Antimony, Arsenic and Chromium Speciation Studies in Biała Przemsza River (Upper Silesia, Poland) Water by HPLC-ICP-MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Researched Area
2.2. Sampling
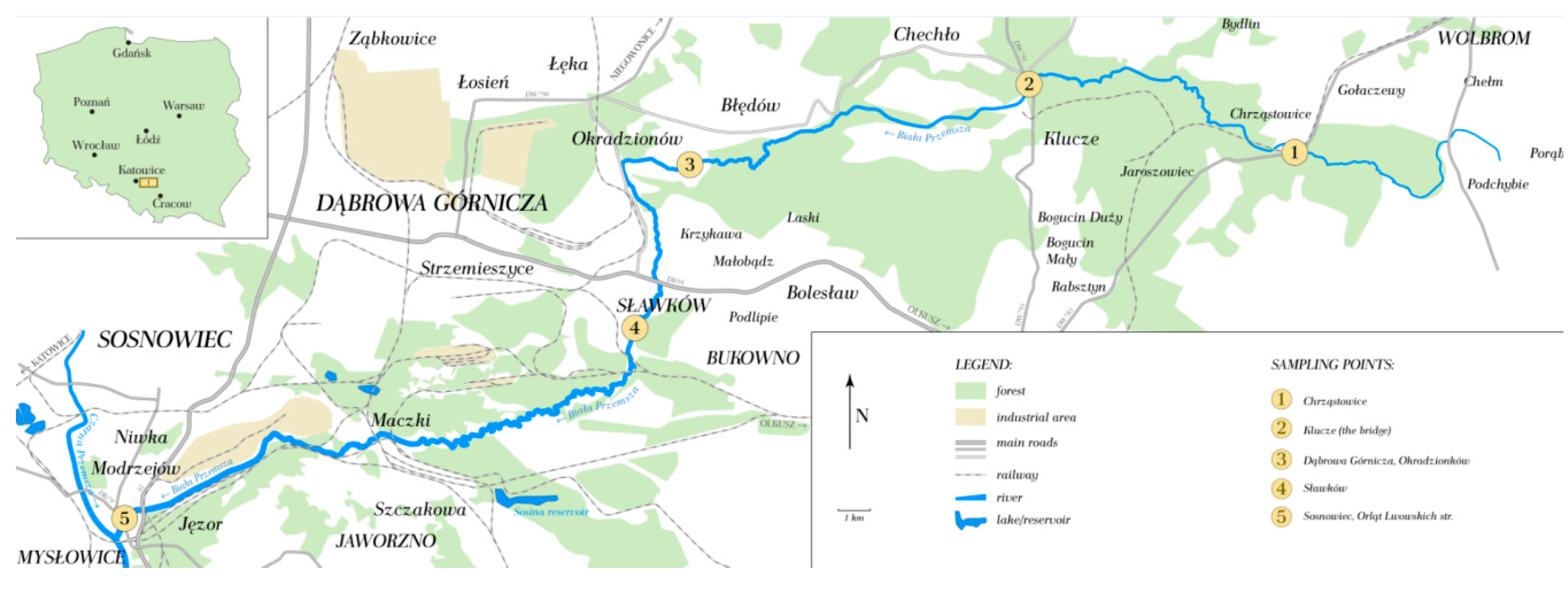
2.3. Sample Preparation
2.4. Research Methodology
| ICP-MS Parameter | Settings |
|---|---|
| Generator power RF (W) | 1125 |
| Plasma gas flow (l/min) | 15 |
| Nebulizer gas flow (l/min) | 0.76–0.82 |
| Auxiliary gas flow (l/min) | 1.15–1.16 |
| Nebulizer | cross |
| Torch | quartz |
| Scanning mode | Peak hopping |
| Dwell time (ms) | 250 |
| Sweeps/Reading | 1 |
| Number of replicates | 830 |
| Fraction | The Extraction Solution | Associated With |
|---|---|---|
| F1 Exchangeable | Acetic acid: 0.11 M CH3COOH, pH = 2.85, shaking for 16 h, the ratio of solid / solution 1:40 Room temperature | Loosely adsorbed cations and anions on sediments and carbonates and very reactive oxy-hydroxides |
| F2 Reducible | Hydroxyl ammonium chloride: 0.1 M NH2OH.HCl, pH = 2.0 shaking for 16 h, the ratio of solid / solution 1:40 Temperature 85 ± 2 °C | Iron/manganese oxides (mostly amorphous or poorly crystallized) |
| F3 Oxidisable | Perhydrol: H2O2 8.8 M, CH3COONH4 1.0 M pH = 2.0 | Organic substance and sulphides |
