, V.; Hradkova, P.; Poustka, J.; Brambilla, G.; De Filipps, S.P.; D’Hollander, W.; Bervoets, L.; Herzke, D.; Huber, S.; de Voogt, P. Occurrence of perfluoroalkyl substances (PFASs) in various food items of animal origin collected in four European countries. Food Addit. Contam. A 2013, 30, 1918–1932. [Google Scholar] [CrossRef] [PubMed]
- Jorundsdottir, H.; Halldorsson, T.I.; Gunnlaugsdottir, H. PFAAs in fish and other seafood products from Icelandic waters. J. Environ. Public Health 2014, 2014, 573607. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Arisawa, K.; Uemura, H.; Katsuura-Kamano, S.; Takami, H.; Sawachika, F.; Nakamoto, M.; Juta, T.; Toda, E.; Mori, K. Consumption of seafood, serum liver enzymes, and blood levels of PFOS and PFOA in the Japanese population. J. Occup. Health 2013, 55, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.J.; Lee, J.W.; Kim, S.K.; Oh, J.E. Foodstuff analyses show that seafood and water are major perfluoroalkyl acids (PFAAs) sources to humans in Korea. J. Hazard. Mater. 2014, 279, 402–409. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, H.W.; Wu, Q.; Zhang, X.Z.; Yun, S.H.; Kannan, K. Perfluorochemicals in meat, eggs and indoor dust in China: Assessment of sources and pathways of human exposure to perfluorochemicals. Environ. Sci. Technol. 2010, 44, 3572–3579. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.N.; Wang, Y.X.; Li, J.G.; Zhao, Y.F.; Guo, F.F.; Liu, J.Y.; Cai, Z.W. Perfluorinated compounds in seafood from coastal areas in China. Environ. Int. 2012, 42, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Lupton, S.J.; Huwe, J.K.; Smith, D.J.; Dearfield, K.L.; Johnston, J.J. Distribution and excretion of perfluorooctane sulfonate (PFOS) in beef cattle (Bos taurus). J. Agric. Food Chem. 2014, 62, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.J.; Clemen, L.A.; Ellefson, M.E.; Johnson, H.O. Compound-specific, quantitative characterization of organic fluorochemicals in biological matrices. Environ. Sci. Technol. 2001, 35, 766–770. [Google Scholar] [CrossRef]
- Wang, J.M.; Shi, Y.L.; Pan, Y.Y.; Cai, Y.Q. Perfluorooctane sulfonate (PFOS) and other fluorochemicals in viscera and muscle of farmed pigs and chickens in Beijing, China. Chin. Sci. Bull. 2010, 55, 3550–3555. [Google Scholar] [CrossRef]
