Occurrence of Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Milk and Yogurt and Their Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
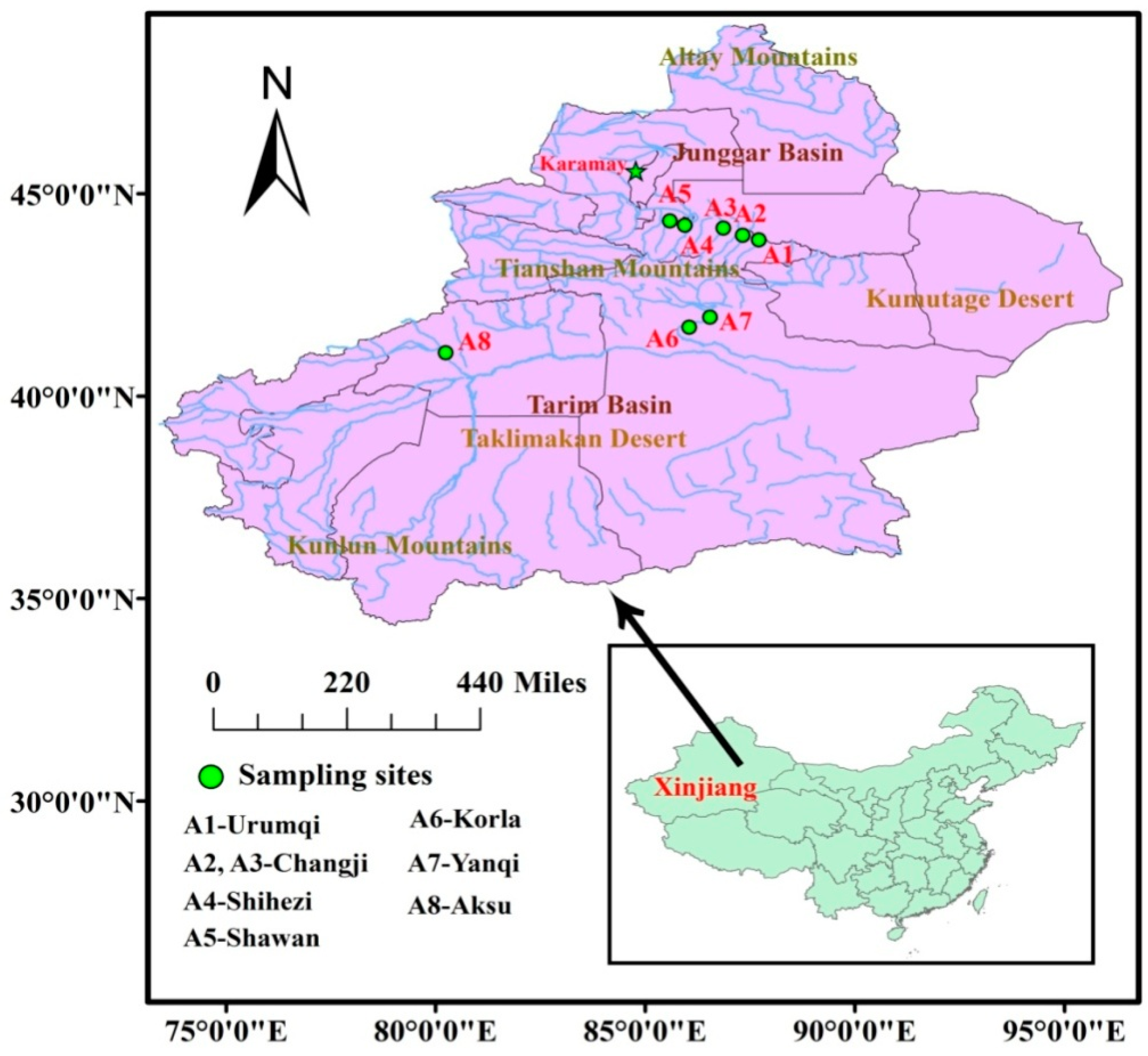
2.1. Samples
2.2. Chemicals and Materials
2.3. Sample Preparation and Instrumental Analysis
2.4. Quality Control and Quality Assurance
2.5. Risk Assessment and Statistical Analysis
3. Results and Discussion
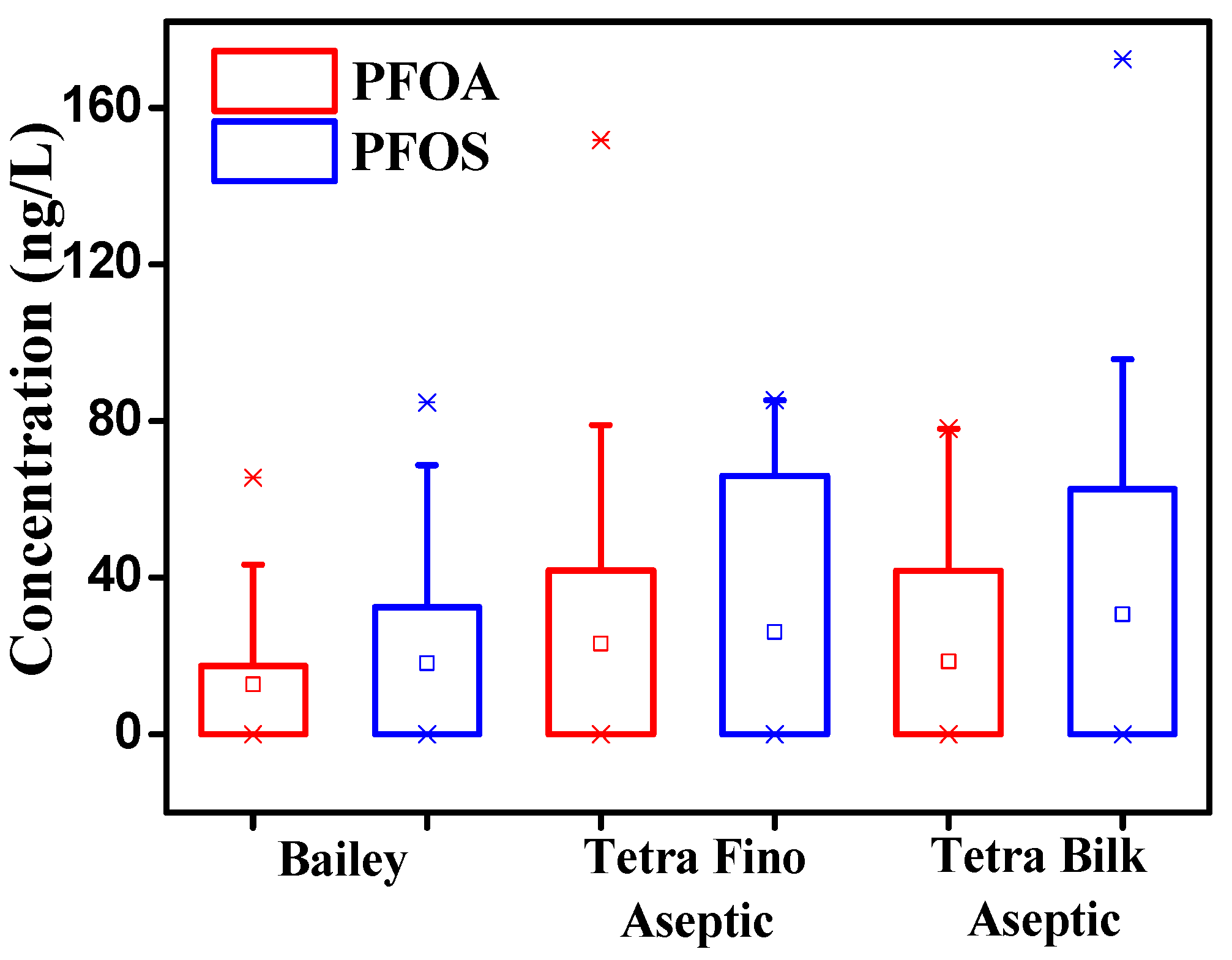
3.1. Concentrations of PFOS and PFOA in Milk and Yogurt
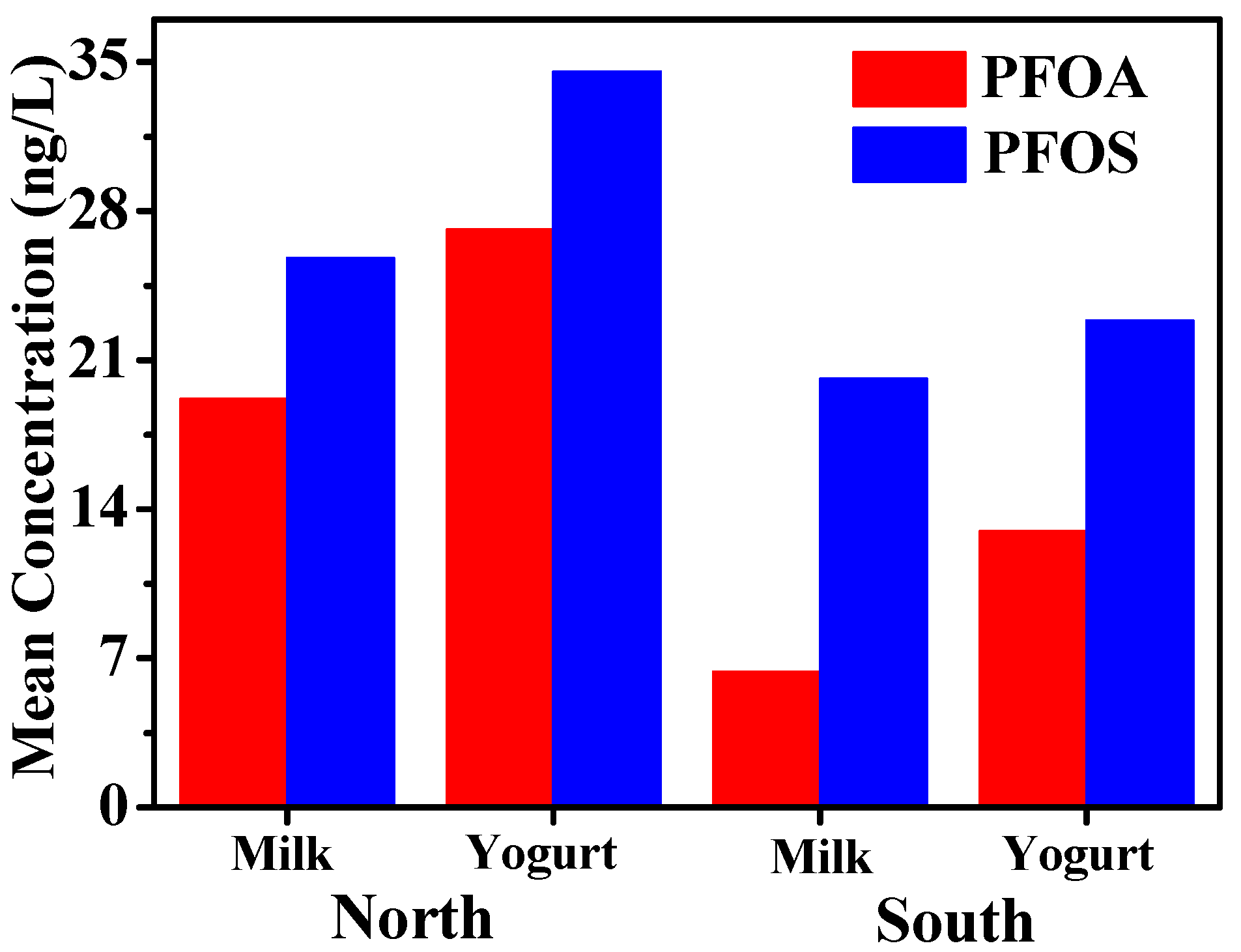
3.2. Comparison of PFOS and PFOA Concentrations in Samples from Different Areas of Xinjiang
3.3. Source Analysis of PFOA and PFOS in Milk and Yogurt
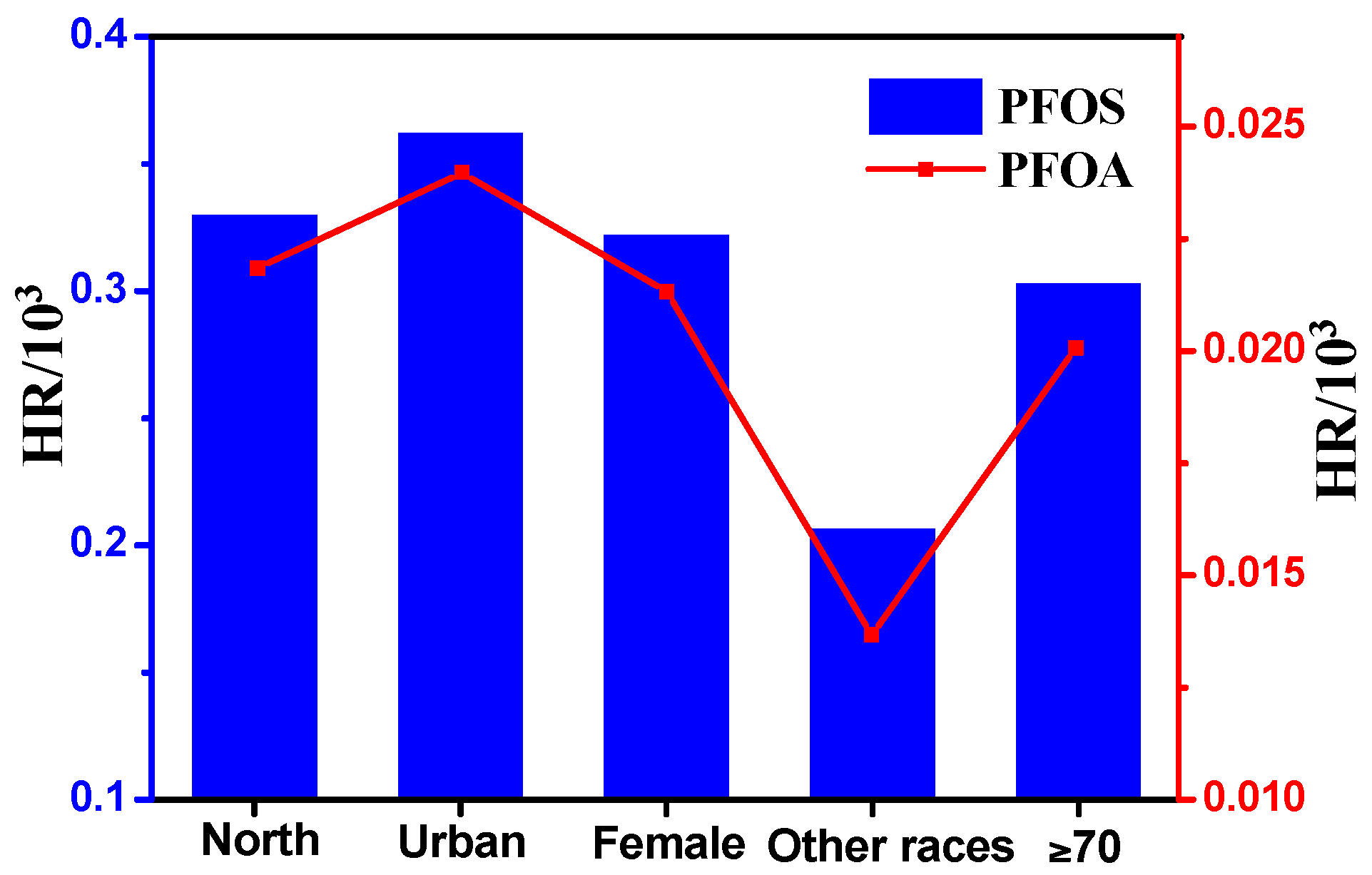
3.4. Preliminary Health Risk Assessment for the General Xinjiang Population
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Surma, M.; Zieliński, H. What do we know about the risk arising from perfluorinated compounds? Pol. J. Environ. Stud. 2015, 24, 449–457. [Google Scholar] [CrossRef]
- Guerranti, C.; Perra, G.; Corsolini, S.; Focardi, S.E. Pilot study on levels of perfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) in selected foodstuffs and human milk from Italy. Food Chem. 2013, 140, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Hlouskova, V.; Hradkova, P.; Poustka, J.; Brambilla, G.; De Filipps, S.P.; D’Hollander, W.; Bervoets, L.; Herzke, D.; Huber, S.; de Voogt, P.; et al. Occurrence of perfluoroalkyl substances (PFASs) in various food items of animal origin collected in four European countries. Food Addit. Contam. 2013, 30, 1918–1932. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Llorca, M.; Kock-Schulmeyer, M.; Skrbic, B.; Oliveira, L.S.; Martinello, K.D.; Al-Dhabi, N.A.; Antic, I.; Farre, M.; Barcelo, D. Assessment of perfluoroalkyl substances in food items at global scale. Environ. Res. 2014, 135, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, S.K.; Chan, H.M.; Moisey, J.; Dabeka, R.; Tittlemier, S.A. Historic dietary exposure to perfluorooctane sulfonate, perfluorinated carboxylates, and fluorotelomer unsaturated carboxylates from the consumption of store-bought and restaurant foods for the Canadian population. J. Agric. Food Chem. 2009, 57, 8534–8544. [Google Scholar] [CrossRef] [PubMed]
- Ashley-Martin, J.; Dodds, L.; Arbuckle, T.E.; Morisset, A.S.; Fisher, M.; Bouchard, M.F.; Shapiro, G.D.; Ettinger, A.S.; Monnier, P.; Dallaire, R.; et al. Maternal and neonatal levels of perfluoroalkyl substances in relation to gestational weight gain. Int. J. Environ. Res. Public Health 2016, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- D’eon, J.C.; Mabury, S.A. Exploring indirect sources of human exposure to perfluoroalkyl carboxylates (PFCAs): Evaluating uptake, elimination, and biotransformation of polyfluoroalkyl phosphate esters (PAPs) in the rat. Environ. Health Perspect. 2011, 119, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Al-sheyab, N.A.; Al-Qudah, K.M.; Tahboub, Y.R. Levels of perfluorinated compounds in human breast milk in Jordan: The impact of sociodemographic characteristics. Environ. Sci. Pollut. Res. 2015, 22, 12415–12423. [Google Scholar] [CrossRef] [PubMed]
- Porpora, M.G.; Lucchini, R.; Abballe, A.; Ingelido, A.M.; Valentini, S.; Fuggetta, E.; Cardi, V.; Ticino, A.; Marra, V.; Fulgenzi, A.R.; et al. Placental transfer of persistent organic pollutants: A preliminary study on mother-newborn pairs. Int. J. Environ. Res. Public Health 2013, 10, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, J.; Ehlers, S.; Oberhausen, A.; Tischer, M.; Furst, P.; Schafft, H.; Lahrssen-Wiederholt, M. Absorption, distribution, and milk secretion of the perfluoroalkyl acids PFBS, PFHxS, PFOS, and PFOA by dairy cows fed naturally contaminated feed. J. Agric. Food Chem. 2013, 61, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Perfluorooctane sulfonate (PFOS), perfluorooctanoic acid (PFOA) and their salts. EFSA J. 2008, 653, 1–131. [Google Scholar]
