The Potential of Sequential Extraction in the Characterisation and Management of Wastes from Steel Processing: A Prospective Review
Abstract
:1. Introduction
1.1. Need for Improvements to Screening Protocols?
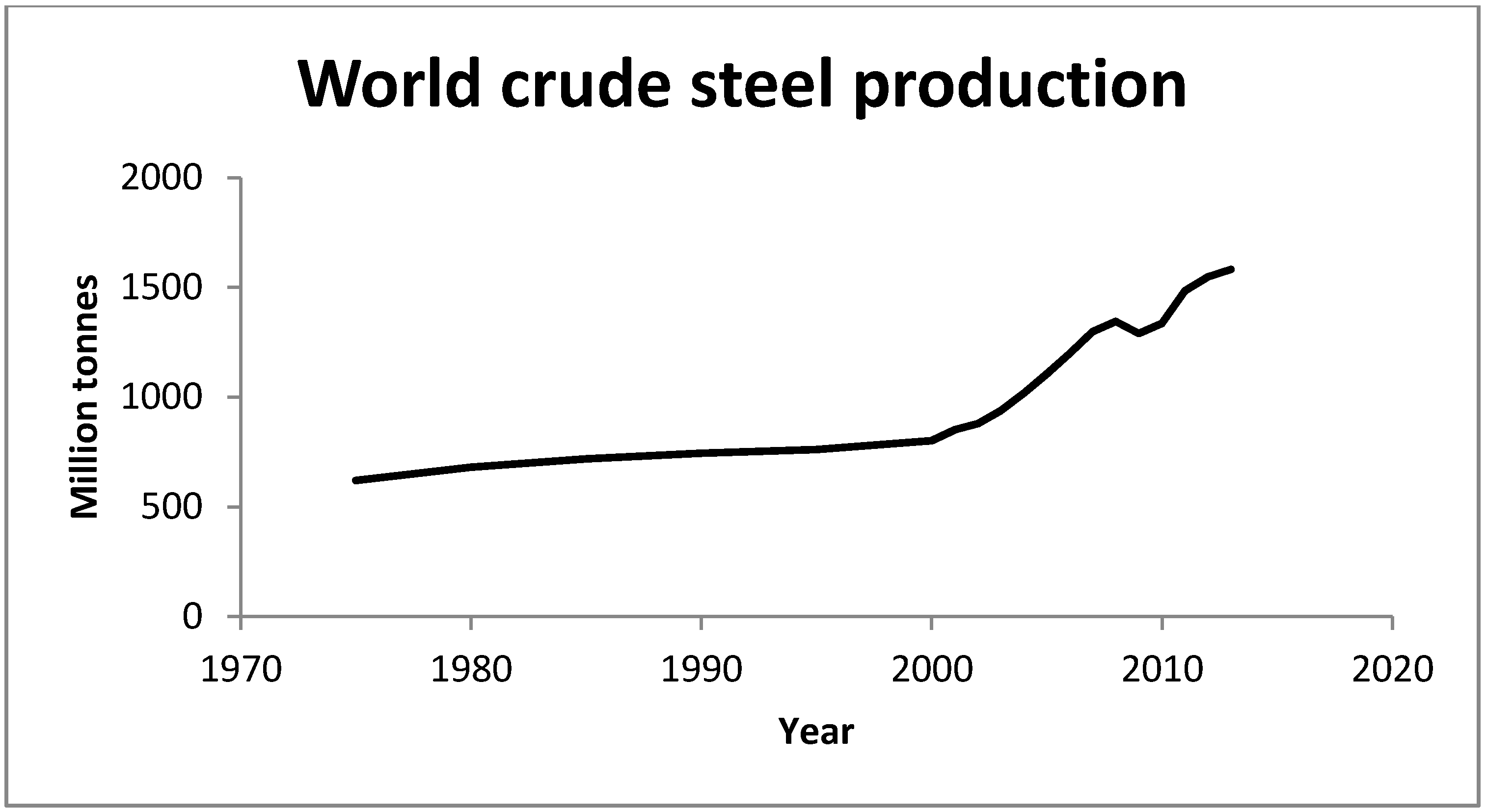
1.2. Regulatory Testing
| Country | Regulation of The Use of Waste Aggregate? | Criteria on Total Content? | Criteria on Leaching Content? | Type of Leaching Test |
|---|---|---|---|---|
| Austria | Guidelines | Yes | Yes | EN 12457-4 (L/S = 10.0 l/kg) |
| Czech republic | Based on landfill legislation | Yes | Yes | EN 12457-4 (L/S = 10.0 l/kg) |
| Denmark | Yes | Yes | Yes | EN 12457-1 |
| Finland | Yes | Yes | Yes | EN 12457-3 and CEN/TS 14405 |
| France | Yes | Yes | Yes | EN 12457-2 and 4 |
| Germany | Yes (new reg in preparation) | Yes | Yes | EN 12457-2 (& new leg. DIN 19528) |
| Italy | Yes | No | Yes | EN 12457-2 |
| Spain | Yes—by region | No | Yes | EN 12457-4 (& Din 38414-s4) |
| United Kingdom | Case by case guidance | No | No | Variable—no set routine |
1.3. Significance of Speciation
- The specific form of an element, such as, its electronic or oxidation state, complexation, molecular structure or isotopic composition.
- The distribution of an element amongst defined chemical species in a system.
- Analytical procedures for identifying and/or measuring the quantities of one or more individual chemical species in a sample.
Sequential Extraction (SE) as a Technique for Waste Characterisation
2. Wastes from the Steel Industry
Nature of Wastes
| Dust [54] | CaO (1–5%) | SiO2 (6%–9%) | MgO (<2%) | Al2O3 (2%–6%) | P2O5 n/a | TiO2 n/a | Fe (48%–52%) | K2O (0.1%–2%) | Na2O n/a | S n/a | C (29%–34%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Slag [43,55] | CaO (30%–60%) | SiO2 (10%–35%) | MgO (1%–6%) | Al2O3 (0.5%–4%) | P2O5 (0.5%–15%) | TiO2 (0.4%–2%) | Fe (7%–80%) | K2O n/a | Na2O n/a | S (<0.1%) | |
| Sludge [54,56,57] | CaO (6%–14%) | SiO2 (2%–10%) | MgO (<0.1%) | Al2O3 (<0.1%) | P2O5 (<0.01%) | TiO2 n/a | Fe (8%–66%) | K2O n/a | Na2O n/a | S (<1.5%) | C (7%–40%) |
| Sinter Dust [62] | BOF Sludge [61] | BF Sludge (Gas Treatment) [61] | BF Sludge (Dry) [63] | BF Sludge (from Landfill) [57] | |
|---|---|---|---|---|---|
| Fe | 43–50 | 48–70 | 7–35 | 21–32 | 5.7–27.5 |
| C | 2.9–6.12 | 0.7–4.6 | 15–47 | 1.0–3.2 | 7–40 |
| Pb | 0.09–5.98 | 0.04–0.14 | 0.8–2 | 0.3–1.2 | 0.1–2 |
| Zn | 0.03–0.34 | 0.2–4.1 | 1–10 | 1.0–3.2 | 1.5–8.6 |
| K | 3–9.07 | n/a | 0.08–0.36 | n/a | 0.1–1.7 |
| Ca | 7.55–7.83 | 3.0–17 | 3.5–18 | n/a | 3.5–13.5 |
3. Sequential Extraction
3.1. Obstacles to the Application of SEP
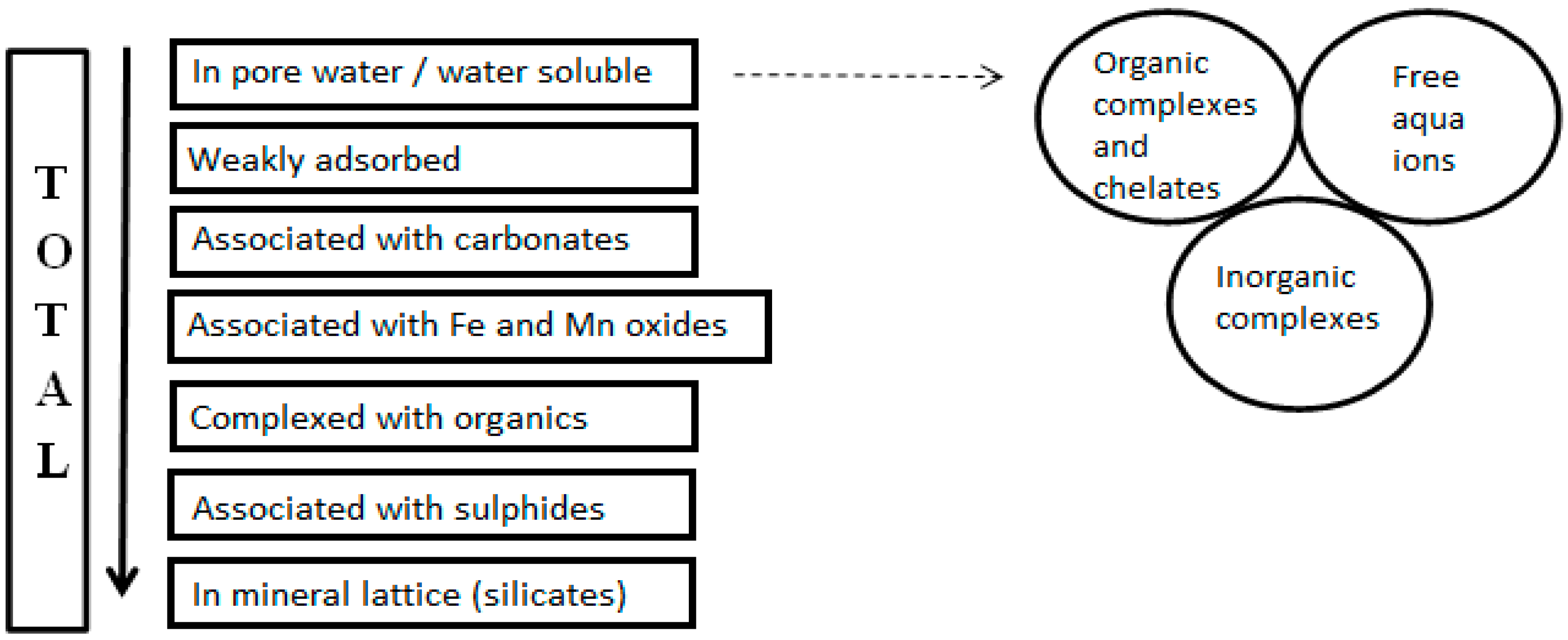
3.2. Defining Fractions
| Sample Type | Fraction 1 (Exchangeable, Water and Acid Soluble) | Fraction 2 (Reducible (Fe and Mn—Oxyhydroxides) | Fraction 3 (Oxidisable—Organic Matter and Sulphides) | Fraction 4 (Residual—Silicate Bound) | |
|---|---|---|---|---|---|
| Reagents | Reagents | Reagents | Reagents | ||
| Soils and sediments [41] | 0.11 moL·L−1 acetic acid 2 h [91] | 0.1 moL·L−1 hydroxyl-ammonium chloride pH 2, 4 h [91] | hydrogen peroxide followed by 1.0 moL·L−1 ammonium acetate at pH 2 | aqua regia | |
| Sewage sludge [92] Sediment [68] | 0.11 moL·L−1 acetic acid | 0.5 moL·L−1 hydroxyl - ammonium chloride pH 1.5 | hydrogen peroxide followed by 1.0 moL·L−1 ammonium acetate at pH 2 | aqua regia | |
| N/A [12] | 0.11 moL·L−1 acetic acid | 0.5 moL·L−1 hydroxyl-ammonium chloride | H2O2, 1.0 moL·L−1 CH3COONH4 | Aqua regia | |
| Sewage sludge [93] | 40 mL 0.11 M Acetic acid | 40 mL 0.10 M hydroxylamine hydrochloride (pH 2 HNO3) | 10 mL 8.8 M H2O2 AND 1 M Acetic acid | HF | |
| Solid wastes [74] | 0.11 moL·L−1 Acetic acid | 0.1 moL·L−1 hydroxylamine hydrochloride (pH 2 HNO3) | 8.8 M H2O2 | 1 moL·L−1 ammonium acetate (adjusted pH HNO3) | Perchloric acid—hydrofluoric acid, hydrochloric acid. |
| Municipal sewage sludge [81] | 0.11 moL·L−1 and CH3COOH | 0.5 moL·L−1 hydroxyl-ammonium chloride pH 1.5 | H2O2, 1.0 moL·L−1 and CH3COONH4 at pH 2 | 7 mL HNO3 + 2 mL HF + 1 mL HClO4 | |
| Marine Sediments [94] | 0.11 M Acetic acid | 0.10 moL·L −1 hydroxylamine hydrochloride (pH 2 HNO3) | 30% H2O2 pH 2 (HNO3) AND 1 M Acetic acid pH 2 (HNO3) | Hot HNO3 conc. | |