- Zhe, W.; Ma, J.P.; Liu, L.X.; Suo, F.Y.; Guan, Y.M.; Liao, P.H. Adult residents dietary structure analysis of five disease monitoring stations in Xinjiang. J. Hyg. Res. 2014, 43, 853–856. [Google Scholar]
- Numata, J.; Kowalczyk, J.; Adolphs, J.; Ehlers, S.; Schafft, H.; Fuerst, P.; Müllergraf, C.; Lahrssenwiederholt, M.; Greiner, M. Toxicokinetics of seven perfluoroalkyl sulfonic and carboxylic acids in pigs fed a contaminated diet. J. Agric. Food Chem. 2014, 62, 6861–6870. [Google Scholar] [CrossRef]
- Kowalczyk, J.; Ehlers, S.; Fürst, P.; Schafft, H.; Lahrssen-Wiederholt, M. Transfer of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from contaminated feed into milk and meat of sheep: Pilot study. Arch. Environ. Contam. Toxicol. 2012, 63, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Wang, P.; Meng, J.; Liu, S.J.; Lu, Y.L.; Khim, J.S.; Giesy, J.P. A review of sources, multimedia distribution and health risks of perfluoroalkyl acids (PFAAs) in China. Chemosphere 2015, 129, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.A.; Liu, J.; Shao, W.L.; Yu, X.J.; Wang, S.L. Climatic characteristics and historical evolution of precipitation in different time scales in Xinjiang from 1961 to 2013. J. Glaciol. 2014, 36, 1363–1375. [Google Scholar]
- Taniyasu, S.; Yamashita, N.; Moon, H.B.; Kwok, K.Y.; Lam, P.K.; Horii, Y.; Petrick, G.; Kannan, K. Does wet precipitation represent local and regional atmospheric transportation by perfluorinated alkyl substances? Environ. Int. 2013, 55, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.B.; Xu, H.L.; Fu, J.Y.; Zhang, Q.Q.; Xu, X.W. Analysis of temporal-spatial variation characteristics of extreme air temperature in Xinjiang, China. Quat. Int. 2012, 282, 14–26. [Google Scholar] [CrossRef]
- Sekiguchi, K.; Kudo, T.; Sankoda, K. Combined sonochemical and short-wavelength UV degradation of hydrophobic perfluorinated compounds. Ultrason. Sonochem. 