Predictors of Urinary 3-Phenoxybenzoic Acid Levels in 50 North Carolina Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Diary Collection
2.3. 24-h Urine Sample Collection
2.4. Analysis of the 24-h Urine Samples
2.5. Statistical Analysis of the Data
3. Results
3.1. Urinary 3-PBA Concentrations
3.2. Predictors of Urinary 3-PBA Concentrations
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Casida, J.E. Pyrethrum flowers and pyrethroid insecticides. Environ. Health Perspect. 1980, 34, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C. Insecticide discovery. An evaluation and analysis. Pestic. Biochem. Physiol. 2013, 107, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Amweg, E.L.; Weston, D.P.; Ureda, N.M. Use and toxicity of pyrethroid pesticides in the central valley, California, USA. Environ. Toxicol. Chem. 2005, 24, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Weston, D.P.; Lydy, M.J. Urban and agricultural sources of pyrethroid insecticides to the Sacramento-San Joaquin Delta of California. Environ. Sci. Technol. 2010, 44, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Horton, M.K.; Jacobson, J.B.; McKelvey, W.; Holmes, D.; Fincher, B.; Quantano, A.; Paez Diaz, B.; Shabbazz, F.; Shepard, P.; Rundle, A.; et al. Characterization of residential control products used in inner city communities in New York City. J. Expo. Sci. Environ. Epidemiol. 2011, 21, 291–301. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency (U.S. EPA). Pyrethrins/Pyrethroids Cumulative Risk Assessment. 2011. Available online: https://www.regulations.gov/document?D=EPA-HQ-OPP-2011-0746-0003 (accessed on 22 November 2016). [Google Scholar]
- Morgan, M.K.; Sheldon, L.S.; Croghan, C.W.; Jones, P.A.; Chuang, J.C.; Wilson, N.K. An observational study of 127 preschool children at their homes and daycare centers in Ohio: Environmental pathways to cis- and trans-permethrin exposure. Environ. Res. 2007, 104, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Starr, J.M.; Graham, S.; Stout, D.; Andrews, K.; Nishioka, M. Pyrethroid pesticides and their metabolites in vacuum cleaner dust collected from homes and day-care centers. Environ. Res. 2008, 108, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Riederer, A.M.; Hunter, R.E.; Hayden, S.W.; Ryan, P.B. Pyrethroid and organophosphorus pesticides in composite diet samples from Atlanta, USA adults. Environ. Sci. Technol. 2010, 44, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Melnyk, L.J.; McCombs, M.; Brown, G.G.; Raymer, J.J.; Nishioka, M.G.; Buehler, S.; Freeman, N.; Michael, L.C. Community duplicate diet methodology: A new tool for estimating dietary exposures to pesticides. J. Environ. Monit. 