- Zeng, X.W.; Qian, Z.M.; Vaughn, M.; Xian, H.; Elder, K.; Rodemich, E.; Bao, J.; Jin, Y.H.; Dong, G.H. Human serum levels of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in Uyghurs from Sinkiang-Uighur autonomous region, China: Background levels study. Environ. Sci. Pollut. Res. 2015, 22, 4736–4746. [Google Scholar] [CrossRef] [PubMed]
- Noorlander, C.W.; van Leeuwen, S.P.J.; Biesebeek, J.D.T.; Mengelers, M.J.B.; Zeilmaker, M.J. Levels of perfluorinated compounds in food and dietary intake of PFOS and PFOA in The Netherlands. J. Agric. Food Chem. 2011, 59, 7496–7505. [Google Scholar] [CrossRef] [PubMed]
- Vestergren, R.; Berger, U.; Glynn, A.; Cousins, I.T. Dietary exposure to perfluoroalkyl acids for the Swedish population in 1999, 2005 and 2010. Environ. Int. 2012, 49, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Tittlemier, S.A.; Volkel, W.; Wilhelm, M.; Twardella, D. Perfluorinated compounds-exposure assessment for the general population in western countries. Int. J. Hyg. Environ. Health 2009, 212, 239–270. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Jogsten, I.E.; Eriksson, U.; Martorell, I.; Perelló, G.; Nadal, M.; van Bavel, B. Human dietary exposure to perfluoroalkyl substances in Catalonia, Spain. Temporal trend. Food Chem. 2012, 135, 1575–1582. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, C.; D’Hollander, W.; Roosens, L.; Covaci, A.; Smolders, R.; Van Den Heuvel, R.; Govarts, E.; Van Campenhout, K.; Reynders, H.; Bervoets, L. First assessment of population exposure to perfluorinated compounds in Flanders, Belgium. Chemosphere 2012, 86, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Halldorsson, T.I.; Fei, C.Y.; Olsen, J.; Lipworth, L.; Mclaughlin, J.K.; Olsen, S.F. Dietary predictors of perfluorinated chemicals: A study from the Danish National Birth Cohort. Environ. Sci. Technol. 