Increasing Rates of Brain Tumours in the Swedish National Inpatient Register and the Causes of Death Register
Abstract
:1. Introduction
1.1. Background
1.2. Incidence
1.3. Aim of the Study
2. Material and Methods
2.1. Study Design
2.2. Statistical Methods
3. Results
3.1. Inpatient Register (IPR) and Causes of Death Register (CDR)
3.1.1. D32 Benign Meningeal Tumours (CNS)
3.1.2. D33 Benign Tumour in the Brain or CNS
3.1.3. D42 Tumour of Unknown Type in the Meninges (CNS)
| ICD-10 | Joinpoint Location | APC 1 (95% CI) | APC 2 (95% CI) | AAPC (95% CI) |
|---|---|---|---|---|
| D32 | ||||
| All (n = 10,962) | No joinpoint detected | - | - | +0.49 (−0.06, +1.04) |
| -Men (n = 3183) | No joinpoint detected | - | - | +0.88 (+0.12, +1.64) |
| -Women (n = 7779) | No joinpoint detected | - | - | +0.37 (−0.32, +1.06) |
| D33 | ||||
| All (n = 6925) | No joinpoint detected | - | - | −0.43 (−0.97, +0.11) |
| -Men (n = 3141) | No joinpoint detected | - | - | −0.63 (−1.40, +0.14) |
| -Women (n = 3784) | No joinpoint detected | - | - | −0.26 (−0.88, +0.37) |
| D42 | ||||
| All (n = 1093) | No joinpoint detected | - | - | −2.10 (−3.60, −0.58) |