| Marine Sediments [95] | 20 mL 0.11 M Acetic acid | 20 mL 0.10M hydroxylamine hydrochloride (pH 1.5 by addition of 2 moL·L−1 HNO3) | 5 mL 8.8 M H2O2 AND 1 M Acetic acid | HNO3 and HF | |
| Marine Sediments [69] | Acetic acid 0.11 moL·L −1 pH 2.85 | Hydroxyl ammonium chloride (NH2OH.HCl 0.1 moL·L−1) pH 2 | 30% H2O2 (8.8 moL·L−1 ) , followed by CH3COONH4 (1 moL·L−1) pH 2 | Mix HNO3 (2 mL) and H2O2 (2 mL) + HF (0.5 mL) | |
| Sample Type | Fraction 1 | Fraction 2 | Fraction 3 | Fraction 4 | Fraction 5 | ||||
|---|---|---|---|---|---|---|---|---|---|
| Reagents | Reagents | Reagents | Reagents | Reagents | |||||
| Tessier (original) fluvial sediments [64] | 8 mL 1M MgCl2, pH 7.0, 1 h | 8 mL 1M NaOAc, acetic acid to pH 5, 6 h | 20 mL 0.04 M NH2OH-HCl in 25% (v/v) HOAc, 6 h | 3 mL 0.02 M HNO3, 5 mL 30% H2O2 (pH 2 with HNO3), 65 °C, 3 h | 3.2 M NH4OAc in 20% (v/v) HNO3 | 3 mL 30% H2O2, pH 2 HSO | HF-HClO4 | ||
| N/A. [12] | 1 M MgCl2 (pH 7.0) | 1 M NaOAc, acetic acid to pH 2 | 0.04 M NH2OH-HCl in 25% (v/v) HOAc, 96 °C | HNO3/H2O2 (85° C) | Then | 3.2 moL·L−1 NH4OAc in 20% (v/v) HNO3 | HF-HClO4 | ||
| MSW [96] | 1 M CH3COOH/CH3COONa pH 5, 5 h | NH2OH-HCl 0.1 M, 40 mL | K4P2O7 0.1 M, 20 mL, pH 9.5, 20 °C, 24 h | NH2OH-HCl 0.04 M in CH3COOH 25%, 20 mL, 60 °C, 6 h | HNO3-HCl Conc 12 h 20 °C, 3 h at 105 °C | ||||
| Soil [91] | 1 M MgCl2, pH 7.0 | 1 M NaOAc/acetic acid pH 5 | 0.04 M NH2OH-HCl in 25% (v/v) HOAc | 0.02M HNO3 in 30% H2O2 pH 2 | 3.2 M NH4OAc in 20% (v/v) HNO3 | ||||
| Marine sediment [94] | 1 M MgCl2, pH 7.0, 1 h | 1 M NaOAc/acetic acid, 5 h | 0.04 M NH2OH-HCl in 25% (v/v) HOAc 6 h—96 °C | 30% H2O2 pH 2 (85 °C), 5 h | 3.2 moL·L−1 NH4OAc in 20% (v/v) HNO3, 0.5 h | Hot HF-HClO4 | |||
| Soil [97] | 8 mL 0.5 M MgCl2, pH 7.0, 20 min | 8 mL 1 M NaOAc, 5 h | 0.04 M NH2OH-HCl in 25% (v/v) HOAc, 6 h—96 °C | 3 mL 0.02 M HNO3 and 5 mL 30% H2O2 Heated 2 h 5 mL 3.2 M NH4Oac, 0.5 h | 4 mL conc. HNO3, and 2 mL HCl Microwave | ||||
| Sediments [98] | 1 M MgCl2, pH 7 | 1 M NaOAc/acetic acid, pH 5 | 0.04 M NH2OH-HCl in 25% (v/v) HOAc | 30% H2O2, pH 2 with HNO3 | HF + HClO4 + HNO3 | ||||
| MSW [99] | 1 M NH4Ac, pH 7 | 1 M NaAc, pH 5 | 0.1 M NH2OH-HCl pH 2, 12 h | 40 mL 0.1 M oxalate buffer, pH 3 | 30% H2O2 (pH 3), 1 M NH4Ac (pH 7), 12 h | ||||
| Soils (Galán) [100] | 1 M NH4OAc, pH 5 | n/a | 0.4 M NH2OH-HCl in CH3OOH 25% | 0.2 M HNO3, 30% H2O2, pH 2 30% H2O2 then another H2O2 | HF, HNO3, HCl, 10:3:1 | ||||
| Sample | Elements | Remarks | |
|---|---|---|---|
| 4 fractions [101] 1990 | Mining wastes | Cu, Cd, Zn, Pb | Low leachability in water was observed with majority of metals found in residual fraction. Zn showed high levels in the acid soluble, reducible and residual fractions. Cu was found in the oxidizable fraction and Pb in the reducible fraction. |
| Adapted Tessier SEP [102] 1995 | Municipal solid waste incinerator ash | As, Cd, Cu, Hg, Pb, S, Zn | The pH of the resulting leachate is the greatest factor governing the concentration of metals in solution. This out ways concentrations in the ash. |
| SE based on Tessier [103] 1996 | Scale, sludge | As, S, Cu, Cr, Zn, Pb | Both scale and sludge consisted mostly of oxides of Si, Al and Fe. The sequential extraction showed that As, Cu and Zn were leachable under extreme conditions. |
| 5 fractions [104] 1996 | Landfill liners | Pb, Ni, Cd, | A new method: combined SE–sorption isotherm analysis. SE data indicated Pb and Ni were principally in the acid soluble fraction, and Cd was in the exchangeable fraction. |
| Sequential extraction [105] 1998 | Dust | Pb | SE revealed Pb in exchangeable fraction was less than 7% and mildly acidic steps for the bulk dusts collected. The finer particle size factions from these areas of smelter showed higher percentages of exchangeable lead. |
| 5-step [106] 2008 | BOF Flying dust | Zn | Reference materials were used to show Zn species ZnCl2 and ZnSO4 extracted from the exchangeable fraction, ZnCO3 in carbonate fraction, and ZnS from the reduced fractions. Complications with selectivity to ZnO as was released during the second and third extraction step. So cant distinguish ZnCO3 from ZnO. |
