Seasonal and Geographic Variation of Pediatric Malaria in Burundi: 2011 to 2012
Abstract
1. Introduction
2. Materials and Methods
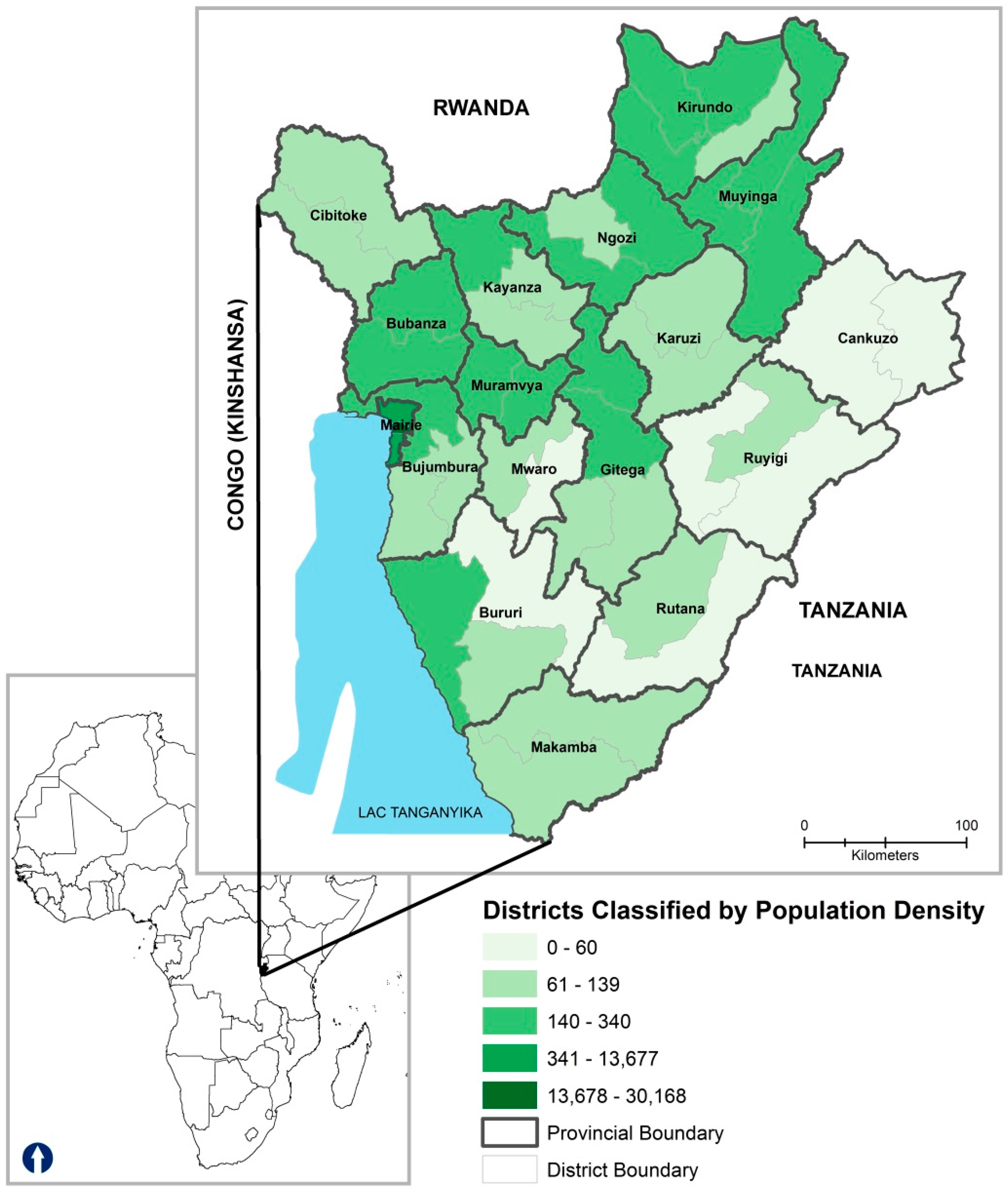
2.1. Study Setting
2.2. Data Source
2.3. Case Definition
2.4. Ethics Statement
2.5. Statistical Analyses
3. Results
3.1. Demographic Characteristics
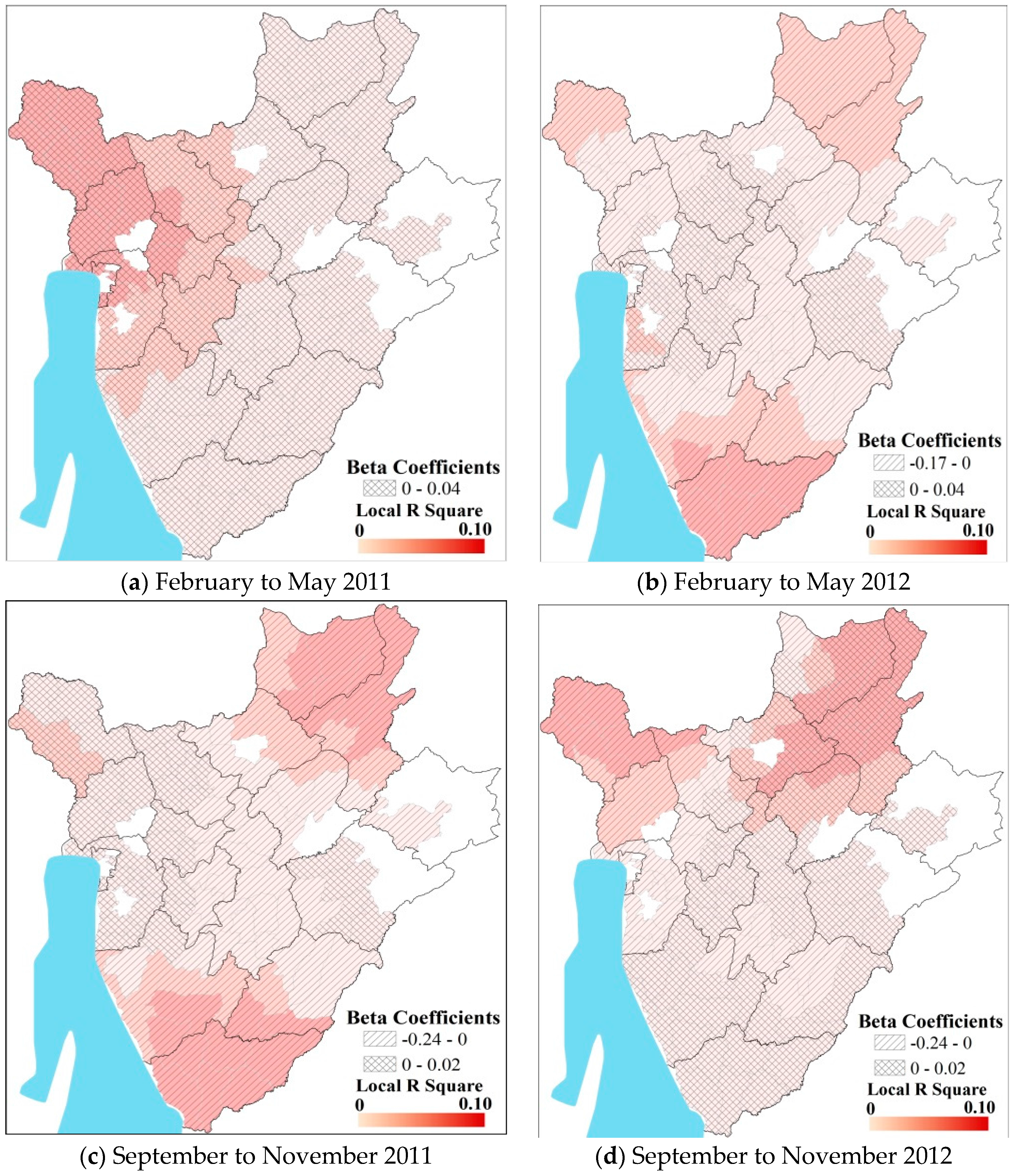
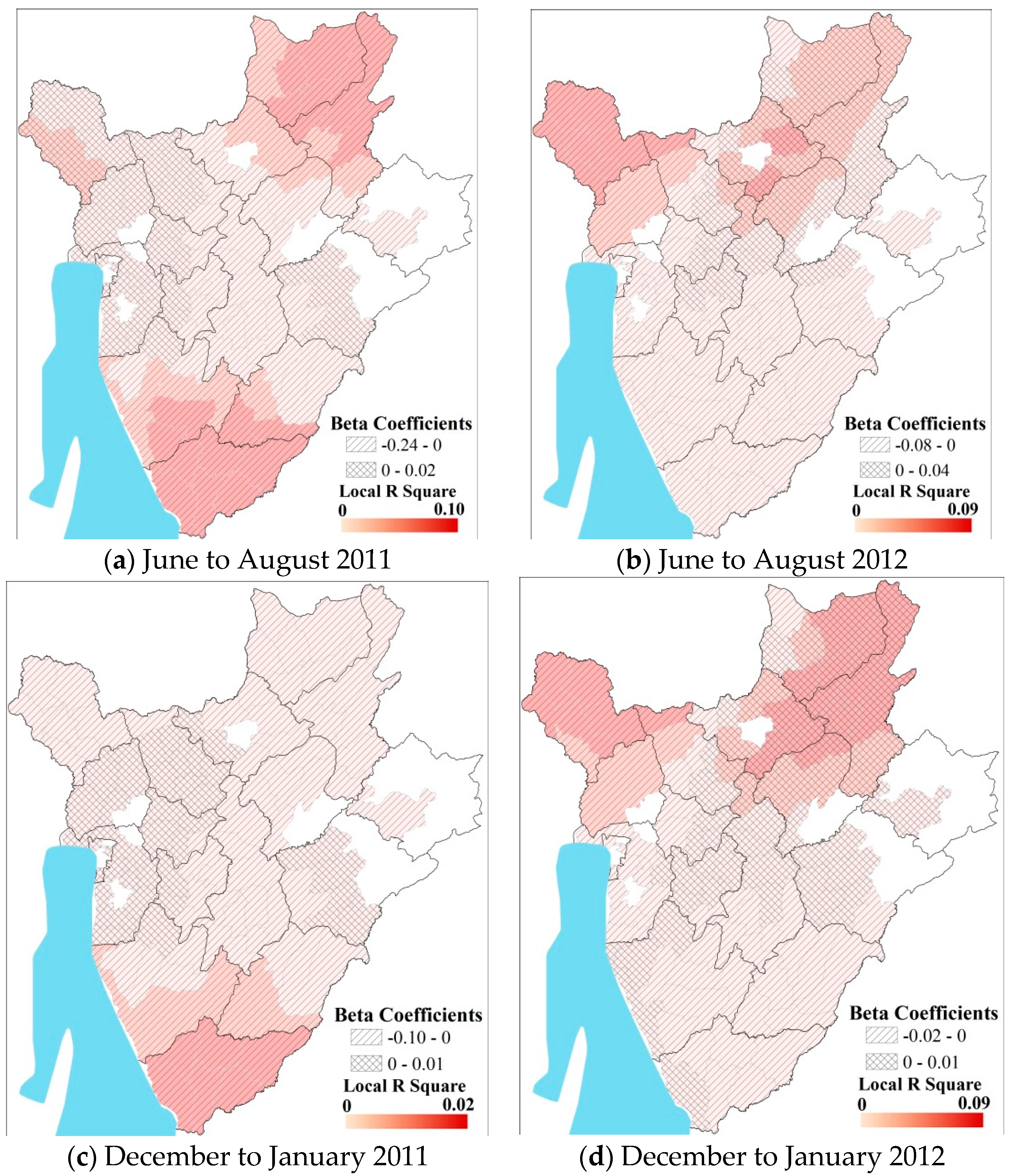
3.2. Predicting Pediatric Malaria Incidence
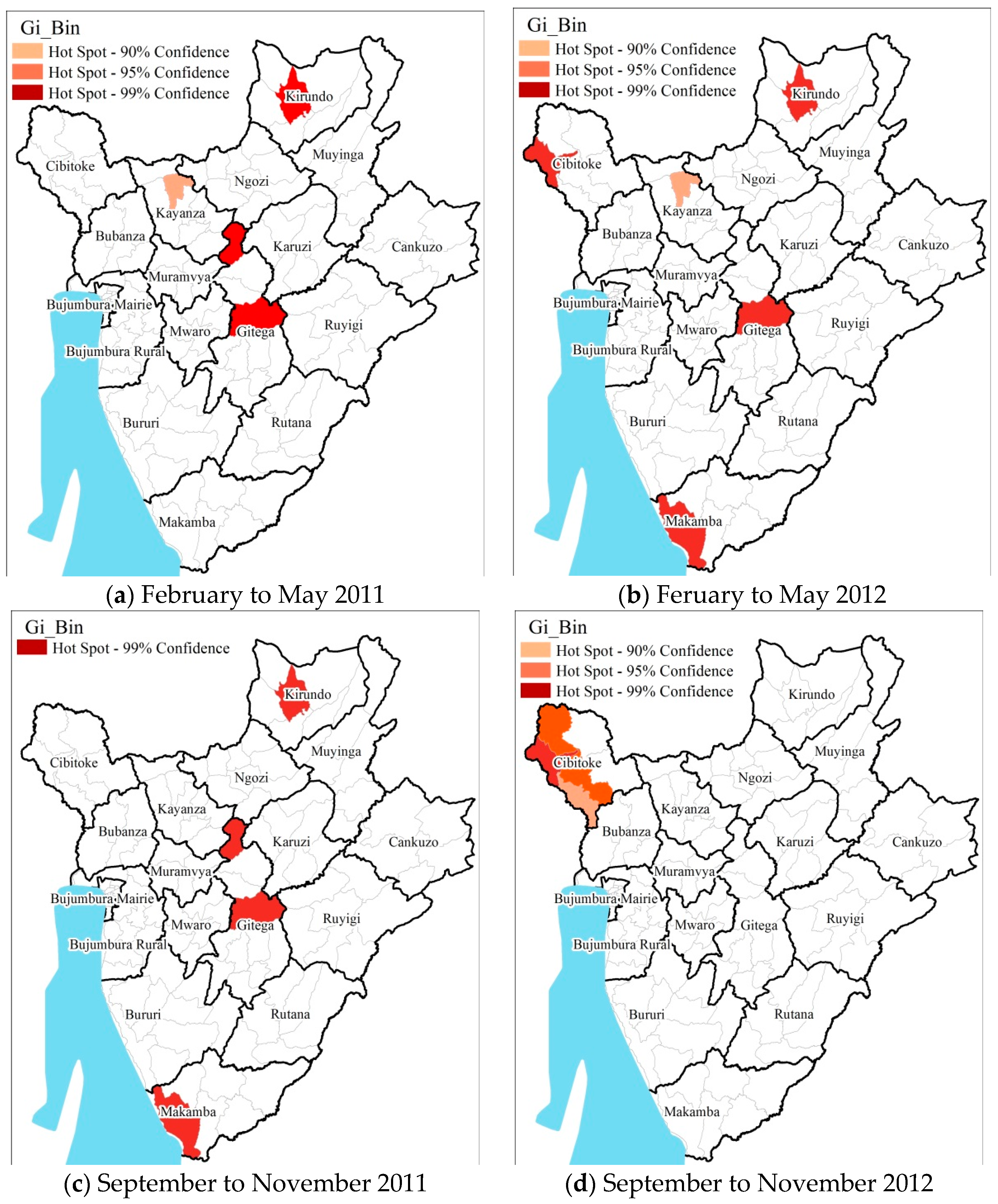
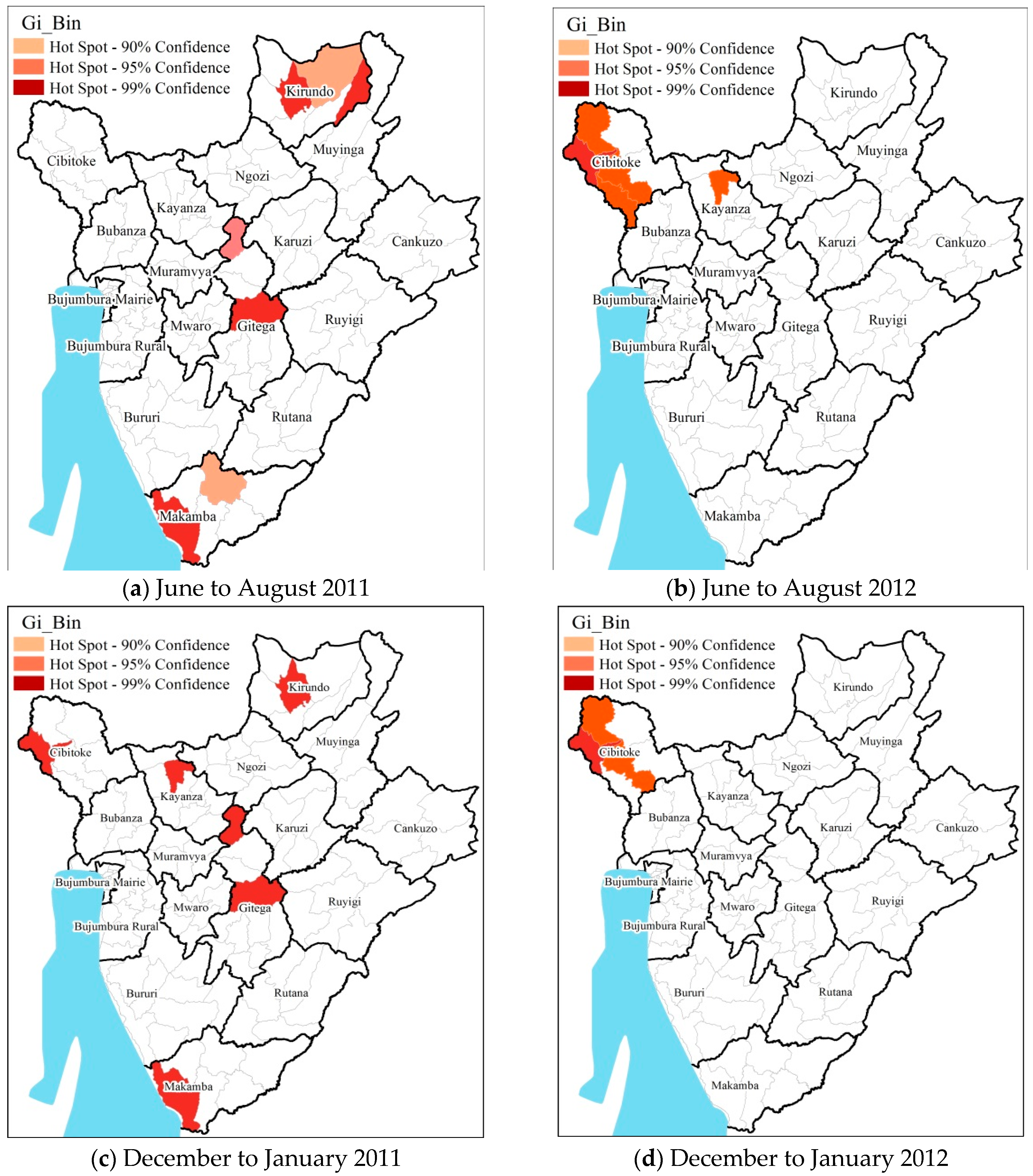
3.3. Hot Spot Cold Spot Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stauffer, W.; Fischer, P.R. Diagnosis and treatment of malaria in children. Clin. Infect. Dis. 2003, 37, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Summer, A.P.; Stauffer, W.M.; Fischer, P.R. Pediatric malaria in the developing world. Semin. Pediatr. Infect. Dis. 2005, 16, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Breman, J.G. The ears of the hippopotamus: Manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 2001, 64, 1–11. [Google Scholar] [PubMed]
- Takem, E.N.; Roca, A.; Cunnington, A. The association between malaria and non-typhoid salmonella bacteraemia in children in Sub-Saharan Africa: A literature review. Malar. J. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- UNICEF. Malaria: A Major Cause of Child Death and Poverty in Africa; Section, H., Division, P., Eds.; UNICEF: New York, NY, USA, 2004. [Google Scholar]
- Snow, R.W.; Korenromp, E.L.; Gouws, E. Pediatric mortality in Africa: Plasmodium falciparum malaria as a cause or risk? Am. J. Trop. Med. Hyg. 2004, 71, 16–24. [Google Scholar] [PubMed]
- Roca-Feltrer, A.; Schellenberg, J.R.; Smith, L.; Carneiro, I. A simple method for defining malaria seasonality. Malar. J. 2009, 8. [Google Scholar] [CrossRef] [PubMed]