| -Men (n = 433) | 2003 | +6.41 (−3.28, +17.07) | −6.71 (−9.76, −3.56) | −2.53 (−5.84, +0.90) |
| -Women (n = 660) | No joinpoint detected | - | - | −1.26 (−2.76, +0.26) |
| D43 | ||||
| All (n = 13,013) | 2007 | +0.17 (−1.01, +1.37) | +4.25 (+1.98, +6.57) | +1.78 (+0.76, +2.81) |
| -Men (n = 6840) | 2007 | +0.13 (−1.62, +1.90) | +4.95 (+1.59, +8.42) | +2.03 (+0.52, +3.56) |
| -Women (n = 6173) | 2008 | +0.36 (−0.41, +1.14) | +4.08 (+1.80, +6.41) | +1.58 (+0.77, +2.40) |
| ICD-10 | Joinpoint Location | APC 1 (95% CI) | APC 2 (95% CI) | AAPC (95% CI) |
|---|---|---|---|---|
| D32 | ||||
| All (n = 845) | No joinpoint detected | - | - | −2.96 (−4.83, −1.05) |
| -Men (n = 282) | 2007 | +3.25 (−1.98, +8.76) | −11.74 (−19.84, −2.82) | −3.03 (−7.21, +1.35) |
| -Women (n = 563) | No joinpoint detected | - | - | −3.26 (−5.39, −1.09) |
| D33 | ||||
| All * (n = 82) | No joinpoint detected | - | - | −7.01 (−12.72, −0.92) |
| D43 | ||||
| All (n = 2374) | 2008 | −7.35 (−10.87, −3.68) | +22.60 (+9.68, +37.03) | +1.72 (−2.29, +5.90) |
| -Men (n = 1166) | 2008 | −8.75 (−12.69, −4.64) | +23.98 (+9.24, +40.71) | +1.06 (−3.46, +5.80) |