| BCR [107] 2008 | Sludge | Cd, Cu, Cr, Ni, Pb, Zn | Different sludges shows BCR recovery between 80%–100%. SE a higher degree of mineralisation and stabilisation can occur by its lowered metal bioavailability – predicted as a result of the associated to the oxidisable and residual fractions. |
| Revised BCR [108] 2013 | Slag | Al, As, Ba, Be, Co, Cr, Cu, Fe, Hg, Mn, Mo, Ni, Pb, Sb, SE, S, V, Zn | Showed significant recoveries 88%–109%. |
| Tessier [109] 2013 | Bottom Ash | Cu, Cd, and Zn | The results showed that the fractionation of Cu, Zn and Cd varied among the different size particles, and was greatly dependent on the intrinsic property of the metal species and their transfer behavior in the furnace. |
| BCR [110] 2015 | BF Slag | Al, Ba, Co, Cr, Cu, Fe, Mn, Mo, Ni, Pb, S, V, Zn | Showed difficulties regarding Zn recovery during step 1 and Cu recovery during step 2 |
| 5-step [42] 2015 | BF Sludge | Hg | Specifically optimised Hg focused SEP that was proven successful for BFS with recoveries 73%–114% despite being optimised for soils. |
3.2.1. Exchangeable Fraction
3.2.2. Reducible Fraction
| Fly Ash | Flue Dust | BOF Sludge | BF Sludge |
|---|---|---|---|
| Size from 0.5 to 300 µm | 0.075–0.250 mm dominated in the flue dust [121] | From less than 5 μm to as large as 1 mm [122] | Up to 1.5 mm fine-grained 1–10 μm coarser part 10–100 μm, where 90% of particles are below 50 μm [53] |
| 0.1–500 μm with majority between 20–60 μm [123] | P50 of 41.468 µm P10 17.57 µm, P90 was 83.6 µm [124] | Average particle size: Fine fraction ~37 μm, Coarse fraction ~210 μm [125] | Percentage distribution [126]: 2–5 mm = 13.65 1.25–2 mm = 32.53 0.8–1.25 mm = 15.56 0.2–0.8 mm = 20.2810.40 0.08–0.2 mm = 10.20 <0.08mm = 7.78 |
| 2 μm–10 μm [127] | <0.7–43 μm range with main fraction falling in 14–22 μm fraction [128] |
3.2.3. Oxidisable Fraction
3.2.4. Residual Fraction
3.3. Contribution of SE to Sample Compositional Assessment
4. The Development of the Sequential Extraction Approach
| Metal species and association | Description | Mobility |
|---|---|---|
| Exchangeable (dissolved) cations | fraction affected by ionic composition, pH, sorption and desorption processes | High. Changes in major cationic composition (e.g., estuarine environment) may cause a release due to ion exchange |
| Metals associated with Fe-Mn oxides (Reducible) | consists of metals attached to iron and manganese oxides and which are unstable under anoxic conditions | Medium. Changes in redox conditions may cause a release but some metals precipitate if sulfide mineral present is insoluble |
| Metals associated with organic matter (Oxidisable) | can be released when the organic matter is degraded leading to release of soluble metals under oxidizing conditions | Medium/High. With time, decomposition/oxidation of organic matter occurs |
| Metals associated with sulfide minerals | The sulfide minerals are a class of minerals containing sulfide (S2−) as the major anion. | Strongly dependent on environmental conditions. Under oxygen-rich conditions, oxidation of sulfide minerals leads to release of metals |
| Metals fixed in crystalline phase (Residual) | Predominantly primary and secondary minerals, which may hold metals within their structure | Low. Only available after weathering or decomposition |
Key Factors Affecting the Effectiveness of SE

| Variable | Soil | Steel Slag | BF Sludge [54] | Fly Ash |
|---|---|---|---|---|
| pH | 3.9 | 12.5 | 9.88 | 13.1 |
5. Application of SE to Different Wastes
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Steel Association. World Steel in Figures 2013; World Steel Association: Brussels, Belgium, 2013. [Google Scholar]
- World Steel Association. World Steel in Figures 2014; World Steel Association: Brussels, Belgium, 2014. [Google Scholar]
- DSTGI. Management of Steel Plant Solid Wastes; Forecasting and Assessment Council: New Delhi, India, 2003. [Google Scholar]
- Department for Environment Food & Rural Affairs. Waste Legislation and Regulations; Department for Environment Food & Rural Affairs: London, UK, 2015. [Google Scholar]
- Environment Media Group Ltd. Lets Recycle; Environment Media Group Ltd.: London, UK, 2015. [Google Scholar]
- Environment Media Group Ltd. Lets Recycle; Environment Media Group Ltd.: London, UK, 2012.