- Midekisa, A.; Beyene, B.; Mihretie, A.; Bayabil, E.; Wimberly, M.C. Seasonal associations of climatic drivers and malaria in the highlands of Ethiopia. Parasites Vectors 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Cazelles, B.; Bouma, M.J.; Chaves, L.F.; Koelle, K. Shifting patterns: Malaria dynamics and rainfall variability in an African highland. Proc. Biol. Sci. 2008, 275, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Minakawa, N.; Githeko, A.K.; Yan, G. Association between climate variability and malaria epidemics in the East African highlands. Proc. Natl. Acad. Sci. USA 2004, 101, 2375–2380. [Google Scholar] [CrossRef] [PubMed]
- Mueller, I.; Namuigi, P.; Kundi, J.; Ivivi, R.; Tandrapah, T.; Bjorge, S.; Reeder, J.C. Epidemic malaria in the highlands of Papua New Guinea. Am. J. Trop. Med. Hyg. 2005, 72, 554–560. [Google Scholar] [PubMed]
- Abeku, T.A.; de Vlas, S.J.; Borsboom, G.; Teklehaimanot, A.; Kebede, A.; Olana, D.; van Oortmarssen, G.J.; Habbema, J.D. Forecasting malaria incidence from historical morbidity patterns in epidemic-prone areas of Ethiopia: A simple seasonal adjustment method performs best. Trop. Med. Int. Health 2002, 7, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Teklehaimanot, H.D.; Lipsitch, M.; Teklehaimanot, A.; Schwartz, J. Weather-based prediction of Plasmodium falciparum malaria in epidemic-prone regions of Ethiopia I. Patterns of lagged weather effects reflect biological mechanisms. Malar. J. 2004, 3. [Google Scholar] [CrossRef]
- Lindsay, S.W.; Birley, M.H. Climate change and malaria transmission. Ann. Trop. Med. Parasitol. 1996, 90, 573–588. [Google Scholar] [PubMed]
- Nkurunziza, H.; Gebhardt, A.; Pilz, J. Geo-additive modelling of malaria in Burundi. Malar. J. 2011, 10. [Google Scholar] [CrossRef] [PubMed]