| Residual | Aqua regia: 3HCl + HNO3 | Non silicate bound metals |
| Parameter | Value |
|---|---|
| Chromium | |
| Column | Ion Pac AG-7; 50 mm × 4 mm, 10 µm |
| Temperature | 35 °C |
| Mobile phase | A: 0.1M NH4NO3 pH = 4;
B: 0.8M HNO3 |
| Elution program | 0–0.5 min 100% A,
1.5–3.5 min 100% B rinsing 3.5–5.0 min. 100% A |
| Flow rate during the analysis (mL/min) | 1.7 |
| Flow rate during the rinsing (mL/min) | 2.0 |
| Volume of sample (µL) | 170 |
| Antimony | |
| Column | Ion Pac AS-7; 200 mm × 4 mm, 10 µm |
| Temperature | 35 °C |
| Mobile phase | 1mM phthalic acid, 10mM EDTANa2; pH = 4.5 |
| Elution time | 3 min |
| Flow rate during the analysis (mL/min) | 1.2 |
| Volume of sample (µL) | 80 |
| Arsenic | |
| Column | Hamilton PRP-X100; 100 mm x 4 mm, 10 µm |
| Temperature | 30 °C |
| Mobile phase | A: 20mM NH4NO3 pH = 8.7
B: 60mM NH4NO3 pH = 8.7 |
| Elution time | 0–2.0 min. 100% A
2.0–3.0 min from 100% A to 100% B 3.0–6.5 min 100% B rinsing 6.5–9.5 min 100% A |
| Flow rate during the analysis (mL/min) | 1.1 |
| Volume of sample (µL) | 100 |
| Analyte | Limit of detection (µg/L) | Recovery (%) | Relative Standard Deviation of Repeatability (%) | Uncertainty (%) |
|---|---|---|---|---|
| Cr (total) | 0.013 | 116 | 5.9 | 23 |
| Cr(III) | 0.19 | 99 | 2.0 | 14 |
