Lifestyle Behaviours Add to the Armoury of Treatment Options for Panic Disorder: An Evidence-Based Reasoning
Abstract
:1. Introduction
“Shift healthcare out of hospitals into communities, spurring innovation through greater competition in delivery, introducing more humanized care into healthcare, and investing in behavioural change and prevention to diminish demand”([1] p. 9)
“We have to be bold because so many of the lifestyle-driven health problems we see today are already at alarming levels. Britain is now the most obese nation in Europe. We have among the worst rates of sexually transmitted infections recorded, a relatively large population of problem drug users and rising levels of harm from alcohol. Smoking alone claims over 80,000 lives every year. Experts estimate that tackling poor mental health could reduce our overall disease burden by nearly a quarter. ... We need a new approach that empowers individuals to make healthy choices and gives communities the tools to address their own, particular needs”([2] p. 2).
2. Background
An incremental Evidence-based Reasoning
- Are MHPs experienced as individualised sequences of symptoms, often with common and identifiable symptom profiles?
- Do genetic variation and epigenetic control mechanisms partially explain how/why system sensitivity may be altered?
- Is there evidence of altered sensitivity within neurotransmitter systems?
- Is there evidence of altered sensitivity within body systems?
- Does altered body system sensitivity lead to lower levels of environmental exposure being required to provoke symptomatic reactions?
- Are such reactions open to misinterpretation if the causes remain unrecognised?
- Can modification of lifestyle behaviours provide an effective intervention?
3. An Incremental Evidence-Based Reasoning
3.1. Are MHPs Experienced as Individualised Sequences of Symptoms, Often with Common and Identifiable Symptom Patterns?
- Palpitations, pounding heart, or accelerated heart rate
- Sweating
- Trembling or shaking
- Sensations of shortness of breath or smothering
- Feeling of choking
- Chest pain or discomfort
- Nausea or abdominal distress
- Feeling dizzy, unsteady, lightheaded or faint
- Derealization (feelings of unreality) or depersonalization (being detached from oneself)
- Fear of losing control or going crazy
- Fear of dying
- Parastheias (numbness or tingling sensations)
- Chills or hot flushes
3.2. Do Genetic Variation and Epigenetic Control Mechanisms Partially Explain How/Why System Sensitivity May Be Altered?
3.3. Is There Evidence of Altered Sensitivity within Neurotransmitter Systems?
3.3.1. Epinephrine
3.3.2. Serotonin (5-HT)
3.4. Is There Evidence of Altered Sensitivity within Body Systems?
3.4.1. Respiratory System
| Author (Year) [reference] | Study Type | PD Patients | Controls | % Lactate Induced Panic Attacks | |
|---|---|---|---|---|---|
| Patients | Controls | ||||
| Pitts & McClure (1967) [90] | RCT | 14 | 10 | 93 | 20 |
| Gorman et al. (1984) [91] | Cohort | 12 | 67 | ||
| Fyer et al. (1985) [92] | RCT | 13 | 58 | ||
| Liebowitz et al. (1985) [93] | RCT | 43 | 20 | 72 | 0 |
| Ehlers et al. (1986) [94] | RCT | 10 | 10 | 90 | 60 |
| Balon et al. (1988) [95] | RCT | 86 | 45 | 85 | 22 |
| Gaffney et al. (1988) [96] | Cohort | 10 | 10 | 80 | 0 |
| Gorman et al. (1988) [97] | Cohort | 31 | 25 | 58 | 20 |
| Aronson et al. (1989) [98] | Cohort | 9 | 9 | 100 | 0 |
| Balon et al. (1989) [99] | Cohort | 45 | 22.2 | ||
| den Boer et al. (1989) [100] | RCT | 15 | 15 | 73 | 0 |
| Russel et al. (1991) [101] | Cohort | 11 | 100 | ||
| Goetz et al. (1996) [102] | Cohort | 202 | 59 | ||
| Binkley & Kutcher (1997) [103] | RCT | 5 | 100 | ||
| Coplan et al. (1998) [104] | RCT | 170 | 44 | 59 | 23 |
| Kellner et al. (1998) [105] | RCT | 10 | 10 | 70 | 20 |
| Strohle et al. (1998) [106] | RCT | 10 | 80 | ||
| Strohle (2000) [107] | RCT | 30 | 23 | 76.6 | 21.7 |
| Total Mean | 40.05882 | 22.16667 | 77.68235 | 17.40833 | |
| Lowest | 5 | 9 | 58 | 0 | |
| Highest | 202 | 45 | 100 | 60 | |
3.4.2. The Relationship between PD and the Immune System
- 1
- By destroying potentially toxic micro-organisms (antigens).
- 2
- By creating an increased reaction to the presence of a previous invading agent.
3.5. Does Altered Body System Sensitivity Lead to Lower Levels of Environmental Exposure Being Required to Provoke Symptomatic Reactions?
3.5.1. Respiratory System Reactivity
- Respiration rate, maintaining O2/CO2 balance, thereby maintaining blood Ph at close to normal.
- Change in metabolic rate, from whatever cause.
3.5.2. Immune System Reactivity
3.6. Are Such Reactions Open to Misinterpretation If the Causes Remain Unrecognised?
- Epigenetic control mechanisms are reactive to external environmental conditions, and therefore influence sensitivity to physiological sensations. Lifestyle behaviours such as diet, fluid intake, exercise and habitual lifestyle drug use (nicotine, caffeine and alcohol), directly influence bodily environmental conditions, and therefore also the function and cognitive appraisal of body systems.
- If the trigger for misinterpretation is from misunderstanding an altered physiological reaction, in addition to a purely psychological response, then the importance of understanding the cause of the panic reaction increases.
- If a rational explanation of the physiological nature of the sensation can be provided, and tested by the patient, through addressing habitual behaviours that may be influencing the physiological environment, then fear of the symptoms and sensations can be reduced.
- If patients can be assisted in recognising lifestyle behaviours to which they have an altered sensitivity, then the impact of change in habitual lifestyle behaviours can improve health status across a broader range of indicators. Control over symptoms can pass back to the patient through positive changes in environmental conditions.