- Devi, N.P.; Jauhari, R.K. Climatic variables and malaria incidence in Dehradun, Uttaranchal, India. J. Vector. Borne. Dis. 2006, 43, 21–28. [Google Scholar] [PubMed]
- Bouma, M.J.; Dye, C.; van der Kaay, H.J. Falciparum malaria and climate change in the northwest frontier province of Pakistan. Am. J. Trop. Med. Hyg. 1996, 55, 131–137. [Google Scholar] [PubMed]
- Grau, G.E.; Taylor, T.E.; Molyneux, M.E.; Wirima, J.J.; Vassalli, P.; Hommel, M.; Lambert, P.H. Tumor necrosis factor and disease severity in children with falciparum malaria. N. Engl. J. Med. 1989, 320, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.E.; Molyneux, M.E.; Wirima, J.J.; Fletcher, K.A.; Morris, K. Blood glucose levels in Malawian children before and during the administration of intravenous quinine for severe falciparum malaria. N. Engl. J. Med. 1988, 319, 1040–1047. [Google Scholar] [CrossRef] [PubMed]
- Walldorf, J.A.; Cohee, L.M.; Coalson, J.E.; Bauleni, A.; Nkanaunena, K.; Kapito-Tembo, A.; Seydel, K.B.; Ali, D.; Mathanga, D.; Taylor, T.E.; et al. School-age children are a reservoir of malaria infection in Malawi. PLoS ONE 2015, 10, e0134061. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, R.J.; Clements, A.C. Mapping the risk of anaemia in preschool-age children: The contribution of malnutrition, malaria, and helminth infections in West Africa. PLoS Med. 2011, 8, e1000438. [Google Scholar] [CrossRef] [PubMed]
- Omumbo, J.; Ouma, J.; Rapuoda, B.; Craig, M.H.; le Sueur, D.; Snow, R.W. Mapping malaria transmission intensity using geographical information systems (GIS): An example from Kenya. Ann. Trop. Med. Parasitol. 