Abstract
The persistent transmission of Japanese encephalitis virus (JEV) in Taiwan necessitates exploring the risk factors of occurrence of Japanese encephalitis (JE). The purpose of this study was to assess the relationship between meteorological factors and the incidence of JE in Taiwan. We collected data for cases of JE reported to the Taiwan Centers for Disease Control (Taiwan CDC) from 2000 to 2014. Meteorological data were obtained from the Taiwan Central Weather Bureau. The relationships between weather variability and the incidence of JE in Taiwan were determined via Poisson regression analysis and a case-crossover methodology. During the 15-year study period, a total of 379 cases of JE were reported. The incidence of JE showed significant seasonality, with the majority of cases occurring in summertime (for oscillation, p < 0.001). The number of JE cases started to increase at temperatures of 22 °C (r2 = 0.88, p < 0.001). Similarly, the number of JE cases began to increase at a relative humidity of 70–74% (r2 = 0.75, p < 0.005). The number of JE cases was positively associated with mean temperature and relative humidity in the period preceding the infection. In conclusion, the occurrence of JE is significantly associated with increasing temperature and relative humidity in Taiwan. Therefore, these factors could be regarded as warning signals indicating the need to implement preventive measures.
1. Introduction
Japanese encephalitis (JE) is caused by the Japanese encephalitis virus (JEV). Japanese encephalitis virus, belonging to the Flaviviridae family, is transmitted by mosquitoes from animals to humans [1]. The main transmission vectors of the JEV are Culex mosquitoes, particularly Culex tritaeniorhynchus (Cx. tritaeniorhynchus), and the main vertebrate hosts for amplifying JEV are pigs and ardeid birds [2,3]. The illness spectrum of JE in humans ranges from asymptomatic infection to a devastating encephalitis syndrome that is associated with appreciable mortality and frequent central nervous system (CNS) sequelae in survivors [4].
JE is an important cause of viral encephalitis in most Asian countries, particularly in South Asia, Southeast Asia, and East Asia [5,6]. Several countries in these regions have significantly reduced the morbidity of JE through several intervention measures, including early diagnosis, prompt treatment, national immunization, and effective vector control [7,8]. However, more than 3 billion people still live in JE-endemic countries, and an estimated 67,900 cases occur annually [5]. Approximately 20–30% of JE cases are fatal and 30–50% of survivors have significant neurologic sequelae [6,9].
In Taiwan, a comprehensive vaccination campaign against JE was launched in the 1960s, during which all children aged less than 3 years old received two doses of the JE vaccine [10,11]. After 1974, the number of vaccination doses was increased to three, with a booster dose administered one year after the two primary doses. After 1976, a fourth booster dose was administered to children during their first year at elementary school. After 1980, the mainly children older than 15 months was target population for JE vaccinations. The vaccination was administered in two doses separated by an interval of 2 weeks, which were administered between March and May, before the epidemic season, and were followed by a booster dose one year later, with a final booster dose (the fourth dose) when the child entered the elementary school. Before 1987, an inactivated vaccine derived from a Nakayama-NIH strain JEV-infected mouse brain was the only vaccine used. A version derived from an inactivated freeze-dried Beijing strain was introduced in 1988, but the Nakayama strain of the vaccine has remained the dominant vaccine on the market. Despite the current vaccination program, cases of JE still occur in Taiwan [10].
The occurrence of vector-borne diseases (VBD) is strongly driven by environmental factors [8,12,13]. Among the most relevant environmental factors are climate, land use and land cover [14]. Several recent studies have shown an increasing trend of the epidemic potential and length of the transmission season of VBDs in temperate regions and tropical highlands under different climate change scenarios [15,16,17]. Although these phenomena have not been adequately explained, the seasonality of JE suggests that meteorological factors might play a key role in its occurrence [10,14,18].
Moreover, the Intergovernmental Panel on Climate Change (IPCC) indicates that local changes in temperature and rainfall will continue to alter the distribution of disease vectors and the risk of VBDs [16,17]. However, heterogeneity in VBD transmission is observed at every spatial scale, ranging from small islands to continents. This heterogeneity is determined by the ecology and biogeography of the vectors, soil types, urbanization, local adaptation to temperature, and host communities [17,19]. The effects of meteorological factors on infectious diseases have attracted global attention in the context of climate change in recent years.