3.7. Can Modification of Lifestyle Behaviours Provide an Effective Intervention?
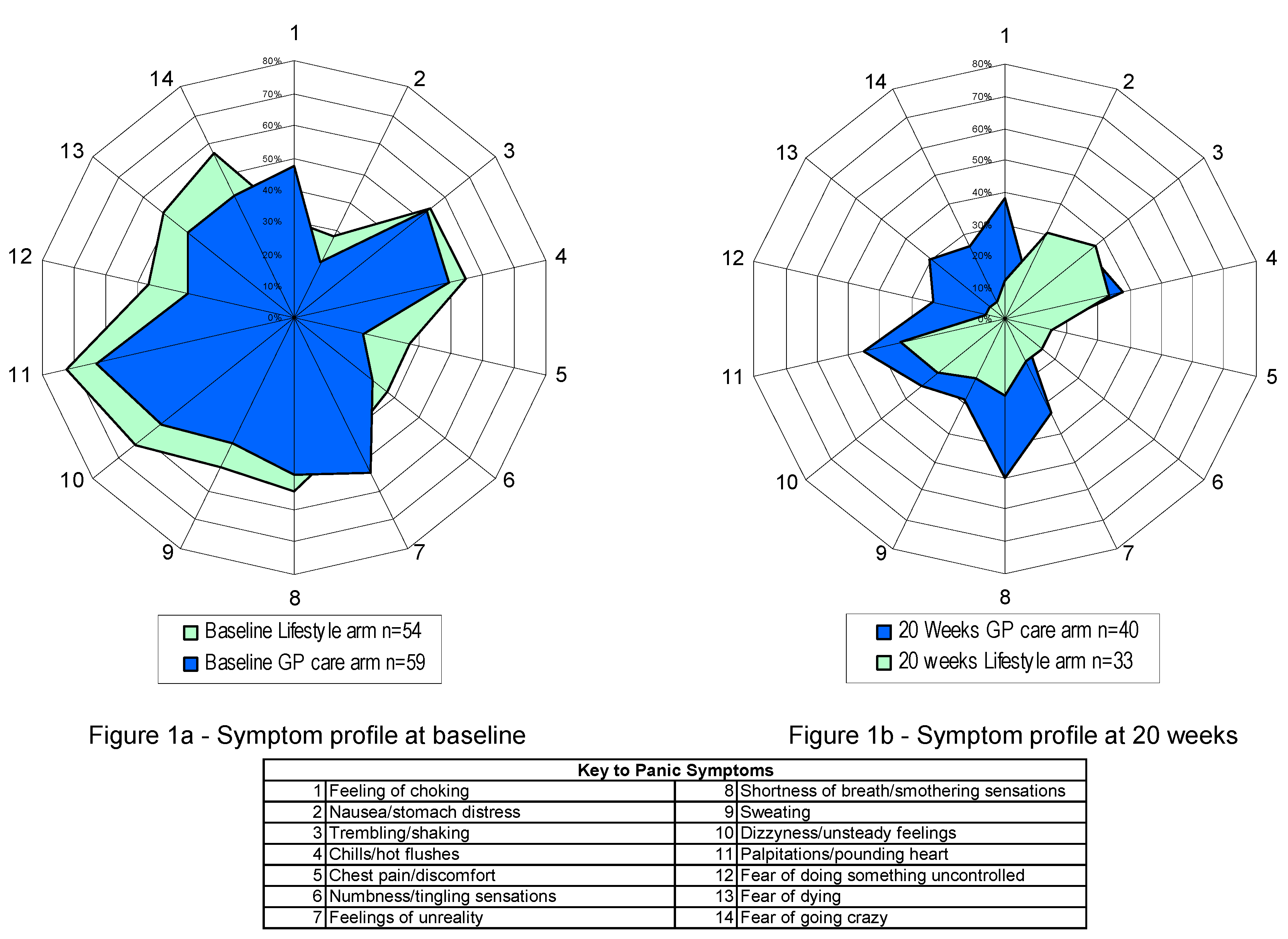
4. Discussion
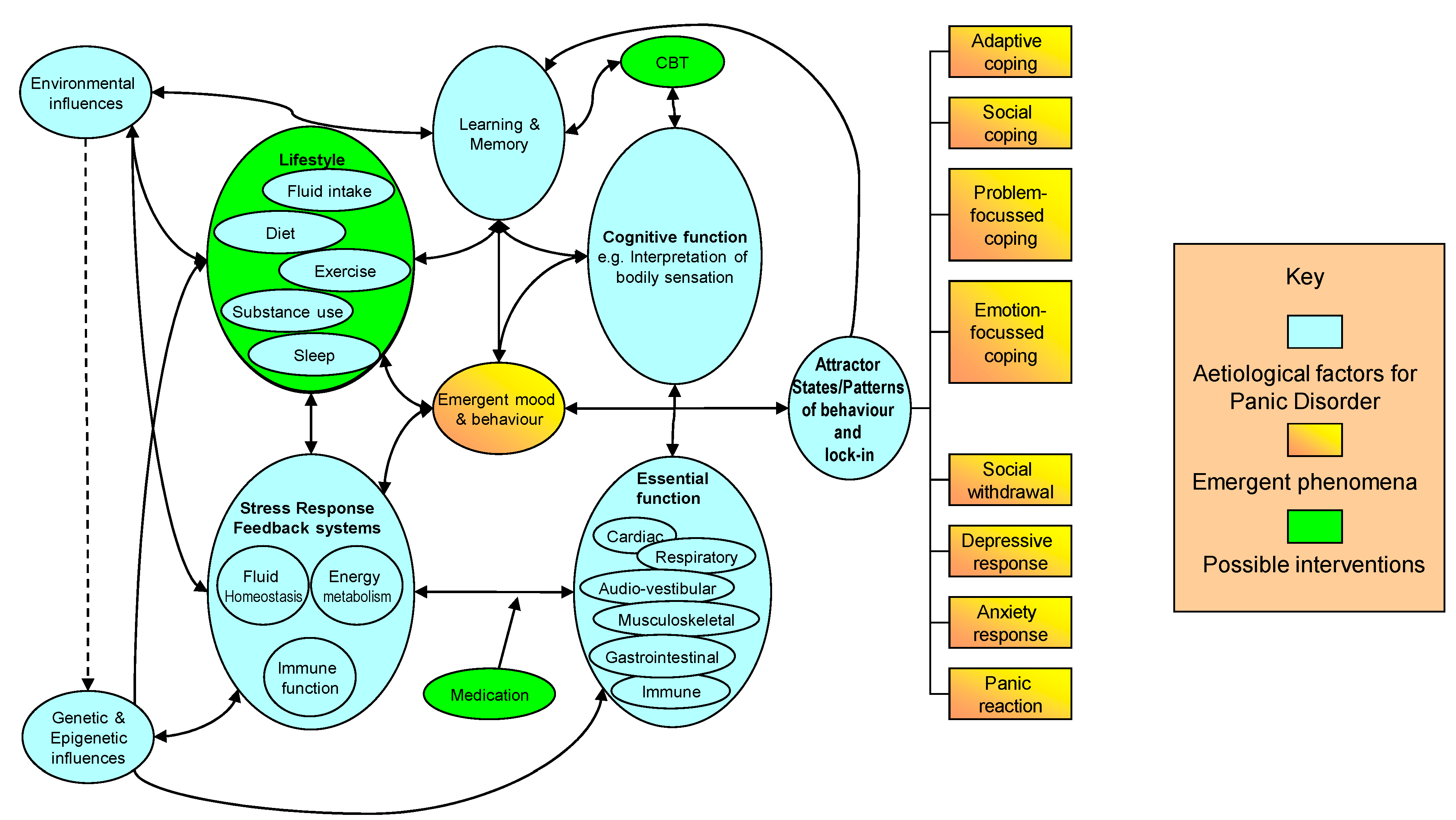
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Economic Forum and McKinsey & Company. Sustainable Health Systems: Visions, Strategies, Critical Uncertainties and Scenarios; World Economic Forum: Geneva, Switzerland, 2013. [Google Scholar]
- Department of Health. Healthy Lives, Healthy People: Our Strategy for Public Health in England; Department of Health: London, UK, 2010.