1998, 92, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Ndiath, M.M.; Cisse, B.; Ndiaye, J.L.; Gomis, J.F.; Bathiery, O.; Dia, A.T.; Gaye, O.; Faye, B. Application of geographically-weighted regression analysis to assess risk factors for malaria hotspots in Keur Soce health and demographic surveillance site. Malar. J. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Ehlkes, L.; Krefis, A.C.; Kreuels, B.; Krumkamp, R.; Adjei, O.; Ayim-Akonor, M.; Kobbe, R.; Hahn, A.; Vinnemeier, C.; Loag, W.; et al. Geographically weighted regression of land cover determinants of Plasmodium falciparum transmission in the Ashanti Region of Ghana. Int. J. Health Geogr. 2014, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Caldas de Castro, M.; Fisher, M.G. Is malaria illness among young children a cause or a consequence of low socioeconomic status? Evidence from the united Republic of Tanzania. Malar. J. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Krefis, A.C.; Schwarz, N.G.; Krüger, A.; Fobil, J.; Nkrumah, B.; Acquah, S.; Loag, W.; Sarpong, N.; Adu-Sarkodie, Y.; Ranft, U.; et al. Modeling the relationship between precipitation and malaria incidence in children from a holoendemic area in Ghana. Am. J. Trop. Med. Hyg. 2011, 84, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Odongo-Aginya, E.; Ssegwanyi, G.; Kategere, P.; Vuzi, P.C. Relationship between malaria infection intensity and rainfall pattern in Entebbe Peninsula, Uganda. Afr. Health Sci. 2005, 5, 238–245. [Google Scholar] [PubMed]
- Sena, L.D.; Deressa, W.A.; Ali, A.A. Analysis of trend of malaria prevalence in south-west Ethiopia: A retrospective comparative study. Malar. J. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. Local spatial autocorrelation statistics: Distributional issues and an application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar]