A further understanding of the relationships between meteorological factors and the occurrence of JE could help improve both disease forecasting and preventive efforts. However, few studies have investigated the effects of meteorological factors on the occurrence of JE in Taiwan. The purpose of this study was to assess the relationships between weather-related factors and the number of JE cases in Taiwan.
2. Methods
2.1. Study Area
Taiwan is an island in East Asia and is located between 21°45’ N and 25°56’ N. The Tropic of Cancer (23.5° N) runs straight through Chiayi City, which is situated in south central Taiwan and divides the entire island into two climate zones. Taiwan includes a total land area of 35,980 km2 and approximately 2.3 million people, giving an average population density of 635 individuals per km2. The northern part of Taiwan belongs to the sub-tropical climate zone, whereas the southern part belongs to the tropical climate zone. Consequently, the weather in Taiwan is relatively warm, and high humidity occurs throughout the year [20].
This study was approved by the Institutional Review Board of the Show Chwan Memorial Hospital, Changhua, Taiwan (SCMH_IRB No. 1040903).
2.2. Surveillance for Japanese Encephalitis Infection
The data used in this study have been previously published by Chang et al. [10]. Briefly, JE has been categorized as a notifiable infectious disease since 1955. Physicians are required to report all cases that meet the case definition of JE, collect samples, and send them to the Centers for Disease Control of Taiwan (Taiwan CDC) within one week of the case being reported for examination [11].
We collected data from all JE-confirmed cases reported to the Taiwan CDC from January 2000 to December 2014. The reported information included patient age, sex, area of residence, geographic location of exposure, travel history, vaccination status, and date of JE onset.
2.3. Case Definitions
A clinical case was defined as a person of any age with an acute onset of fever and a change in mental status and/or a new onset of seizures (excluding simple febrile seizures) at any time of the year [6,10].
A confirmed case was defined as a clinical case with a positive laboratory test (presence of IgM antibodies specific to the JE virus in a single sample of CSF or serum; and/or a four-fold increase in IgG antibodies; and/or the detection of JE virus antigens in tissue via immunohistochemistry; or the detection of the JE virus genome in serum, plasma, blood, CSF or tissue samples; or that met the clinical case definition and was epidemiologically linked to a confirmed case [10,11,21].
2.4. Meteorological Data
Complete meteorological data, including the maximum and minimum daily mean temperatures, relative humidity, vapor pressure, precipitation, and daylight hours, between January 2000 and December 2014 were obtained from the Taiwan Central Weather Bureau (http://www.cwb.gov.tw). We used the regional meteorological data value for each calendar week obtained from all 17 weather stations across the island, excluding stations in isolated islands and areas in mountains, linked to each case depending on their regions of residence.
2.5. Statistical Analysis
We calculated the annual incidence of JE by dividing the number of reported JE cases by the mid-year population of individuals of the same age, as reported between 2000 and 2014 in Taiwan census data. This was expressed as the number of JE cases per 1,000,000 individuals. Seasonal trends in the occurrence of JE were assessed using Poisson regression models that incorporated terms for the calendar year, as well as sine and cosine to assess time trends and seasonality of JE using the monthly aggregate case number as the response variable. In addition to the time trends and seasonality, we added the temperature, relative humidity, vapor pressure, precipitation, and daylight hours into the models [20,22], such that:
in which E[Yi(t)] denotes the expected case counts at month t in year i. α is a constant value, each β term denotes a regression coefficient for a year or month, t indicates the months between January 2000 and December 2014, and i indicates the 15 years during the years 2000 and 2014. The function yeari(t) denotes whether it is yeari (1 = yes, 0 = no). The function month (t) indicates a month number (i.e., 1 to 12 for January to December). Temperaturei(t) is the temperature at month t in year i; likewise, relative humidityi(t) is the relative humidity at month t in yeari. We used the construction of univariable and multivariable Poisson regression models to evaluate the correlation between monthly number of JE cases and weather exposure. We also used oscillatory seasonal smoothers for smoothing to account for annual variations during the 15-year study period. We used Akaike’s information criterion (AIC) to optimize the knots within the spline model to avoid the pitfalls associated with both overfitting and underfitting [23]. A backwards-elimination algorithm was conducted in multivariable models, in which covariates were retained at p ≤ 0.20 [23].