2012, 14, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Trunnelle, K.J.; Bennett, D.H.; Tulve, N.S.; Clifton, M.S.; Davis, M.D.; Calafat, A.M.; Moran, R.; Tancredi, D.J.; Hertz-Picciotto, I. Urinary Pyrethroid and Chlorpyrifos Metabolite Concentrations in Northern California Families and Their Relationship to Indoor Residential Insecticide Levels, Part of the Study of Use of Products and Exposure Related Behavior (SUPERB). Environ. Sci. Technol. 2014, 48, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Riederer, A.M.; Bartell, S.M.; Barr, D.B.; Ryan, P.B. Diet and nondiet predictors of urinary 3-phenoxybenzoic acid in NHANES 1999–2002. Environ. Health Perspect. 2008, 116, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Olsson, A.O.; Wong, L.Y.; Udunka, S.O.; Baker, S.E.; Whitehead, R.D.; Magsumbol, M.S.; Williams, B.L.; Needham, L.L. Urinary concentrations of metabolites of pyrethroid insecticides in the general US population: National Health and Nutrition Examination Survey 1999–2002. Environ. Health Perspect. 2010, 118, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Leng, G.; Kuhn, K.H.; Idel, H. Biological monitoring of pyrethroids in blood and pyrethroid metabolites in urine; Applications and limitations. Sci. Total Environ. 1997, 199, 173–181. [Google Scholar] [CrossRef]
- Ratelle, M.; Cote, J.; Bouchard, M. Toxicokinetics of permethrin biomarkers of exposure in orally exposed volunteers. Toxicol. Lett. 2015, 232, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Fourth National Report on Human Exposure to Environmental Chemicals. 2009. Available online: https://www.cdc.gov/exposurereport/pdf/fourthreport.pdf (accessed on 24 June 2016). [Google Scholar]
- Berkowitz, G.S.; Obel, J.; Deych, E.; Lapinski, R.; Godbold, J.; Liu, Z.; Landrigan, P.J.; Wolff, M.S. Exposure to indoor pesticides during pregnancy in a multiethnic urban cohort. Environ. Health Perspect. 2003, 111, 79–84. [Google Scholar] [CrossRef] [PubMed]
- McKelvey, W.; Jacobson, J.B.; Kass, D.; Barr, D.B.; Davis, M.; Calafat, A.M.; Aldous, K.M. Population-based biomonitoring of exposure to organophosphate and pyrethroid pesticides in New York City. Environ. Health Perspect. 2013, 121, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.K. Predictors of urinary levels of 2,4-dichlorophenoxyacetic acid, 3,5,6-trichloro-2-pyridinol, 3-phenoxybenzoic acid, and pentachlorophenol in 121 adults from Ohio. Int. J. Hyg. Environ. Health 2015, 218, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.K.; Sobus, J.R.; Boyd-Barr, D.; Croghan, C.W.; Chen, F.; Walker, R.; Alston, L.; Andersen, E.; Clifton, M.S. Temporal variability of pyrethroid metabolite levels in bedtime, morning, and 24-h urine samples for 50 adults in North Carolina. Environ. Res. 2016, 144, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.M.; Sobus, J.R.; Strynar, M.J.; Pleil, J.D.; Nakayama, S.F. Evaluating an alternative method for rapid urinary creatinine determination. J. Toxicol. Environ. Health A 2014, 77, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Verbovsek, T. A comparison of parameters below the limit of detection in geochemical analyses by substitution methods. Mater. Geoenviron. 2011, 58, 393–404. [Google Scholar]