- Carr, S.; Lhussier, M.; Forster, N.; Geddes, L.; Deane, K.; Pennington, M.; Visram, S.; White, M.; Michie, S.; Donaldson, C.; et al. An evidence synthesis of qualitative and quantitative research on component intervention techniques, effectiveness, cost-effectiveness, equity and acceptability of different versions of health-related lifestyle advisor role in improving health. Health Technol. Assess 2011, 15, 1–284. [Google Scholar] [CrossRef] [PubMed]
- MRC. A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health; Medical Research Council: London, UK, 2000.
- MRC. Developing and Evaluating Complex Interventions: New Guidance; Medical Research Council: London, UK, 2008.
- College of Occupational Therapists. Recovering Ordinary Lives: The Strategy for Occupational Therapy in Mental Health Services 2007–2017: A Vision for the Next Ten Years; College of Occupational Therapists: London, UK, 2010. [Google Scholar]
- Lambert, R.A.; Lorgelly, P.; Harvey, I.; Poland, F. Cost-effectiveness analysis of an occupational therapy-led lifestyle approach and routine general practitioner’s care for panic disorder. Soc. Psychiatry Psychiatr. Epidemiol. 2010, 45, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.; Caan, W.; McVicar, A. Influences of lifestyle and general practice (GP) care on the symptom profile of people with panic disorder. J. Public Ment. Health 2008, 7, 18–24. [Google Scholar] [CrossRef]
- Lambert, R.A.; Harvey, I.; Poland, F. A pragmatic, unblinded randomised controlled trial comparing an occupational therapy-led lifestyle approach and routine GP care for panic disorder treatment in primary care. J. Affect. Disord. 2007, 99, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Lambert, R.A. Intervention in panic and anxiety disorders through lifestyle modification. In International Handbook of Occupational Therapy Interventions, 2nd ed.; Soderback, I., Ed.; Springer: London, UK, 2015; pp. 541–552. [Google Scholar]
- Lambert, R.A. Routine general practice care for panic disorder within the lifestyle approach to managing panic study. Ment. Illn. 2012, 4, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G. Australian and new zealand clinical practice guidelines for the treatment of panic disorder and agoraphobia. Aust. N. Z. J. Psychiatry 2003, 37, 641–656. [Google Scholar]
- American Psychiatric Association. Practice Guidelines for the Treatment of Psychiatric Disorders; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- NICE. Anxiety (Amended): Management of Anxiety (Panic Disorder, with or without Agoraphobia, and Generalised Anxiety Disorder) in Adults in Primary, Secondary and Community Care; NICE Clinical Guideline 22 (Amended); National Institute for Health and Care Excellence: London, UK, 2007. [Google Scholar]
- Schoen, C.; Osborn, R.; How, S.K.; Doty, M.M.; Peugh, J. In chronic condition: Experiences of patients with complex health care needs, in eight countries, 2008. Health Aff. 2009, 28, w1–w16. [Google Scholar] [CrossRef] [PubMed]
- Chwastiak, L.; Vanderlip, E.; Katon, W. Treating complexity: Collaborative care for multiple chronic conditions. Int. Rev. Psychiatry 2014, 26, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Sterling, M.; Pedler, A. A neuropathic pain component is common in acute whiplash and associated with a more complex clinical presentation. Man. Ther. 2009, 14, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Kleinknecht-Dolf, M.; Grand, F.; Spichiger, E.; Muller, M.; Martin, J.S.; Spirig, R. Complexity of nursing care in acute care hospital patients: Results of a pilot study with a newly developed questionnaire. Scand. J. Caring Sci. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- De Silva, M.J.; Breuer, E.; Lee, L.; Asher, L.; Chowdhary, N.; Lund, C.; Patel, V. Theory of Change: A theory-driven approach to enhance the Medical Research Council’s framework for complex interventions. Trials 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.M. Oxford English Dictionary. Available online: http://www.pubfacts.com/detail/18959196/Oxford-English-Dictionary (accessed on 6 May 2015).