2017, 39, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Alsberg, T.; Vestergren, R.; Berger, U. Determination of perfluoroalkyl carboxylic, sulfonic, and phosphonic acids in food. Anal. Bioanal. Chem. 2012, 404, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Guerranti, C.; Perra, G.; Corsolini, S.; Focardi, S.E. Pilot study on levels of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) in selected foodstuffs and human milk from Italy. Food Chem. 2013, 140, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.B.; Bailey, V.A.; Routledge, A.; Lloyd, A.S.; Hird, S.; Mortimer, D.N.; Gem, M. Dietary intake estimate for perfluorooctanesulphonic acid (PFOS) and other perfluorocompounds (PFCs) in UK retail foods following determination using standard addition LC–MS/MS. Food Addit. Contam. A 2010, 27, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Haug, L.S.; Salihovic, S.; Jogsten, I.E.; Thomsen, C.; Bavel, B.V.; Lindström, G.; Becher, G. Levels in food and beverages and daily intake of perfluorinated compounds in Norway. Chemosphere 2010, 80, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Ericson, I.; Martícid, R.; Nadal, M.; Bavel, B.V.; Lindström, G.; Domingo, J.L. Human exposure to perfluorinated chemicals through the diet: Intake of perfluorinated compounds in foods from the Catalan (Spain) market. J. Agric. Food Chem. 2008, 56, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Zareitalabad, P.; Siemens, J.; Hamer, M.; Amelung, W. Perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS) in surface waters, sediments, soils and wastewater—A review on concentrations and distribution coefficients. Chemosphere 2013, 91, 725–732. [Google Scholar] [CrossRef]
- Guruge, K.S.; Manage, P.M.; Yamanaka, N.; Miyazaki, S.; Taniyasu, S.; Yamashita, N. Species-specific concentrations of perfluoroalkyl contaminants in farm and pet animals in Japan. Chemosphere 2008, 73, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Vestergren, R.; Orata, F.; Berger, U.; Cousins, I.T. Bioaccumulation of perfluoroalkyl acids in dairy cows in a naturally contaminated environment. Environ. Sci. Pollut. Res. 2013, 20, 7959–7969. [Google Scholar] [CrossRef]
- Zafeiraki, E.; Costopoulou, D.; Vassiliadou, I.; Leondiadis, L.; Dassenakis, E.; Hoogenboom, R.L.; van Leeuwen, S.P. Perfluoroalkylated substances (PFASs) in home and commercially produced chicken eggs from The Netherlands and Greece. Chemosphere 2016, 144, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Stahl, T.; Falk, S.; Failing, K.; Berger, J.; Georgii, S.; Brunn, H. Perfluorooctanoic acid and perfluorooctane sulfonate in liver and muscle tissue from wild boar in Hesse, Germany. Arch. Environ. Contam. Toxicol. 