- Morgan, M.K.; Sheldon, L.S.; Thomas, K.W.; Egeghy, P.P.; Croghan, C.W.; Jones, P.A.; Chuang, J.C.; Wilson, N.K. Adult and children’s exposure to 2,4-D from multiple sources and pathways. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary creatinine concentrations in the US population: Implications for urinary biologic monitoring measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Fourth National Report on Human Exposures to Environmental Chemicals. Updated Tables 2015. Available online: http://www.cdc.gov/biomonitoring/pdf/FourthReport_UpdatedTables_Feb2015.pdf (accessed on 12 July 2016).
- Ueyama, J.; Kimata, A.; Kamijima, M.; Hamajima, N.; Ito, Y.; Suzuki, K.; Inoue, T.; Yamamoto, K.; Takagi, K.; Saito, I.; et al. Urinary excretion of 3-phenoxybenzoic acid in middle-aged and elderly general population of Japan. Environ. Res. 2009, 109, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zheng, M.; Wu, C.; Wang, G.; Feng, C.; Zhou, Z. Urinary pyrethroid metabolites among pregnant women in an agricultural area of the Province of Jiangsu, China. Int. J. Hyg. Environ. Health 2012, 215, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Wielgomas, B.; Piskunowicz, M. Biomonitoring of pyrethroid exposure among rural and urban populations in northern Poland. Chemosphere 2013, 93, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.C.; Cantonwine, D.E.; Del Toro, A.L.V.; Calafat, A.M.; Valentin-Blasini, L.; Davis, M.D.; Baker, S.D.; Alshawabkeh, A.N.; Cordero, J.F.; Meeker, J.D. Urinary biomarkers of exposure to insecticides, herbicides, and one insect repellent among pregnant women in Puerto Rico. Environ. Health 2014, 13, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Dewailly, E.; Forde, M.; Robertson, L.; Kaddar, N.; Sidi, E.A.; Cote, S.; Gaudreau, E.; Drescher, O.; Ayotte, P. Evaluation of pyrethroid exposure in pregnant women from 10 Caribbean countries. Environ. Int. 2014, 63, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Bradman, A.; Kogut, K.; Eisen, A.; Jewell, N.; Quirós-Alcalá, L.; Castorina, R.; Chevrier, J.; Holland, N.; Boyd Barr, D.; Kavanagh-Baird, G.; et al. Variability of organophosphorus pesticide metabolite levels in spot and 24-hr urine samples collected from young children during 1 week. Environ. Health Perspect. 2013, 121, 119–124. [Google Scholar]