- Rogers, J.C. Eleanor Clarke Slagle Lectureship—1983; clinical reasoning: The ethics, science, and art. Am. J. Occup. Ther. 1983, 37, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Tiffen, J.; Corbridge, S.J.; Slimmer, L. Enhancing clinical decision making: Development of a contiguous definition and conceptual framework. J. Prof. Nurs. 2014, 30, 399–405. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic & Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Rucci, P.; Miniati, M.; Oppo, A.; Mula, M.; Calugi, S.; Frank, E.; Shear, M.K.; Mauri, M.; Pini, S.; Cassano, G.B. The structure of lifetime panic-agoraphobic spectrum. J. Psychiatr. Res. 2009, 43, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Cannon, D.M.; Klaver, J.M.; Klug, S.A.; Carlson, P.J.; Luckenbaugh, D.A.; Ichise, M.; Drevets, W.C. Gender-specific abnormalities in the serotonin transporter system in panic disorder. Int. J. Neuropsychopharmacol. 2013, 16, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Kelly, M.M.; Forsyth, J.P.; Karekla, M. Sex differences in response to a panicogenic challenge procedure: An experimental evaluation of panic vulnerability in a non-clinical sample. Behav. Res. Ther. 2006, 44, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Siegert, M.; David, A.S. Depersonalization and individualism: The effect of culture on symptom profiles in panic disorder. J. Nerv. Ment. Dis. 2007, 195, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Tibi, L.; van Oppen, P.; Aderka, I.M.; van Balkom, A.J.; Batelaan, N.M.; Spinhoven, P.; Penninx, B.W.; Anholt, G.E. Examining determinants of early and late age at onset in panic disorder: An admixture analysis. J. Psychiatr. Res. 2013, 47, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.; Zvolensky, M.J.; Sachs-Ericsson, N.; Schmidt, N.B.; Bonn-Miller, M.O. Associations between age of onset and lifetime history of panic attacks and alcohol use, abuse, and dependence in a representative sample. Compr. Psychiatry 2006, 47, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Preter, M.; Klein, D.F. Lifelong opioidergic vulnerability through early life separation: A recent extension of the false suffocation alarm theory of panic disorder. Neurosci. Biobehav. Rev. 2014, 46, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tsuchiya, M.; Kawakami, N.; Furukawa, T.A. Non-fearful vs. fearful panic attacks: A general population study from the national comorbidity survey. J. Affect. Disord. 2009, 112, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Sardinha, A.; Freire, R.C.; Zin, W.A.; Nardi, A.E. Respiratory manifestations of panic disorder: Causes, consequences and therapeutic implications. J. Bras. Pneumol. 2009, 35, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Wollburg, E.; Roth, W.T.; Kim, S. Effects of breathing training on voluntary hypo- and hyperventilation in patients with panic disorder and episodic anxiety. Appl. Psychophysiol. Biofeedback 2011, 36, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.; Westen, D. Personality subtypes in patients with panic disorder. Compr. Psychiatry 2009, 50, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Petty, C.R.; Hirshfeld-Becker, D.R.; Henin, A.; Faraone, S.V.; Fraire, M.; Henry, B.; McQuade, J.; Rosenbaum, J.F. Developmental trajectories of anxiety disorders in offspring at high risk for panic disorder and major depression. Psychiatry Res. 2007, 153, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Detzel, T.; Wesner, A.C.; Fritz, A.; da Silva, C.T.; Guimaraes, L.; Heldt, E. Family burden and family environment: Comparison between patients with panic disorder and with clinical diseases. Psychiatry Clin. Neurosci. 2015, 69, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Larsen, D.K.; Asmundson, G.J.; Stein, M.B. Effect of a novel environment on resting heart rate in panic disorder. Depress. Anxiety 1998, 8, 24–28. [Google Scholar] [CrossRef]
- Roy-Byrne, P.P.; Craske, M.G.; Stein, M.B. Panic disorder. Lancet 2006, 368, 1023–1032. [Google Scholar] [CrossRef]
- Bentley, D.R. The Human Genome Project—An overview. Med. Res. Rev. 2000, 20, 189–196. [Google Scholar] [CrossRef]
- Wain, L.V.; Armour, J.A.; Tobin, M.D. Genomic copy number variation, human health, and disease. Lancet 2009, 374, 340–350. [Google Scholar] [CrossRef]
- Wenner, M. Too little, too much: A new sense for how variable numbers of genes cause disease. Sci. Am. 2009, 300, 14–15. [Google Scholar] [CrossRef]
- Dawkins, R. The Selfish Gene; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Lohmueller, K.E.; Albrechtsen, A.; Li, Y.; Kim, S.Y.; Korneliussen, T.; Vinckenbosch, N.; Tian, G.; Huerta-Sanchez, E.; Feder, A.F.; Grarup, N.; et al. Natural selection affects multiple aspects of genetic variation at putatively neutral sites across the human genome. PLoS. Genet. 2011, 7. [Google Scholar] [CrossRef] [PubMed]
- Na, H.R.; Kang, E.H.; Lee, J.H.; Yu, B.H. The genetic basis of panic disorder. J. Korean Med. Sci. 2011, 26, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.P.; Fyer, A.J.; Durner, M.; Heiman, G.A.; Baisre, D.L.; Hodge, S.E.; Knowles, J.A.; Weissman, M.M. Further genetic evidence for a panic disorder syndrome mapping to chromosome 13q. Proc. Natl. Acad. Sci USA 2003, 100, 2550–2555. [Google Scholar] [CrossRef] [PubMed]
- Smoller, J.W.; Gardner-Schuster, E.; Covino, J. The genetic basis of panic and phobic anxiety disorders. Am. J. Med. Genet. C Semin. Med. Genet. 2008, 148C, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T. Epigenetics in psychiatry. Neuropsychobiology 2009, 60. [Google Scholar] [CrossRef] [PubMed]
- Lipton, B.H. The Biology of Belief: Unleashing the Power of Consciousness, Matter and Miracles; Hay House Inc.: Santa Monica, CA, USA, 2008. [Google Scholar]
- Delcuve, G.P.; Rastegar, M.; Davie, J.R. Epigenetic control. J. Cell. Physiol. 2009, 219, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.A. Stressed and depressed? Check your GDNF for epigenetic repression. Neuron 2011, 69, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.; Hill, L.E.; Chandramohan, Y.; Whitcomb, D.; Droste, S.K.; Reul, J.M. Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLoS ONE 2009, 4. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.; Eikelis, N.; Schlaich, M.; Lambert, G.; Alvarenga, M.; Kaye, D.; El-Osta, A.; Guo, L.; Barton, D.; Pier, C.; et al. Human sympathetic nerve biology: Parallel influences of stress and epigenetics in essential hypertension and panic disorder. Ann. N. Y. Acad. Sci. 2008, 1148, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, R.; Greenwood, P.M.; Kumar, R.; Fossella, J. Beyond heritability: Neurotransmitter genes differentially modulate visuospatial attention and working memory. Psychol. Sci. 2005, 16, 200–207. [Google Scholar] [CrossRef] [PubMed]