To investigate the relationship between JEV infections and various temperatures and relative humidity levels, we estimated the incidence of JE at various temperatures and relative humidity levels. According to previous studies [18,24,25], we assumed that the survival and transmission of the JE virus would change as temperature and relative humidity changed, and this might therefore affect the infectivity of the JE virus in a defined population. The average incidence of JE (NT) in various temperature domains (T to T + ΔT) was estimated using the following formula [20,22]:
Here, i denotes an index from 0 to n, ti is the average temperature for the ith 7-day period, Ci is the total cases of JE for the i + 2nd 7-day period, and f (ti) is a function with the following results:
The numerator on the right side of the equation is the sum of all Ci comprising the 7-day average temperatures (ti) within the temperature domain of T to T+ΔT during the study period. The denominator is the total number of JE cases with T < ti ≤ T + ΔT during the same study period.
Similarly, the average incidence of JE (Nh) in various relative humidity domains (H to H + ΔH) was assessed using the following formula:
Here, i is a sequence from 0 to n, hi is the average relative humidity for theith 7-day period, Ci is the total cases of JE from the i + 2nd 7-day period, and f (hi) is a function with the following results:
We used a case-crossover analysis to further investigate the acute effects of meteorological exposure on the occurrence of JE [26]. This design is characterized by self-matching, wherein cases serve as their own controls [27,28]. This specifically provides a means to evaluate the acute effects of brief exposures [26,29]. In this way, each subject’s exposure prior to a case-defining event was compared with his or her own exposure during a control period when he/she had not yet been diagnosed as a case. A case day was defined as the day on which the first symptom of JE presented [28,30], and the case period was defined as 0–14 days prior to that day. The control day was selected two to four weeks before the case date (14–29 days prior to the case day). The possible effect period was estimated according to the incubation period of JE, which is approximately 7 days (range: 5–15 days) [8]. The average daily values of the meteorological factors were used as exposures, as were aggregated or mean values of the meteorological factors.
The analysis of case-crossover data is an application of standard methods for stratified data analysis. A conditional logistic regression analysis was performed to determine exposure odds ratios (ORs) as estimates of incidence rate ratios and 95% confidence intervals (CIs) associated with meteorological variables [31].
To study the possibility of interactions (effect modification) from the demographic characteristics of patients, we developed multiplicative interaction terms and incorporated them into a logistic regression model [32]. We used SAS software Version 9.2 (SAS Institute Inc., Cary, NC, USA) to perform all statistical analyses. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Epidemiological Characteristics of Patients with Japanese Encephalitis
Table 1 shows the number and annual incidence rate of JE cases by gender, age, region, and season. Between January 2000 and December 2014, a total of 379 patients with JE were diagnosed by physicians in Taiwan. All cases were indigenous. The incidence rate (cases per 1,000,000 individuals per year) of JE was 1.105 (range: 0.59 to 1.61). JEV predominantly affected men compared to women (1.366 vs. 0.839). The incidence of JE increased as patient age increased, with a peak (1.491) in the age range of 20–59 years. The highest and lowest incidences of JE were 4.829 and 0.492 in the eastern and the northern region, respectively, in Taiwan. The highest incidence of JE was 0.902 in the summer (June to August), and followed by 0.114 in the spring, and 0.090 in the autumn.

Table 1.
Demographic characteristics of patients with Japanese encephalitis in Taiwan, 2000–2014.
3.2. Seasonality and Effects of Weather Factors
After oscillatory or cubic spline smoothers were incorporated into the model, mean temperature and mean relative humidity were the only factors found to be independently associated with JE infection.
Figure 1 shows the actual and predicted monthly reported cases of JE. There was a seasonal pattern of JE infection (for seasonal oscillation, p < 0.001), but no distinct annual trends in this model. In general, the overall trends of the predicted monthly reported case numbers fit the actual trends well (Pearson chi-squared = 50.78; p > 0.05).