- Wielgomas, B. Variability of urinary excretion of pyrethroid metabolites in seven persons over seven consecutive days—Implications for observational studies. Toxicol. Lett. 2013, 221, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.D.; McCann, T.; Naleway, C.; Woods, J.S.; Leroux, B.G.; Bollen, A.M. The validity of spot urine samples for low-level occupational mercury exposure assessment and relationship to porphyrin and creatinine excretion rates. J. Pharmacol. Exp. Ther. 1996, 277, 239–244. [Google Scholar] [PubMed]
- Kimata, A.; Takaaki, K.; Ueyama, J.; Yamamoto, K.; Kamijima, M.; Suzuki, K.; Inoue, T.; Ito, Y.; Hamajima, N. Relationship between dietary habits and urinary concentrations of 3-phenoxybenzoic acid in a middle-aged and elderly general population in Japan. Environ. Health Prev. Med. 2009, 14, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Fortes, C.; Mastroeni, S.; Pilla, M.A.; Antonelli, G.; Lunghini, L.; Aprea, C. The relation between dietary habits and urinary levels of 3-phenoxybenzoic acid, a pyrethroid metabolite. Food Chem. Toxicol. 2013, 52, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Preedy, V.R. Pesticide Residues in Coffee Agroecosystems. In Coffee in Health and Disease Prevention; Academic Press: London, UK, 2014; p. 1045. [Google Scholar]
- Chen, L.; Zhao, T.; Pan, C.; Ross, J.H.; Krieger, R.L. Preformed biomarkers including dialkylphosphate (DAPs) in produce may confound biomonitoring in pesticide exposure and risk assessment. J. Agric. Food Chem. 2012, 60, 9342–9351. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Morgan, M.K.; Graham, S.; Starr, J.M. Measurement of Pyrethroids and Their Environmental Degradates in Fresh Fruits and Vegetables using a Modification of the Quick Easy Cheap Effective Rugged Safe (QuEChERS) Method. Talanta 2016, 151, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.K.; MacMillan, D.K.; Zehr, D.; Sobus, J.R. Pyrethroid insecticides and their environmental degradates in repeated duplicate-diet solid food samples of 50 adults. J. Expo. Sci. Environ. Epidemiol. 2016, 9, 2964–2985. [Google Scholar]
| 3-PBA | n c | % d | Mean ± SD e | GM f | Minimum | Percentiles | Maximum | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 25th | 50th | 75th | 95th | |||||||
| ng/mL | 50 | 98 | 2.43 ± 2.59 | 1.68 | <0.25 | 0.99 | 1.41 | 2.87 | 8.85 | 13.7 |
| ng/mg | 50 | 98 | 2.85 ± 3.04 | 1.74 | <0.25 | 0.94 | 1.53 | 3.57 | 10.1 | 13.6 |
| Factors | n a | % b | 3-PBA | |
|---|---|---|---|---|
| GM (95% CI) c | p-Value | |||
| Demographic factors | ||||
| Sex | ||||
| Female | 30 | 60 | 1.73 (1.18–2.53) | 0.769 |
| Male | 20 | 40 | 1.60 (1.28–2.01) | |
| Age | ||||
| ≤30 years old | 27 | 54 | 1.59 (1.27–1.99) | 0.64 |
| >30 years old | 23 | 46 | 1.78 (1.22–2.61) | |
| Race d | ||||
| White | 25 | 69 | 1.86 (1.49–2.33) | 0.699 |
| Black | 11 | 31 | 1.64 (1.12–2.40) | |
| BMI e | ||||
| Underweight/Normal (<25.0) | 17 | 34 | 1.78 (1.11–2.84) | 0.734 |
| Overweight (25.0–29.9) | 13 | 26 | 1.85 (1.26–2.72) | |