- McGowan, P.O.; Szyf, M. The epigenetics of social adversity in early life: Implications for mental health outcomes. Neurobiol. Dis. 2010, 39, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M. Beyond the usual suspects: A cholinergic route for panic attacks. Mol. Psychiatry 2002, 7, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Medlock, C.; Dzemidzic, M.; Shin, Y.W.; Goddard, A.W.; Dydak, U. Decreased GABA levels in anterior cingulate cortex/medial prefrontal cortex in panic disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 44, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Maron, E.; Nutt, D.J.; Kuikka, J.; Tiihonen, J. Dopamine transporter binding in females with panic disorder may vary with clinical status. J. Psychiat. Res. 2010, 44, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Hohoff, C.; Domschke, K.; Sand, P.; Kuhlenbaumer, G.; Schirmacher, A.; Freitag, C.M.; Meyer, J.; Stober, G.; Franke, P.; et al. Norepinephrine transporter (NET) promoter and 5′-UTR polymorphisms: Association analysis in panic disorder. Neurosci. Lett. 2005, 377, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Mueller, E.M.; Panitz, C.; Nestoriuc, Y.; Stemmler, G.; Wacker, J. Panic disorder and serotonin reuptake inhibitors predict coupling of cortical and cardiac activity. Neuropsychopharmacology 2014, 39, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Koszycki, D.; Torres, S.; Swain, J.E.; Bradwejn, J. Central cholecystokinin activity in irritable bowel syndrome, panic disorder, and healthy controls. Psychosom. Med. 2005, 67, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Kvetnansky, R.M.; Adrenal, M.R. Encyclopedia of Stress; Fink, G., Ed.; Academic Press: London, UK, 2000; Volume 1, pp. 63–70. [Google Scholar]
- Zigmond, M.J.; Bloom, F.E.; Landis, S.C.; Roberts, J.L.; Squire, L.R. Fundamental Neuroscience; Academic Press: London, UK, 1999. [Google Scholar]
- Veltman, D.J.; van Zijderveld, G.A.; van Dyck, R. Epinephrine infusions in Panic Disorder: A double-blind placebo-controlled study. J. Affect. Disord. 1996, 39, 133–140. [Google Scholar] [CrossRef]
- Van Zijderveld, G.A.; Veltman, D.J.; van Dyck, R.; Van Doornen, L.J.P. Epinephrine-induced panic attacks and hyperventilation. J. Psychiatr. Res. 1999, 33, 73–78. [Google Scholar] [CrossRef]
- Veltman, D.J.; van Zijderveld, G.A.; van Dyck, R.; Bakker, A. Predictability, controllability, and fear of symptoms of anxiety in epinephrine-induced panic. Biol. Psychiatry 1998, 44, 1017–1026. [Google Scholar] [CrossRef]
- Abelson, J.L.; Weg, J.G.; Nesse, R.M.; Curtis, G.C. Neuroendocrine responses to laboratory panic: Cognitive intervention in the doxapram model. Psychoneuroendocrinology 1996, 21, 375–390. [Google Scholar] [CrossRef]
- Oh, J.; Yu, B.; Heo, J.; Yoo, I.; Song, H.; Jeon, H.J. Plasma catecholamine levels before and after paroxetine treatment in patients with panic disorder. Psychiatry Res. 2014, 225, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Delaney, C.; Gien, J.; Roe, G.; Isenberg, N.; Kailey, J.; Abman, S.H. Serotonin contributes to high pulmonary vascular tone in a sheep model of persistent pulmonary hypertension of the newborn. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L894–L901. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.G. Guy, food intake and stress, human. In Encyclopedia of Stress; Fink, G., Ed.; Academic Press: London, UK, 2000; Volume 2, pp. 158–162. [Google Scholar]
- Bell, C.; Abrams, J.; Nutt, D. Tryptophan depletion and its implications for psychiatry. Br. J. Psychiatry 2001, 178, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Kesic, M.; Tvrdeic, A.; Kolaric, D.; Stojkovic, R.; Cicin-Sain, L. Serotonergic modulation of pain and analgesic responses: A study in rats with constitutionally altered serotonin transporters. Eur. J. Pain 2014, 19, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Otto, M.W.; Tuby, K.S.; Gould, R.A.; McLean, R.Y.S.; Pollack, M.H. An effect-size analysis of the relative efficacy and tolerability of serotonin selective reuptake inhibitors for panic disorder. Am. J. Psychiatry 2001, 158, 1989–1992. [Google Scholar] [CrossRef] [PubMed]
- Van Apeldoorn, F.J.; van Hout, W.J.; Mersch, P.P.; Huisman, M.; Slaap, B.R.; Hale, W.W., III; Visser, S.; van Dyck, R.; den Boer, J.A. Is a combined therapy more effective than either CBT or SSRI alone? Results of a multicenter trial on panic disorder with or without agoraphobia. Acta Psychiatr. Scand. 2008, 117, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, X.; Zhang, N.; Ma, Q. Effects of exercise on stress-induced changes of norepinephrine and serotonin in rat hippocampus. Chin. J. Physiol. 2013, 56, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Maron, E.; Shlik, J. Serotonin function in panic disorder: Important, but why? Neuropsychopharmacology 2006, 31, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.J.; Nutt, D.J. Serotonin and panic. Br. J. Psychiatry 1998, 172, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Toru, I.; Shlik, J.; Maron, E.; Vasar, V.; Nutt, D.J. Tryptophan depletion does not modify response to CCK-4 challenge in patients with panic disorder after treatment with citalopram. Psychopharmacology 2006, 186, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Schruers, K.; Klaassen, T.; Pols, H.; Overbeek, T.; Deutz, N.E.; Griez, E. Effects of tryptophan depletion on carbon dioxide provoked panic in panic disorder patients. Psychiatry Res. 2000, 93, 179–187. [Google Scholar] [CrossRef]
- Maron, E.; Toru, I.; Hirvonen, J.; Tuominen, L.; Lumme, V.; Vasar, V.; Shlik, J.; Nutt, D.J.; Helin, S.; Nagren, K.; et al. Gender differences in brain serotonin transporter availability in panic disorder. J. Psychopharmacol. 2011, 25, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.K.; Yang, J.C.; Lee, H.J.; Kim, Y.K. The association between serotonin-related gene polymorphisms and panic disorder. J. Anxiety Disord 2008, 22, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Le Francois, B.; Czesak, M.; Steubl, D.; Albert, P.R. Transcriptional regulation at a HTR1A polymorphism associated with mental illness. Neuropharmacology 2008, 55, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Graeff, F.G. New perspective on the pathophysiology of panic: Merging serotonin and opioids in the periaqueductal gray. Braz. J. Med. Biol. Res. 2012, 45, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Fullana, M.A.; Vilagut, G.; Ortega, N.; Bruffaerts, R.; de Girolamo, G.; de Graaf, R.; Haro, J.M.; Kovess, V.; Matschinger, H.; Bulbena, A.; et al. Prevalence and correlates of respiratory and non-respiratory panic attacks in the general population. J. Affect. Disord. 2011, 131, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Nardi, A.E.; Nascimento, I.; Valenca, A.M.; Lopes, F.L.; Mezzasalma, M.A.; Zin, W.A.; Versiani, M. Respiratory panic disorder subtype: Acute and long-term response to nortriptyline, a noradrenergic tricyclic antidepressant. Psychiatry Res. 2003, 120, 283–293. [Google Scholar] [CrossRef]
