Adaptive Response in Animals Exposed to Non-Ionizing Radiofrequency Fields: Some Underlying Mechanisms
Abstract
:1. Introduction
2. Experimental Section
2.1. RF Exposure
2.2. Animals and Treatment
3. Results and Discussion
- (i)
- Microscope slides prepared from the bone marrow and spleen tissues showed a significant and progressive decrease in damage as the time progressed after irradiation.
- (ii)
- There were increased numbers of colony forming units (CFU-BM) in the bone marrow.
- (iii)
- There were increased levels of colony stimulating factor (CSF) and interleukin-3 (IL-3) in the serum. These indices suggested RF-induced AR helping in faster regeneration and restoration of hematopoietic tissue damaged by subsequent γ-radiation [6].
- (1)
- Kunming mice were exposed to RF at 12, 120 and 1200 μW/cm2 power density for 1 h/day for 14 days and then subjected to lethal dose of 8.0 Gy γ-radiation. After 15 days, the numbers of surviving mice were 11, 43 and 25%, respectively (18% in mice exposed to γ-radiation alone) (Table 1). Since RF exposure at 120 μW/cm2 power density resulted in maximum survival advantage, another experiment was conducted at this power density and there was a significant increase in weights of spleen and thymus compared with those exposed to 8.0 Gy γ-radiation alone.Table 1. Survival study in mice which were pre-exposed to 900 MHz RF with and without subsequent exposure to γ-radiation.
Table 1. Survival study in mice which were pre-exposed to 900 MHz RF with and without subsequent exposure to γ-radiation. Group Treatment Power Density γ-Radiation # Mice Studied # Mice Alive * % Survival 1 γ-Radiation - 8 Gy 28 5 18 2 900 MHz RF 12 µW/cm2 8 Gy 28 3 11 3 900 MHz RF 120 µW/cm2 8 Gy 28 12 43 ** 4 900 MHz RF 1200 µW/cm2 8 Gy 28 7 25 5 Amifostine - 8 Gy 28 8 29 Notes: *: 15 days after exposure to 8 Gy γ-radiation. **: p ≤ 0.05. Data from Cao et al. [7]. - (2)
- The same RF exposure protocol and a sub-lethal dose of 5.0 Gy γ-radiation was used in the next set of experiments. Mice exposed to RF+5.0 Gy γ-radiation showed:
- (i)
- significantly increased CFU-BM,
- (ii)
- significantly increased expression levels of genes related to the cell cycle, viz., cyclin-D1, cyclin-E, cyclin-DK4 and cyclin-DK2 in the spleen, and
- (iii)
- lethally irradiated “recipient” mice injected with bone marrow cells from RF + 5.0 Gy γ-radiation exposed mice showed increased numbers of colony forming units in their spleen (CFU-S). Overall, these observations provided some mechanistic evidence for RF-induced AR in mitigating the damage induced by subsequent γ-radiation [7].
- (i)
- an accelerated repair (% damage that is remaining at different repair times) of BLM-induced primary DNA damage,
- (ii)
- significantly reduced level of oxidative damage assessed from malondialdehyde (MDA) levels, and
- (iii)
- considerable increase in SOD, an anti-oxidant enzyme [10].