- Boots, B. Local measures of spatial association. Ecoscience 2002, 9, 168–176. [Google Scholar]
- Brunsdon, C.; Fotheringham, A.; Charlton, M. Geographically weighted regression: A method for exploring spatial nonstationarity. Geogr. Anal. 1996, 28, 281–289. [Google Scholar] [CrossRef]
- Brunsdon, C.; Fotheringham, S.; Charlton, M. Geographically weighted regression-modelling spatial non-stationarity. J. Royal Statisti. Soc. 1998, 47, 431–443. [Google Scholar]
- Fotheringham, A.; Brunsdon, C.; Charlton, M. Geographically Weighted Regression: The Analysis of Spatially Varying Relationships; Wiley: Chichester, UK, 2002. [Google Scholar]
- Institut de Statistiques et d’Études Économiques (ISTEEBU) du Burundi et ICF International. Burundi Enquête Démographique et de Santé 2010: Rapport de synthèse; ISTEEBU et ICF: Calverton, MA, USA, 2010.
- Checchi, F.; Cox, J.; Balkan, S.; Tamrat, A.; Priotto, G.; Alberti, K.P.; Guthmann, J.P. Malaria epidemics and interventions, Kenya, Burundi, Southern Sudan, and Ethiopia, 1999–2004. Emerg. Infect. Dis. 2006, 12, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Guthmann, J.P.; Bonnet, M.; Ahoua, L.; Dantoine, F.; Balkan, S.; van Herp, M.; Tamrat, A.; Legros, D.; Brown, V.; Checchi, F. Death rates from malaria epidemics, Burundi and Ethiopia. Emerg. Infect. Dis. 2007, 13, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Etuk, E.U.; Egua, M.A.; Muhammad, A.A. Prescription pattern of antimalarial drugs in children below 5 years in a tertiary health institution in Nigeria. Ann. Afr. Med. 2008, 7, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Nyarko, S.H.; Cobblah, A. Sociodemographic Determinants of malaria among under-five children in Ghana. Malar. Res. Treat. 2014, 2014, 304361. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, L.; Ou, C.Q.; Li, L.; Zhuang, Y.; Yang, J.; Zhou, Y.X.; Qian, J.; Chen, P.Y.; Liu, Q.Y. Malaria incidence from 2005–2013 and its associations with meteorological factors in Guangdong, China. Malar. J. 2015, 14, 116. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Statistics and Information Systems. In Global Health Estimates; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Siraj, A.S.; Santos-Vega, M.; Bouma, M.J.; Yadeta, D.; Ruiz Carrascal, D.; Pascual, M. Altitudinal changes in malaria incidence in highlands of Ethiopia and Colombia. Science 2014, 343, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Alonso, D.; Bouma, M.J.; Pascual, M. Epidemic malaria and warmer temperatures in recent decades in an East African highland. Proc. Biol. Sci. 2011, 278, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Himeidan, Y.E.; Kweka, E.J. Malaria in East African highlands during the past 30 years: Impact of environmental changes. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Bødker, R.; Akida, J.; Shayo, D.; Kisinza, W.; Msangeni, H.A.; Pedersen, E.M.; Lindsay, S.W. Relationship between altitude and intensity of malaria transmission in the Usambara Mountains, Tanzania. J. Med. Entomol. 2003, 40, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Protopopoff, N.; Van Bortel, W.; Speybroeck, N.; Van Geertruyden, J.P.; Baza, D.; D’Alessandro, U.; Coosemans, M. Ranking malaria risk factors to guide malaria control efforts in African highlands. PLoS ONE 2009, 4, e8022. [Google Scholar] [CrossRef] [PubMed]
- Minakawa, N.; Omukunda, E.; Zhou, G.; Githeko, A.; Yan, G. Malaria vector productivity in relation to the highland environment in Kenya. Am. J. Trop. Med. Hyg. 2006, 75, 448–453. [Google Scholar] [PubMed]