- Freire, R.C.; Lopes, F.L.; Valenca, A.M.; Nascimento, I.; Veras, A.B.; Mezzasalma, M.A.; de-Melo-Neto, V.L.; Zin, W.A.; Nardi, A.E. Panic disorder respiratory subtype: A comparison between responses to hyperventilation and CO2 challenge tests. Psychiatry Res. 2008, 157, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Nardi, A.E.; Valenca, A.M.; Lopes, F.L.; Nascimento, I.; Mezzasalma, M.A.; Zin, W.A. Clinical features of panic patients sensitive to hyperventilation or breath-holding methods for inducing panic attacks. Braz. J. Med. Biol. Res. 2004, 37, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Song, H.M.; Kim, J.H.; Heo, J.Y.; Yu, B.H. Clinical characteristics of the respiratory subtype in panic disorder patients. Psychiatry Investig. 2014, 11, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Schruers, K.; Griez, E. The effects of tianeptine or paroxetine on 35% CO2 provoked panic in panic disorder. J. Psychopharmacol. 2004, 18, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, G.; Diaz-Galvis, J.; Schruers, K.; Berlanga, C.; Lara-Munoz, C.; Griez, E. Acute exercise reduces the effects of a 35% CO2 challenge in patients with panic disorder. J. Affect. Disord. 2008, 107, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Pitts, F.N., Jr.; McClure, J.N., Jr. Lactate metabolism in anxiety neurosis. N. Engl. J. Med. 1967, 277, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.M.; Askanazi, J.; Liebowitz, M.R.; Fyer, A.J.; Stein, J.; Kinney, J.M.; Klein, D.F. Response to hyperventilation in a group of patients with panic disorder. Am. J. Psychiatr. 1984, 141, 857–861. [Google Scholar] [PubMed]
- Fyer, A.J.; Liebowitz, M.R.; Gorman, J.M.; Davies, S.O.; Klein, D.F. Lactate vulnerability of remitted panic patients. Psychiatr. Res. 1985, 14, 143–148. [Google Scholar] [CrossRef]
- Liebowitz, M.R.; Gorman, J.M.; Fyer, A.J.; Levitt, M.; Dillon, D.; Levy, G.; Appleby, I.L.; Anderson, S.; Palij, M.; Davies, S.O. Lactate provocation of panic attacks. II. Biochemical and physiological findings. Arch. Gen. Psychiatr. 1985, 42, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, A.; Margraf, J.; Roth, W.T.; Taylor, C.B.; Maddock, R.J.; Sheikh, J.; Kopell, M.L.; McClenahan, K.L.; Gossard, D.; Blowers, G.H. Lactate infusions and panic attacks: Do patients and controls respond differently? Psychiatry Research 1986, 17, 295–308. [Google Scholar] [CrossRef]
- Balon, R.; Pohl, R.; Yeragani, V.K.; Rainey, J.M.; Weinberg, P. Lactate- and isoproterenol-induced panic attacks in panic disorder patients and controls. Psychiatry Research 1988, 23, 153–160. [Google Scholar] [CrossRef]
- Gaffney, F.A.; Fenton, B.J.; Lane, L.D.; Lake, C.R. Hemodynamic, ventilatory, and biochemical responses of panic patients and normal controls with sodium lactate infusion and spontaneous panic attacks. Arch. Gen. Psychiatr. 1988, 45, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.M.; Fyer, M.R.; Goetz, R.; Askanazi, J.; Liebowitz, M.R.; Fyer, A.J.; Kinney, J.; Klein, D.F. Ventilatory physiology of patients with panic disorder. Arch Gen Psychiatry 1988, 45, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Aronson, T.A.; Carasiti, I.; McBane, D.; Whitaker-Azmitia, P. Biological correlates of lactate sensitivity in panic disorder. Biol Psychiatry 1989, 26, 463–477. [Google Scholar] [CrossRef]
- Balon, R.; Jordan, M.; Pohl, R.; Yeragani, V.K. Family history of anxiety disorders in control subjects with lactate-induced panic attacks. Am. J. Psychiatr. 1989, 146, 1304–1306. [Google Scholar] [PubMed]
- Den Boer, J.A.; Westenberg, H.G.M.; Klompmakers, A.; van Lint, L.E.M. Behavioral biochemical and neuroendocrine concomitants of lactate-induced panic anxiety. Biol. Psychiat. 1989, 26, 612–622. [Google Scholar] [CrossRef]
- Russell, J.L.; Kushner, M.G.; Beitman, B.D.; Bartels, K.M. Nonfearful panic disorder in neurology patients validated by lactate challenge. Am. J. Psychiatr. 1991, 148, 361–364. [Google Scholar] [PubMed]
- Goetz, R.R.; Klein, D.F.; Gorman, J.M. Symptoms Essential to the Experience of Sodium Lactate-Induced Panic. Neuropsychopharmacology 1996, 14, 355–366. [Google Scholar] [CrossRef]
- Binkley, K.E.; Kutcher, S. Panic response to sodium lactate infusion in patients with multiple chemical sensitivity syndrome. J. Allergy Clin. Immunol. 1997, 99, 570–574. [Google Scholar] [CrossRef]
- Coplan, J.D.; Goetz, R.; Klein, D.F.; Papp, L.A.; Fyer, A.J.; Liebowitz, M.R.; Davies, S.O.; Gorman, J.M. Plasma cortisol concentrations preceding lactate-induced panic. Psychological, biochemical, and physiological correlates. Arch. Gen. Psychiatr. 1998, 55, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.; Knaudt, K.; Jahn, H.; Holsboer, F.; Wiedemann, K. Atrial natriuretic hormone in lactate-induced panic attacks: Mode of release and endocrine and pathophysiological consequences. J. Psychiatr. Res. 1998, 32, 37–48. [Google Scholar] [CrossRef]
- Strohle, A.; Kellner, M.; Yassouridis, A.; Holsboer, F.; Wiedemann, K. Effect of Flumazenil in Lactate-Sensitive Patients With Panic Disorder. Am. J. Psychiatr. 1998, 155, 610–612. [Google Scholar] [CrossRef] [PubMed]
- Strohle, A. Increased response to a putative panicogenic nocebo administration in female patients with panic disorder. J. Psychiatr. Res. 2000, 34, 439–442. [Google Scholar] [CrossRef]
- Johnson, P.L.; Truitt, W.A.; Fitz, S.D.; Lowry, C.A.; Shekhar, A. Neural pathways underlying lactate-induced panic. Neuropsychopharmacology 2008, 33, 2093–2107. [Google Scholar] [CrossRef] [PubMed]
- Putnam, R.W.; Filosa, J.A.; Ritucci, N.A. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am. J. Physiol. Cell Physiol. 2004, 287, C1493–C1526. [Google Scholar] [CrossRef] [PubMed]
- Maddock, R.J.; Buonocore, M.H.; Copeland, L.E.; Richards, A.L. Elevated brain lactate responses to neural activation in panic disorder: A dynamic 1H-MRS study. Mol. Psychiatry 2009, 14, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Nillni, Y.I.; Rohan, K.J.; Bernstein, A.; Zvolensky, M.J. Premenstrual distress predicts panic-relevant responding to a CO2 challenge among young adult females. J. Anxiety Disord. 2010, 24, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Telch, M.J.; Smits, J.A.; Brown, M.; Dement, M.; Powers, M.B.; Lee, H.; Pai, A. Effects of threat context and cardiac sensitivity on fear responding to a 35% CO2 challenge: A test of the context-sensitivity panic vulnerability model. J. Behav. Ther. Exp. Psychiatry 2010, 41, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Fadda, E.; Galimberti, E.; Cammino, S.; Bellodi, L. Smoking, physical activity and respiratory irregularities in patients with panic disorder. Riv. Psichiatr. 2013, 48, 293–300. [Google Scholar] [PubMed]
