L-Asparaginase Isolated from Phaseolus vulgaris Seeds Exhibited Potent Anti-Acute Lymphoblastic Leukemia Effects In-Vitro and Low Immunogenic Properties In-Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enzymes and Reagents
2.2. Cell Line and Culture Condition
2.3. Cell Viability Assay
2.4. DNA Cell Cycle Assay
2.5. Annexin V Apoptosis Detection Assay
2.6. Asparaginase Sensitization Protocol
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pui, C.H.; Mullighan, C.G.; Evans, W.E.; Relling, M.V. Pediatric acute lymphoblastic leukemia: Where are we going and how do we get there? Blood 2012, 120, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Pui, C.H.; Carroll, W.L.; Meshinchi, S.; Arceci, J. Biology, risk stratification, and therapy of pediatric acute leukemias: An update. J. Clin. Oncol. 2011, 2, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.E.; Crom, W.R.; Abromowitch, M.; Dodge, R.; Look, A.T.; Bowman, W.P.; George, S.L.; Pui, C.H. Clinical pharmacodynamics of high-dose methotrexate in acute lymphocytic leukemia. N. Engl. J. Med. 1986, 314, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Conter, V.; Schrappe, M.; Arico, M.; Reiter, A.; Rizzari, C.; Dördelmann, M.; Valsecchi, M.; Zimmermann, M.; Ludwig, W.; Basso, G. Role of cranial radiotherapy for childhood T-cell acute lymphoblastic leukemia with high WBC count and good response to prednisone: Associazione Italiana ematologia oncologia pediatrica and the Berlin-Frankfurt-Münster groups. J. Clin. Oncol. 1997, 15, 2786–2791. [Google Scholar] [PubMed]
- Slavin, S.; Nagler, A.; Naparstek, E.; Kapelushnik, Y.; Aker, M.; Cividalli, G.; Varadi, G.; Kirschbaum, M.; Ackerstein, A.; Samuel, S. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 1998, 91, 756–763. [Google Scholar] [PubMed]
- Reiter, A.; Schrappe, M.; Ludwig, W.D.; Hiddemann, W.; Sauter, S.; Henze, G.; Zimmermann, M.; Lampert, F.; Havers, W.; Niethammer, D. Chemotherapy in 998 unselected childhood acute lymphoblastic leukemia patients: Results and conclusions of the multicenter trial ALL-BFM 86. Blood 1994, 84, 3122–3133. [Google Scholar] [PubMed]
- Cooper, S.L.; Brown, P.A. Treatment of pediatric acute lymphoblastic leukemia. Pediatr. Clin. N. Am. 2015, 62, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Broome, J.D. Evidence that the L-asparaginase of guinea pig serum is responsible for its antilymphoma effects I: Properties of the L-asparaginase of guinea pig serum in relation to those of the antilymphoma substance. J. Exp. Med. 1963, 118, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, B.; Madras, B.K.; Meister, A.; Old, L.J.; Boyse, E.A.; Stockert, E. Asparagine synthetase activity of mouse leukemias. Science 1968, 160, 533. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Ohtawa, K.; Mitsui, K.; Kodera, Y.; Hiroto, M.; Matsushima, A.; Inada, Y.; Nishimura, H. Cell cycle arrest and apoptosis of leukemia cells induced by L-asparaginase. Leukemia 1997, 11, 1858–1861. [Google Scholar] [CrossRef] [PubMed]
- Batool, T.; Makky, E.A.; Jalal, M.; Yusoff, M.M. A comprehensive review on L-asparaginase and its applications. Appl. Biochem. Biotechnol. 2016, 178, 900–923. [Google Scholar] [CrossRef] [PubMed]