- Siraj, A.S.; Bouma, M.J.; Santos-Vega, M.; Yeshiwondim, A.K.; Rothman, D.S.; Yadeta, D.; Sutton, P.C.; Pascual, M. Temperature and population density determine reservoir regions of seasonal persistence in highland malaria. Proc. Biol. Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, C.A.; Chambo, W.; Mwaimu, M.; Magogo, F.; Carneiro, I.A.; Curtis, C.F. Variation of malaria transmission and morbidity with altitude in Tanzania and with introduction of alphacypermethrin treated nets. Malar. J. 2003, 2. [Google Scholar] [CrossRef] [PubMed]
- Kamugisha, M.L.; Gesase, S.; Mlwilo, T.D.; Mmbando, B.P.; Segeja, M.D.; Minja, D.T.; Massaga, J.J.; Msangeni, H.A.; Ishengoma, D.R.; Lemnge, M.M. Malaria specific mortality in lowlands and highlands of Muheza district, North-Eastern Tanzania. Tanzan Health Res. Bull. 2007, 9, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.M. The control of epidemic malaria in the highlands of Western Kenya: After the campaign. J. Trop. Med. Hyg. 1964, 67, 230–237. [Google Scholar] [PubMed]
- Muturi, E.J.; Shililu, J.I.; Jacob, B.G.; Mwangangi, J.M.; Mbogo, C.M.; Githure, J.I.; Novak, R.J. Diversity of riceland mosquitoes and factors affecting their occurrence and distribution in Mwea, Kenya. J. Am. Mosq. Control Assoc. 2008, 24, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, M.O.; Tedesco, C.; McTighe, T.J.; Austin, C.; Kitron, U. Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area, 2002. Int. J. Health Geogr. 2004, 3. [Google Scholar] [CrossRef] [PubMed]
| Variable | 2010 | 2011 | 2012 | Total |
|---|---|---|---|---|
| Male | 3262 | 5847 | 4361 | 13,470 |
| Female | 2968 | 5281 | 3775 | 12,024 |
| Total Cases | 6230 | 11,128 | 8136 | 25,494 |
| Urban | 21 | 42 | 31 | 94 |
| Rural | 4245 | 7154 | 4366 | 15,765 |
| Rural-Urban | 1913 | 3730 | 3692 | 9335 |