- Cowley, D.S.; Roy-Byrne, P.P. Hyperventilation and panic disorder. Am. J. Med. 1987, 83, 929–937. [Google Scholar] [CrossRef]
- Klein, D.F. Panic disorder and agoraphobia: Hypothesis hothouse. J. Clin. Psychiatry 1996, 57, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Preter, M.; Klein, D.F. Panic, suffocation false alarms, separation anxiety and endogenous opioids. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Nardi, A.E.; Valenca, A.M.; Nascimento, I.; Mezzasalma, M.A.; Zin, W.A. Hyperventilation in panic disorder and social phobia. Psychopathology 2001, 34, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Strohle, A.; Kellner, M.; Holsboer, F.; Wiedemann, K. Anxiolytic activity of atrial natriuretic peptide in patients with panic disorder. Am. J. Psychiatry 2001, 158, 1514–1516. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H. Basilar artery blood flow velocity changes in patients with panic disorder following 35% carbon dioxide challenge. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 1343–1344. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.M.; Coplan, J.D.; Browne, S.T.; Goetz, R.; Welkowitz, L.A.; Papp, L.A.; Klein, D.F.; Gorman, J.M. Hemodynamic response to respiratory challenges in panic disorder. J. Psychosom. Res. 1998, 44, 153–161. [Google Scholar] [CrossRef]
- Sikter, A.; Frecska, E.; Braun, I.M.; Gonda, X.; Rihmer, Z. The role of hyperventilation: Hypocapnia in the pathomechanism of panic disorder. Rev. Bras. Psiquiatr. 2007, 29, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Beck, J.G.; Ohtake, P.J.; Shipherd, J.C. Exaggerated anxiety is not unique to CO2 in panic disorder: A comparison of hypercapnic and hypoxic challenges. J. Abnorm. Psychol. 1999, 108, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Horwath, E.; Adams, P.; Wickramaratne, P.; Pine, D.; Weissman, M.M. Panic disorder with smothering symptoms: Evidence for increased risk in first-degree relatives. Depress. Anxiety 1997, 6, 147–153. [Google Scholar] [CrossRef]
- Niccolai, V.; van Duinen, M.A.; Griez, E.J. Respiratory patterns in panic disorder reviewed: A focus on biological challenge tests. Acta Psychiatr. Scand. 2009, 120, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Psychoneuroimmunology. In Encyclopedia of Stress; Fink, G., Ed.; Academic Press: London, UK, 2000; Volume 3, pp. 294–298. [Google Scholar]
- Vander, A.; Sherman, J.; Luciano, D. Human Physiology: The Mechanics of Body Function, 8th ed.; McGraw-Hill: London, UK, 2001. [Google Scholar]
- Shimada-Sugimoto, M.; Otowa, T.; Miyagawa, T.; Khor, S.S.; Kashiwase, K.; Sugaya, N.; Kawamura, Y.; Umekage, T.; Kojima, H.; Saji, H.; et al. Immune-related pathways including HLA-DRB1(*)13:02 are associated with panic disorder. Brain Behav. Immun. 2015, 46, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Foldager, L.; Kohler, O.; Steffensen, R.; Thiel, S.; Kristensen, A.S.; Jensenius, J.C.; Mors, O. Bipolar and panic disorders may be associated with hereditary defects in the innate immune system. J. Affect. Disord. 2014, 164, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Van Duinen, M.A.; Schruers, K.R.J.; Griez, E.J.L.; Maes, M. Neuroimmunological parameters in panic disorder. Acta Neuropsychiatr. 2004, 16, 94–100. [Google Scholar] [CrossRef]
- Park, J.E.; Kim, S.W.; Park, Q.; Jeong, D.U.; Yu, B.H. Lymphocyte subsets and mood states in panic disorder patients. J. Korean Med. Sci. 2005, 20, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Van Duinen, M.A.; Schruers, K.R.; Kenis, G.R.; Wauters, A.; Delanghe, J.; Griez, E.J.; Maes, M.H. Effects of experimental panic on neuroimmunological functioning. J. Psychosom. Res. 2008, 64, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.J.; Klein, D.F.; Mannuzza, S.; Moulton, J.L., III; Pine, D.S.; Klein, R.G. Relationship between separation anxiety disorder, parental panic disorder, and atopic disorders in children: A controlled high-risk study. J. Am. Acad. Child Adolesc. Psychiatry 2002, 41, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, M.; Pesenti-Gritti, P.; Medland, S.E.; Ogliari, A.; Tambs, K.; Spatola, C.A. A genetically informed study of the association between childhood separation anxiety, sensitivity to CO2, panic disorder, and the effect of childhood parental loss. Arch. Gen. Psychiatry 2009, 66, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Bovasso, G.; Eaton, W. Types of panic attacks and their association with psychiatric disorder and physical illness. Compr. Psychiatry 1999, 40, 469–477. [Google Scholar] [CrossRef]
- Passik, S.D.; Roth, A.J. Anxiety symptoms and panic attacks preceding pancreatic cancer diagnosis. Psychooncology 1999, 8, 268–272. [Google Scholar] [CrossRef]
- Slaughter, J.R.; Jain, A.; Holmes, S.; Reid, J.C.; Bobo, W.; Sherrod, N.B. Panic disorder in hospitalized cancer patients. Psychooncology 2000, 9, 253–258. [Google Scholar] [CrossRef]
- Griffeth, B.T.; Mehra, A. Panic as a harbinger of pancreatic cancer. Psychosomatics 2008, 49, 538–539. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kinoshita, H.; Akechi, T.; Uchitomi, Y.; Andoh, M. First panic attack episodes in head and neck cancer patients who have undergone radical neck surgery. J. Pain Symptom Manag. 2007, 34, 575–578. [Google Scholar] [CrossRef] [PubMed]
- Osorio, F.L.; Lima, M.P.; Chagas, M.H. Assessment and screening of panic disorder in cancer patients: Performance of the PHQ-PD. J. Psychosom. Res. 2015, 78, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Sansone, R.A.; Sansone, L.A. Panic disorder subtypes: Deceptive somatic impersonators. Psychiatry 2009, 6, 33–37. [Google Scholar] [PubMed]
- Kircanski, K.; Craske, M.G.; Epstein, A.M.; Wittchen, H.U. Subtypes of panic attacks: A critical review of the empirical literature. Depress. Anxiety 2009, 26, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Roberson-Nay, R.; Kendler, K.S. Panic disorder and its subtypes: A comprehensive analysis of panic symptom heterogeneity using epidemiological and treatment seeking samples. Psychol. Med. 2011, 41, 2411–2421. [Google Scholar] [CrossRef] [PubMed]
- Stavile, K.L.; Sinert, R. Metabolic Acidosis Medicine: Instant Access to the Minds of Medicine. Available online: http://www.emedicine.com/emerg/topic312.htm (accessed on 4 August 2014).