| Cr(VI) | 0.37 | 102 | 2.3 | 11 |
| Sb (total) | 0.005 | 106 | 3.2 | 12 |
| Sb(III) | 0.009 | 105 | 1.9 | 8 |
| Sb(V) | 0.012 | 101 | 2.4 | 8 |
| As (total) | 0.096 | 88 | 4.8 | 29 |
| As(III) | 0.08 | 96 | 2.9 | 12 |
| As(V) | 0.12 | 104 | 2.4 | 11 |
| AB | 0.16 | 93 | 3.7 | 17 |
| MMA | 0.08 | 95 | 3.1 | 12 |
| DMA | 0.09 | 95 | 2.7 | 11 |
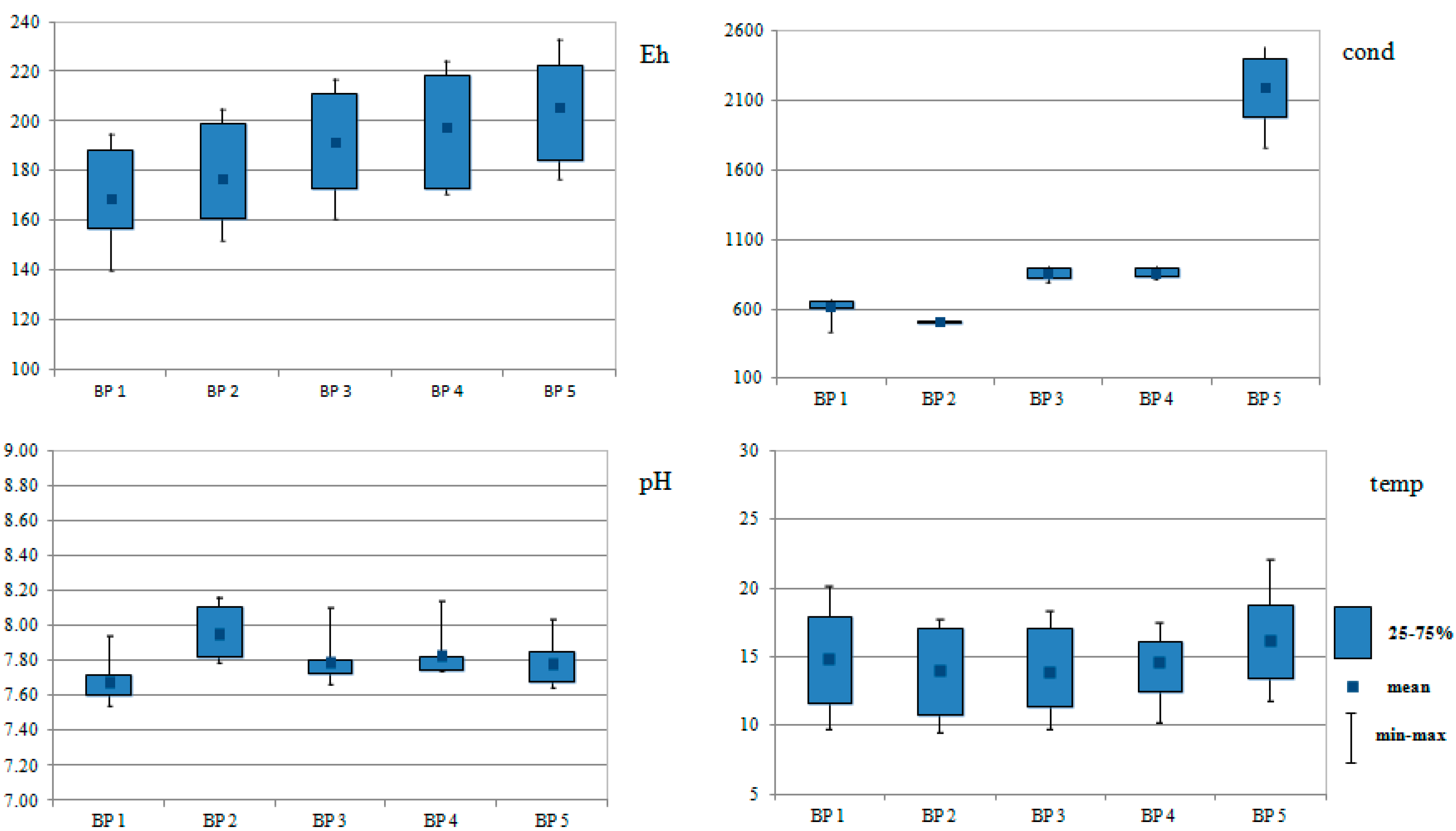
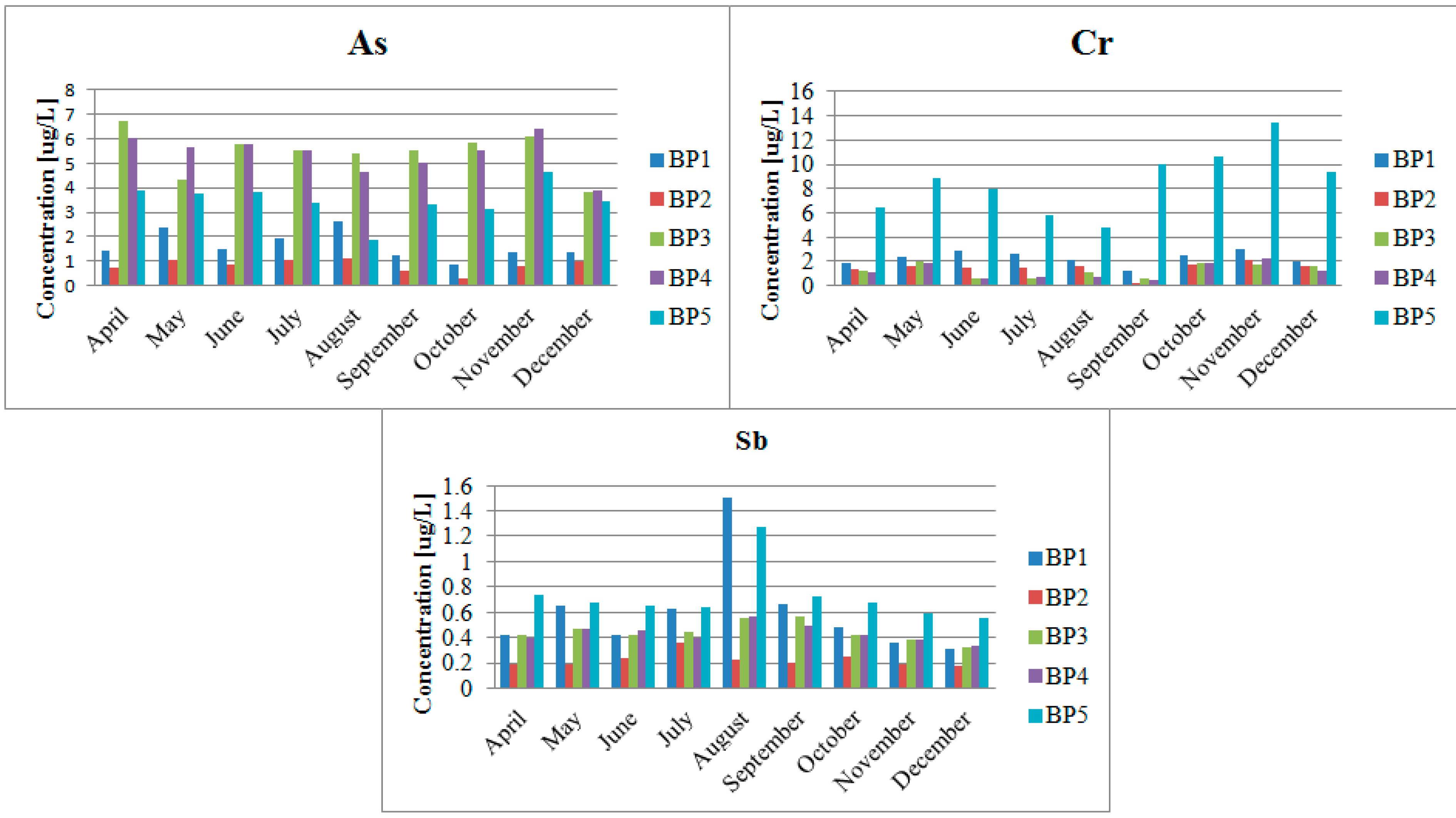
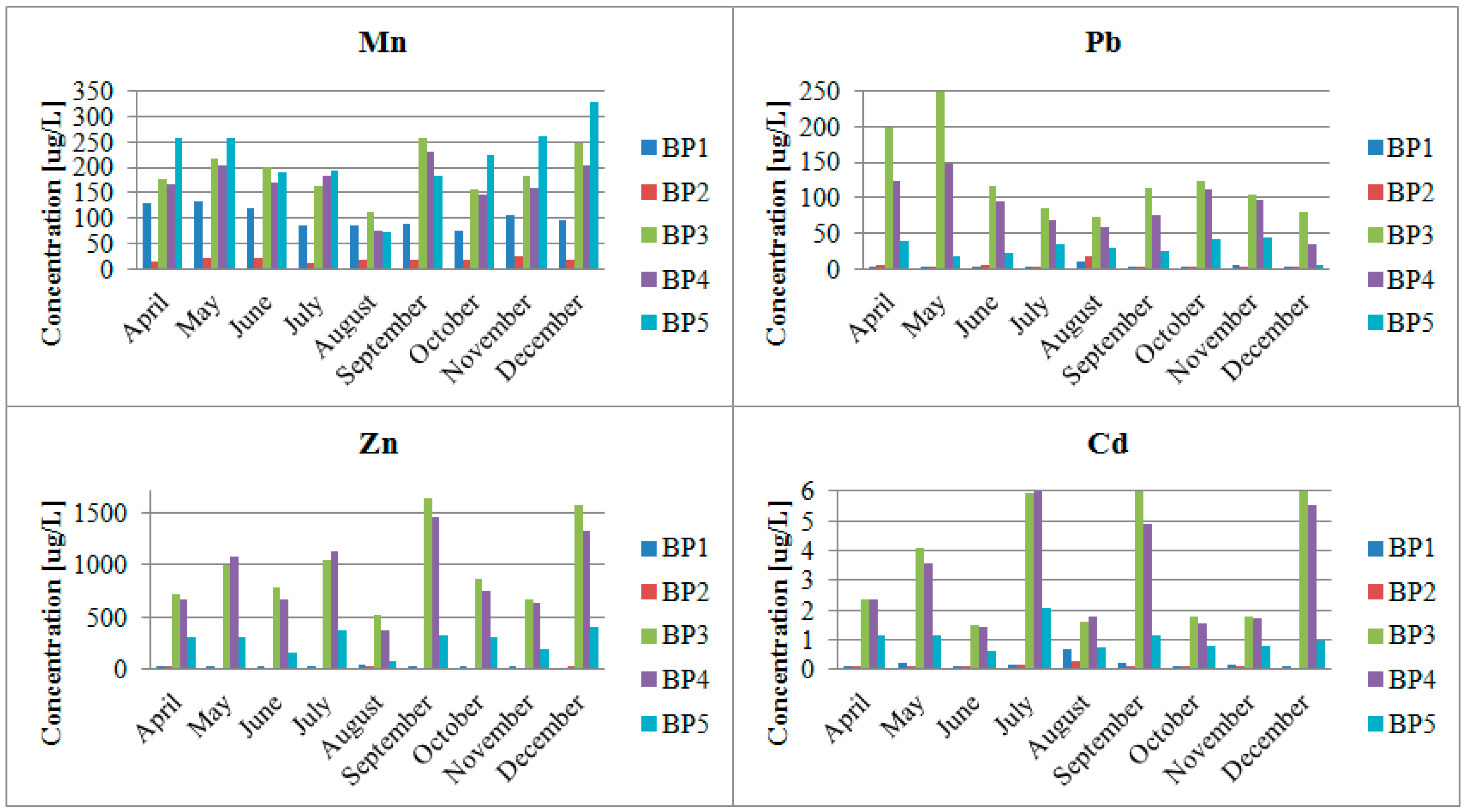
3. Results
| Date of Sampling | Sampling Point | >2.0 mm | 2.0–1.0 mm | 1.0–0.5 mm | 0.5–0.2 mm | 0.2–0.1 mm | <0.1 mm | Unit |
|---|---|---|---|---|---|---|---|---|
| April | BP1 | 0.16 | 0.22 | 15.79 | 77.85 | 3.64 | 0.17 | % |
| BP2 | 0.29 | 0.17 | 15.54 | 73.77 | 10.06 | 0.18 | % | |
| BP3 | 5.51 | 11.94 | 12.47 | 31.89 | 25.85 | 12.34 | % | |
| BP4 | 0.00 | 0.01 | 2.27 | 88.87 | 8.78 | 0.08 | % | |
| BP5 | 0.07 | 0.23 | 2.00 | 80.85 | 15.44 | 1.41 | % | |
| July | BP1 | 0.16 | 0.22 | 15.79 | 77.85 | 3.64 | 0.17 | % |
| BP2 | 2.43 | 2.45 | 6.32 | 63.08 | 23.17 | 3.68 | % | |
| BP3 | 1.30 | 0.75 | 1.02 | 36.36 | 34.93 | 26.94 | % | |
| BP4 | 0.00 | 0.00 | 0.39 | 78.43 | 20.22 | 0.96 | % | |
| BP5 | 1.26 | 1.48 | 8.77 | 80.18 | 7.55 | 0.76 | % | |
| October | BP1 | 0.07 | 0.11 | 9.25 | 84.21 | 6.09 | 0.28 | % |