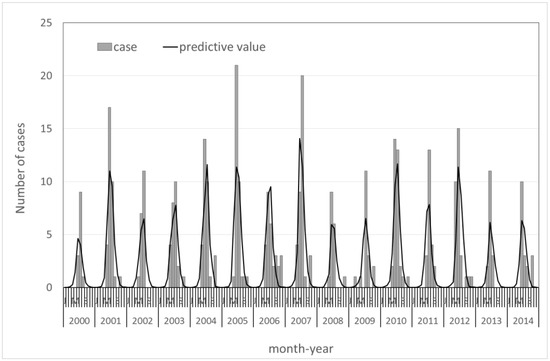
Figure 1.
Trends in monthly Japanese encephalitis cases from 2000–2014 in Taiwan.
The association between meteorological factors and the incidence of JE is presented in Table 2. Univariate analysis using Poisson models showed several meteorological factors associated with the incidence of JE; however, after annual trends and oscillatory seasonal smoothers were incorporated into the models, only the mean temperature and relative humidity were independently associated with the incidence of JE (Table 2). Age and sex did not modify temperature or relative humidity effects.

Table 2.
Weekly weather patterns 8–14 days prior to symptom onset and the incidence of JE virus infection in Taiwan, 2000–2014.
3.3. Temperature, Relative Humidity, and Occurrence of JE
The relationship between occurrence (case count) of JE and temperature is shown in Figure 2A. The occurrence of JE changes with different temperatures. The number of JE cases began to rise at a temperature of 22 °C (r2 = 0.88, p < 0.001). The average case count (NT) increased by 14.4% (95% CI: 7.4–21.4%) for each 1 °C increase in temperature.
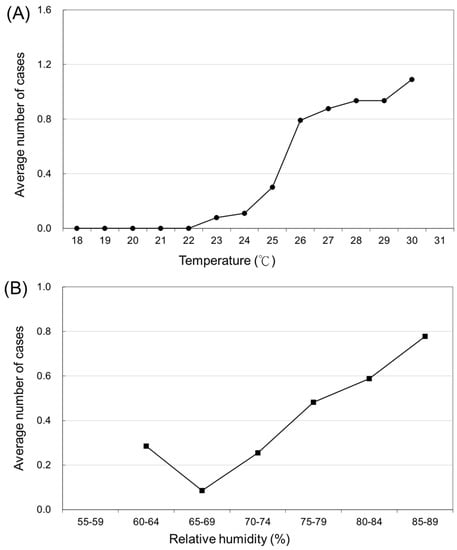
Figure 2.
(A) The average number of Japanese encephalitis virus infections in various temperature domains. (B) The average number of Japanese encephalitis virus infections in various relative humidity domains.
Figure 2B presents the association between the variation in the number of JE cases and the various relative humidity domains (Nh). The number of JE cases began to increase at a relative humidity of 70–74% (r2 = 0.75, p < 0.05). An increase in relative humidity of 5% was correlated with a 9.8% increase in JE cases (95% CI: 1.0–18.6%).
3.4. Acute Meteorological Effects Using Case-Crossover Analysis
Figure 3A presents the relationship between the risk of JE virus infection and per degree increase in temperature from days 3–7, plateau till 12 days, and then decline. Figure 3B shows the risk of JE infection to steadily drop till day 9, and increase till day 12, and then decline, as per unit (5%) increase in relative humidity. The increased occurrence of JE was significantly associated with increased mean temperature and relative humidity.
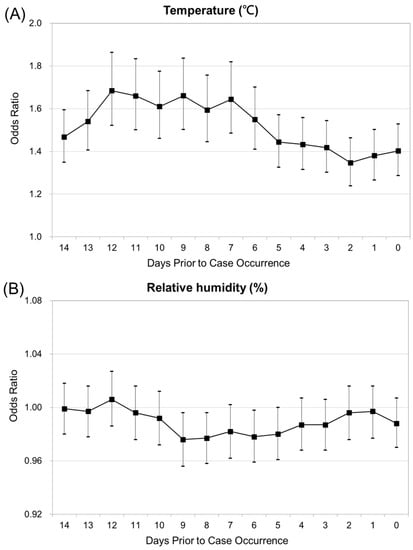
Figure 3.