| -Women (n = 1208) | 2008 | −5.95 (−10.58, −1.07) | +19.91 (+3.70, +38.65) | +1.99 (−3.23, +7.48) |
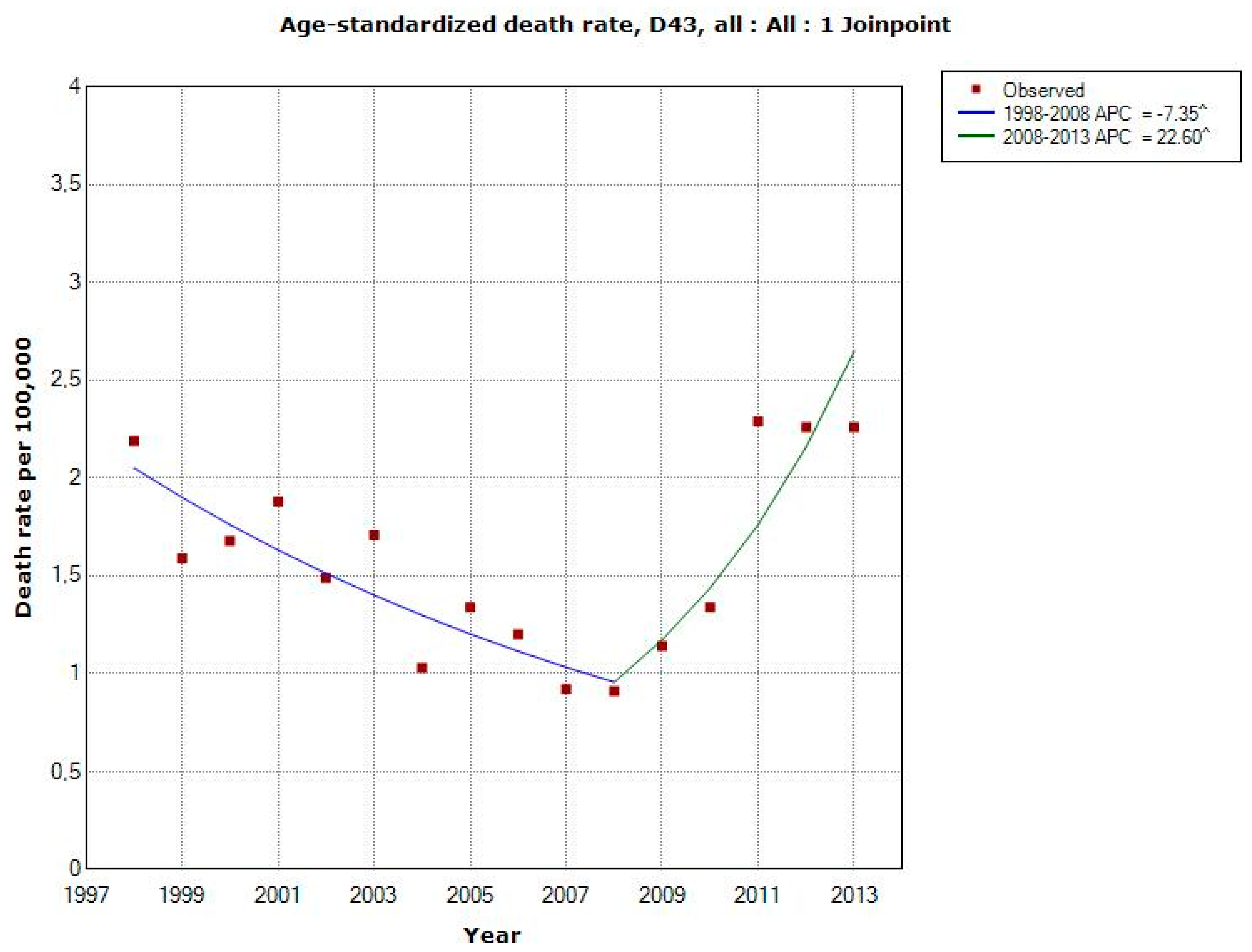
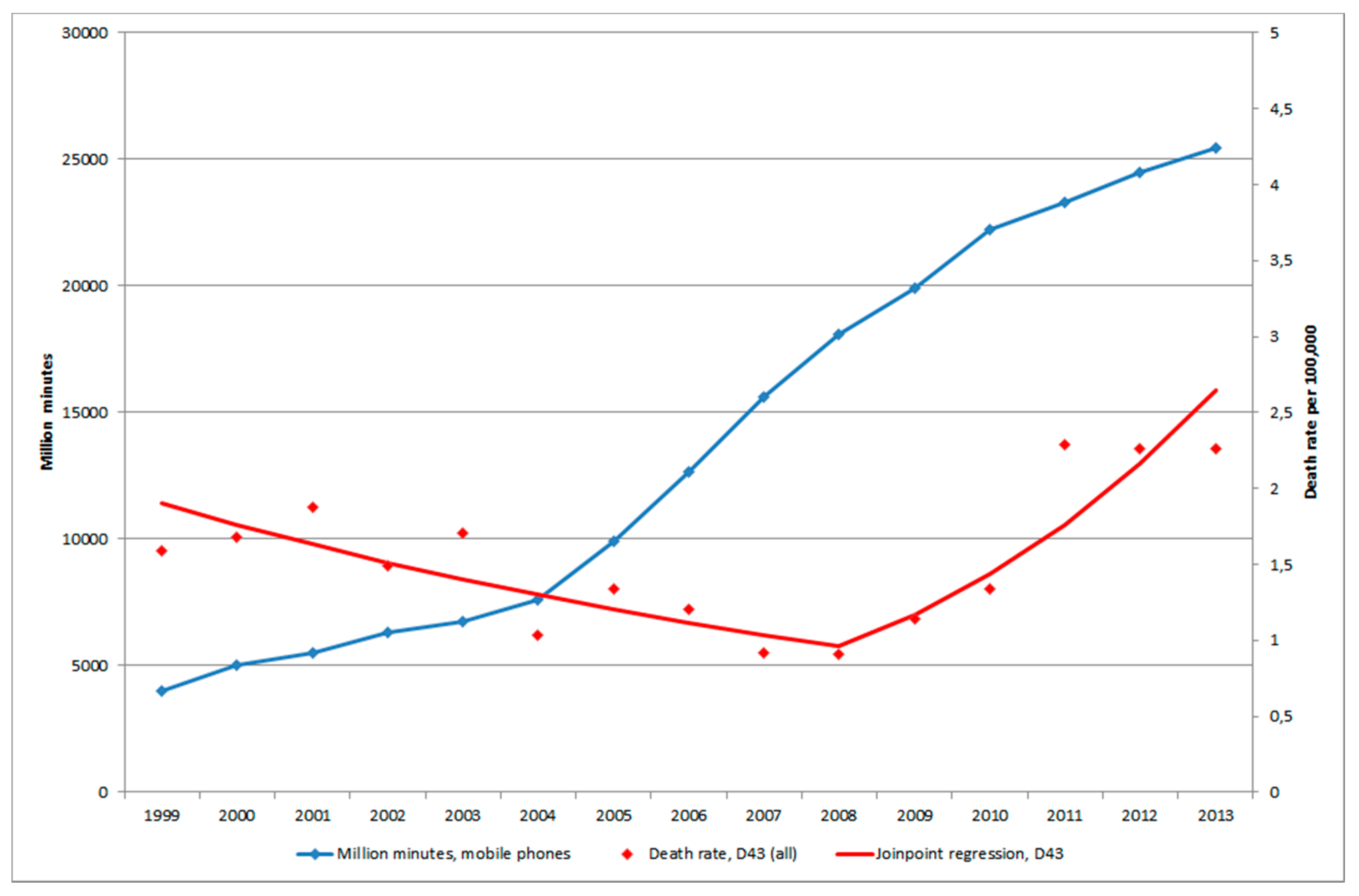
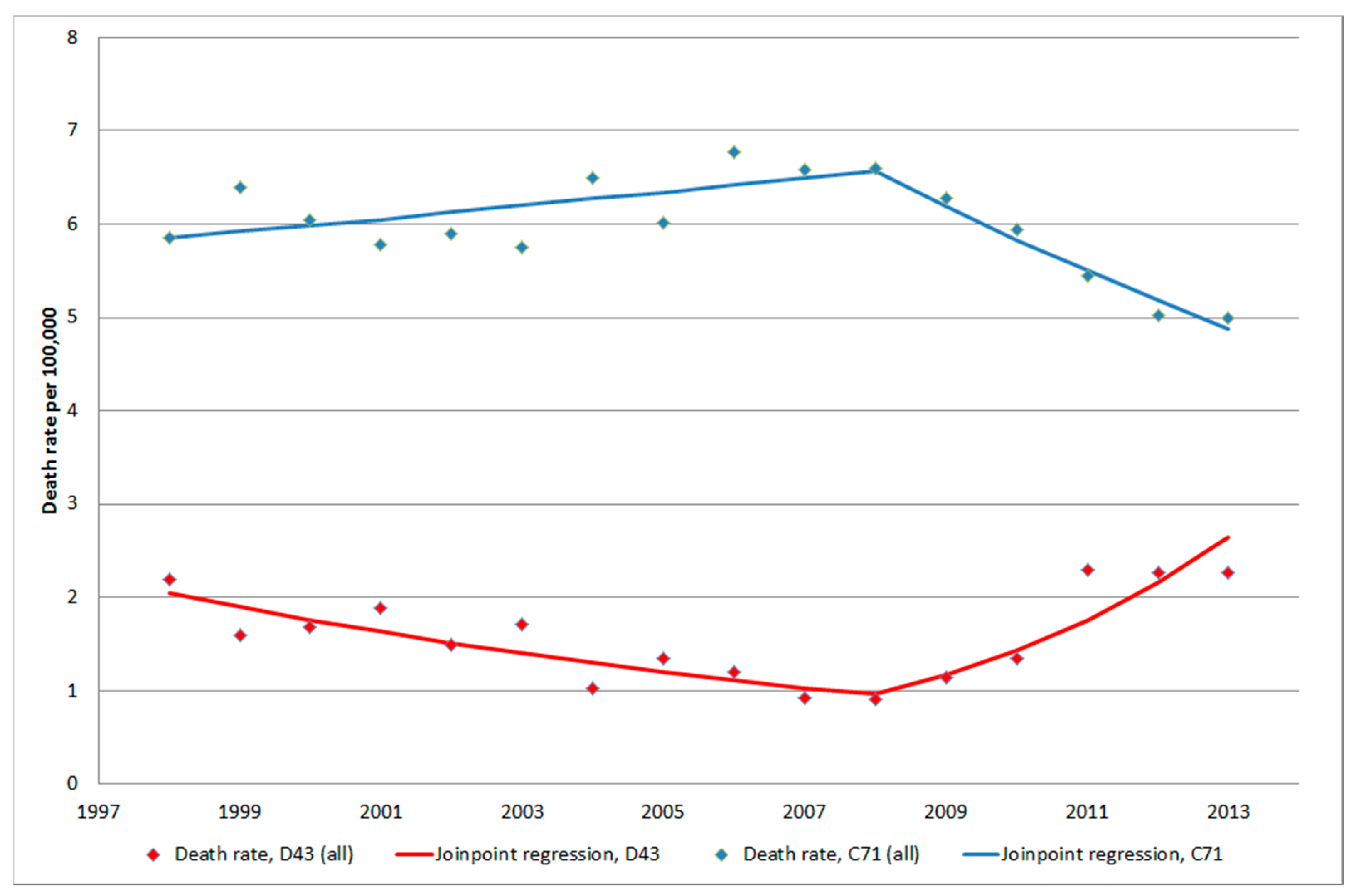
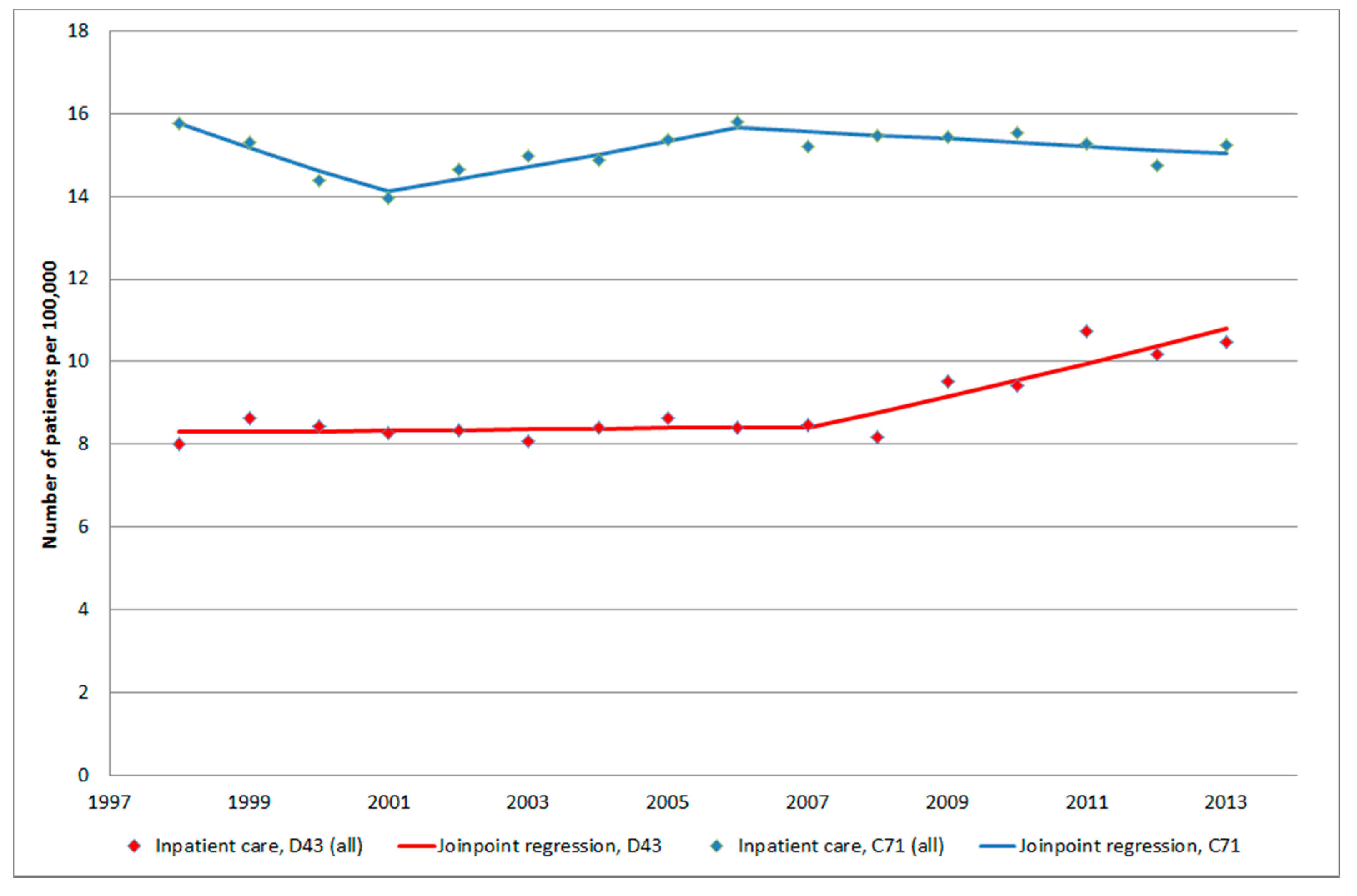
3.1.4. D43 Tumour of Unknown Type in the Brain or CNS
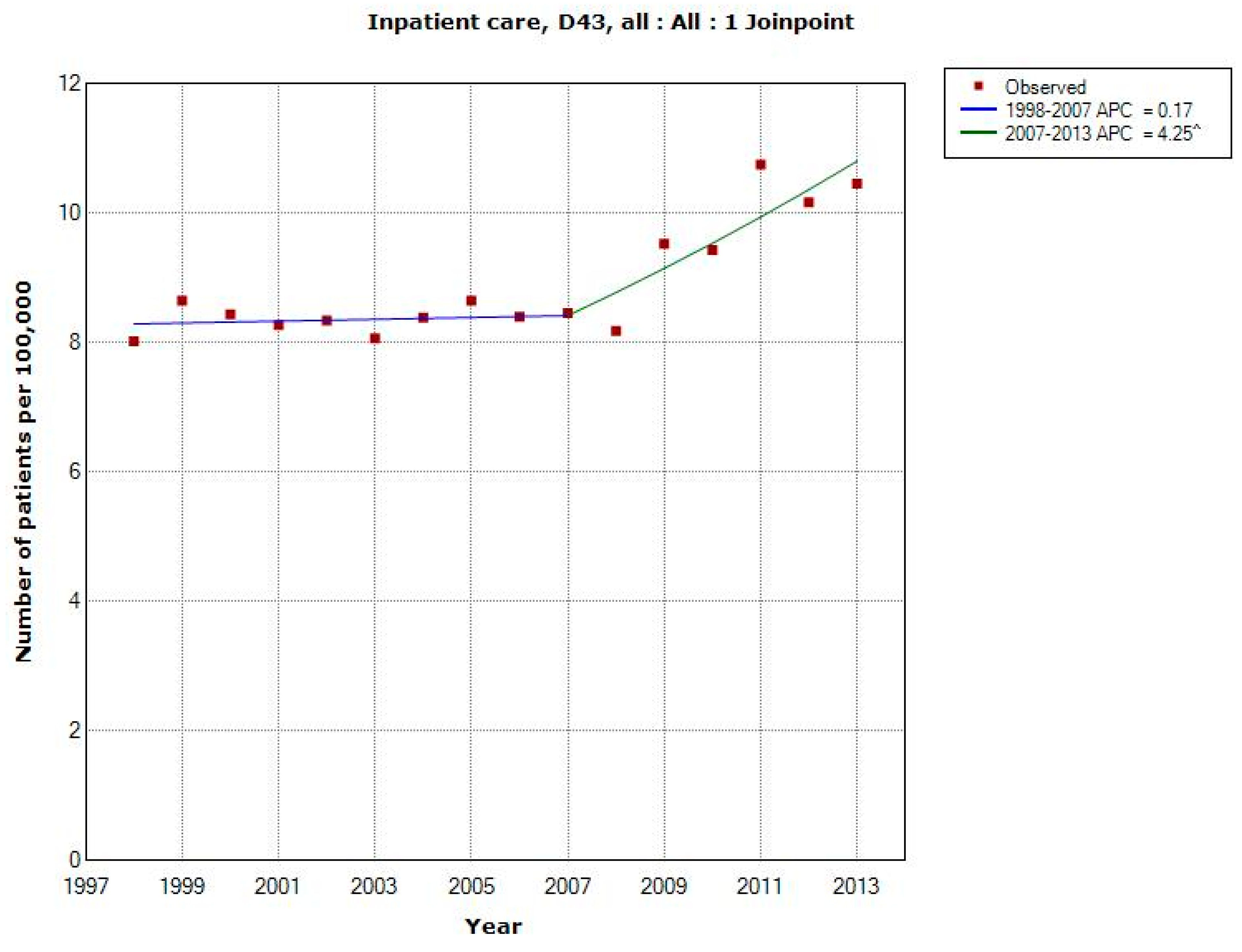
3.1.5. C71 = Malignant Brain Tumours
3.2. The Swedish Cancer Register
3.2.1. ICD-7 Code 193.0 = Brain Tumours (Including Brain, Meninges, CNS Nerves)