| BP2 | 11.97 | 3.19 | 5.74 | 38.08 | 31.42 | 9.60 | % | |
| BP3 | 0.08 | 0.00 | 0.92 | 59.91 | 33.03 | 6.06 | % | |
| BP4 | 0.18 | 0.11 | 0.31 | 32.10 | 30.28 | 37.02 | % | |
| BP5 | 0.52 | 0.76 | 4.46 | 82.38 | 10.82 | 1.05 | % |
| (µg/L) N = 30 | BP1 | BP2 | BP3 | BP4 | BP5 | |
|---|---|---|---|---|---|---|
| As(III) | minimum | <0.08 | <0.08 | <0.08 | 0.26 | <0.08 |
| maximum | 0.21 | 0.08 | 0.99 | 0.56 | 3.83 | |
| median | 0.11 | 0.08 | 0.72 | 0.39 | 1.24 | |
| As(V) | minimum | <0.12 | <0.12 | 0.13 | 0.16 | 0.17 |
| maximum | 1.35 | 0.56 | 3.67 | 3.83 | 2.22 | |
| median | 0.55 | 0.26 | 1.71 | 2.05 | 1.45 | |
| Cr(VI) | minimum | <0.37 | <0.37 | <0.37 | <0.37 | <0.37 |
| maximum | 0.95 | 0.97 | 0.93 | 1.19 | 1.07 | |
| median | <0.37 | <0.37 | <0.37 | 0.51 | 0.67 | |
| Cr(III) | minimum | 0.44 | 0.41 | 0.86 | 0.51 | 1.07 |
| maximum | 2.59 | 4.63 | 3.33 | 3.41 | 3.65 | |
| median | 2.24 | 1.74 | 2.13 | 2.51 | 1.92 | |
4. Discussion
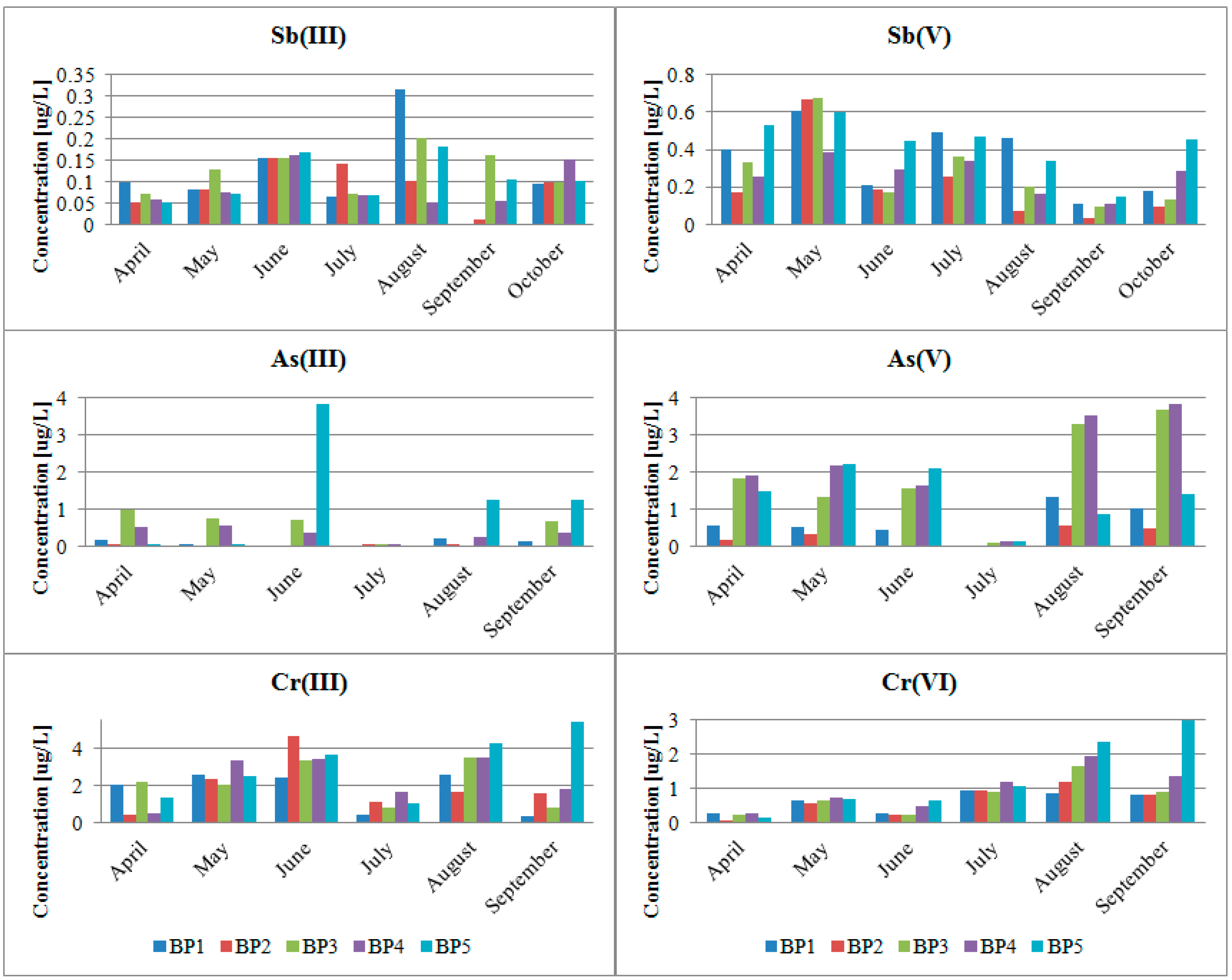
4.1. Impact of the Physicochemical Parameters on the Concentration of Metal (Loid)s and their Forms in Biała Przemsza River Waters
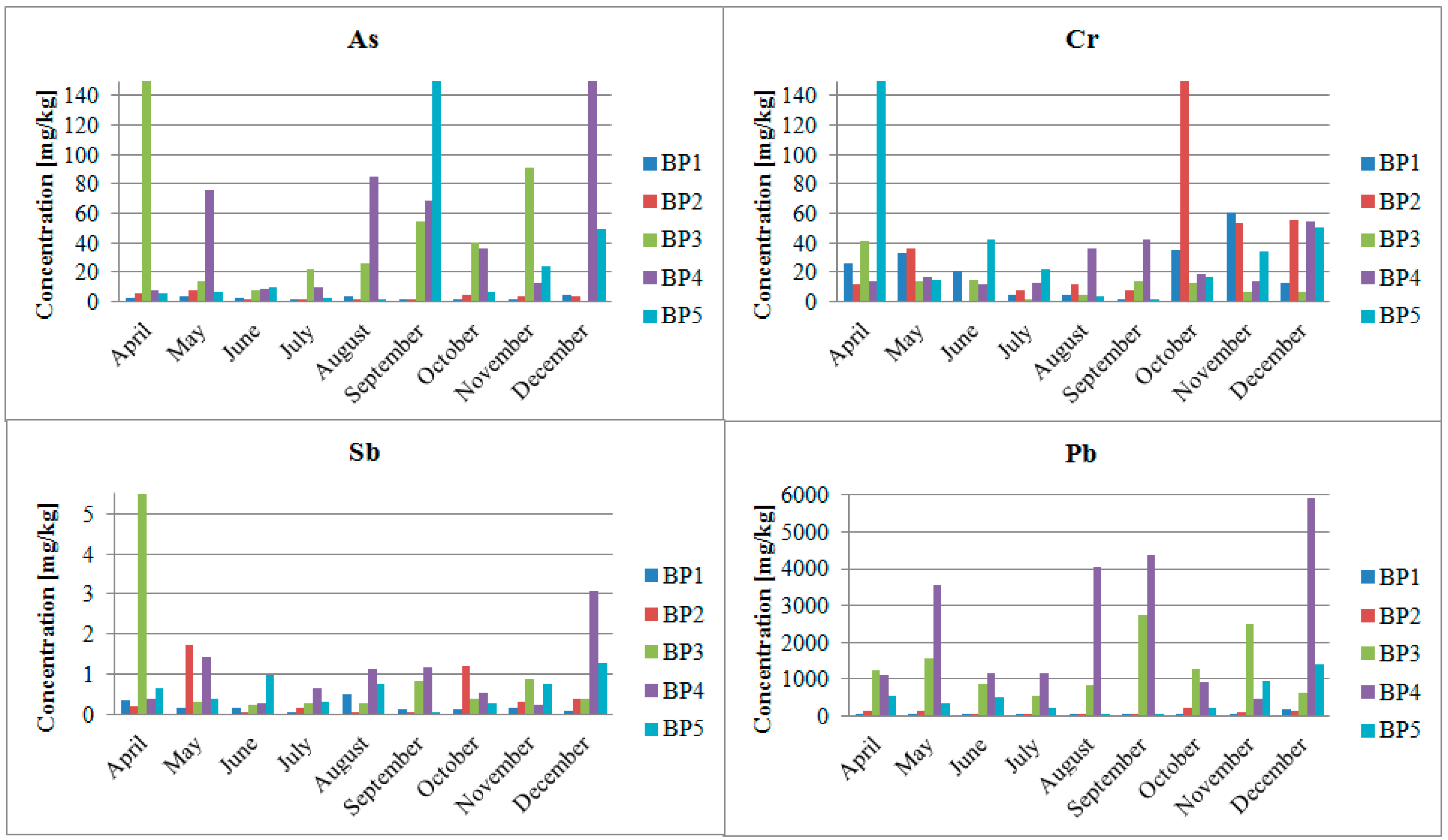
4.2. Impact of the Pb and Zn Ore Mining Industry on the Pollution of the Biała Przemsza River