| Obese (≥30.0) | 20 | 40 | 1.49 (0.97–2.30) | |
| Lifestyle factors | ||||
| Sampling season f | ||||
| Spring | 16 | 32 | 1.53 (0.96–2.43) | 0.803 |
| Summer/fall | 15 | 30 | 1.63 (1.04–2.57) | |
| Winter | 19 | 38 | 1.85 (1.21–2.82) | |
| Insecticide use at home g | ||||
| Yes | 28 | 56 | 1.41 (1.12–2.40) | 0.102 |
| No | 22 | 44 | 2.09 (1.67–2.62) | |
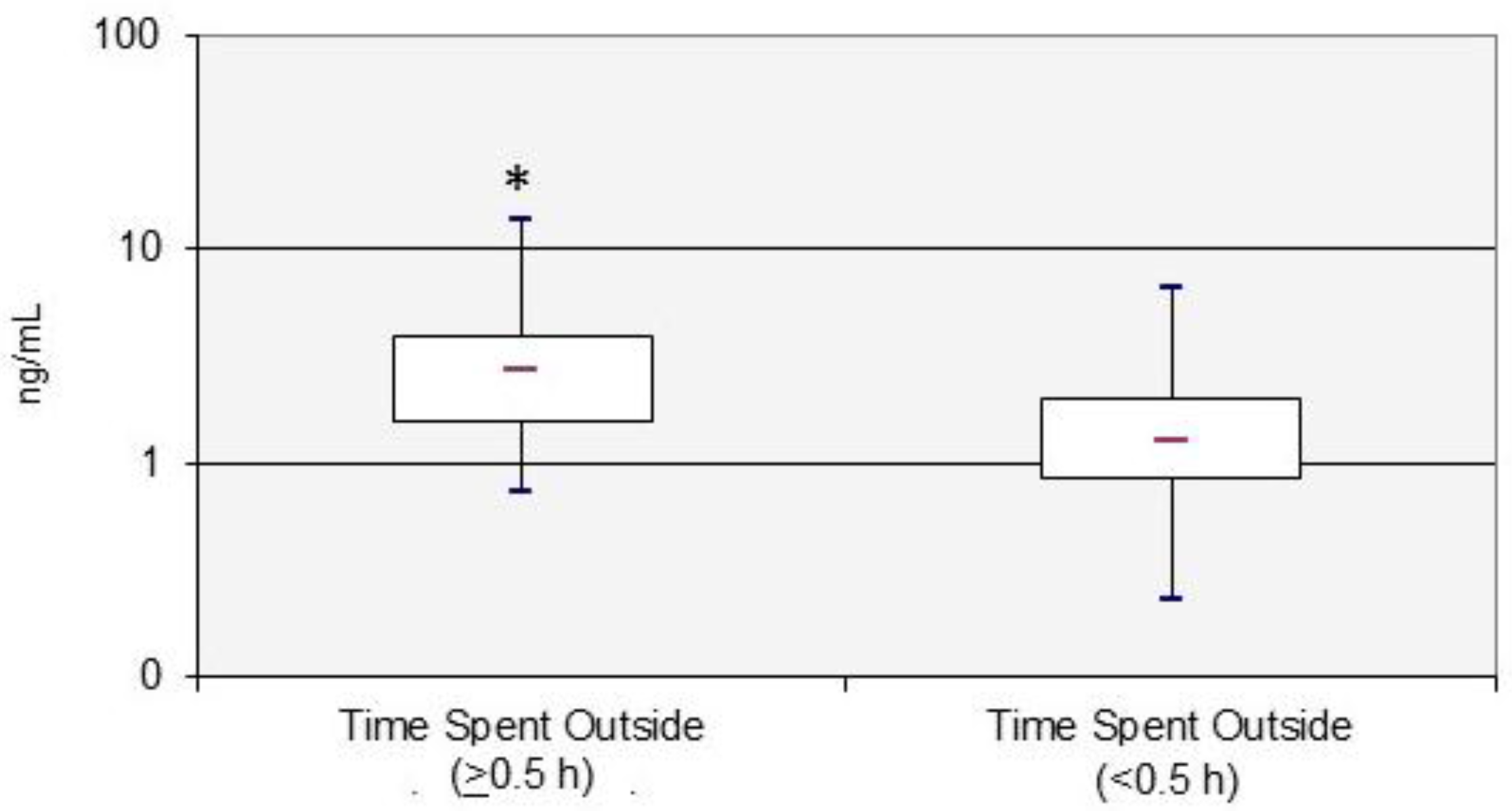
| Time spent outside at home | ||||
| <0.5 h | 34 | 68 | 1.32 (1.05–1.65) | 0.002 |
| ≥0.5 h | 16 | 32 | 2.80 (1.91–4.10) | |
| Dietary Factors | n a | % b | 3-PBA | |
|---|---|---|---|---|
| GM (95% CI) c | p-Value | |||
| Fruits | ||||
| Apples | ||||
| 0 times | 35 | 70 | 1.56 (1.25–1.96) | 0.383 |
| ≥1 time | 15 | 30 | 1.97 (1.34–2.89) | |
| Bananas | ||||
| 0 times | 35 | 70 | 1.46 (1.16–1.83) | 0.074 |
| ≥1 time | 15 | 30 | 2.32 (1.58–3.40) | |
| Berries d | ||||
| 0 times | 30 | 60 | 1.83 (1.46–2.29) | 0.385 |
| ≥1 time | 20 | 40 | 1.47 (1.01–2.16) | |
| Strawberries | ||||
| 0 times | 36 | 72 | 1.69 (1.35–2.11) | 0.94 |
| ≥1 time | 14 | 28 | 1.65 (1.13–2.42) | |
| Vegetables | ||||
| Beans e | ||||
| 0 times | 34 | 68 | 1.49 (1.19–1.86) | 0.141 |
| ≥1 time | 16 | 32 | 2.17 (1.48–3.18) | |
| Carrots | ||||
| 0 times | 32 | 64 | 1.75 (1.40–2.19) | 0.626 |
| ≥1 time | 18 | 36 | 1.55 (1.06–2.27) | |
| Leafy f | ||||
| 0 times | 23 | 46 | 1.82 (1.46–2.28) | 0.518 |
| ≥1 time | 27 | 54 | 1.56 (1.06–2.29) | |
| Onions | ||||
| 0 times | 32 | 64 | 1.56 (1.25–1.96) | 0.439 |
| ≥1 times | 18 | 36 | 1.90 (1.30–2.78) | |
| Potatoes g | ||||
| 0 times | 34 | 68 | 1.72 (1.38–2.16) | 0.745 |
| ≥1 times | 16 | 32 | 1.58 (1.08–2.32) | |
| Peppers h | ||||
| 0 times | 33 | 66 | 1.84 (1.47–2.30) | 0.286 |
| ≥1 times | 17 | 34 | 1.40 (0.96–2.05) | |
| Tomatoes | ||||
| 0 times | 20 | 40 | 1.62 (1.30–2.03) | 0.831 |
| ≥1 times | 30 | 60 | 1.71 (1.17–2.51) | |
| Meats | ||||
| Baked or grilled chicken/turkey | ||||
| 0 times | 37 | 74 | 1.87 (1.49–2.34) | 0.127 |
| ≥1 times | 13 | 26 | 1.23 (0.84–1.80) | |
| Fried chicken or chicken nuggets | ||||
| 0 times | 40 | 80 | 1.60 (1.28–2.01) | 0.458 |
| ≥1 times | 10 | 20 | 2.01 (1.37–2.94) | |
| Ham or lunch meats | ||||
| 0 times | 35 | 70 | 1.66 (1.33–2.08) | 0.923 |
| ≥1 times | 15 | 30 | 1.71 (1.16–2.50) | |
| Dairy | ||||
| Cheeses | ||||
| 0 times | 33 | 66 | 1.53 (1.22–1.91) | 0.285 |
| ≥1 time | 17 | 34 | 2.01 (1.37–2.94) | |
| Yogurts | ||||
| 0 times | 32 | 64 | 1.65 (1.32–2.07) | 0.876 |
| ≥1 time | 18 | 36 | 1.72 (1.17–2.52) | |
| Grains | ||||
| Breads i | ||||
| 0 times | 27 | 54 | 2.01 (1.61–2.52) | 0.1 |
| ≥1 time | 23 | 46 | 1.35 (0.92–1.98) | |
| Cereals | ||||
| 0 times | 36 | 72 | 1.66 (1.33–2.08) | 0.896 |
| >1 time | 14 | 28 | 1.72 (1.17–2.52) | |
| Spaghetti and other pastas | ||||
| 0 times | 33 | 66 | 1.63 (1.30–2.04) | 0.754 |