2012, 62, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.; Knapp, H. Carryover of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from soil to plant and distribution to the different plant compartments studied in cultures of carrots (Daucus carota ssp. Sativus), potatoes (Solanum tuberosum), and cucumbers (Cucumis sativus). J. Agric. Food Chem. 2011, 59, 11011–11018. [Google Scholar] [PubMed]
- Washington, J.W.; Jenkins, T.M.; Rankin, K.; Naile, J.E. Decades-scale degradation of commercial, side-chain, fluorotelomer-based polymers in soils and water. Environ. Sci. Technol. 2015, 49, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Asselt, E.D.V.; Kowalczyk, J.; Eijkeren, J.C.H.V.; Zeilmaker, M.J.; Ehlers, S.; Fürst, P.; Lahrssen-Wiederholt, M.; Fels-Klerx, H.J.V.D. Transfer of perfluorooctane sulfonic acid (PFOS) from contaminated feed to dairy milk. Food Chem. 2013, 141, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Winkens, K.; Koponen, J.; Schuster, J.; Shoeib, M.; Vestergren, R.; Berger, U.; Karvonen, A.M.; Pekkanen, J.; Kiviranta, H.; Cousins, I.T. Perfluoroalkyl acids and their precursors in indoor air sampled in children’s bedrooms. Environ. Pollut. 2016, 222, 423–432. [Google Scholar] [CrossRef] [PubMed]
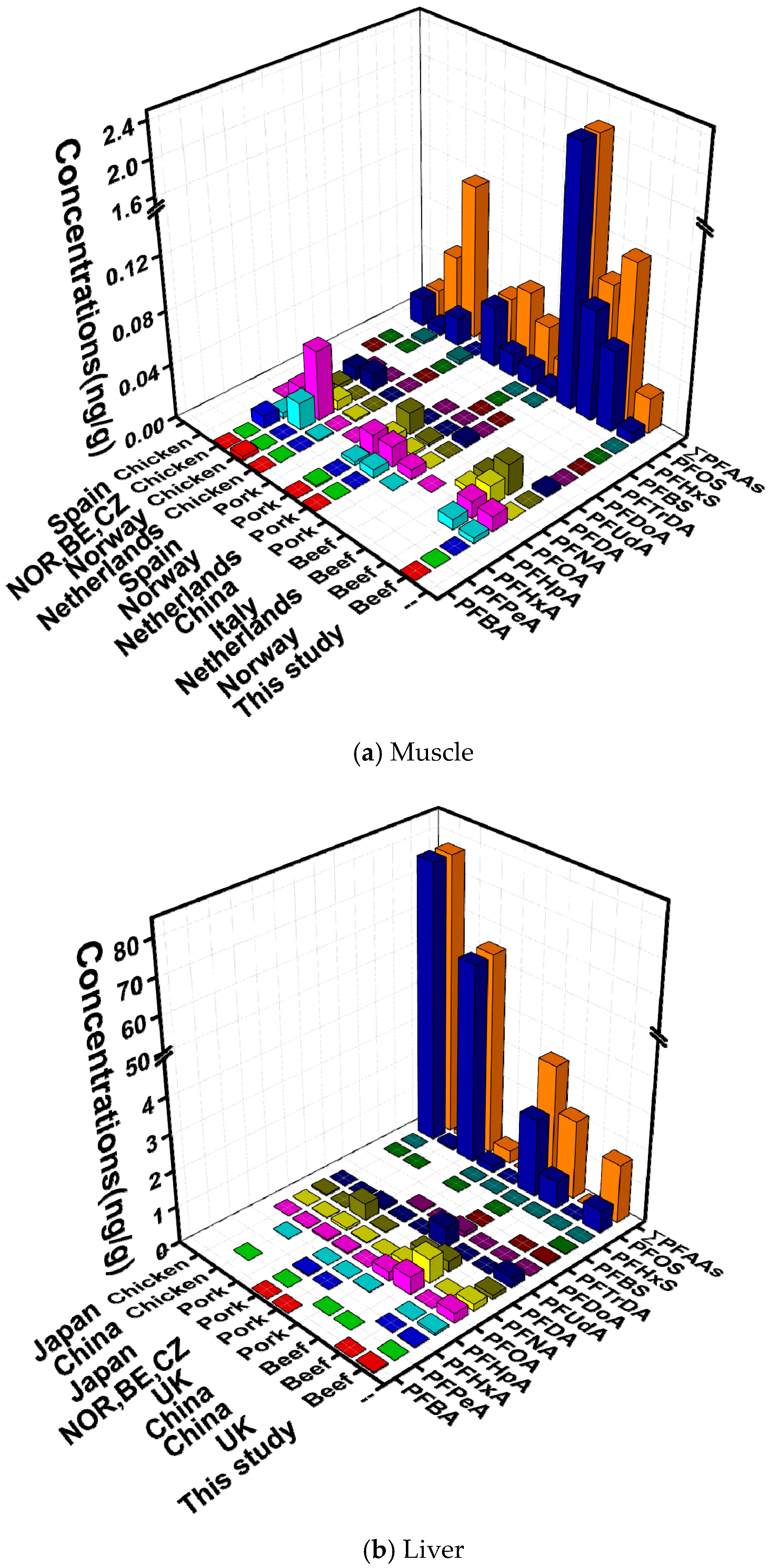
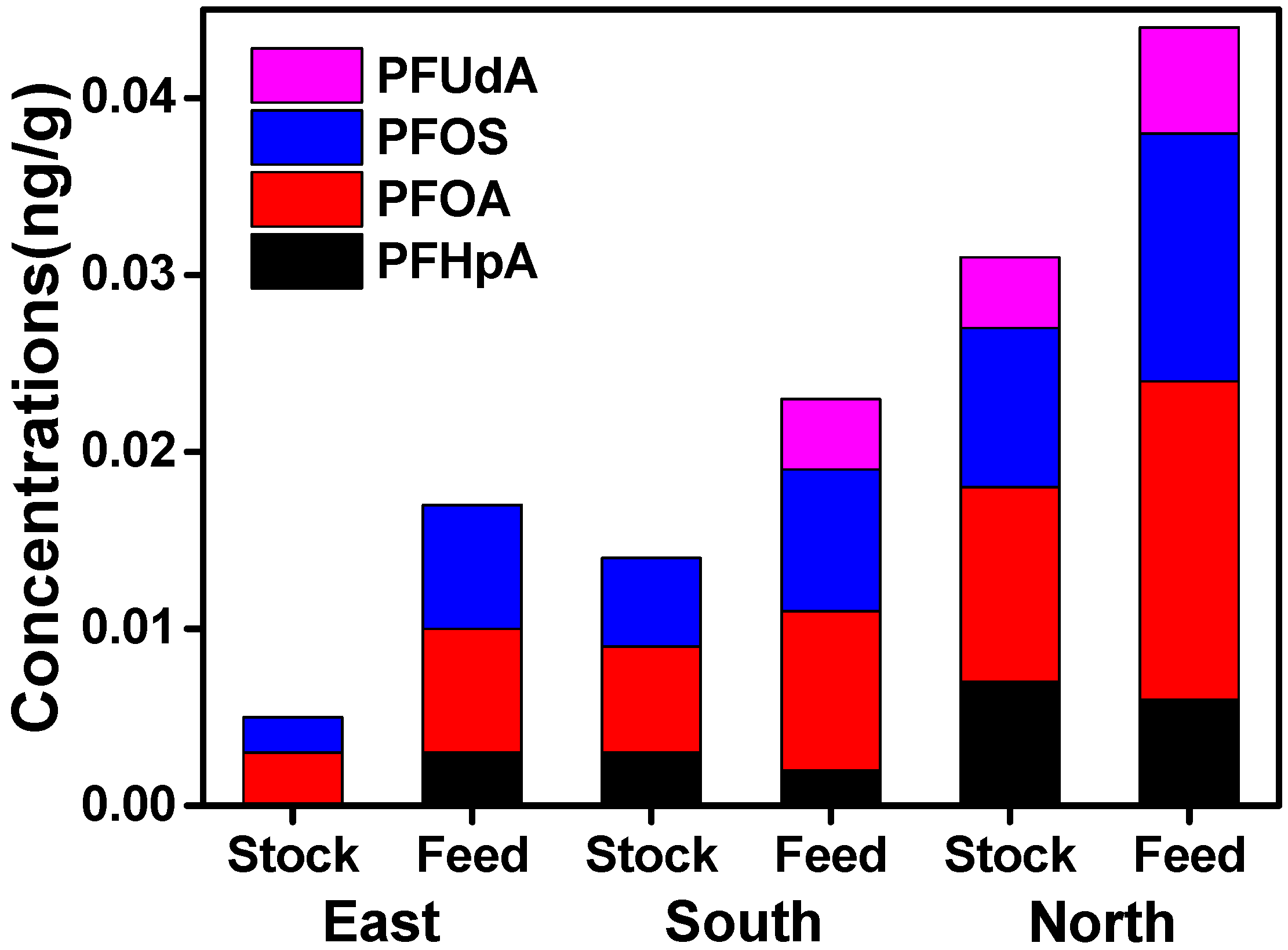

| Analytes | Muscle (n = 176) | Liver (n = 117) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Mean ± SE | Median | DF. (%) | Min. | Max. | Mean ± SE | Median | DF. (%) | |
| PFBA | ND | ND | 0.511 | 0.032 ± 0.00067 | 21 | |||||
| PFBS | ND | ND | 0.320 | 0.020 ± 0.00061 | 19 | |||||
| PFPeA | ND | ND | 0.734 | 0.003 ± 0.00001 | 3 | |||||
| PFHxA | ND | ND | 0.253 | 0.016 ± 0.00021 | 12 | |||||
| PFHxS | ND | ND | ||||||||
| PFHpA | ND | 0.061 | 0.004 ± 0.00001 | 17 | ND | 0.803 | 0.056 ± 0.00172 | 39 | ||
| PFOA | ND | 0.103 | 0.011 ± 0.00007 | 24 | ND | 1.532 | 0.264 ± 0.00896 | 0.221 | 75 | |
| PFOS | ND | 0.082 | 0.008 ± 0.00003 | 30 | ND | 4.221 | 0.617 ± 0.05131 | 0.439 | 82 | |
| PFNA | ND | ND | 1.243 | 0.184 ± 0.00691 | 0.056 | 56 | ||||
| PFDA | ND | ND | 0.847 | 0.106 ± 0.00326 | 0.061 | 58 | ||||