4.3. Metal (Loid)s and their Forms in Biała Przemsza Bottom Sediments
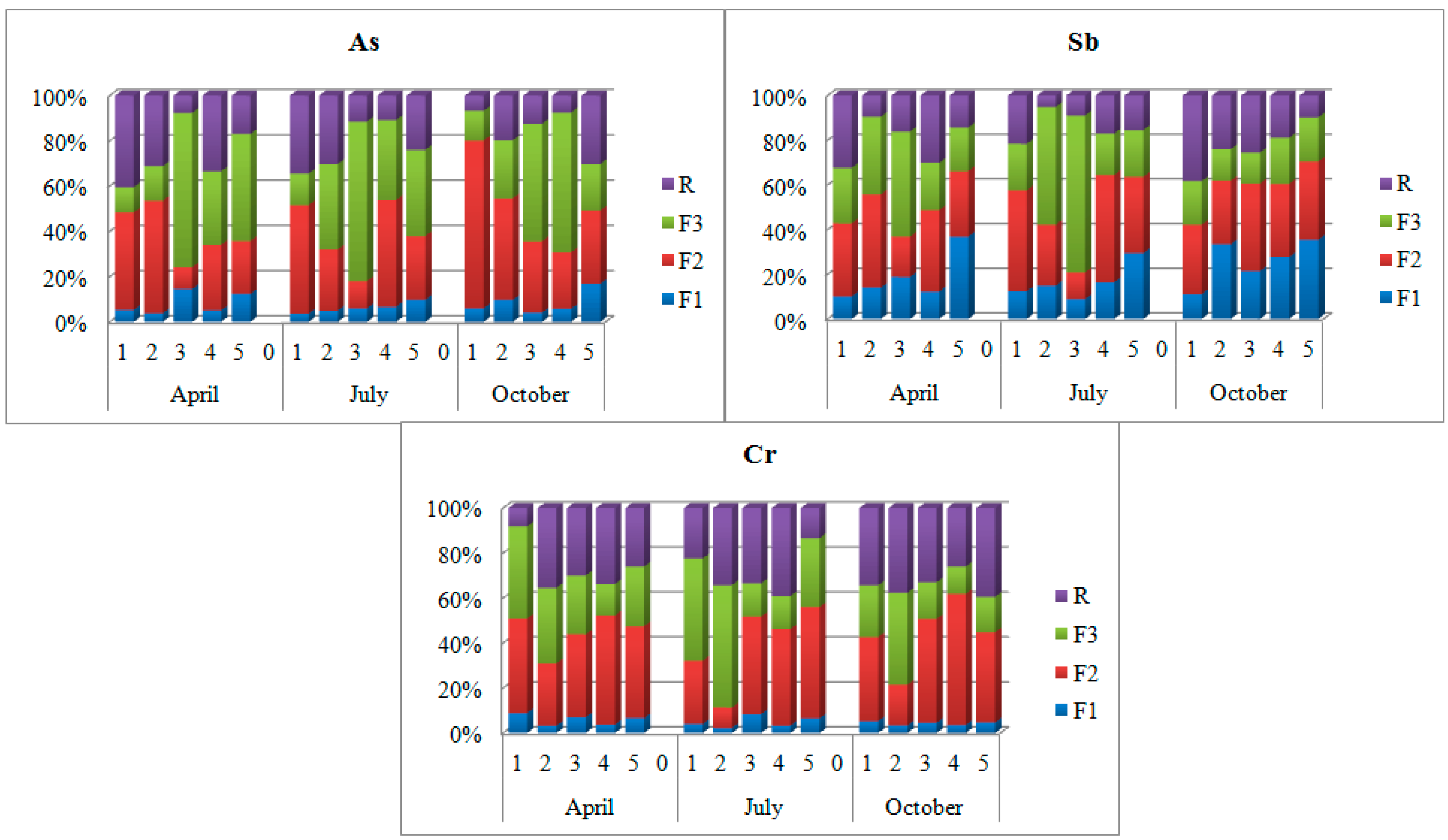
4.3.1. BCR Extraction Procedure
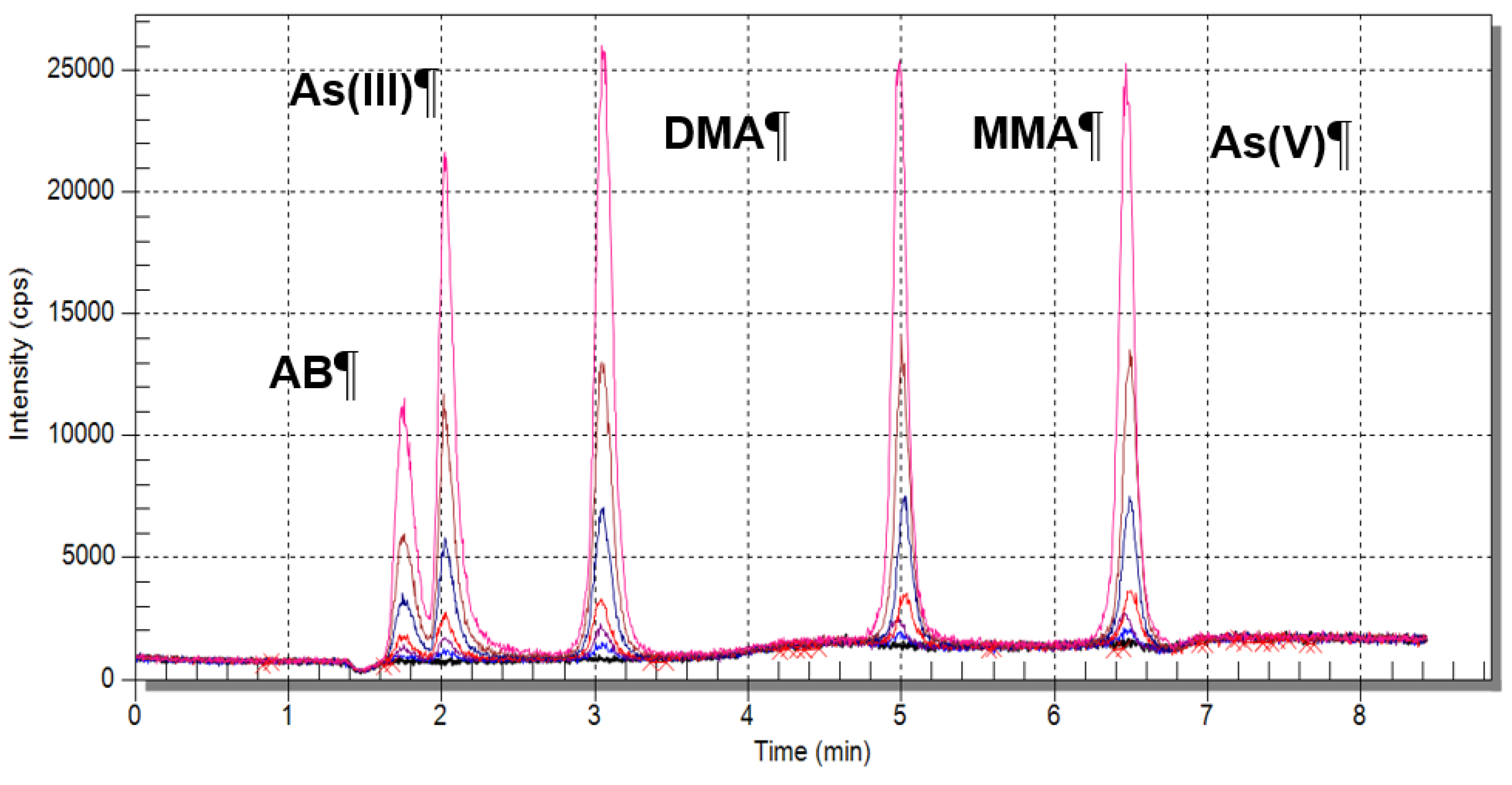
4.3.2. Speciation Analysis Using HPLC-ICP-MS Technique
5. Conclusions
Acknowledgments
Author Contributions
Conflict of Interests
References
- Cornelis, R.; Caruso, J.; Crews, H.; Heumann, K. Handbook of Elemental Speciation: Techniques and Methodology; John Wiley & Sons Ltd.: Chichester, UK, 2005; pp. 47–85. [Google Scholar]
- Templeton, D.M.; Ariese, F.; Cornelis, R.; Danielsson, L.; Muntau, G.H.; Van Leeuwen, H.P.; Lobinski, R. Guidelines for terms related to chemical speciation and fractionation of elements definitions, structural aspects, and methodological approaches. Pure Appl. Chem. 2000, 72, 1453–1470. [Google Scholar] [CrossRef]
- Jabłońska-Czapla, M.; Szopa, S.; Grygoyć, K.; Łyko, A.; Michalski, R. Development and validation of HPLC-ICP-MS method for the determination inorganic Cr, As and Sb speciation forms and its application for Pławniowice reservoir (Poland) water and bottom sediments variability study. Talanta 2014, 120, 475–483. [Google Scholar] [CrossRef]
- Das, A.K.; Guardia, M.; Cervera, M.L. Literature survey of on-line elemental speciation in aqueous solutions. Talanta 2001, 55, 1–28. [Google Scholar] [CrossRef]