- Boos, J.; Werber, G.; Ahlke, E.; Schulze-Westhoff, P.; Nowak-Göttl, U.; Würthwein, G.; Verspohl, E.; Ritter, J.; Jürgens, H. Monitoring of asparaginase activity and asparagine levels in children on different asparaginase preparations. Eur. J. Cancer 1996, 32, 1544–1550. [Google Scholar] [CrossRef]
- Pieters, R.; Carroll, W.L. Biology and treatment of acute lymphoblastic leukemia. Pediatr. Clin. N. Am. 2008, 55, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, L.J.; Kurtzberg, J.; Voute, P.; Jurgens, H.; Halpern, S.L. An open-label, multicenter study of polyethylene glycol-L-asparaginase for the treatment of acute lymphoblastic leukemia. Cancer 1995, 75, 1176–1181. [Google Scholar] [CrossRef]
- Duval, M.; Suciu, S.; Ferster, A.; Rialland, X.; Nelken, B.; Lutz, P.; Benoit, Y.; Robert, A.; Manel, A.M.; Vilmer, E. Comparison of Escherichia coli–asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: Results of a randomized European Organisation for Research and Treatment of Cancer—Children‘s leukemia group phase 3 trial. Blood 2002, 99, 2734–2739. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.L. Pegaspargase: A review of clinical studies. Adv. Drug Deliv. Rev. 2003, 55, 1293–1302. [Google Scholar] [CrossRef]
- Fernandez, C.; Stewart, E.; Panetta, J.; Wilkinson, M.; Morrison, A.; Finkelman, F.; Sandlund, J.; Pui, C.; Jeha, S.; Relling, M. Successful challenges using native E. coli asparaginase after hypersensitivity reactions to PEGylated E. coli asparaginase. Cancer Chemother. Pharmacol. 2014, 73, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Elshal, M.F.; Kumosani, T.A.; Aldahlawi, A.M. Purification and characterization of asparaginase from Phaseolus vulgaris seeds. Evid. Based Complement. Altern. Med. 2015, 309, 214. [Google Scholar]
- Niazi, S.K. Handbook of Pharmaceutical Manufacturing Formulations, 1st ed.; CRC: Boca Raton, FL, USA, 2009. [Google Scholar]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun. 1999, 36, 47–50. [Google Scholar] [CrossRef]
- Attallah, A.; Abdel-Wahab, M.; Elshal, M.; Zalata, K.; Ibrahim, N.; Ezzat, F. Apoptosis in chronic gastritis: Evaluation of the gastric mucosa by DNA flow cytometry and the expression of the high molecular weight cytokeratin. Hepato-Gastroenterology 1995, 43, 1305–1312. [Google Scholar]
- Osman, A.M.M.; Bayoumi, H.M.; Al-Harthi, S.E.; Damanhouri, Z.A.; ElShal, M.F. Modulation of doxorubicin cytotoxicity by resveratrol in a human breast cancer cell line. Cancer Cell Int. 2012, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.A.; Smith, C.; Karol, S.E.; Ramsey, L.B.; Liu, C.; Pui, C.H.; Jeha, S.; Evans, W.E.; Finkelman, F.D.; Relling, M.V. Effect of premedications in a murine model of asparaginase hypersensitivity. J. Pharmacol. Exp. Ther. 2015, 352, 541–551. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health (USA). Office for Protection from Research Risks Public Health Service Policy on Humane Care and Use of Laboratory Animals, 1st ed.National Institutes of Health: Bethesda, MD, USA, 1986.