- CEWEP. “Landfill Taxes & Bans” Confederation of European Waste-to-Energy Plants. Available online: http://www.cewep.eu/media/www.cewep.eu/org/med_557/955_2012-04-27_cewep_-_landfill_taxes__bans_website.pdf (accessed on 1 June 2012).
- Cointreau, S. Landfill ER Revenue versus Landfill Costs; World Bank: Washington, DC, USA, 2008. [Google Scholar]
- Beratungsgesellschaft für Integrierte Problemlösungen. Services to Support Member States—Enforcement Actions and Inspections Concerning the Application of EU Waste Legislation; European Commission: Brussels, Belgium, 2010. [Google Scholar]
- Treating Waste as a Resource for the EU Industry. Analysis of Various Waste Streams and the Competitiveness of Their Client Industries; The European Competitiveness and Sustainable Industrial Policy Consortium: Rotterdam/Copenhagen, The Netherlands, 2013.
- Kretzschmar, R.; Mansfeldt, T.; Mandaliev, P.N.; Barmettler, K.; Marcus, M.A.; Voegelin, A. Speciation of Zn in blast furnace sludge from former sedimentation ponds using synchrotron X-ray diffraction, fluorescence, and absorption spectroscopy. Environ. Sci. Technol. 2012, 46, 12381–12390. [Google Scholar] [CrossRef] [PubMed]
- Bacon, J.R.; Davidson, C.M. Is there a future for sequential chemical extraction? Analyst 2008, 133, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Van der Sloot, H.A. Horizontal standardisation of test methods for waste, secondary raw materials, construction materials, sludge, biowaste and (contaminated) soil. Waste Manag. 2003. [Google Scholar] [CrossRef] [PubMed]
- Review of Scientific Literature on the Use of Stabilisation/Solidification for the Treatment of Contaminated Soil, Solid Waste and Sludges; Environment Agency: Bristol, UK, 2004.
- Van der Sloot, H.A.; Kosson, D.S. Leaching Assessment Methodologies for Disposal and Use of Bauxite Residues; Hans van der Sloot Consultancy: Nashville, TN, USA, 2010. [Google Scholar]
- Van der Sloot, H.A.; Meeussen, J.C.L.; van Zomeren, A.; Kosson, D.S. Developments in the characterisation of waste materials for environmental impact assessment purposes. J. Geochem. Explor. 2006, 88, 72–76. [Google Scholar] [CrossRef]
- Marguı́, E.; Salvadó, V.; Queralt, I.; Hidalgo, M. Comparison of three-stage sequential extraction and toxicity characteristic leaching tests to evaluate metal mobility in mining wastes. Anal. Chim. Acta 2004, 524, 151–159. [Google Scholar] [CrossRef]
- Corus. Corus Corporate responsibility report 2007/08. In Sustainable Construction Solutions; Tata Steel Group: London, UK, 2008. [Google Scholar]
- Steel industry by-products. Available online: http://www.worldsteel.org/publications/fact-sheets/content/01/text_files/file/document/Fact_By-products_2014.pdf (accessed on 12 April 2015).
- Okoro, H.K.; Fatoki, O.S.; Adekola, F.A.; Ximba, B.J.; Snyman, R.G. A review of sequential extraction procedures for heavy metals speciation in soil and sediments. Open Access Sci. Rep. 2012. [Google Scholar] [CrossRef]
- Esakku, S.; Selvam, A.; Joseph, K.; Palanivelu, K. Assessment of heavy metal species in decomposed municipal solid waste. Chem. Speciat. Bioavailab. 2005, 17, 95–102. [Google Scholar] [CrossRef]
- Landreth, R.E.; Rebers, P.A. Municipal Solid Wastes: Problems and Solutions; Taylor & Francis Inc.: London, UK, 1996. [Google Scholar]
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.-G.; Muntau, H.; van Leeuwen, H.P.; Lobinski, R. Guidelines for terms related to chemical speciation and fractionation of elements. Definitions, structural aspects, and methodological approaches (IUPAC Recommendations 2000). Pure Appl. Chem. 2000, 72, 1453–1470. [Google Scholar] [CrossRef]
- Environment Agency. SI 2004/1375; Environmental Protection: England and Wales, UK, 2004. [Google Scholar]
- Environmental Protection. S.S.I. 2003/185; Environmental Protection: England and Wales, UK, 2003. [Google Scholar]
- European Commission. Standard E12457; European Commission: Munich, Germany, 2002. [Google Scholar]
- European Union. EU Council Decision, Annex 2003/33/EC; European Union: Maastricht, The Netherlands, 2003. [Google Scholar]
- Environmental Permitting Guidance: The Landfill Directive; Department for Environment, Food & Rural Affairs: Bristol, United Kingdom, 2010.
- British Standards Institue. BS EN 12457-3: Characterisation of waste. Leaching. In Compliance Test for Leaching of Granular Waste Materials and Sludges. Two Stage Batch Test at a Liquid to Solid Ratio of 2 l/kg and 8 l/kg for Materials with a High Solid Content and with a Particle Size below 4 mm (without or with Size Reduction); British Standards Institue: London, UK, 2002. [Google Scholar]
- Saveyn, H.; Eder, P.; Garbarino, E.; Muchova, L.; Hjelmar, O.; van der Sloot, H.; Comans, R.; van Zomeren, A.; Hyks, J.; Oberender, A. Study on Methodological Aspects Regarding Limit Values for Pollutants in Aggregates in the Context of the Possible Development of End-of-Waste Criteria under the EU Waste Framework Directive; EUR—Scientific and Technical Research Reports; Institute for Prospective Technological Studies: Seville, Spain, 2014. [Google Scholar]
- AWE International. Available online: http://www.aweimagazine.com/article.php?article_id=1081 (accessed on 14 September 2015).
- Williams, P.T. Waste Treatment and Disposal, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Kumpiene, J.; Mench, M.; Bes, C.M.; Fitts, J.P. Assessment of aided phytostabilization of copper-contaminated soil by X-ray absorption spectroscopy and chemical extractions. Environ. Pollut. 2011, 159, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, W.; Monteny, F.; Leermakers, M.; Bouillon, S. Evalution of sequential extractions on dry and wet sediments. Anal. Bioanal. Chem. 2003, 376, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, C.L.; Williamson, C.A.; Collins, W.K.; Dahlin, D.C. Sequential extraction versus comprehensive characterization of heavy metal species in brownfield soils. Environ. Forensics 2002, 3, 191–201. [Google Scholar] [CrossRef]
- CMP. Electric Arc Furnace Dust. In 1993 Overview; Electric Power Research Institute, Center for Materials Production: Palo Alto, CA, USA, 1993. [Google Scholar]
- Chen, J.; Yan, X.; Wang, X.; Zhang, J.; Huang, J.; Zhao, J. Heavy metal chemical extraction from industrial and municipal mixed sludge by ultrasound-assisted citric acid. J. Ind. Eng. Chem. 2015, 27, 368–372. [Google Scholar]
- Salomons, W.; Forstner, U. Trace-metal analysis on polluted sediments: Evaluation of environmental impact. Environ. Technol. Lett. 1980, 1, 506–517. [Google Scholar] [CrossRef]
- Baruah, N.K.; Kotoky, P.; Bhattacharyya, K.G.; Borah, G.C. Metal speciation in Jhanji River sediments. Sci. Total Environ. 1996, 193, 1–12. [Google Scholar] [CrossRef]
- Li, X.; Thornton, I. Chemical partitioning of trace and major elements in soils contaminated by mining and smelting activities. Appl. Geochem. 2001, 16, 1693–1706. [Google Scholar] [CrossRef]
- Ure, A.M.; Quevauviller, P.; Muntau, H.; Griepink, B. Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BRC of the Commission of the European Communities. Int. J. Environ. Anal. Chem. 1993, 51, 135–151. [Google Scholar] [CrossRef]
- Foldi, C.; Andree, C.A.; Mansfeldt, T. Sequential extraction of inorganic mercury in dumped blast furnace sludge. Environ. Sci. Pollut. Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, I.Z.; Prezzi, M. Chemical, mineralogical, and morphological properties of steel slag. Adv. Civil Eng. 2011. [Google Scholar] [CrossRef]
- Fatoki, O.S.; Mathabatha, S. An assessment of heavy metal pollution in the East London and Port Elizabeth harbours. Impact Factor Descr. 2002, 27. [Google Scholar] [CrossRef]
- Shawabkeh, R.A. Hydrometallurgical extraction of zinc from Jordanian electric arc furnance dust. Hydrometallurgy 2010, 104, 61–65. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Available and Emerging Technologies for Reducing Greenhouse Gas Emissions from the Iron and Steel Industry; Environmental Protection Agency: Washington, DC, USA, 2012. [Google Scholar]
- Hasanbeigi, A.; Price, L.K.; McKane, A.T. The State–of-the-Art Clean Technologies (SOACT) for Steelmaking Handbook, 2nd ed.; Asia Pacific Partnership on Clean Development and Climate: Washington, DC, USA, 2010. [Google Scholar]
- Ricketts, J.A. How a Blast Furnace Works in Making Steel; American Iron and Steel Institute: Washington, DC, USA, 2014. [Google Scholar]
- American Iron and Steel Institue. 2014, Volume 2014. Available online: http://www.steel.org/making-steel/how-its-made/steelmaking-flowlines.aspx (accessed on 18 January 2015).