2008, 42, 8971–8977. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, J.; Ehlers, S.; Furst, P.; Schafft, H.; Lahrssen-Wiederholt, M. Transfer of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from contaminated feed into milk and meat of sheep: Pilot study. Arch. Environ. Contam. Toxicol. 2012, 63, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Haug, L.S.; Salihovic, S.; Jogsten, I.E.; Thomsen, C.; van Bavel, B.; Lindstrom, G.; Becher, G. Levels in food and beverages and daily intake of perfluorinated compounds in Norway. Chemosphere 2010, 80, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Shi, Y.L.; Pan, Y.Y.; Cai, Y.Q. Perfluorinated compounds in milk, milk powder and yoghurt purchased from markets in China. Chin. Sci. Bull. 2010, 55, 1020–1025. [Google Scholar] [CrossRef]
- Yang, L.; Wang, M.; Li, J.G.; Wang, Y.X.; Zhao, Y.F.; Wu, Y.N. Analysis of perfluoroalkyl substances and their precursors in eighteen market milk. Chin. J. Prev. Med. 2015, 49, 524–528. [Google Scholar]
- Still, M.; Schlummer, M.; Gruber, L.; Fiedler, D.; Wolz, G. Impact of industrial production and packaging processes on the concentration of per- and polyfluorinated compounds in milk and dairy products. J. Agric. Food Chem. 2013, 61, 9052–9062. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.L.; Wang, J.M.; Pan, Y.Y.; Cai, Y.Q. Tissue distribution of perfluorinated compounds in farmed freshwater fish and human exposure by consumption. Environ. Toxicol. Chem. 2012, 31, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Zhe, W.; Ma, J.P.; Liu, L.X.; Suo, F.Y.; Guan, Y.M.; Liao, P.H. Adult residents dietary structure analysis of five disease monitoring stations in Xinjiang. J. Hyg. Res. 2014, 43, 853–856. [Google Scholar]