- Chou, J.; De Rosa, C.T. Case studies—Arsenic. Int. J. Hyg. Environ. Health 2003, 206, 381–386. [Google Scholar] [CrossRef]
- Kotaś, J.; Stasicka, Z. Chromium occurrence in the environment and methods of its speciation. Environ. Pollut. 2000, 107, 263–283. [Google Scholar] [CrossRef]
- Nriagu, J.O.; Nioboer, E. Chromium in Natural and Human Environments; Wiley Interscience: New York, NY, USA, 1988; pp. 51–62. [Google Scholar]
- Kabata-Pendias, A.; Pendias, H. Biogeochemia Pierwiastków Śladowych; Wydawnictwo Naukowe Polskie Wydawnictwo Naukowe: Warszawa, Poland, 1999; pp. 246–257. [Google Scholar]
- Michalski, R.; Jabłońska-Czapla, M.; Łyko, A.; Szopa, S. Hyphenated methods for speciation analysis. In Encyclopedia of Analytical Chemistry; John Wiley & Sons. Ltd.: Chechester, UK, 2013; pp. 1–17. [Google Scholar]
- Michalski, R.; Jabłońska, M.; Szopa, S. Role and importance of hyphenated techniques in speciation analysis. In Speciation Studies in Soil Sediment and Environmental Samples; Bakirdere, S., Ed.; CRC Press: Boca Raton, FA, USA, 2013; pp. 242–262. [Google Scholar]
- Michalski, R.; Jabłońska, M.; Szopa, S.; Łyko, A. Application of ion chromatography with ICP-MS or MS detection to the determination of selected halides and metal/metalloids species. Crit. Rev. Anal. Chem. 2011, 41, 133–150. [Google Scholar] [CrossRef]