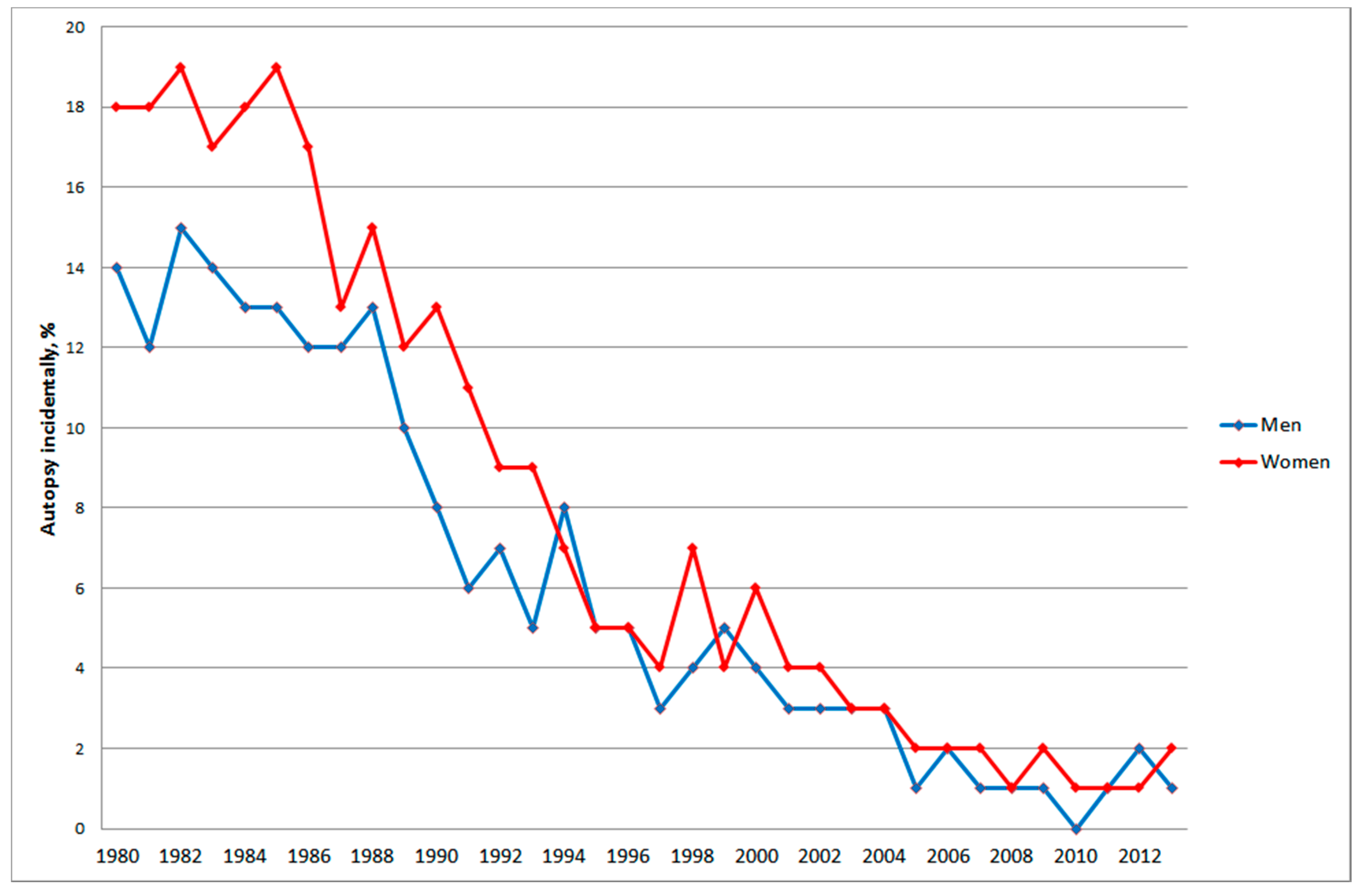
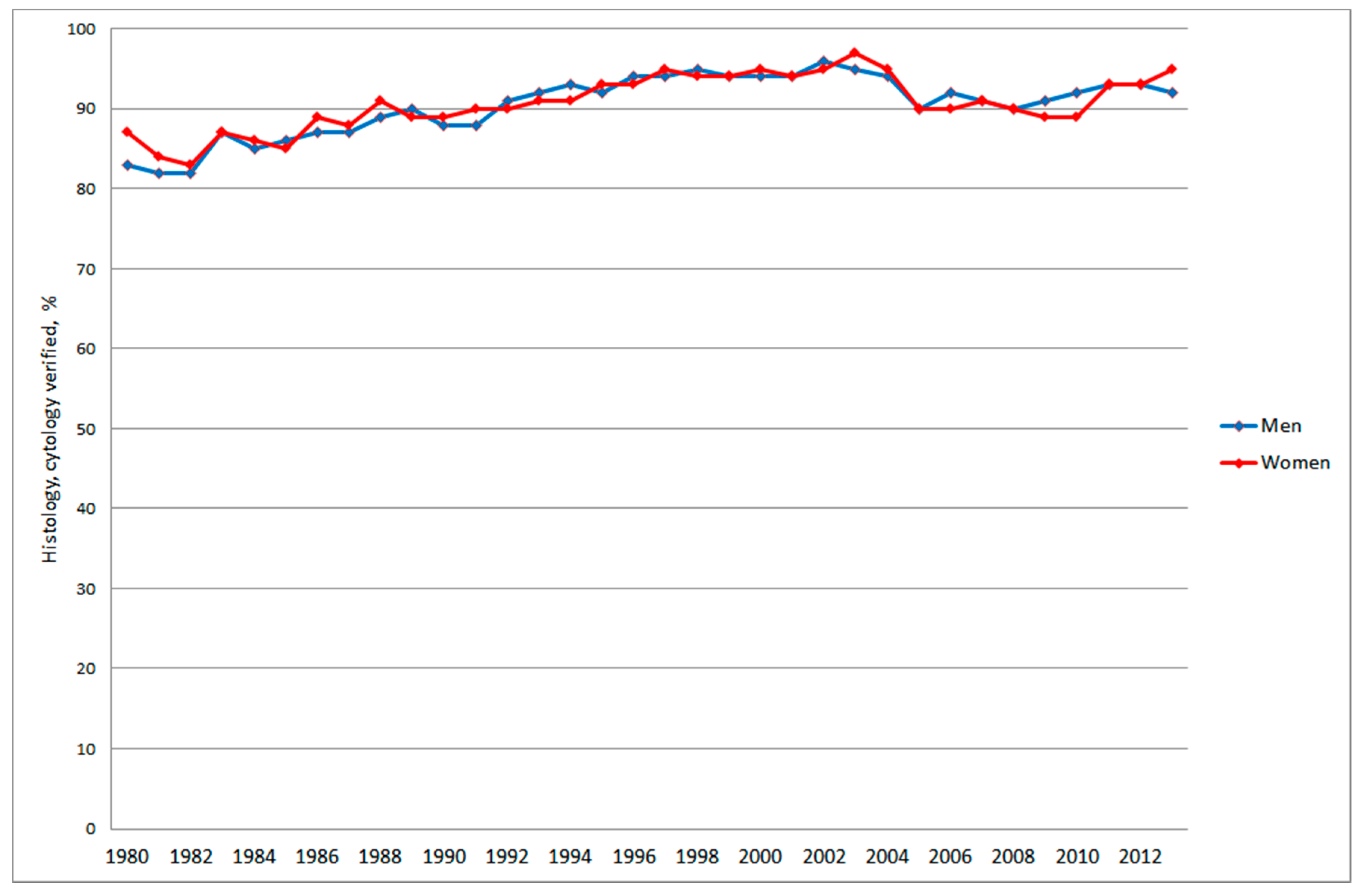
3.2.2. Autopsy, Histology and Cytology
3.3. Mobile Phone Communication
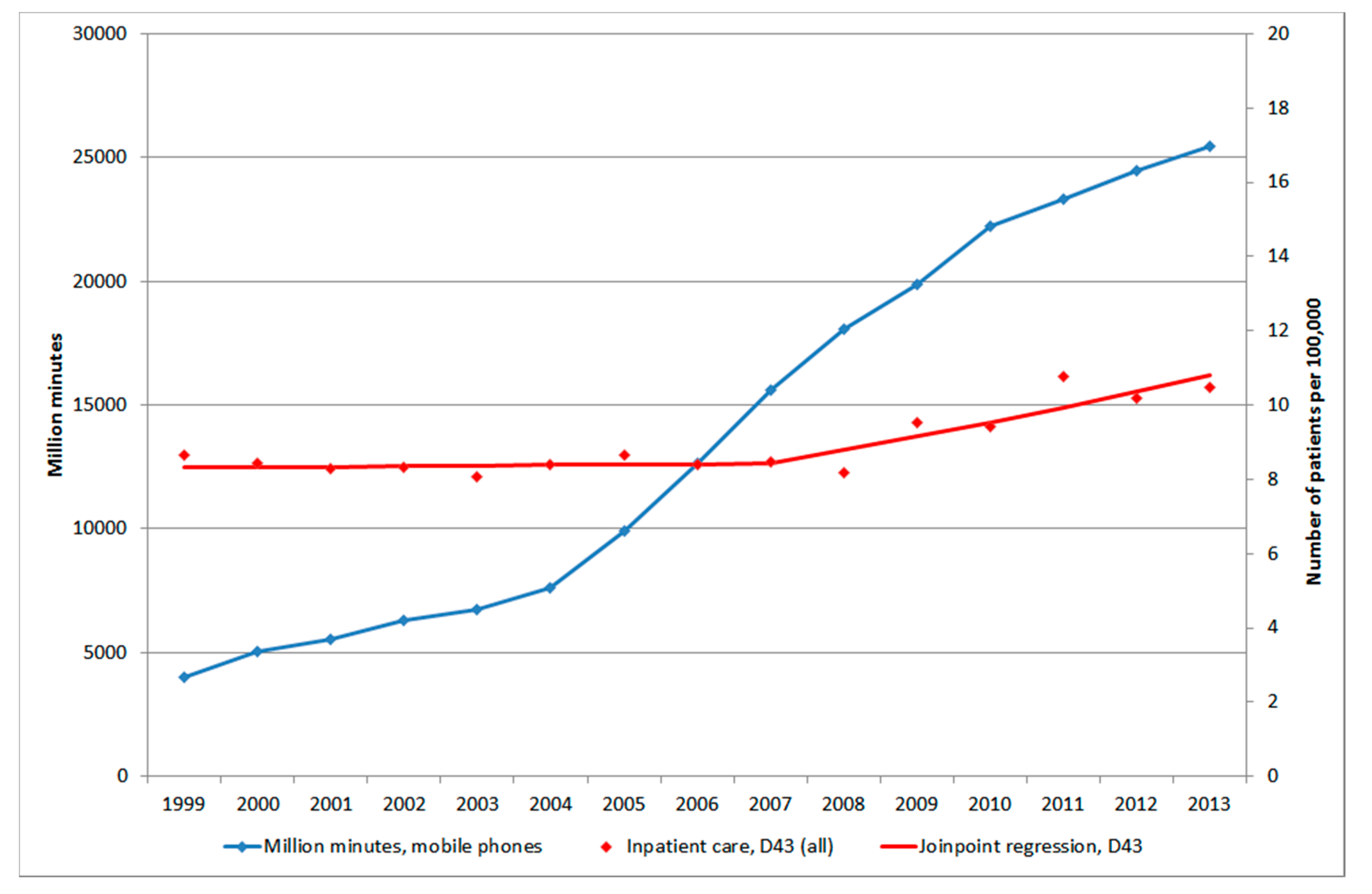
3.4. Restricted Cubic Spline Plot
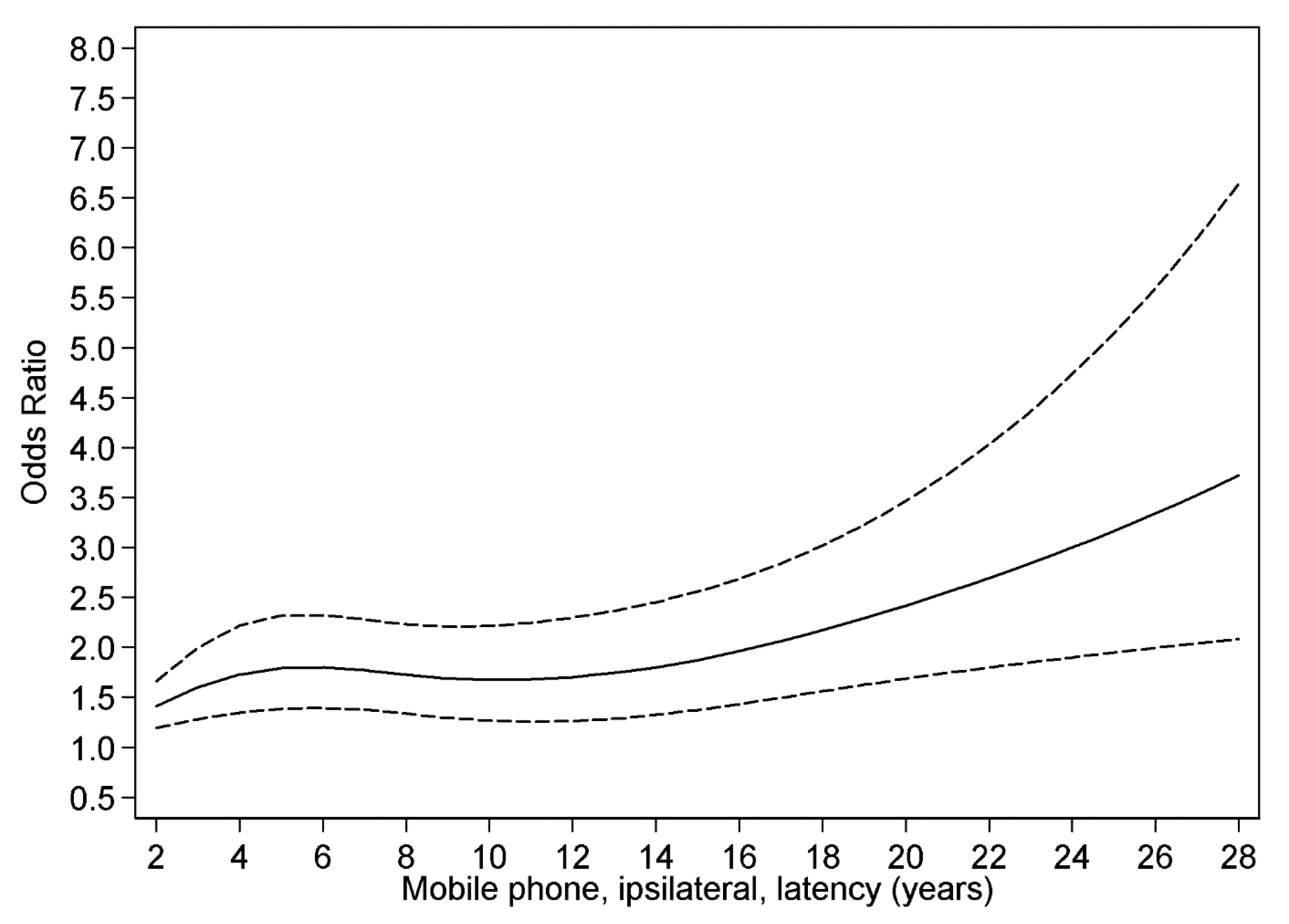
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- International Telecommunication Union (ITU). Measuring the Information Society Report. 2014. Available online: https://www.itu.int/en/ITU-D/Statistics/Documents/publications/mis2014/MIS2014_without_Annex_4.pdf (accessed on 1 April 2015).