- Vermes, I.; Haanen, C.; Steffens-Nakken, H.; Reutellingsperger, C. A novel assay for apoptosis flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J. Immunol. Methods 1995, 184, 39–51. [Google Scholar] [CrossRef]
- Zhang, G.; Gurtu, V.; Kain, S.R.; Yan, G. Early detection of apoptosis using a fluorescent conjugate of annexin V. Biotechniques 1997, 23, 525–531. [Google Scholar] [PubMed]
- Avramis, V.I.; Tiwari, P.N. Asparaginase (native ASNase or pegylated ASNase) in the treatment of acute lymphoblastic leukemia. Int. J. Nanomed. 2006, 1, 241–254. [Google Scholar]
- Panosyan, E.H.; Seibel, N.L.; Martin-Aragon, S.; Gaynon, P.S.; Avramis, I.A.; Sather, H.; Franklin, J.; Nachman, J.; Ettinger, L.J.; La, M. Asparaginase antibody and asparaginase activity in children with higher-risk acute lymphoblastic leukemia: Children‘s Cancer Group Study CCG-1961. J. Pediatr. Hematol. Oncol. 2004, 26, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.; Hak, L.; Storm, M.; Evans, W.; Sandlund, J.; Rivera, G.; Wang, B.; Pui, C.; Relling, M. Anti-asparaginase antibodies following E. coli asparaginase therapy in pediatric acute lymphoblastic leukemia. Leukemia 1998, 12, 1527–1533. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.; Hunger, S.P.; Boos, J.; Rizzari, C.; Silverman, L.; Baruchel, A.; Goekbuget, N.; Schrappe, M.; Pui, C.H. L-asparaginase treatment in acute lymphoblastic leukemia. Cancer 2011, 117, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Kawedia, J.D.; Cheng, C.; Pei, D.; Fernandez, C.A.; Cai, X.; Crews, K.R.; Kaste, S.C.; Panetta, J.C.; Bowman, W.P. Clinical utility and implications of asparaginase antibodies in acute lymphoblastic leukemia. Leukemia 2012, 26, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.H. Mast cell tryptases and chymases in inflammation and host defense. Immunol. Rev. 2007, 217, 141–154. [Google Scholar] [CrossRef] [PubMed]

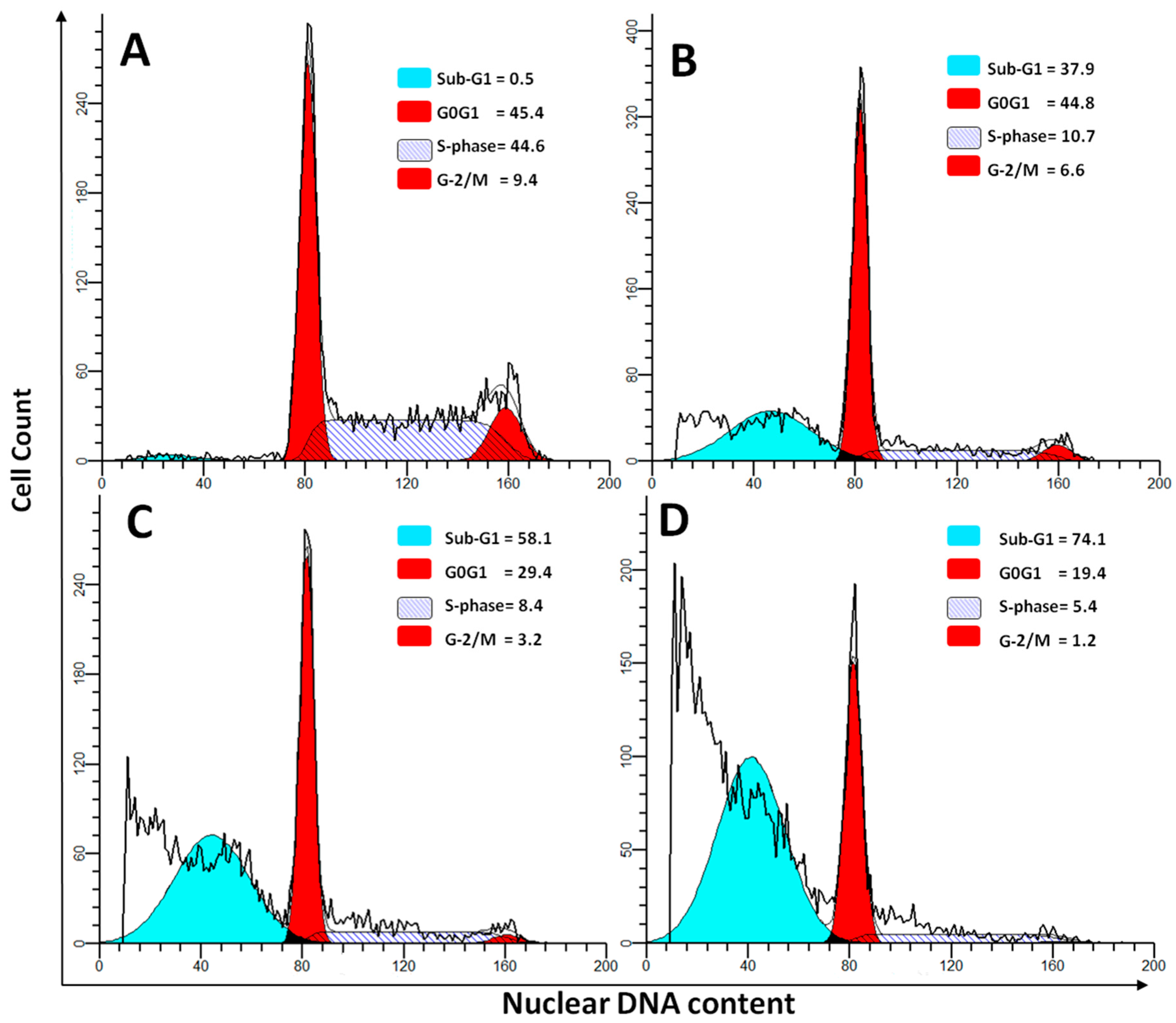
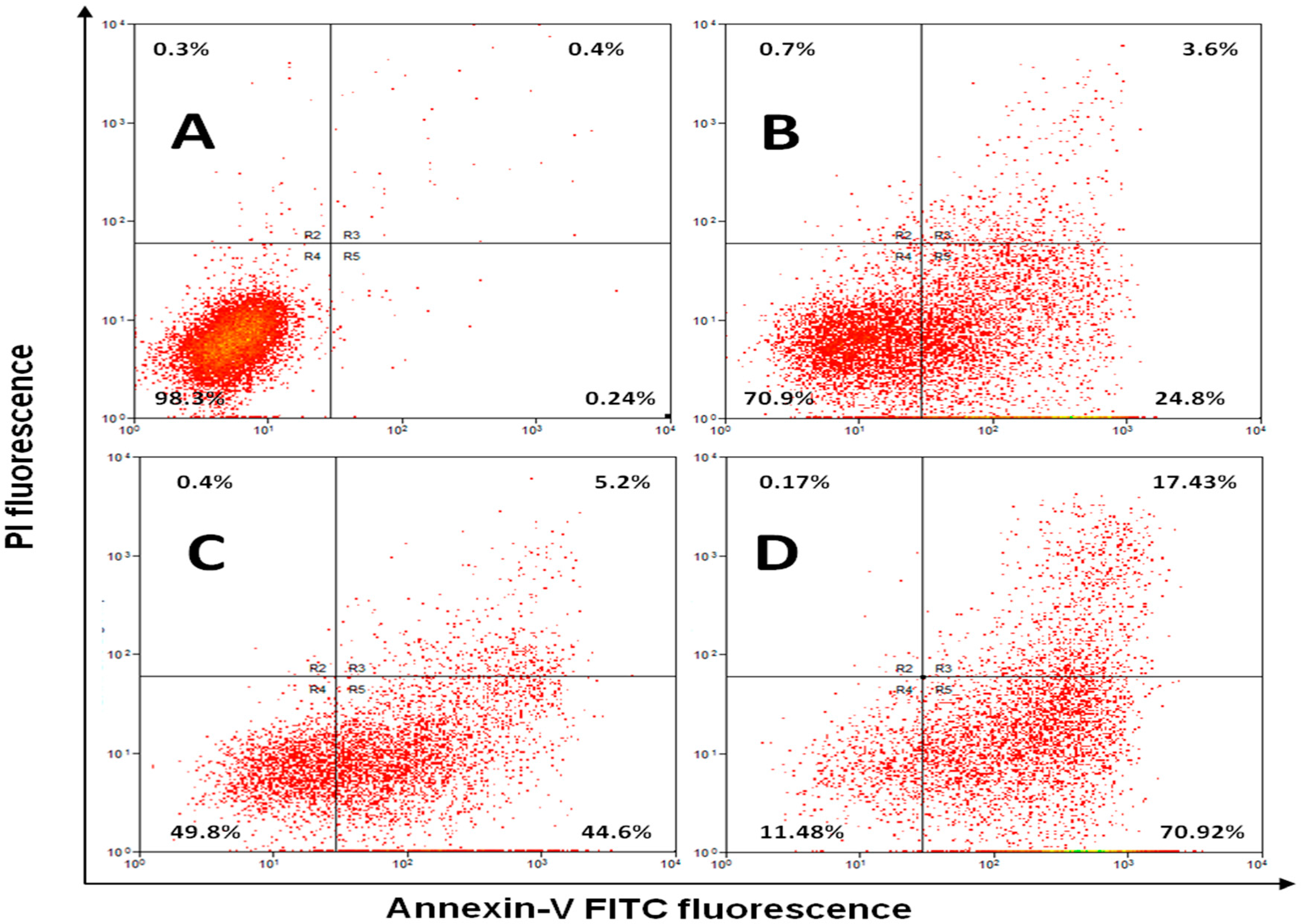
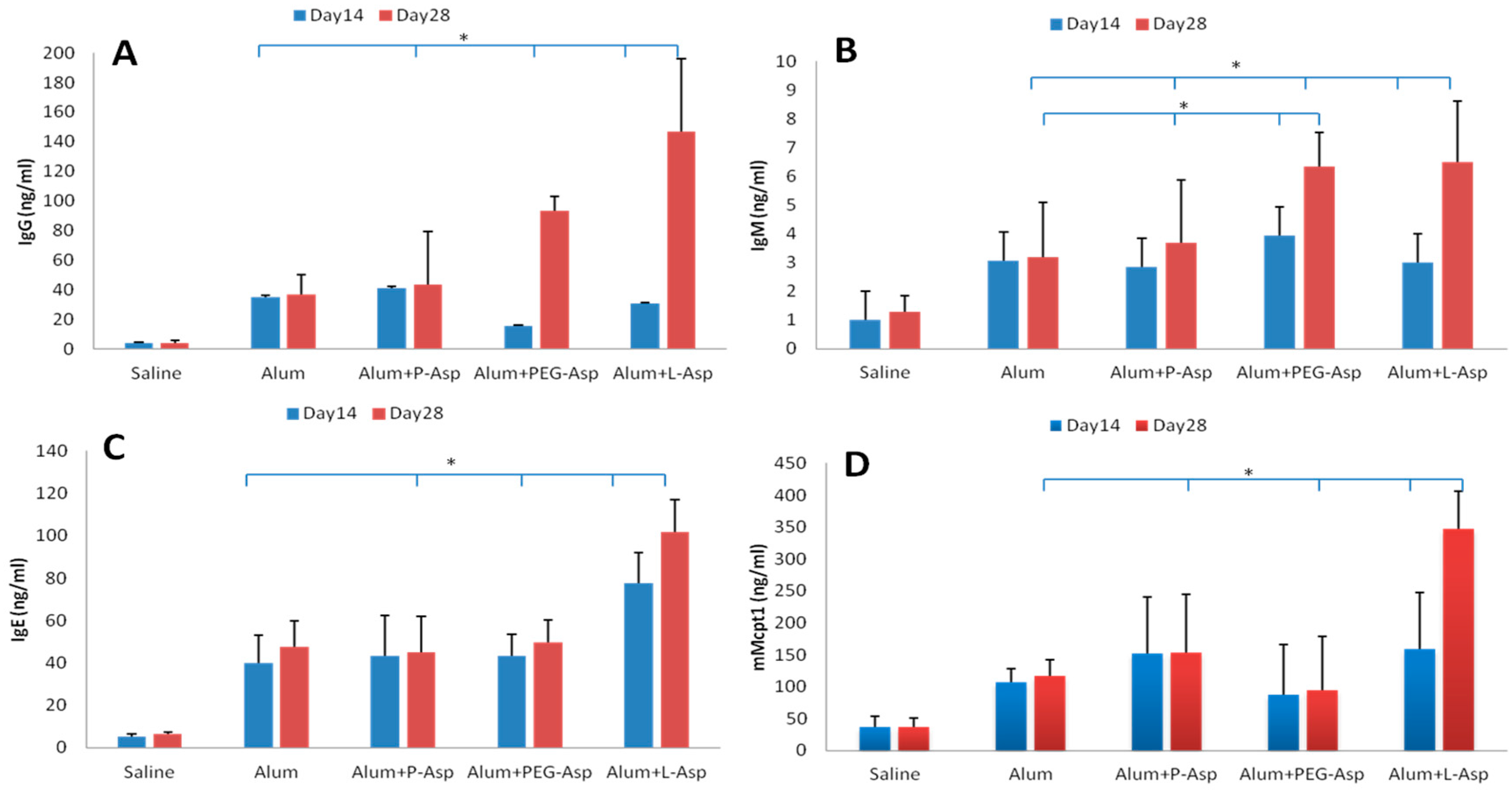
| Phase | Untreated | P-Asp | L-Asp | PEG-Asp |
|---|---|---|---|---|
| Sub-G1 | 0.76 ± 0.04 | 58.43 ± 6.42 a,b,c | 33.75 ± 6.45 a | 29.58 ± 2.74 a |
| G0G1 | 49.57 ± 7.35 | 29.43 ± 2.54 a,b,c | 43.54 ± 7.45 | 42.4 ± 6.72 |