- Barbarossa, A.; Gazzotti, T.; Zironi, E.; Serraino, A.; Pagliuca, G. Short communication: Monitoring the presence of perfluoroalkyl substances in Italian cow milk. J. Dairy Sci. 2014, 97, 3339–3343. [Google Scholar] [CrossRef] [PubMed]
- Van Asselt, E.D.; Kowalczyk, J.; van Eijkeren, J.C.H.; Zeilmaker, M.J.; Ehlers, S.; Fürst, P.; Lahrssen-Wiederholt, M.; van der Fels-Klerx, H.J. Transfer of perfluorooctane sulfonic acid (PFOS) from contaminated feed to dairy milk. Food Chem. 2013, 141, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, Q.; Zeng, G.M.; Chen, Z.M.; Jiang, M.; Yu, J. Photochemical degradation of PFOA using UV irradiation: A critical review. Sci. Sin. Chim. 2011, 41, 964–975. [Google Scholar] [CrossRef]
- Filipovic, M.; Berger, U.; McLachlan, M.S. Mass balance of perfluoroalkyl acids in the Baltic Sea. Environ. Sci. Technol. 2013, 47, 4088–4095. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jin, Y.H.; Quan, X.; Sasaki, K.; Saito, N.; Nakayama, S.F.; Sato, I.; Tsuda, S. Perfluorosulfonates and perfluorocarboxylates in snow and rain in Dalian, China. Environ. Int. 2009, 35, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Erkejan, A.; Yin, J.X.; Li, L.J. Characteristic analysis the precipitation in Xinjiang in the past 50 years. J. Shihezi Univ. 2011, 29, 737–741. (In Chinese) [Google Scholar]
- Xie, S.W.; Wang, T.Y.; Liu, S.J.; Jones, K.C.; Sweetman, A.J.; Lu, Y.L. Industrial source identification and emission estimation of perfluorooctane sulfonate in China. Environ. Int. 2013, 52, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Ma, J.; Kunisue, T.; Libelo, E.L.; Tanabe, S.; Kannan, K. Perfluorinated compounds in human breast milk from several Asian countries, and in infant formula and dairy milk from the United States. Environ. Sci. Technol. 2008, 42, 8597–8602. [Google Scholar] [CrossRef] [PubMed]