| Unclassified | 51 | 202 | 47 | 300 |
| Total Cases | 6230 | 11,128 | 8136 | 25,494 |
| Season | Total Cases | Range of Cases Per Season | Average No. of Cases per Season | St. Dev |
|---|---|---|---|---|
| Major Wet a | 8155 | 0–364 | 37.41 | 90.295 |
| Minor Wet b | 3433 | 0–518 | 15.745 | 60.3 |
| Major Dry c | 4048 | 0–485 | 39.1 | 39.0 |
| Minor Dry d | 2738 | 0–364 | 12.58 | 37.04 |
| Season | β | R2 |
|---|---|---|
| OLS│GWR | OLS│GWR | |
| Major Wet a | −0.045│−0.005 | 0.01│0.013 |
| Minor Wet b | −0.030│−0.260 | 0.018│0.030 |
| Major Dry c | −0.030│−0.021 | 0.03│0.030 |
| Minor Dry d | −0.015│−0.010 | 0.015│0.020 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moise, I.K.; Roy, S.S.; Nkengurutse, D.; Ndikubagenzi, J. Seasonal and Geographic Variation of Pediatric Malaria in Burundi: 2011 to 2012. Int. J. Environ. Res. Public Health 2016, 13, 425. https://doi.org/10.3390/ijerph13040425
Moise IK, Roy SS, Nkengurutse D, Ndikubagenzi J. Seasonal and Geographic Variation of Pediatric Malaria in Burundi: 2011 to 2012. International Journal of Environmental Research and Public Health. 2016; 13(4):425. https://doi.org/10.3390/ijerph13040425
Chicago/Turabian StyleMoise, Imelda K., Shouraseni Sen Roy, Delphin Nkengurutse, and Jacques Ndikubagenzi. 2016. "Seasonal and Geographic Variation of Pediatric Malaria in Burundi: 2011 to 2012" International Journal of Environmental Research and Public Health 13, no. 4: 425. https://doi.org/10.3390/ijerph13040425
APA StyleMoise, I. K., Roy, S. S., Nkengurutse, D., & Ndikubagenzi, J. (2016). Seasonal and Geographic Variation of Pediatric Malaria in Burundi: 2011 to 2012. International Journal of Environmental Research and Public Health, 13(4), 425. https://doi.org/10.3390/ijerph13040425