- Policht-Latawiec, A.; Kanownik, W.; Grubka, K. Characteristics of selected physicochemical indices of upland carbonate stream water with coarse-grained substrate. J. Ecol. Eng. 2014, 4, 23–28. [Google Scholar]
- Nocoń, W.; Nocoń, K.; Barbusiński, K.; Kernert, J. The influence of zinc-lead ore mining industry on the level of the Biała Przemsza bottom sediments contamination. ACEE 2012, 1, 1–6. [Google Scholar] [CrossRef]
- Lis, J.; Pasieczna, A. Detailed Geochemical Map of Upper Sile—Sia. Pilot Sheet Sławków; Państwowy Instytut Geologiczny: Warszawa, Poland, 1999; pp. 1–21. [Google Scholar]
- Tokalioglu, S.; Kartal, S.; Birol, G. Application of three stage sequential extraction procedure for the determination of extractable metal contents in highway soils. Turk. J. Chem. 2003, 27, 333–346. [Google Scholar]
- Geotechnical studies—laboratory testing soil—Part 4: Determination of particle size. Available online: http://sklep.pkn.pl/pkn-cen-iso-ts-17892-4-2009p.html (accessed on 23 April 2015).
- Regulation of Minister of Environment on 22 October 2014. Available online: http://isap.sejm.gov.pl/DetailsServlet?id=WDU20140001482 (accessed on 30 October 2014).