- Tittlemier, S.A.; Pepper, K.; Seymour, C.; Moisey, J.; Bronson, R.; Cao, X.; Dabeka, R.W. Dietary exposure of Canadians to perfluorinated carboxylates and perfluorooctane sulfonate via consumption of meat, fish, fast foods, and food items prepared in their packaging. J. Agric. Food Chem. 2007, 55, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, C.S.; Zhang, Y.; Meng, W. Bioaccumulation and trophic transfer of perfluorinated compounds in a eutrophic freshwater food web. Environ. Pollut. 2014, 184, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Loi, E.I.H.; Yeung, L.W.Y.; Taniyasu, S.; Lam, P.K.S.; Kannan, K.; Yamashita, N. Trophic magnification of poly- and perfluorinated compounds in a subtropical food web. Environ. Sci. Technol. 2011, 45, 5506–5513. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.E.; De Silva, A.O.; Small, J.; Williamson, M.; Wang, X.W.; Morris, A.; Katz, S.; Gamberg, M.; Muir, D.C.G. Biomagnification of perfluorinated compounds in a remote terrestrial food chain: Lichen-caribou-wolf. Environ. Sci. Technol. 2011, 45, 8665–8673. [Google Scholar] [CrossRef] [PubMed]
- Vestergren, R.; Orata, F.; Berger, U.; Cousins, I.T. Bioaccumulation of perfluoroalkyl acids in dairy cows in a naturally contaminated environment. Environ. Sci. Pollut. Res. 2013, 20, 7959–7969. [Google Scholar] [CrossRef] [PubMed]
- Gebbink, W.A.; Glynn, A.; Darnerud, P.O.; Berger, U. Perfluoroalkyl acids and their precursors in Swedish food: The relative importance of direct and indirect dietary exposure. Environ. Pollut. 2015, 198, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Taxvig, C.; Rosenmai, A.K.; Vinggaard, A.M. Polyfluorinated alkyl phosphate ester surfactants—Current knowledge and knowledge gaps. Basic Clin. Pharmacol. 