| S-Phase | 41.51 ± 9.47 | 8.47 ± 1.1 a,b,c | 16.25 ± 3.75 a | 19.3 ± 2.01 a |
| G2/M | 8.14 ± 1.21 | 3.62 ± 0.52 a,b,c | 6.42 ± 2.02 | 5.65 ± 2.51 |
| Phase | Untreated | P-Asp | L-Asp | PEG-Asp |
|---|---|---|---|---|
| Early Apoptosis | 0.65 ± 0.11 | 49.3 ± 11.08 a,b.c | 31.42 ± 11.57 a | 27.36 ± 8.35 a |
| Late Apoptosis | 0.58 ± 0.13 | 5.28 ± 1.54 a,b.c | 17.82 ± 9.31 | 13.75 ± 4.39 |
| Necrosis | 0.49 ± 0.09 | 1.03 ± 0.31 a,b.c | 5.71 ± 3.75 a | 9.14 ± 1.91 a |
| Live cells | 98.14 ± 1.87 | 44.32 ± 9.28 a | 44.86 ± 12.37 | 49.41 ± 11.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, S.A.; Elshal, M.F.; Kumosani, T.A.; Aldahlawi, A.M.; Basbrain, T.A.; Alshehri, F.A.; Choudhry, H. L-Asparaginase Isolated from Phaseolus vulgaris Seeds Exhibited Potent Anti-Acute Lymphoblastic Leukemia Effects In-Vitro and Low Immunogenic Properties In-Vivo. Int. J. Environ. Res. Public Health 2016, 13, 1008. https://doi.org/10.3390/ijerph13101008