- Martinsen, E.W.; Raglin, J.S.; Hoffart, A.; Friis, S. Tolerance to intensive exercise and high levels of lactate in panic disorder. J. Anxiety Disord. 1998, 12, 333–342. [Google Scholar] [CrossRef]
- Griez, E.; Schruers, K. Experimental pathophysiology of panic. J. Psychosom. Res. 1998, 45, 493–503. [Google Scholar] [CrossRef]
- Ueda, Y.; Aizawa, M.; Takahashi, A.; Fujii, M.; Isaka, Y. Exaggerated compensatory response to acute respiratory alkalosis in panic disorder is induced by increased lactic acid production. Nephrol. Dial. Transplant. 2009, 24, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Strohle, A.; Graetz, B.; Scheel, M.; Wittmann, A.; Feller, C.; Heinz, A.; Dimeo, F. The acute antipanic and anxiolytic activity of aerobic exercise in patients with panic disorder and healthy control subjects. J. Psychiatr. Res. 2009, 43, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Caldirola, D.; Namia, C.; Micieli, W.; Carminati, C.; Bellodi, L.; Perna, G. Cardiorespiratory response to physical exercise and psychological variables in panic disorder. Rev. Bras. Psiquiatr. 2011, 33, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Sizer, F.; Whitney, E. Nutrition: Concepts and Controversies, 8th ed.; Wadsworth: London, UK, 2000. [Google Scholar]
- Shiels, M.S.; Katki, H.A.; Freedman, N.D.; Purdue, M.P.; Wentzensen, N.; Trabert, B.; Kitahara, C.M.; Furr, M.; Li, Y.; Kemp, T.J.; et al. Cigarette smoking and variations in systemic immune and inflammation markers. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Cook, R.T. Alcohol abuse, alcoholism, and damage to the immune system—A review. Alcohol Clin. Exp. Res. 1998, 22, 1927–1942. [Google Scholar] [PubMed]
- Sher, L. Effects of heavy alcohol consumption on the cardiovascular system may be mediated in part by the influence of alcohol-induced depression on the immune system. Med. Hypotheses 2003, 60, 702–706. [Google Scholar] [CrossRef]
- Curtis, B.J.; Zahs, A.; Kovacs, E.J. Epigenetic targets for reversing immune defects caused by alcohol exposure. Alcohol Res. 2013, 35, 97–113. [Google Scholar] [PubMed]
- Pohl, J.; Sheppard, M.; Luheshi, G.N.; Woodside, B. Diet-induced weight gain produces a graded increase in behavioral responses to an acute immune challenge. Brain Behav. Immun. 2014, 35, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Horn, P.L.; West, N.P.; Pyne, D.B.; Koerbin, G.; Lehtinen, S.J.; Fricker, P.A.; Cripps, A.W. Routine exercise alters measures of immunity and the acute phase reaction. Eur. J. Appl. Physiol. 2015, 115, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Sahun, I.; Gallego, X.; Gratacos, M.; Murtra, P.; Trullas, R.; Maldonado, R.; Estivill, X.; Dierssen, M. Differential responses to anxiogenic drugs in a mouse model of panic disorder as revealed by Fos immunocytochemistry in specific areas of the fear circuitry. Amino Acids 2007, 33, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Vilarim, M.M.; Rocha Araujo, D.M.; Nardi, A.E. Caffeine challenge test and panic disorder: A systematic literature review. Expert Rev. Neurother. 2011, 11, 1185–1195. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.M. A cognitive approach to panic. Behav. Res. Ther. 1986, 24, 461–470. [Google Scholar] [CrossRef]
- Pauli, P.; Dengler, W.; Wiedemann, G.; Montoya, P.; Flor, H.; Birbaumer, N.; Buchkremer, G. Behavioral and neurophysiological evidence for altered processing of anxiety-related words in panic disorder. J. Abnorm. Psychol. 1997, 106, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, N.G.; Oei, T.P. Catastrophic cognitions in panic disorder with and without agoraphobia. Clin. Psychol. Rev. 1998, 18, 341–365. [Google Scholar] [CrossRef]
- Molarius, A.; Berglund, K.; Eriksson, C.; Eriksson, H.G.; Linden-Bostrom, M.; Nordstrom, E.; Persson, C.; Sahlqvist, L.; Starrin, B.; Ydreborg, B. Mental health symptoms in relation to socio-economic conditions and lifestyle factors—A population-based study in Sweden. BMC Public Health 2009, 9. [Google Scholar] [CrossRef] [PubMed]
- Chaney, E.H.; Chaney, J.D.; Wang, M.Q.; Eddy, J.M. Lifestyle behaviors and mental health of American adults. Psychol. Rep. 2007, 100, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Rucklidge, J.J. Successful treatment of OCD with a micronutrient formula following partial response to Cognitive Behavioral Therapy (CBT): A case study. J. Anxiety Disord. 2009, 23, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, A.; Deane, F.P.; Williams, P. Dietitians and exercise physiologists in primary care: Lifestyle interventions for patients with depression and/or anxiety. J. Allied Health 2009, 38, e63–e68. [Google Scholar] [PubMed]
- Lambert, R.; Brown, C. Health and complexity. In Complexity Science and Society; Bogg, J., Geyer, R., Eds.; Radcliffe Publishing: Oxford, UK, 2007. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambert, R. Lifestyle Behaviours Add to the Armoury of Treatment Options for Panic Disorder: An Evidence-Based Reasoning. Int. J. Environ. Res. Public Health 2015, 12, 7017-7043. https://doi.org/10.3390/ijerph120607017
Lambert R. Lifestyle Behaviours Add to the Armoury of Treatment Options for Panic Disorder: An Evidence-Based Reasoning. International Journal of Environmental Research and Public Health. 2015; 12(6):7017-7043. https://doi.org/10.3390/ijerph120607017
Chicago/Turabian StyleLambert, Rod. 2015. "Lifestyle Behaviours Add to the Armoury of Treatment Options for Panic Disorder: An Evidence-Based Reasoning" International Journal of Environmental Research and Public Health 12, no. 6: 7017-7043. https://doi.org/10.3390/ijerph120607017
APA StyleLambert, R. (2015). Lifestyle Behaviours Add to the Armoury of Treatment Options for Panic Disorder: An Evidence-Based Reasoning. International Journal of Environmental Research and Public Health, 12(6), 7017-7043. https://doi.org/10.3390/ijerph120607017