2014, 115, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Zafeiraki, E.; Costopoulou, D.; Vassiliadou, I.; Bakeas, E.; Leondiadis, L. Determination of perfluorinated compounds (PFCs) in various foodstuff packaging materials used in the Greek market. Chemosphere 2014, 94, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ericson, I.; Marti-Cid, R.; Nadal, M.; Van Bavel, B.; Lindstrom, G.; Domingo, J.L. Human exposure to perfluorinated chemicals through the diet: Intake of perfluorinated compounds in foods from the Catalan (Spain) market. J. Agric. Food Chem. 2008, 56, 1787–1794. [Google Scholar] [CrossRef] [PubMed]

| Analyte | Mass Transition a | Fragment (V) | CE (V) | RT (min) | LOD (ng/L) | LOQ (ng/L) |
|---|---|---|---|---|---|---|
| PFOS | 499.0→99.0 | 160 | 55 | 6.7 | 10 | 30 |
| 499.0→80.0 | 160 | 72 | ||||
| PFOA | 412.8→368.8 | 70 | 4 | 2.0 | 5 | 15 |
| 412.8→168.9 | 70 | 16 |
| Type | PFOA 1 | PFOS 1 |
|---|---|---|
| Retail milk, n = 91 | ||
| Range | <5.0–151.8 | <10.0–172.9 |
| Mean | 16.2 | 24.5 |
| Frequency values > LOD (%) | 33.0 | 39.6 |
| Raw milk, n = 24 | ||
| Range | <10.0–25.1 | |
| Mean | 2.2 | |
| Frequency values > LOD (%) | 12.5 | |
| Yogurt, n = 54 | ||
| Range | <5.0–279.9 | <10.0–200.6 |
| Mean | 22.6 | 31.8 |
| Frequency values > LOD (%) | 37.0 | 48.1 |
| Areas | Type | Number | PFOA | PFOS | ||||
|---|---|---|---|---|---|---|---|---|
| Max | Mean | Frequency | Max | Mean | Frequency | |||
| Urumqi | aM | 36 | 151.8 | 27.1 | 47.2 | 172.9 | 35.7 | 50.0 |
| bY | 10 | 279.9 | 39.1 | 50.0 | 200.6 | 48.2 | 60.0 | |
| 1Changji | M | 10 | 79.0 | 16.6 | 40.0 | 95.8 | 20.8 | 40.0 |
| Y | 14 | 199.9 | 27.7 | 42.9 | 117.3 | 36.3 | 50.0 | |
| Shihezi | M | 12 | 65.6 | 9.3 | 25.0 | 74.8 | 13.7 | 25.0 |
| Y | 6 | 54.0 | 15.7 | 33.3 | 91.4 | 24.0 | 33.3 | |
| Shawan | M | 12 | 56.8 | 7.5 | 16.7 | 53.8 | 12.1 | 25.0 |
| Y | 6 | 77.0 | 12.8 | 16.7 | 84.3 | 22.2 | 33.3 | |
| Korla | M | 7 | 42.3 | 16.9 | 42.9 | 88.2 | 32.0 | 57.1 |
| Y | 6 | 94.9 | 23.1 | 50.0 | 152.7 | 34.7 | 50.0 | |