- Cardis, E.; Deltour, I.; Mann, S.; Moissonnier, M.; Taki, M.; Varsier, N.; Wake, K.; Wiart, J. Distribution of RF energy emitted by mobile phones in anatomical structures of the brain. Phys. Med. Biol. 2008, 53, 2771–2783. [Google Scholar] [CrossRef] [PubMed]
- Christ, A.; Gosselin, M.C.; Christopoulou, M.; Kühn, S.; Kuster, N. Age-dependent tissue-specific exposure of cell phone users. Phys. Med. Biol. 2010, 55, 1767–1783. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, O.P.; Morgan, L.L.; de Salles, A.A.; Han, Y.Y.; Herberman, R.B.; Davis, D.L. Exposure limits: The underestimation of absorbed cell phone radiation, especially in children. Electromagn. Biol. Med. 2012, 31, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Linde, T.; Hansson Mild, K. Measurement of low frequency magnetic fields from digital cellular telephones. Bioelectromagnetics 1997, 18, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Calderón, C.; Addison, D.; Mee, T.; Findlay, R.; Maslanyj, M.; Conil, E.; Kromhout, H.; Lee, A.K.; Sim, M.R.; Taki, M.; et al. Assessment of extremely low frequency magnetic field exposure from GSM mobile phones. Bioelectromagnetics 2014, 35, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Baan, R.; Grosse, Y.; Lauby-Secretan, B.; el Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Islami, F.; Galichet, L.; Straif, K. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011, 12, 624–626. [Google Scholar] [CrossRef] [PubMed]
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. In Non-Ionizing Radiation, Part 2: Radiofrequency Electromagnetic Fields; IARC: Lyon, France, 2013; Volume 102, Available online: http://monographs.iarc.fr/ENG/Monographs/vol102/mono102.pdf (accessed on 3 February 2015).
- Hardell, L.; Carlberg, M.; Hansson Mild, K. Pooled analysis of two case-control studies on use of cellular and cordless telephones and the risk for malignant brain tumours diagnosed in 1997–2003. Int. Arch. Occup. Environ. Health 2006, 79, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Carlberg, M.; Hansson Mild, K. Pooled analysis of two case-control studies on the use of cellular and cordless telephones and the risk of benign brain tumours diagnosed during 1997–2003. Int. J. Oncol. 2006, 28, 509–518. [Google Scholar] [PubMed]
- Hardell, L.; Carlberg, M.; Hansson Mild, K. Pooled analysis of case-control studies on malignant brain tumours and the use of mobile and cordless phones including living and deceased subjects. Int. J. Oncol. 2011, 38, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Interphone Study Group. Brain tumour risk in relation to mobile telephone use: Results of the INTERPHONE international case-control study. Int. J. Epidemiol. 2010, 39, 675–694. [Google Scholar]
- Interphone Study Group. Acoustic neuroma risk in relation to mobile telephone use: Results of the INTERPHONE international case-control study. Cancer Epidemiol. 2011, 35, 453–464. [Google Scholar]
- Cardis, E.; Armstrong, B.K.; Bowman, J.D.; Giles, G.G.; Hours, M.; Krewski, D.; McBride, M.; Parent, M.E.; Sadetzki, S.; Woodward, A.; et al. Risk of brain tumours in relation to estimated RF dose from mobile phones: Results from five Interphone countries. Occup. Environ. Med. 2011, 68, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, M.; Hardell, L. Decreased survival of glioma patients with astrocytoma grade IV (glioblastoma multiforme) associated with long-term use of mobile and cordless phones. Int. J. Environ. Res. Public Health 2014, 11, 10790–10805. [Google Scholar] [CrossRef] [PubMed]
- Akhavan-Sigari, R.; Baf, M.M.; Ariabod, V.; Rohde, V.; Rahighi, S. Connection between cell phone use, p53 gene expression in different zones of glioblastoma multiforme and survival prognoses. Rare Tumors 2014, 6. [Google Scholar] [CrossRef]
- Gennaro, V.; Tomatis, L. Business bias: How epidemiologic studies may underestimate or fail to detect increased risks of cancer and other diseases. Int. J. Occup. Environ. Health 2005, 11, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Aydin, D.; Feychting, M.; Schüz, J.; Tynes, T.; Andersen, T.V.; Schmidt, L.S.; Poulsen, A.H.; Johansen, C.; Prochazka, M.; Lannering, B.; et al. Mobile phone use and brain tumors in children and adolescents: A multicenter case-control study. J. Natl. Cancer Inst. 2011, 103, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Söderqvist, F.; Carlberg, M.; Hansson Mild, K.; Hardell, L. Childhood brain tumour risk and its association with wireless phones: A commentary. Environ. Health 2011, 10. [Google Scholar] [CrossRef]
- Deltour, I.; Auvinen, A.; Feychting, M.; Johansen, C.; Klaeboe, L.; Sankila, R.; Schüz, J. Mobile phone use and incidence of glioma in the Nordic countries 1979–2008: Consistency check. Epidemiology 2012, 23, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Statens Serum Institut (SSI). Cancerregisteret. Tal og Analyse. 2012. Available online: http://www.ssi.dk/Aktuelt/Nyheder/2013/~/media/Indhold/DK%20-%20dansk/Sundhedsdata%20og%20it/NSF/Registre/Cancerregisteret/Cancerregisteret%202012.ashx (accessed on 1 April 2015).
- Kræftens Bekæmpelse. Voldsom Stigning i Nye Tilfælde af Aggressiv Hjernekræft. 2012. Available online: https://web.archive.org/web/20121128153253/http://www.cancer.dk/Nyheder/nyhedsartikler/2012kv4/Kraftig+stigning+i+hjernesvulster.htm (accessed on 2 April 2015).
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Preliminary Opinion on Potential Health Effects of Exposure to Electromagnetic Fields (EMF). 2013. Available online: http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_041.pdf (accessed on 1 April 2015).
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). Opinion on Potential Health Effects of Exposure to Electromagnetic Fields (EMF). 2015. Available online: http://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_041.pdf (accessed on 1 April 2015).
- World Health Organization (WHO). Radio Frequency fields: Environmental Health Criteria Monograph. 2015. Available online: http://www.who.int/peh-emf/research/rf_ehc_page/en/ (accessed on 1 April 2015).
- Swedish Radiation Protection Foundation. Sharp Increase in Patients Treated for Brain Tumors with Unclear Diagnosis in Sweden. 2014. Available online: http://www.stralskyddsstiftelsen.se/2014/10/increase-brain-tumors/ (accessed on 1 April 2015).