Mohamed SA, Elshal MF, Kumosani TA, Aldahlawi AM, Basbrain TA, Alshehri FA, Choudhry H. L-Asparaginase Isolated from Phaseolus vulgaris Seeds Exhibited Potent Anti-Acute Lymphoblastic Leukemia Effects In-Vitro and Low Immunogenic Properties In-Vivo. International Journal of Environmental Research and Public Health. 2016; 13(10):1008. https://doi.org/10.3390/ijerph13101008
Chicago/Turabian StyleMohamed, Saleh A., Mohamed F. Elshal, Taha A. Kumosani, Alia M. Aldahlawi, Tasneem A. Basbrain, Fauziah A. Alshehri, and Hani Choudhry. 2016. "L-Asparaginase Isolated from Phaseolus vulgaris Seeds Exhibited Potent Anti-Acute Lymphoblastic Leukemia Effects In-Vitro and Low Immunogenic Properties In-Vivo" International Journal of Environmental Research and Public Health 13, no. 10: 1008. https://doi.org/10.3390/ijerph13101008
APA StyleMohamed, S. A., Elshal, M. F., Kumosani, T. A., Aldahlawi, A. M., Basbrain, T. A., Alshehri, F. A., & Choudhry, H. (2016). L-Asparaginase Isolated from Phaseolus vulgaris Seeds Exhibited Potent Anti-Acute Lymphoblastic Leukemia Effects In-Vitro and Low Immunogenic Properties In-Vivo. International Journal of Environmental Research and Public Health, 13(10), 1008. https://doi.org/10.3390/ijerph13101008