| Yanqi | M | 7 | <LOD | <LOD | 0 | 49.8 | 13.6 | 28.6 |
| Y | 6 | 28.4 | 4.7 | 16.7 | 45.0 | 14.9 | 33.3 | |
| Aksu | M | 7 | 15.1 | 2.2 | 14.3 | 81.5 | 14.7 | 28.6 |
| Y | 6 | 66.7 | 11.1 | 16.7 | 88.1 | 19.0 | 50.0 | |
| Group | DCmilk g/day | ADI ng/kg/day a | ||
|---|---|---|---|---|
| PFOA | PFOS | |||
| Area1 | North | 123 | 0.0328 | 0.0495 |
| South | 15 | 0.0040 | 0.0060 | |
| Area2 | Urban | 135 | 0.0360 | 0.0543 |
| Rural | 54 | 0.0144 | 0.0217 | |
| Gender | Male | 71 | 0.0190 | 0.0286 |
| Female | 77 | 0.0205 | 0.0310 | |
| Race | Han Chinese | 118 | 0.0314 | 0.0474 |
| Uighur | 32 | 0.0086 | 0.0129 | |
| Others | 120 | 0.0320 | 0.0483 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, Z.; Lu, J.; Liu, Z.; Li, S.; Wang, G.; Wang, X. Occurrence of Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Milk and Yogurt and Their Risk Assessment. Int. J. Environ. Res. Public Health 2016, 13, 1037. https://doi.org/10.3390/ijerph13101037
Xing Z, Lu J, Liu Z, Li S, Wang G, Wang X. Occurrence of Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Milk and Yogurt and Their Risk Assessment. International Journal of Environmental Research and Public Health. 2016; 13(10):1037. https://doi.org/10.3390/ijerph13101037
Chicago/Turabian StyleXing, Zhenni, Jianjiang Lu, Zilong Liu, Shanman Li, Gehui Wang, and Xiaolong Wang. 2016. "Occurrence of Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Milk and Yogurt and Their Risk Assessment" International Journal of Environmental Research and Public Health 13, no. 10: 1037. https://doi.org/10.3390/ijerph13101037
APA StyleXing, Z., Lu, J., Liu, Z., Li, S., Wang, G., & Wang, X. (2016). Occurrence of Perfluorooctanoic Acid and Perfluorooctane Sulfonate in Milk and Yogurt and Their Risk Assessment. International Journal of Environmental Research and Public Health, 13(10), 1037. https://doi.org/10.3390/ijerph13101037