- World Coal Association. Available online: http://www.worldcoal.org/coal/uses-of-coal/coal-steel/ (accessed on 5 May 2014).
- Daněk, T. Physical and chemical properties of sludge from iron and steel industry stabilised with coal fly ash. Zesz. Naukowe ATH—Inzynieria Wlokiennicza Ochr. Srodowiska 2006, 24, 34–41. [Google Scholar]
- Swindley, S.P.; Charles, J.; Shillaker, D.; Williams, K. Control of effluents in steel production: Wastewater discharges at British Steel Port Talbot. Ironmak. Steelmak. 1998, 25, 29–33. [Google Scholar]
- Vereš, J.; Lovás, M.; Jakabský, Š.; Šepelák, V.; Hredzák, S. Characterization of blast furnace sludge and removal of zinc by microwave assisted extraction. Hydrometallurgy 2012, 129–130, 67–73. [Google Scholar] [CrossRef]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Biswal, S.K.; Mohapatra, B.K.; Misra, V.N. Effective Utilization of Blast Furnace Flue Dust of Integrated Steel Plants. Eur. J. Miner. Process. Environ. Prot. 2002, 2, 61–68. [Google Scholar]
- Emery, J.J. Slag Utilization in Pavement Construction. In Extending Aggregate Resources—ASTM Special Technical Publication; American Society for Testing and Materials: Washington, DC, USA, 1982. [Google Scholar]
- Dakun, V.I.; Esezobor, D.E.; Rostovsky, V.I. Reclamation of dumped sludge in steel industry. J. Repub. Environ. Control. Ration. Util. Nat. Resour. 1993, 1719, 92–96. [Google Scholar]
- Mansfeldt, T.; Dohrmann, R. Chemical and mineralogical characterization of blast-furnace sludge from an abandoned landfill. Environ. Sci. Technol. 2004, 38, 5977–5984. [Google Scholar] [CrossRef] [PubMed]
- Navarro, C.; Díaz, M.; Villa-García, M.A.A. Physico-chemical characterization of steel slag. study of its behavior under simulated environmental conditions. Environ. Sci. Technol. 2010, 44, 5383–5388. [Google Scholar] [CrossRef] [PubMed]
- Proctor, D.M.; Fehling, K.A.; Shay, E.C.; Wittenborn, J.L.; Green, J.J.; Avent, C.; Bigham, R.D.; Connolly, M.; Lee, B.; Shepker, T.O.; et al. Physical and Chemical Characteristics of Blast furnace, basic oxygen furnace, and electric arc furnace steel industry slags. Environ. Sci. Technol. 2000, 34, 1576–1582. [Google Scholar] [CrossRef]
- Das, B.; Prakash, S.; Reddy, P.S.R.; Misra, V.N. An overview of utilization of slag and sludge from steel industries. Resour. Conserv. Recycl. 2007, 50, 40–57. [Google Scholar] [CrossRef]
- Besta, P.; Janovská, K.; Samolejová, A.; Beránková, A.; Vozňáková, I.; Hendrych, M. The cycle and effect of zinc in the blast-furnace process. Metalurgija 2013, 52, 197–200. [Google Scholar]
- Integrated Pollution Prevention and Control. Best Available Techniques (BAT) Reference Document for Iron and Steel Production; European Parliament, Institute for Prospective Technological Studies: Seville, Spain, 2010. [Google Scholar]
- Vereš, J.; Kajabský, Š.; Šepelák, V. Chemical, physical, morphological and structural characterization of blast furnace sludge. J. Basic Princ. Diffus. Theory Exp. Appl. 2010. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–850. [Google Scholar] [CrossRef]
- Coetzee, P. Determination and speciation of heavy metals in sediments of the Hartebeespoort Dam By sequential extraction. Water SA 1993, 19, 291–300. [Google Scholar]
- Stephens, S.R.; Alloway, B.J.; Parker, A.; Carter, J.E.; Hodson, M.E. Changes in the leachability of metals from dredged canal sediments during drying and oxidation. Environ. Pollut. 2001, 114, 407–413. [Google Scholar] [CrossRef]
- Svete, P.; Milacic, R.; Pihlar, B. Partitioning of Zn, Pb and Cd in river sediments from a lead and zinc mining area using the BCR three-step sequential extraction procedure. J. Environ. Monit. 2001, 3, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Riba, A.; Sahuquillo, A.; Rubio, R.; Rauret, G. Assessment of metal mobility in dredged harbour sediments from Barcelona, Spain. Sci. Total Environ. 2004, 321, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.G.; Shi, J.B.; He, B.; Liu, J.F.; Liang, L.N.; Jiang, G.B. Speciation of heavy metals in marine sediments from the East China Sea by ICP-MS with sequential extraction. Environ. Int. 2004, 30, 769–783. [Google Scholar] [CrossRef] [PubMed]
- Mossop, K.F.; Davidson, C.M. Comparison of original and modified BCR sequential extraction procedures for the fractionation of copper, iron, lead, manganese and zinc in soils and sediments. Anal. Chim. Acta 2003, 478, 111–118. [Google Scholar] [CrossRef]
- Davidson, C.M.; Duncan, A.L.; Littlejohn, D.; Ure, A.M.; Garden, L.M. A critical evaluation of the three-stage BCR sequential extraction procedure to assess the potential mobility and toxicity of heavy metals in industrially-contaminated land. Anal. Chim. Acta 1998, 363, 45–55. [Google Scholar] [CrossRef]
- Fernandez, E.; Jimenez, R.; Lallena, A.M.; Aguilar, J. Evaluation of the BCR sequential extraction procedure applied for two unpolluted Spanish soils. Environ. Pollut. 2004, 131, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, E.A.; Mochon, M.C.; Sanchez, J.C.J.; Rodriguez, M.T. Heavy metal extractable forms in sludge from wastewater treatment plants. Chemosphere 2002, 47, 765–775. [Google Scholar] [CrossRef]
- Bruder-Hubscher, V.; Lagarde, F.; Leroy, M.J.F.; Coughanowr, C.; Enguehard, F. Application of a sequential extraction procedure to study the release of elements from municipal solid waste inceration bottom ash. Anal. Chim. Acta 2002, 451, 285–295. [Google Scholar] [CrossRef]
- Thornton, I.; Farago, M.E.; Thums, C.R.; Parrish, R.R.; McGill, R.A.; Breward, N.; Fortey, N.J.; Simpson, P.; Young, S.D.; Tye, A.M.; et al. Urban geochemistry: Research strategies to assist risk assessment and remediation of brownfield sites in urban areas. Environ. Geochem. Health 2008, 30, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Kirpichtchikova, T.A.; Manceau, A.; Spadini, L.; Panfili, F.; Marcus, M.A.; Jacquet, T. Speciation and solubility of heavy metals in contaminated soil using X-ray microfluorescence, EXAFS spectroscopy, chemical extraction, and thermodynamic modeling. Geochim. Cosmochim. Acta 2006, 70, 2163–2190. [Google Scholar] [CrossRef]
- Li, J. Risk Assessment of Heavy Metals in Surface Sediments from the Yanghe River, China. Int. J. Environ. Res. Public Health 2014, 11, 12441–12453. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, J.G. Modern Analytical Chemistry, 1st Edition (Harvey, David). J. Chem. Educ. 2000, 77, 705. [Google Scholar] [CrossRef]
- Sharmin, S.; Zakir, H.M.; Shikazono, N. Fractionation profile and mobility pattern of trace metals in sediments of Nomi River, Tokyo, Japan. J. Soil Sci. Environ. Manag. 2010, 1, 1–14. [Google Scholar]