(A) Conditional logistic regression analysis for Japanese encephalitis, with temperature as explanatory variables. (B) Conditional logistic regression analysis for Japanese encephalitis, with relative humidity explanatory variables.
4. Discussion
The epidemiological evidence demonstrated that JE remains an important public health problem in Taiwan [5,10]. We analyzed JE data reported to the Taiwan CDC from 2000 to 2014 using Poisson regression analysis and a case-crossover methodology. We found that the incidence of JE was highest during the summer months, but that the incidence was positively correlated with increased mean temperature and mean relative humidity in both long-term and acute effect analyses. In this study, we also identified the importance of meteorological exposure in determining the incidence and seasonality of JE in developed countries, such as Taiwan.
JEV is an arthropod-borne virus (arbovirus) that is transmitted in an enzootic cycle among mosquito vectors and vertebrate hosts [33]. The emergence of JE is driven by environmental factors, such as climate [8]. Various patterns of seasonality in the occurrence of JE have been reported in various countries. In temperate countries (e.g., China, Japan, Nepal, and Korea), seasonal JE outbreaks occur in conjunction with the temperature and rainfall increase in the summer months, when JEV is detectable in mosquitoes, pigs, and birds. On the other hand, in tropical and subtropical countries (e.g., Indonesia, Malaysia, the Philippines, and Vietnam), sporadic JE cases occur year-round, with a peak during the rainy season [8,14]. Our results showed a distinct seasonal pattern of JEV infection, with a peak occurrence in the summer (June, July, August) months. This result was similar to Southern China [18,34], India [15] and Nepal [24].
In this study, the occurrence of JEV infection began to increase at 22 °C. We also found that the number of JE cases started to increase at a relative humidity of 70–74%, with transmission assumed to be due to biting by an infected mosquito. It has been reported that the activity of the JEV is affected by the temperature and relative humidity [8,33]. The mechanism underlying the effects of temperature and relative humidity in the transmission of JEV remains unclear.
In our study, male patients had a higher annual incidence rate of JE than female patients (1.37 vs. 0.84 per 1,000,000). Hsu’s study [35] showed the odds of having JE neutralizing antibodies are higher in men than in women (ratio = 1.25, 95% CI: 1.12–1.40) in Taiwanese people. However, no sex differences were reported in the incidence of JE cases in India [13,21]. We are unable to definitively explain this epidemiological finding in Taiwan, but postulate that contributing factors may include different behavioral patterns or different reporting patterns.
Children less than 15 years of age were considered a high-risk group for JE in South, East, Southeast Asia and Australasia because most adults are immune [5]. Since childhood JE vaccination programs have not been fully implemented across the geographic range of JEV, the target population for infections has shifted from children to adults in several countries, in contrast to the pre-vaccination era [36,37]. In our study, the highest incidence rate of JE occurred in the age range of 20–59 years old.
In our study, a high percentage (42.7%) of JE cases occurred in the southern region of Taiwan. There are several possible reasons for this, which are discussed below: (1) In Taiwan, most confirmed cases of JE involved patients who lived near paddy fields or pig farms [38], and southern Taiwan is an agricultural region. Compared with other regions, there are more pig farms near rice paddy fields as well as wetland habitats for water birds in southern Taiwan. These provide suitable environments for maintaining the JEV infection cycle [39,40]. (2) In Taiwan, almost all dengue fever cases (approximately >95%) over the last decade occurred in the southern region of Taiwan [11]. This fact, along with the high proportion of JE cases, suggests that mosquitoes and the JE virus are well-adapted to this region. (3) Finally, the higher proportion of cases in the southern region of Taiwan is also a reflection of the population numbers.