- Socialstyrelsen. Statistikdatabas för Diagnoser i Sluten Vård. Available online: http://www.socialstyrelsen.se/statistik/statistikdatabas/diagnoserislutenvard (accessed on 1 April 2015).
- Ludvigsson, J.F.; Andersson, E.; Ekbom, A.; Feychting, M.; Kim, J.L.; Reuterwall, C.; Heurgren, M.; Olausson, P.O. External review and validation of the Swedish national inpatient register. BMC Public Health 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Socialstyrelsen. Statistikdatabas för Dödsorsaker. Available online: http://www.socialstyrelsen.se/statistik/statistikdatabas/dodsorsaker (accessed on 1 April 2015).
- Socialstyrelsen. Statistikdatabas för Cancer. Available online: http://www.socialstyrelsen.se/statistik/statistikdatabas/cancer (accessed on 1 April 2015).
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Carlberg, M. Mobile phone and cordless phone use and the risk for glioma—Analysis of pooled case-control studies in Sweden, 1997–2003 and 2007–2009. Pathophysiology 2015, 22, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Post-Och Telestyrelsen (PTS). Svensk Telemarknad 2013. 2014. Available online: http://statistik.pts.se/pts2013/download/Svensk%20Telemarknad%202013.pdf (accessed on 1 April 2015).
- Post-Och Telestyrelsen (PTS). Prisutveckling för Telefoni Och Bredband till Och Med Första Halvåret. 2009. Available online: http://www.pts.se/upload/Rapporter/Tele/2009/2009-30-prisutvecklingen-telefoni-bredband-halvar-09.pdf (accessed on 1 April 2015).
- Perry, A.; Scheithauer, B.W.; Stafford, S.L.; Lohse, C.M.; Wollan, P.C. “Malignancy” in meningiomas: A clinicopathologic study of 116 patients, with grading implications. Cancer 1999, 85, 2046–2056. [Google Scholar] [PubMed]
- Kondziolka, D.; Patel, A.D.; Kano, H.; Flickinger, J.C.; Lunsford, L.D. Long-term Outcomes after Gamma Knife Radiosurgery for Meningiomas. Am. J. Clin. Oncol. 2014. [CrossRef]
- Vertosick, F.T., Jr.; Selker, R.G.; Arena, V.C. Survival of patients with well-differentiated astrocytomas diagnosed in the era of computed tomography. Neurosurgery 1991, 4, 496–501. [Google Scholar]
- Ohgaki, H.; Kleihues, P. Population-based studies on incidence, survival rates, and genetic alterations in astocytic and oligodendroglial gliomas. J. Neuropathol. Exp. Neurol. 2005, 64, 479–489. [Google Scholar] [PubMed]
- Ohgaki, H.; Dessen, P.; Jourde, B.; Horstmann, S.; Nishikawa, T.; di Patre, P.L.; Burkhard, C.; Schüler, D.; Probst-Hensch, N.M.; Maiorka, P.C.; et al. Genetic pathways of glioblastoma: A population-based study. Cancer Res. 2004, 64, 6892–6899. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Sato, K.; Biernat, W.; Tachibana, O.; von Ammon, K.; Ogata, N.; Yonekawa, Y.; Kleihues, P.; Ohgaki, H. Incidence and timing of p53 mutations during astrocytoma progression in patients with multiple biopsies. Clin. Cancer Res. 1997, 3, 523–530. [Google Scholar] [PubMed]
- Ohgaki, H.; Kleihues, P. Epidemiology and etiology of gliomas. Acta Neuropathol. 2005, 109, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Official Statistics of Sweden. Statistics—Health and Medical Care. Causes of Death 2013. 2015. Available online: http://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/19736/2015-2-42.pdf (accessed on 1 April 2015).
- Barlow, L.; Westergren, K.; Holmberg, L.; Talbäck, M. The completeness of the Swedish Cancer Register: A sample survey for year 1998. Acta Oncol. 2009, 48, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Tavelin, B.; Axelsson, B. A study of patients not registered in the Swedish Cancer Register but reported to the Swedish Register of Palliative Care 2009 as deceased due to cancer. Acta Oncol. 2014, 53, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Kilander, C.; Mattsson, F.; Ljung, R.; Lagergren, J.; Sadr-Azodi, O. Systematic underreporting of the population-based incidence of pancreatic and biliary tract cancers. Acta Oncol. 2014, 53, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, T.; Ernst, H.; Streckert, J.; Zhou, Y.; Taugner, F.; Hansen, V.; Dasenbrock, C.T. Indication of cocarcinogenic potential of chronic UMTS-modulated radiofrequency exposure in an ethylnitrosourea mouse model. Int. J. Radiat. Biol. 2010, 86, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Lerchl, A.; Klose, M.; Grote, K.; Wilhelm, A.F.; Spathmann, O.; Fiedler, T.; Streckert, J.; Hansen, V.; Clemens, M. Tumor promotion by exposure to radiofrequency electromagnetic fields below exposure limits for humans. Biochem. Biophys. Res. Commun. 2015. [Google Scholar] [CrossRef]
- Carlberg, M.; Hardell, L. Pooled analysis of Swedish case-control studies 1997–2003 and 2007–2009 on meningioma risk associated with use of mobile and cordless phones. Oncol. Rep. 2015, in press. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardell, L.; Carlberg, M. Increasing Rates of Brain Tumours in the Swedish National Inpatient Register and the Causes of Death Register. Int. J. Environ. Res. Public Health 2015, 12, 3793-3813. https://doi.org/10.3390/ijerph120403793
Hardell L, Carlberg M. Increasing Rates of Brain Tumours in the Swedish National Inpatient Register and the Causes of Death Register. International Journal of Environmental Research and Public Health. 2015; 12(4):3793-3813. https://doi.org/10.3390/ijerph120403793
Chicago/Turabian StyleHardell, Lennart, and Michael Carlberg. 2015. "Increasing Rates of Brain Tumours in the Swedish National Inpatient Register and the Causes of Death Register" International Journal of Environmental Research and Public Health 12, no. 4: 3793-3813. https://doi.org/10.3390/ijerph120403793
APA StyleHardell, L., & Carlberg, M. (2015). Increasing Rates of Brain Tumours in the Swedish National Inpatient Register and the Causes of Death Register. International Journal of Environmental Research and Public Health, 12(4), 3793-3813. https://doi.org/10.3390/ijerph120403793