- Moore, F.; Nematollahi, M.J.; Keshavarzi, B. Heavy metals fractionation in surface sediments of Gowatr bay—Iran. Environ. Monit Assess. 2015, 187, 4117. [Google Scholar] [CrossRef] [PubMed]
- Hanay, Ö.; Hasar, H.; Kocer, N.N.; Aslan, S. Evaluation for Agricultural Usage with Speciation of Heavy Metals in a Municipal Sewage Sludge. Bull. Environ. Contam. Toxicol. 2008, 81, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Abu-Obaid, A.A. The Influence of Surfactants on the adsorption of heavy metal ions using inorganic legands in selected contaminated soil samples in Palestine. Ph.D. Thesis, An-Najah National University, Nablus, Palestine, 2010. [Google Scholar]
- Elder, J.F. Metal biogeochemistry in surface-water systems—A review of principles and concepts. Available online: http://pubs.usgs.gov/circ/1988/1013/report.pdf (accessed on 11 september 2015).
- David, J.A.; Leventhal, J.S. Bioavailabilty of Metals, Chapter 2: Preliminarycompilation of Descriptive Geoenvironmental Mineral Deposit Models; Department of the Interior U.S Geological Survey: Denver, CO, USA, 1995. [Google Scholar]
- Fadiran, A.O.; Tiruneh, A.T.; Mtshali, J.S. Assessment of Mobility and Bioavailability of Heavy Metals in Sewage Sludge from Swaziland through Speciation Analysis. Am. J. Environ. Prot. 2014, 3, 198–208. [Google Scholar] [CrossRef]
- Wilson, C.A.; Cresser, M.S.; Davidson, D.A. Sequential element extraction of soils from abandoned farms: An investigation of the partitioning of anthropogenic element inputs from historic land use. J. Environ. Monit. 2006, 8, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.E.; Ottosen, L.M.; Pedersen, A.J. Speciation of Pb in industrially polluted soils. Water Air Soil Pollut. 2006, 170, 359–382. [Google Scholar] [CrossRef]
- Boenke, A. The standards, measurements and testing programme (SMT), the European support to standardisation, measurements and testing projects. In Modern Developments and Applications in Microbeam Analysis; Love, G., Nicholson, W.A.P., Armigliato, A., Eds.; Springer: Brussels, Belgium, 1998. [Google Scholar]
- Bacon, J.R.; Hewitt, I.J.; Cooper, P. Reproducibility of the BCR sequential extraction procedure in a long-term study of the association of heavy metals with soil components in an upland catchment in Scotland. Sci. Total Environ. 2005, 337, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.A.; Tack, F.M.G. Fractionation of Cu, Pb and Zn in certified reference soils SRM 2710 and SRM 2711 using the optimized BCR sequential extraction procedure. Environ. Res. 2003, 8, 37–50. [Google Scholar] [CrossRef]
- Maiz, I.; Arambarri, I.; Garcia, R.; Millán, E. Evaluation of heavy metal availability in polluted soils by two sequential extraction procedures using factor analysis. Environ. Pollut. 2000, 110, 3–9. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Barahona, E.; Lachica, M.; Ure, A.M.; Davidson, C.M.; Gomez, A.; Lück, D.; Bacon, J. Application of a modified BCR sequential extraction (three-step) procedure for the determination of extractable trace metal contents in a sewage sludge amended soil reference material (CRM 483), complemented by a three-year stability study of acetic acid and EDTA extractable metal content. J. Environ. Monit. 2000, 2, 228–233. [Google Scholar] [PubMed]
- Nyamangara, J. Use of sequential extraction to evaluate zinc and copper in a soil amended with sewage sludge and inorganic metal salts. Agric. Ecosyst. Environ. 1998, 69, 135–141. [Google Scholar] [CrossRef]
- Usero, J.; Gamero, M.; Morillo, J.; Gracia, I. Comparative study of three sequential extraction procedures for metals in marine sediments. Environ. Int. 1998, 24, 487–496. [Google Scholar] [CrossRef]
- Cuong, D.T.; Obbard, J.P. Metal speciation in coastal marine sediments from Singapore using a modified BCR-sequential extraction procedure. Appl. Geochem. 2006, 21, 1335–1346. [Google Scholar] [CrossRef]
- Prudent, P.; Domeizel, M.; Massiani, C. Chemical sequential extraction as decision-making tool: Application to municipal solid waste and its individual constituents. Sci. Total Environ. 1996, 178, 55–61. [Google Scholar] [CrossRef]
- Emmerson, R.H.; Birkett, J.W.; Scrimshaw, M.; Lester, J.N. Solid phase partitioning of metals in managed retreat soils: Field changes over the first year of tidal inundation. Sci. Total Environ. 2000, 254, 75–92. [Google Scholar] [CrossRef]
- Wepener, V.; Vermeulen, L. A note on the concentrations and bioavailability of selected metals in sediments of Richards Bay Harbour, South Africa. S. Afr. Water Res. Comm. 2005, 31, 589–596. [Google Scholar] [CrossRef]
- Flyhammar, P. Use of sequential extraction on anaerobically degraded municipal solid waste. Sci. Total Environ. 1998, 212, 203–215. [Google Scholar] [CrossRef]
- Zimmerman, A.J.; Weindorf, D.C. Heavy metal and Trace metal analysis in soil by sequential extraction: A review of procedures. Int. J. Anal. Chem. 2010, 2010, 7. [Google Scholar] [CrossRef] [PubMed]
- Clevenger, T.E. Use of sequential extraction to evaluate the heavy metals in mining wastes. Water Air Soil Pollut. 1990, 50, 241–254. [Google Scholar] [CrossRef]
- Buchholz, B.A.; Landsberger, S. Leaching dynamics studies of municipal solid waste incinerator ash. Air Waste Manag. Assoc. 1995, 45, 579–590. [Google Scholar] [CrossRef]
- Peralta, G.L.; Graydon, J.W.; Kirk, D. Physicochemical characteristics and leachability of scale and sludge from Bulalo geothermal system, Philippines. Geothermics 1996, 25, 17–35. [Google Scholar] [CrossRef]
- Salim, I.; Miller, C.J.; Howard, J.L. Sorption isotherm-sequential extraction analysis of heavy metal retention in landfill liners. Soil Sci. Soc. Am. J. 1996, 60, 107–114. [Google Scholar] [CrossRef]
- Spear, T.M.; Svee, W.; Vincent, J.H.; Stanisich, N. Chemical speciation of lead dust associated with primary lead smelting. Environ. Health Perspect. 1998, 106, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Sammut, M.L.; Rose, J.; Masion, A.; Fiani, E.; Depoux, M.; Ziebel, A.; Hazemann, J.L.; Proux, O.; Borschneck, D.; Noack, Y. Determination of zinc speciation in basic oxygen furnace flying dust by chemical extracions and X-ray spectroscopy. Chemosphere 2008, 70, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, A.; Lloréns, M.; Sáez, J.; Isabel Aguilar, M.A.; Ortuño, J.F.; Meseguer, V.F. Comparitive study of six different sludges by sequential speciation of heavy metals. Bioresour. Technol. 2008, 99, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.; Välimäki, I.; Pöykiö, R.; Nurmesniemi, H.; Dahl, O. Evaluation of trace element availability from secondary metallurgical slag generated in steelmaking by sequential chemical extraction. Environ. Sci. Technol. 2013, 10, 1193–1208. [Google Scholar] [CrossRef]