This study has several limitations. First, the public health surveillance data may be incomplete. It is believed that many notifiable infectious diseases (e.g., JE) are underreported [41]. A reporting bias may occur anywhere in the reporting chain. This bias would occur if weather effects were somehow correlated with the likelihood of disease reporting [23]. Since doctors may be more likely to test for JE when they consider it high season for it, and thus mild cases in the beginning of the season are more likely to be missed. Second, it was difficult to obtain weather data in all counties in Taiwan. After excluding stations in isolated islands and areas in the mountains, only 17 weather stations were ultimately included in our analysis. These weather data may not represent the true status of weather exposure in the individual areas in Taiwan. The results supported the null hypothesis due to non-differential misclassification. The effects of meteorological exposure on the occurrence of JE in this study were most likely underestimated [27].
In summary, the seasonal pattern of JEV infection in Taiwan was confirmed, and meteorological factors that might contribute to the observed seasonality, including the mean temperature and relative humidity, were evaluated. Public health authorities could regard the results of the threshold estimation as a warning signal, and by applying these results with a prediction model for long-term trends, they could develop and deploy public health interventions before early summer to reduce the risk of infection and spread of the JE virus. JE has attracted growing attention because several unexpected outbreaks occurred in many countries in the 2000s [15,24]. Our findings demonstrate the importance of meteorological factors in determining JE case occurrence and can help explain the notable seasonal pattern of JE.
5. Conclusions
The occurrence of JE is significantly associated with increasing temperature and relative humidity in Taiwan. Therefore, these factors could be regarded as alert signals indicating the need to implement preventive strategies.
Acknowledgments
This study was supported by a grant (Most-104-314-B-217-001) from the Ministry of Science and Technology, Taiwan.
Author Contributions
Che-Liang Lin contributed to the study design, data collection, data analysis, and drafting; Hsiao-Ling Chang contributed to the study design, data collection, and data analysis; Chuan-Yao Lin contributed to the study design and data collection; Kow-Tong Chen served as the principal investigator of this study and contributed to the conception, study design, drafting, and revision; all authors approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leinikki, P. Japanese encephalitis. In Principles and Practice of Clinical Virology, 5th ed.; Zuckerman, A.J., Banatvala, J.E., Pattison, J.R., Eds.; Wiley: Chichester, UK, 2004; pp. 459–466. [Google Scholar]
- Lindenbach, B.; Rice, C. Flaviviridae: The viruses and their replication. In Fields Virology, 4th ed.; Fields, B.N., Howley, P.M., Griffin, D.E., Lamb, R.A., Martin, M.A., Roizman, B., Straus, S.E., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 991–1041. [Google Scholar]
- Vaughn, D.W.; Hoke, C.J. The epidemiology of Japanese encephalitis prospects for prevention. Epidemiol. Rev. 1992, 14, 197–221. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.; Basu, A. Japanese encephalitis—A pathological and clinical perspective. PLoS Negl. Trop. Dis. 2009, 3, e437. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Hill, S.; Fischer, M.; Jacobson, J.; Hoke, C.; Hombach, J.; Marfin, A.A.; Solomon, T.; Tsai, T.F.; Tsu, V.D.; et al. Estimated global incidence of Japanese encephalitis: A systemic review. Bull. World Health Organ. 2011, 89, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Japanese encephalitis surveillance and immunization—Asia and Western pacific, 2012. MMWR Morb. Mortal. Wkly. Rep. 2013, 62, 658–662. [Google Scholar]
- Heffelfinger, J.D.; Li, X.; Batmunkh, N.; Grabovac, V.; Diorditsa, S.; Liyanage, J.B.; Pattamadilok, S.; Kirsten, S.B.; Bahl, S.; Vannice, K.S.; et al. Japanese encephalitis: Surveillance and immunization in Asia and the Western Pacific, 2016. Wkly. Epidemiol. Rep. 2017, 92, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Le Flohic, G.; Porphyre, V.; Barbazan, P.; Gonzalez, J.P. Review of climate, landscape, and viral genetics as drivers of the Japanese encephalitis virus ecology. PLoS Negl. Trop. Dis. 2013, 7, e2208. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Hills, S.; Staples, E.; Johnson, B.; Yaich, M.; Solomon, T. Japanese encephalitis prevention and control: Advances, challenges, and new initiatives. In Emerging Infections 8; Scheld, W.M., Hammer, S.M., Hughes, J.M., Eds.; ASM Press: Washington, DC, USA, 2008; pp. 93–124. [Google Scholar]
- Chang, Y.K.; Chang, H.L.; Wu, H.S.; Chen, K.T. Epidemiological features of Japanese encephalitis in Taiwan from 2000 to 2014. Am. J. Trop. Med. Hyg. 2017, 96, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control, Taiwan. Notifiable Infectious Disease Statistical System. Available online: http://nidss.cdc.gov.tw (accessed on 15 February 2017).