| ≥1 time | 17 | 34 | 1.77 (1.21–2.59) | |
| Other Foods | ||||
| Salty snacks j | ||||
| 0 times | 24 | 48 | 1.69 (1.35–2.11) | 0.956 |
| ≥1 time | 26 | 52 | 1.67 (1.14–2.44) | |
| Sweet snacks k | ||||
| 0 times | 27 | 54 | 1.53 (1.23–1.92) | 0.425 |
| ≥1 time | 23 | 46 | 1.86 (1.27–2.73) | |
| Nuts and nut butters | ||||
| 0 times | 34 | 68 | 1.54 (1.23–1.93) | 0.308 |
| ≥1 time | 16 | 32 | 2.01 (1.37–2.94) | |
| Beverages | ||||
| Soft drinks (sugar) | ||||
| 0 times | 35 | 70 | 1.50 (1.14–1.98) | 0.169 |
| ≥1 time | 15 | 30 | 2.16 (1.40–3.33) | |
| Soft drinks (sugarless) | ||||
| 0 times | 38 | 76 | 1.76 (1.35–2.29) | 0.486 |
| ≥1 time | 12 | 24 | 1.44 (0.87–2.38) | |
| Coffee | ||||
| 0 times | 27 | 54 | 1.42 (1.00–2.02) | 0.137 |
| ≥1 time | 23 | 46 | 2.03 (1.52–2.72) | |
| Fruit juices | ||||
| 0 times | 37 | 74 | 1.50 (1.19–1.90) | 0.122 |
| ≥1 time | 13 | 26 | 2.29 (1.27–4.14) | |
| Milk | ||||
| 0 times | 31 | 62 | 1.87 (1.45–2.41) | 0.247 |
| ≥1 time | 19 | 38 | 1.40 (0.89–2.21) | |
| Water l | ||||
| ≤1 time | 13 | 26 | 1.66 (0.99–2.79) | 0.963 |
| >1 time | 37 | 74 | 1.68 (1.29–2.19) | |
| Factors | Factor Type | β Coefficient | Standard Error | p-Value |
|---|---|---|---|---|
| Intercept | --- | −1.90 | 0.922 | 0.046 b |
| 24-h time spent outside c | Lifestyle | 0.0006 | ||
| ≥0.5 h | 0.778 | 0.211 | ||
| <0.5 h | 0 (ref.) | |||
| Beans | Dietary | 0.100 | ||
| Yes | 0.404 | 0.240 | ||
| No | 0 (ref.) | |||
| Breads | Dietary | 0.019 | ||
| Yes | −0.490 | 0.200 | ||
| No | 0 (ref.) | |||
| Coffee | Dietary | 0.007 | ||
| Yes | 0.661 | 0.232 | ||
| No | 0 (ref.) | |||
| Fruit juices | Dietary | 0.066 | ||
| Yes | 0.442 | 0.234 | ||
| No | 0 (ref.) | |||
| Creatinine level (mg/dL) d | --- | 0.403 | 0.187 | 0.037 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, M.; Jones, P.; Sobus, J.; Boyd Barr, D. Predictors of Urinary 3-Phenoxybenzoic Acid Levels in 50 North Carolina Adults. Int. J. Environ. Res. Public Health 2016, 13, 1172. https://doi.org/10.3390/ijerph13111172
Morgan M, Jones P, Sobus J, Boyd Barr D. Predictors of Urinary 3-Phenoxybenzoic Acid Levels in 50 North Carolina Adults. International Journal of Environmental Research and Public Health. 2016; 13(11):1172. https://doi.org/10.3390/ijerph13111172
Chicago/Turabian StyleMorgan, Marsha, Paul Jones, Jon Sobus, and Dana Boyd Barr. 2016. "Predictors of Urinary 3-Phenoxybenzoic Acid Levels in 50 North Carolina Adults" International Journal of Environmental Research and Public Health 13, no. 11: 1172. https://doi.org/10.3390/ijerph13111172
APA StyleMorgan, M., Jones, P., Sobus, J., & Boyd Barr, D. (2016). Predictors of Urinary 3-Phenoxybenzoic Acid Levels in 50 North Carolina Adults. International Journal of Environmental Research and Public Health, 13(11), 1172. https://doi.org/10.3390/ijerph13111172