- Yao, J.; Kong, Q.; Zhu, H.; Long, Y.; Shen, D. Content and fractionation of Cu, Zn and Cd in size fractionated municipal solid waste incineration bottom ash. Ecotoxicol. Environ. Saf. 2013, 94, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.; Heikinheimo, E.; Välimäki, I.; Dahl, O. Characterization of industrial secondary desulphurization slag by chemical fractionation with supportive X-ray diffraction and scanning electron microscopy. Int. J. Miner. Process. 2015, 134, 29–35. [Google Scholar] [CrossRef]
- Anju, M.; Banerjee, D.K. Comparison of two sequential extraction procedures for heavy metal partitioning in mine tailings. Chemosphere 2010, 78, 1393–1402. [Google Scholar] [CrossRef] [PubMed]
- Brumer, G.W.; Gerth, J.; Herms, U. Heavy Metal Species, Mobility and Availability in Soils; Bodenk, Z.P., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 1986. [Google Scholar]
- Salomons, W. Environmental impact of metals derived from mining activities: Processes, predictions, prevention. J. Geochem. Explor. 1995, 52, 5–23. [Google Scholar] [CrossRef]
- Rauret, G. Extraction procedures for the determination of heavy metals in contaminated soil and sediment. Talanta 1998, 46, 449–455. [Google Scholar] [CrossRef]
- Camobreco, V.; Richards, B.K.; Steenhuis, T.S.; Peverly, J.H.; McBride, M.B. Movement of heavy metals through undisturbed and homogenized soil columns. Soil Sci. 1996, 161, 740–750. [Google Scholar] [CrossRef]
- Bohn, H.L.; Myer, R.A.; O’Connor, G.A. Soil Chemistry, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Matichenkov, V.V.; Bocharnikova, E.A. Studies in Plant. Science; Datnoff, L.E., Snyder, G.H., Korndörfer, G.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2001; Volume 8, pp. 209–219. [Google Scholar]
- Radosz, M.; Gawdzik, J. Impact assessment of microwave hygenization of sewage sludge on heavy metal mobility. Tech. Sanit. 2005. [Google Scholar] [CrossRef]
- Hall, G.P.; Pelchat, P. Comparability of results obtained by the use of different extraction schemes for the determination of element froms in soils. Water Air Soil Pollut. 1999, 112, 41–53. [Google Scholar] [CrossRef]
- Luoma, S.N. Can we determine the biological availability of sediment-bound trace elements? Hydrobiologia 1989, 176–177, 379–396. [Google Scholar] [CrossRef]
- Leimalm, U.; Lundgren, M.; Ökvist, L.S.; Björkman, B. Off-gas dust in an experimental blast furnace, Part 1: Characterization of flue dust, sludge and shaft fines. ISIJ Int. 2010, 50, 1560–1569. [Google Scholar] [CrossRef]
- Kelebek, S.; Yörük, S.; Davis, B. Characterization of basic oxygen furnace dust and zinc removal by acid leaching. Miner. Eng. 2004, 17, 285–291. [Google Scholar] [CrossRef]
- Sarkar, A.; Rano, R.; Mishra, K.K.; Sinha, I.N. Particle size distribution profile of some Indian fly ash—A comparative study to assess their possible uses. Fuel Process. Technol. 2005, 86, 1221–1238. [Google Scholar] [CrossRef]
- Zhan, G.; Guo, Z. Study on basic properties of sintering dust from iron and steel plant and potassium recovery. J. Environ. Sci. 2013, 25, 1226–1234. [Google Scholar] [CrossRef]
- Cantarino, M.V.; de Carvalho Filho, C.; Borges Mansur, M. Selective removal of zinc from basic oxygen furnace sludges. Hydrometallurgy 2012, 111–112, 124–128. [Google Scholar] [CrossRef]
- Mihaiescu, D.C.; Predeanu, G.; Panaitescu, C. Characterisation of some blast furnace waste dusts. U.P.B. Sci. Bull. 2014, 76, 227–234. [Google Scholar]
- Ismail, K.N.; Hussin, K.; Idris, M.S. Physical, cheimcal and mineralogical properties of fly ash. J. Nucl. Relat. Technol. 2007, 4, 47–51. [Google Scholar]
- Rauret, G.; López-Sánchez, J.F. New sediment and soil CRMs for extractable trace metal content. Int. J. Environ. Anal. Chem. 2001, 79, 81–95. [Google Scholar] [CrossRef]
- Snoeyink, V.L.; Jenkins, D. Water Chemistry; John Wiley and Sons: New York, NY, USA, 1980. [Google Scholar]
- Marin, B.; Valladon, M.; Polve, M.; Monaco, A. Reproducibility testing of a sequential extraction scheme for the determination of trace metal speciation in a marine reference sediment by inductively coupled plasma-mass spectrometry. Anal. Chim. Acta 1997, 342, 91–112. [Google Scholar] [CrossRef]
- Klock, P.R.; Czamanske, G.K.; Foose, M.; Pesek, J. Selective chemical dissolution of sulfides: An evaluation of six methods applicable to assaying sulfide-bound nickel. Chem. Geol. 1986, 54, 157–162. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Lavilla, I.; Bendicho, C. Chemical sequential extraction for metal partitioning in environmental solid samples. J. Environ. Monit. 2002, 4, 823–857. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wang, W.X.; Chen, J. Geochemistry of Cd, Cr, and Zn in highly contaminated sediments and its influences on assimilation by marine bivalves. Environ. Sci. Technol. 2002, 36, 5164–5171. [Google Scholar] [CrossRef] [PubMed]
- Gleyzes, C.; Tellier, S.M.; Astruc, M. Fractionation studies of trace elements in Contaminated soils and Sediments: A review of sequential extraction procedure. Trend Anal. Chem. 2002, 21, 451–467. [Google Scholar] [CrossRef]
- Dollar, N.L.; South, G.M.; Filipelli, G.M.; Mastalerz, M. Chemical fractionation of metals in wetland sediments: Indiana dunes national lakeshore. Environ. Sci. Technol. 2001, 35, 3608–3615. [Google Scholar] [CrossRef] [PubMed]
- Borovec, Z.; Tolar, V.; Mraz, L. Distribution of some metals in sediments of the central part of the Labe (Eibe) River: Czeech Republic. Ambio 1993, 22, 200–205. [Google Scholar]
- Kullgren, A.; Campanella, L.; D’Orazio, D.; Petronio, B.M.; Pietrantonio, E. Proposal for a metal speciation study in sediments. Anal. Chim. Acta 1995, 309, 387–393. [Google Scholar]
- Borovec, Z. Evaluation of the concentrations of trace elements in stream sediments by factor and cluster analysis and the sequential extraction procedure. Sci. Total Environ. 1996, 177, 237–250. [Google Scholar] [CrossRef]
- Gomez-Ariza, J.L.; Giraldez, I.; Sanchez-Rodas, D.; Rodas, E. Morales. Metal sequential Extraction Procedure optimized for heavy metal polluted and iron oxide rich sediments. Anal. Chim. Acta 2000, 414, 151–164. [Google Scholar] [CrossRef]
- Chang, A.C.; Page, A.L.; Warneke, J.E.; Grgurevic, E. Sequential extraction of soil heavy metals following a sludge applications. J. Environ. Q. 1984, 13. [Google Scholar] [CrossRef]
- Salomons, W. Adoption of common schemes for single and sequential extractions of trace metal in soils and sediments. Int. J. Environ. Anal. Chem. 1993, 51, 3–4. [Google Scholar] [CrossRef]