- Jabłońska-Czapla, M. Arsenic, antimony and chromium speciation using HPLC-ICP-MS technique in selected rivers ecosystems of Upper Silesia. In Proceedings of Poland-Validation of Methodology—the 38th International Symposium on Environmental Analytical Chemistry, Lausanne, Switzerland, 17–20 June 2014.
- Policht-Latawiec, A.; Kapica, A. Influence of hard coal mine on water quality in the vistula river. Rocz. Ochr. Sr. 2013, 15, 2640–2651. [Google Scholar]
- Regulation of Minister of Environment on 11 February 2004. Available online: http://isap.sejm.gov.pl/DetailsServlet?id=WDU20040320284 (accessed on 11 February 2004).
- Pasieczna, A.; Dusza-Dobek, A.; Markowski, W. Effect of coal mining and steel metal water quality in Biała Przemsza and Bobrek River. Min. Geol. 2010, 5, 181–190. [Google Scholar]
- Wang, J.; Li, A.; Wang, Q.; Zhou, Y.; Fu, L.; Li, Y. Assessment of the manganese content of the drinking water source in Yancheng, China. J. Hazard. Mater. 2010, 182, 259–265. [Google Scholar] [CrossRef]
- Landrum, J.T.; Bennett, P.C.; Engel, A.S.; Alsina, M.A.; Pasten, P.A.; Miliken, K. Partitioning geochemistry of arsenic and antimony, El Tatio Geyser, Chile. App. Geochem. 2009, 24, 664–676. [Google Scholar] [CrossRef]
- Ciszewski, D. Heavy metals in vertical profiles of the middle Odra River overbank sediments: Evidence for pollution changes. Water Air Soil Pollut. 2003, 143, 81–98. [Google Scholar] [CrossRef]
- Giri, S.; Singh, A.K. Assessment of surface water quality using heavy metal pollution index in Subarnarekha River, India. Water Qual. Expos. Health 2014, 5, 173–182. [Google Scholar] [CrossRef]
- Yang, X.; Duan, J.; Wang, L.; Wei, L.; Guan, J.; Beecham, S.; Mulcahy, D. Heavy metal pollution and health risk assessment in the Wei River in China. Environ. Monit. Assess. 2015, 187, 111–121. [Google Scholar] [CrossRef]
- Ciszewski, D. Flood-related changes in heavy metal concentrations within sediments of the Biała Przemsza River. Geomorphology 2001, 40, 205–218. [Google Scholar] [CrossRef]
- Wardas, M.; Budek, L.; Helios-Rybicka, E. Variability of heavy metals content in bottom sediments of the Wilga River, a tributary of the Vistula River (Kraków area, Poland). Appl. Geochem. 1996, 11, 197–202. [Google Scholar] [CrossRef]
- Taylor, M.P.; Kesterton, R.G.H. Heavy metal contamination of arid river environment: Gruben River, Namibia. Geomorphology 2002, 42, 311–327. [Google Scholar] [CrossRef]
- Samarina, V.P. The effect of a mining and smelting plant on the dynamics of heavy metals in small river basins in the zone of Kursk-Belgorod magnetic anomaly. Water Resour. 2003, 30, 550–558. [Google Scholar] [CrossRef]
- Jiang, B.F.; Sun, W.L. Assessment of heavy metal pollution in sediment from Xiangjiang River (China) using sequential extraction and lead isotope analysis. J. Cent. South Univ. Technol. 2014, 21, 2349–2358. [Google Scholar] [CrossRef]
- Wang, L.; Yu, R.; Hu, G.; Tu, X. Speciation an assessment of heavy metals in surface sediments of Jinjiang River tidal reach, southeast of China. Environ. Monit. Assess. 2010, 165, 491–499. [Google Scholar] [CrossRef]
- Lasheen, M.R.; Ammar, N.S. Speciation of some heavy metals in River Nile sediments, Cairo, Egypt. Environmentalist 2009, 29, 8–16. [Google Scholar] [CrossRef]
- Asoaka, S.; Taakahashi, Y.; Araki, Y.; Tanimizu, M. Comparison of antimony and arsenic behavior in an Ichinokawa River water-sediment system. Chem.Geol. 2012, 334, 1–8. [Google Scholar] [CrossRef]
- Miyashita, S.; Shimoya, M.; Kamidate, Y.; Kuroiwa, T.; Shikino, O.; Fujiwara, S.; Francesconi, K.A.; Kaise, T. Rapid determination of arsenic species in freshwater organisms from the arsenic-rich Hayakawa River in Japan using HPLC-ICP-MS. Chemosphere 2009, 75, 1065–1073. [Google Scholar] [CrossRef]
- Bednar, A.J.; Kirgan, R.A.; Jones, W.T. Comparison of standard and reaction cell inductively coupled plasma mass spectrometry in the determination of chromium and selenium species by HPLC-ICP-MS. Anal.Chim. Acta 2009, 632, 27–34. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabłońska-Czapla, M. Antimony, Arsenic and Chromium Speciation Studies in Biała Przemsza River (Upper Silesia, Poland) Water by HPLC-ICP-MS. Int. J. Environ. Res. Public Health 2015, 12, 4739-4757. https://doi.org/10.3390/ijerph120504739
Jabłońska-Czapla M. Antimony, Arsenic and Chromium Speciation Studies in Biała Przemsza River (Upper Silesia, Poland) Water by HPLC-ICP-MS. International Journal of Environmental Research and Public Health. 2015; 12(5):4739-4757. https://doi.org/10.3390/ijerph120504739
Chicago/Turabian StyleJabłońska-Czapla, Magdalena. 2015. "Antimony, Arsenic and Chromium Speciation Studies in Biała Przemsza River (Upper Silesia, Poland) Water by HPLC-ICP-MS" International Journal of Environmental Research and Public Health 12, no. 5: 4739-4757. https://doi.org/10.3390/ijerph120504739
APA StyleJabłońska-Czapla, M. (2015). Antimony, Arsenic and Chromium Speciation Studies in Biała Przemsza River (Upper Silesia, Poland) Water by HPLC-ICP-MS. International Journal of Environmental Research and Public Health, 12(5), 4739-4757. https://doi.org/10.3390/ijerph120504739