- Tian, H.Y.; Bi, P.; Cazelles, B.; Zhou, S.; Huang, S.Q.; Yang, J.; Pei, Y.; Wu, X.X.; Fu, S.H.; Tong, S.L.; et al. How environmental conditions impact mosquito ecology and Japanese encephalitis: An co-epidemiological approach. Environ. Int. 2015, 79, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Kovats, R.S.; Campbell-Lendrum, D.H.; McMichael, A.J.; Woodward, A.; Cox, J.S. Early effects of climatechange: Do they include changes in vector-borne disease? Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.L.; Lee, Y.M. Japanese encephalitis: The virus and vaccines. Hum. Vaccin. Immunother. 2014, 10, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Borah, J.; Dutta, P.; Khan, S.A.; Mahanta, J. Association of weather and anthropogenic factors for transmission of Japanese encephalitis in an endemic area of India. Ecohealth 2013, 10, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change: The Physical Science Basis. Contribution of Working Group I tothe Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1335p. [Google Scholar]
- Woodward, A.; Smith, K.R.; Campbell-Lendrum, D.; Chadee, D.D.; Honda, Y.; Liu, Q.; Olwoch, J.; Revich, B.; Sauerborn, R.; Chafe, Z.; et al. Climate change and health: On the latest IPCC report. Lancet 2014, 383, 1185–1189. [Google Scholar] [CrossRef]
- Wang, L.; Hu, W.; Soares Magalhaes, R.J.; Bi, P.; Ding, F.; Sun, H.; Li, S.; Yin, W.; Wei, L.; Liu, Q.; et al. The role of environmental factors in the spatial distribution of Japanese encephalitis in mainland China. Environ. Int. 2014, 73, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, E.D.; Thomas, M.B. Local adaptation to temperature and the implications for vector-borne diseases. Trends. Parasitol. 2014, 30, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.C.; Su, B.H.; Su, H.J.; Chang, H.L.; Lin, C.Y.; Chen, H.; Chen, K.T. The association between the incidence of mumps and meteorological parameters in Taiwan. Hum. Vaccin. Immunother. 2015, 11, 1406–1412. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. A Cohort Study to Assess the New WHO Japanese Encephalitis Surveillance Standards. 2007. Available online: http://www.who.int/bulletin/Volumes/86/3/07-04330/en/ (accessed on 21 February 2016).
- Chang, H.L.; Chio, C.P.; Su, H.J.; Liao, C.M.; Lin, C.Y.; Shau, W.Y.; Chi, Y.C.; Cheng, Y.T.; Chou, Y.L.; Li, C.Y.; et al. The association between enterovirus 71 infections and meteorological parameters in Taiwan. PLoS ONE 2012, 7, e46845. [Google Scholar] [CrossRef] [PubMed]
- Kinlin, L.M.; Spain, C.V.; Ng, V.; Johnson, C.C.; White, A.N.; Fisman, D.N. Environmental exposures and invasive meningococcal disease: An evaluation of effect on varying time scales. Am. J. Epidemiol. 2009, 169, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Kumar Pant, D.; Tenzin, T.; Chand, R.; Kumar Sharma, B.; Raj Bist, P. Spatio-temporal epidemiology of Japanese encephalitis in Nepal, 2007–2015. PLoS ONE 2017, 12, e0180591. [Google Scholar] [CrossRef] [PubMed]
- Bi, P.; Zhang, Y.; Parton, K.A. Weather variables and Japanese encephalitis in the metropolitan area of Jinan city, China. J. Infect. 2007, 55, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Maclure, M. The case-crossover design: A method for studying transient effects on the risk of acute events. Am. J. Epidemiol. 1991, 133, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.A.; Schulz, K.F. Bias and causal associations in observational research. Lancet 2002, 359, 248–252. [Google Scholar] [CrossRef]