- Fiedler, H.D.; López-Sánchez, J.F.; Rubio, R.; Rauret, G.; Quevauviller, Ph.; Ure, A.M.; Muntau, H. Study of the stability of extractable trace metal contents in a river sediment using sequential extraction. Analyst 1994, 119, 1109–1114. [Google Scholar] [CrossRef]
- Ho, M.D.; Evans, G.J. Operational Speciation of Cadmium, Copper, Lead and Zinc in the NIST Standard Reference Materials 2710 and 2711 (Montana Soil) by the BCR Sequential Extraction Procedure and Flame Atomic Absorption Spectrometry. Anal. Commun. 1997, 34, 363–364. [Google Scholar] [CrossRef]
- López-Sánchez, J.F.; Sahuquillo, A.; Fiedler, H.D.; Rubio, R.; Rauret, G.; Muntau, H.; Quevauviller, P. CRM 601, A stable material for its extractable content of heavy metals. Analyst 1998, 123, 1675–1677. [Google Scholar] [CrossRef]
- Smeda, A.; Zyrnicki, W. Application of sequential extraction and the ICP-AES method for study of the partitioning of metals in fly ashes. Microchem. J. 2002, 72, 9–16. [Google Scholar] [CrossRef]
- Ramos, L.; Hernandez, L.M.; Gonzalez, M.J. sequential fractionation of copper, lead, cadmium and zinc in soils from or near Doñana National Park. J. Environ. Qual. 1994, 23, 50–57. [Google Scholar] [CrossRef]
- Qiang, T.; Xiao-quan, S.; Zhe-ming, N. Evaluation of a sequential extraction procedure for the fractionation of amorphous iron and manganese oxides and organic matter in soils. Sci. Total Environ. 1994, 151, 159–165. [Google Scholar] [CrossRef]
- Benitez, L.N.; Dubois, J.P. Evaluation of the selectivity of sequential extraction procedures applied to the speciation of cadmium in soils. Int. J. Environ. Anal. Chem. 1999, 74, 289–303. [Google Scholar] [CrossRef]
- Atkins, P.; Paulo, J.D. Physical Chemistry, 10th ed.; OUP Oxford: Oxford, UK, 2014. [Google Scholar]
- Martin, J.M.; Nirel, P.; Thomas, A.J. Sequential extraction techniques: Promises and problems. Mar. Chem. 1987, 22, 311–341. [Google Scholar] [CrossRef]
- Hlavay, J.; Prohaska, T.; Weisz, M.; Wenzel, W.W.; Stingeder, G.J. Determination of trace elements bound to soils and sediment fractions. Pure Appl. Chem. 2004, 76, 415–442. [Google Scholar] [CrossRef]
- Van der Sloot, H.; Dijkstra, J. Development of Horizontally Standardized Leaching Tests for Construction Materials: A Material Based or Release Based Approach? Identical Leaching Mechanisms for Different Materials; Energy Research Center of the Netherlands: Petten, Netherlands, 2004. [Google Scholar]
- Van Herck, P.; van der Bruggen, B.; Vogels, G.; Vandecasteele, C. Application of computer modelling to predict the leaching behaviour of heavy metals from MSWI fly ash and comparison with a sequential extraction method. Waste Manag. 2000, 20, 203–210. [Google Scholar] [CrossRef]
- Van Herck, P.; Vandecasteele, C. Evaluation of the use of a sequential extraction procedure for the characterization and treatment of metal containing solid waste. Waste Manag. 2001, 21, 685–694. [Google Scholar] [CrossRef]
- Malviya, R.; Chaudhary, R. Leaching behavior and immobilization of heavy metals in solidified/stabilized products. J. Hazard. Mater. 2006, 137, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Fällman, A.M. Leaching of chromium and barium from steel slag in laboratory and field tests—A solubility controlled process? Waste Manag. 2000, 20, 149–154. [Google Scholar] [CrossRef]
- Qiu, H.; Gu, H.-H.; He, E.-K.; Wang, S.-Z.; Qiu, R.-L. Attenuation of metal bioavailability in acidic multi-metal contaminated soil treated with fly ash and steel slag. Pedosphere 2012, 22, 544–553. [Google Scholar] [CrossRef]
- Cappuyns, V.; Swennen, R.; Niclaes, M. Application of the BCR sequential extraction scheme to dredged pond sediments contaminated by Pb-Zn mining: A combined geochemical and mineralogical approach. J. Geochem. Explor. 2007, 93, 78–90. [Google Scholar] [CrossRef]
- Nemati, K.; Bakar, N.K.A.; Abas, M.R.; Sobhanzadeh, E. Speciation of heavy metals by modified BCR sequential extraction procedure in different depths of sediments from Sungai Buloh, Selangor, Malaysia. J. Hazard. Mater. 2011, 192, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Bódog, I.; Polyák, K.; Csikós-Hartyányi, Z.; Hlavay, J. Sequential extraction procedure for the speciation of elements in Fly Ash Samples. Microchem. J. 1996, 54, 320–330. [Google Scholar] [CrossRef]
- Kirk, D.W.; Chan, C.C.Y.; Marsh, H. Chromium behavior during thermal treatment of MSW fly ash. J. Hazard. Mater. 2002, 90, 39–49. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Ye, T.; Guo, Y.; Gao, X. A study on the chemical and mineralogical characterization of MSWI fly ash using a sequential extraction procedure. J. Hazard. Mater. 2006, 134, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Sukandar, S.; Yasuda, K.; Tanaka, M.; Aoyama, I. Metals leachability from medical waste incinerator fly ash: A case study on particle size comparison. Environ. Pollut. 2006, 144, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Leinz, R.W.; Sutley, S.J.; Desborough, G.A.; Briggs, P.H. ICARD 2000. In Proceedings of the Fifth International Conference on Acid Rock Drainage, Denver, CO, USA, 24–27 January 2000; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2000; Volume II. [Google Scholar]
- Laforest, G.; Duchesne, J. Characterization and leachability of electric arc furnace dust made from remelting of stainless steel. J. Hazard. Mater. 2006, 135, 156–164. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodgers, K.J.; Hursthouse, A.; Cuthbert, S. The Potential of Sequential Extraction in the Characterisation and Management of Wastes from Steel Processing: A Prospective Review. Int. J. Environ. Res. Public Health 2015, 12, 11724-11755. https://doi.org/10.3390/ijerph120911724
Rodgers KJ, Hursthouse A, Cuthbert S. The Potential of Sequential Extraction in the Characterisation and Management of Wastes from Steel Processing: A Prospective Review. International Journal of Environmental Research and Public Health. 2015; 12(9):11724-11755. https://doi.org/10.3390/ijerph120911724
Chicago/Turabian StyleRodgers, Kiri J., Andrew Hursthouse, and Simon Cuthbert. 2015. "The Potential of Sequential Extraction in the Characterisation and Management of Wastes from Steel Processing: A Prospective Review" International Journal of Environmental Research and Public Health 12, no. 9: 11724-11755. https://doi.org/10.3390/ijerph120911724
APA StyleRodgers, K. J., Hursthouse, A., & Cuthbert, S. (2015). The Potential of Sequential Extraction in the Characterisation and Management of Wastes from Steel Processing: A Prospective Review. International Journal of Environmental Research and Public Health, 12(9), 11724-11755. https://doi.org/10.3390/ijerph120911724