| PFUdA | ND | 0.066 | 0.003 ± 0.00002 | 8 | ND | 2.523 | 0.277 ± 0.02267 | 0.181 | 73 | |
| PFDoA | ND | ND | 0.266 | 0.007 ± 0.00016 | 9 | |||||
| PFTrDA | ND | ND | 0.532 | 0.036 ± 0.00112 | 21 | |||||
| ∑PFAAs | 0.026 | 47 | 1.632 | 94 | ||||||
| Group | DCmuscle/liver (g/day) | ADI (ng/kg bw/day) | HR/103 | |||
|---|---|---|---|---|---|---|
| PFOA | PFOS | PFOA | PFOS | |||
| Region | North | 75.0 (1.9) a | 0.034 | 0.039 | 0.023 | 0.257 |
| South | 63.0 (1.6) | 0.016 | 0.025 | 0.011 | 0.165 | |
| East b | 75.0 (1.9) | 0.013 | 0.019 | 0.008 | 0.127 | |
| Urban/rural | Urban | 90.0 (2.3) | 0.026 | 0.035 | 0.018 | 0.234 |
| Rural | 62.0 (1.6) | 0.018 | 0.024 | 0.012 | 0.161 | |
| Gender | Male | 71.0 (1.8) | 0.021 | 0.028 | 0.014 | 0.185 |
| Female | 64.0 (1.6) | 0.019 | 0.025 | 0.013 | 0.167 | |
| Ethnicity | Han Chinese | 69.0 (1.7) | 0.020 | 0.027 | 0.014 | 0.179 |
| Uighur | 66.0 (1.7) | 0.019 | 0.026 | 0.013 | 0.172 | |
| Others | 70.0 (1.8) | 0.021 | 0.027 | 0.014 | 0.182 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Lu, J.; Xing, Z.; Li, S.; Liu, Z.; Tong, Y. Occurrence, Distribution, and Risk Assessment of Perfluoroalkyl Acids (PFAAs) in Muscle and Liver of Cattle in Xinjiang, China. Int. J. Environ. Res. Public Health 2017, 14, 970. https://doi.org/10.3390/ijerph14090970
Wang G, Lu J, Xing Z, Li S, Liu Z, Tong Y. Occurrence, Distribution, and Risk Assessment of Perfluoroalkyl Acids (PFAAs) in Muscle and Liver of Cattle in Xinjiang, China. International Journal of Environmental Research and Public Health. 2017; 14(9):970. https://doi.org/10.3390/ijerph14090970
Chicago/Turabian StyleWang, Gehui, Jianjiang Lu, Zhenni Xing, Shanman Li, Zilong Liu, and Yanbin Tong. 2017. "Occurrence, Distribution, and Risk Assessment of Perfluoroalkyl Acids (PFAAs) in Muscle and Liver of Cattle in Xinjiang, China" International Journal of Environmental Research and Public Health 14, no. 9: 970. https://doi.org/10.3390/ijerph14090970
APA StyleWang, G., Lu, J., Xing, Z., Li, S., Liu, Z., & Tong, Y. (2017). Occurrence, Distribution, and Risk Assessment of Perfluoroalkyl Acids (PFAAs) in Muscle and Liver of Cattle in Xinjiang, China. International Journal of Environmental Research and Public Health, 14(9), 970. https://doi.org/10.3390/ijerph14090970