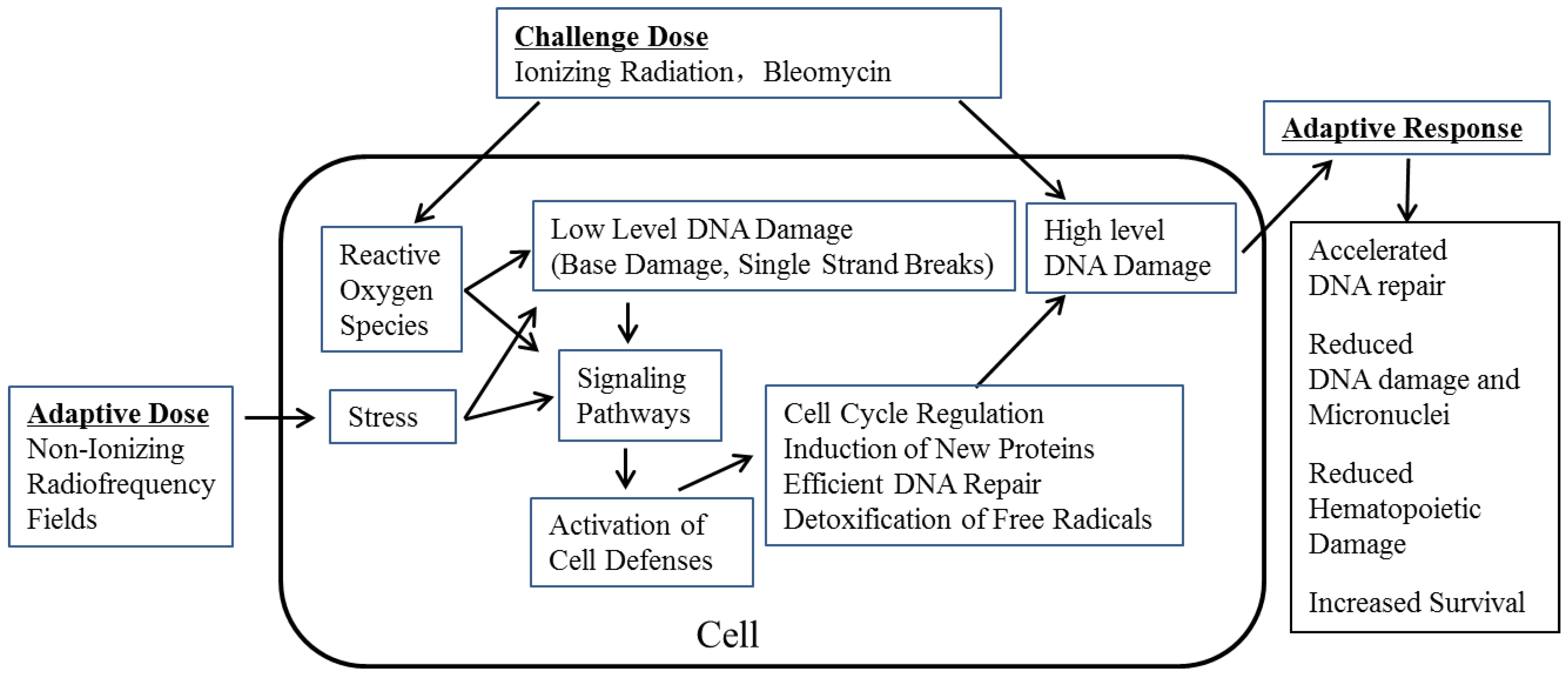
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IARC. Non-Ionizing Radiation, Part II: Radiofrequency Electromagnetic Fields. In IARC Monograph on the Evaluation of Carcinogenic Risk to Humans; IARC: Lyon, France, 2013. [Google Scholar]
- Samson, L.; Cairns, J. A new pathway for DNA repair in Escherichia coli. Nature 1977, 267, 281–293. [Google Scholar] [CrossRef]
- Dimova, E.G.; Bryant, P.E.; Chankova, S.G. Adaptive response: Some underlying mechanisms and open questions. Genet. Mol. Biol. 2008, 31, 396–408. [Google Scholar]
- Mcintosh, R.L.; Anderson, V. A comprehensive tissue properties database provided for the thermal assessment of a human at rest. Biophys. Rev. Lett. 2010, 5, 129–151. [Google Scholar] [CrossRef]
- McIntosh, R.L.; Deppeler, L.; Oliva, M.; Parente, J.; Tambuwala, F.; Turner, S.; Winship, D.; Wood, A.W. Comparison of radiofrequency exposure of a mouse dam and foetuses at 900 MHz. Phys. Med. Biol. 2010, 55, N111–N122. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, Q.; Jin, Z.D.; Zhang, J.; Lu, M.X.; Nie, J.H.; Tong, J. Effects of 900-MHz microwave radiation on γ-ray-induced damage to mouse hematopoietic system. J. Toxicol. Environ. Health 2010, 73, 507–513. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, Q.; Jin, Z.D.; Zhou, Z; Nie, J.H.; Tong, J. Induction of adaptive response: Pre-exposure of mice to 900 MHz radiofrequency fields reduces hematopoietic damage caused by subsequent exposure to ionising radiation. Int. J. Radiat. Biol. 2011, 87, 720–728. [Google Scholar] [CrossRef]
- Jiang, B.C; Nie, J.H.; Zhou, Z.; Zhang, J.; Tong, J.; Cao, Y. Adaptive response in mice exposed to 900 MHz radiofrequency fields: primary DNA damage. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Jiang, B.C.; Zong, C.Y.; Zhao, H.; Ji, Y.; Tong, J.; Cao, Y. Induction of adaptive response in mice exposed to 900 MHz radiofrequency fields: Application of micronucleus assay. Mutat. Res. 2013, 751, 127–129. [Google Scholar] [CrossRef]
- Zong, C.Y.; Cao, Y. Adaptive response in mice exposed to 900 MHz radiofrequency fields: Bleomycin-induced DNA and oxidative damage/repair. (Unpublished work).