- Levy, D.; Lumley, T.; Sheppard, L.; Kaufman, J.; Checkoway, H. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology 2001, 12, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Née, M.; Avalos, M.; Luxcey, A.; Contrand, B.; Salmi, L.R.; Fourrier-Réglat, A.; Gadegbeku, B.; Lagarde, E.; Orriols, L. Prescription medicine use by pedestrians and the risk of injurious road traffic crashes: A case-crossover study. PLoS Med. 2017, 14, e1002347. [Google Scholar] [CrossRef] [PubMed]
- Murray, E.L.; Klein, M.; Brondi, L.; McGowan, J.E., Jr.; van Mels, C.; Brooks, W.A.; Kleinbaum, D.; Goswami, D.; Ryan, P.B.; Bridges, C.B. Rainfall, household crowding, and acute respiratory infections in the tropics. Epidemiol. Infect. 2012, 140, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M. Epidemiology: Study Design and Data Analysis, 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2005; pp. 515–600. [Google Scholar]
- Cameron, A.C.; Trivedi, P.K. Regression Analysis of Count Data, 1st ed.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Miller, R.H.; Masuoka, P.; Klein, T.A.; Kim, H.C.; Somer, T.; Grieco, J. Ecological niche modeling to estimate the distribution of Japanese encephalitis virus in Asia. PLoS Negl. Trop. Dis. 2012, 6, e1678. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cui, S.; Gao, X.; Wang, H.; Song, M.; Li, M.; Fu, S.; Lv, Z.; He, Y.; Lei, W.; et al. The Spatio-temporal distribution of Japanese encephalitis cases in different age groups in mainland China, 2004–2014. PLoS Negl. Trop. Dis. 2016, 10, e0004611. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.C.; Chen, Y.J.; Hsu, F.K.; Huang, J.H.; Chang, C.M.; Chou, P.; Lin, I.F.; Chang, F.Y. The incidence of Japanese encephalitis in Taiwan—A population-based study. PLOS Negl. Trop. Dis. 2014, 8, e3030. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Choe, Y.J.; Kim, J.H.; Song, K.M.; Cho, H.; Bae, G.R.; Lee, J.K. Epidemiology of Japanese encephalitis in South Korea, 2007–2010. Int. J. Infect. Dis. 2012, 16, e448–e452. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Matsunaga, Y.; Takasaki, T.; Tanaka-Taya, K.; Taniguchi, K.; Okabe, N.; Kurane, I. Japanese encephalitis: Surveillance and elimination efforts in Japan from 1982 to 2004. Jpn. J. Infect. Dis. 2008, 61, 333–338. [Google Scholar] [PubMed]
- Mathur, A.; Kumar, R.; Sharma, S.; Kulshreshtha, R.; Kumar, A.; Chaturvedi, U.C. Rapid diagnosis of Japanese encephalitis by immunofluorescent examination of cerebrospinal fluid. Indian J. Med. Res. 1990, 91, 1–4. [Google Scholar] [PubMed]
- Su, C.L.; Yang, C.F.; Teng, H.J.; Lu, L.C.; Lin, C.; Tsai, K.H.; Chen, Y.Y.; Chen, L.Y.; Chang, S.F.; Shu, P.Y. Molecular epidemiology of Japanese encephalitis virus in mosquitoes in Taiwan during 2005–2012. PLoS Negl. Trop. Dis. 2014, 8, e3122. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, A.; Mulyatno, K.C.; Susilowati, H.; Hendrianto, E.; Utsumi, T.; Amin, M.; Lusida, M.I.; Soegijanto, S.; Konishi, E. Prevalence of antibodies to Japanese encephalitis virus among pigs in Bali and East Java, Indonesia, 2008. Jpn. J. Infect. Dis. 2010, 63, 58–60. [Google Scholar] [PubMed]
- Doyle, T.J.; Glynn, M.K.; Groseclose, S.L. Completeness of notifiable infectious disease reporting in the United States: An analytical literature review. Am. J. Epidemiol. 2002, 155, 866–874. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).