- Burkhardt, M.; Pokovic, K.; Gnos, M.; Schmid, T.; Kuster, N. Numerical and experimental dosimetry of Petri dish exposure setups. Bioelectromagnetics 1996, 17, 483–493. [Google Scholar] [CrossRef]
- Jin, Z.D.; Zong, C.Y.; Jiang, B.C.; Zhou, Z.; Tong, J.; Cao, Y. The effect of combined exposure of 900 MHz radiofrequency fields and doxorubicin in HL-60 cells. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- Mortazavi, S.; Mosleh-Shirazi, M.; Tavassoli, A.; Taheri, M.; Bagheri, Z.; Ghalandari, R.; Bonyadi, S.; Shafie, M.; Haghani, M. A comparative study on the increased radioresistance to lethal doses of gamma rays after exposure to microwave radiation and oral intake of flaxseed oil. Iran J. Radiat. Res. 2011, 9, 9–14. [Google Scholar]
- Mortazavi, S.; Motamedifar, M.; Mehdizadeh, A.; Namdari, G.; Taheri, M. The effect of pre-exposure to radiofrequency radiations emitted from a GSM mobile phone on the susceptibility of BALB/c mice to Escherichia coli. J. Biomed. Phys. Eng. 2012, 2, 139–146. [Google Scholar]
- Mortazavi, S.M.; Mosleh-Shirazi, M.; Tavassoli, A.; Taheri, M.; Mehdizadeh, A.; Namazi, S.; Jamali, A.; Ghalandari, R.; Bonyadi, S.; Haghani, M.; Shafie, M. Increased radioresistance to lethal doses of gamma rays in mice and rats after exposure to microwave radiation emitted by a GSM mobile phone simulator. Dose Response 2012, 11, 281–292. [Google Scholar]
- Haghani, M.; Mortazavi, S.; Sardari, D.; Mosleh-Shirazi, M.; Mansouri, A. Assessment of the role of specific absorption rate of mobile phones on the induction of microwave-induced survival adaptive responses after exposure to lethal doses of gamma radiation. Int. J. Radiat. Res. 2013, 11, 167–173. [Google Scholar]
- Sannino, A.; Sarti, M.; Reddy, S.B.; Prihoda, T.J.; Scarfi, M.R. Induction of adaptive response in human blood lymphocytes exposed to radiofrequency radiation. Radiat. Res. 2009, 171, 735–742. [Google Scholar] [CrossRef]
- Sannino, A.; Zeni, O.; Sarti, M.; Romeo, S.; Reddy, S.B.; Belisario, M.A.; Prihoda, T.J.; Vijayalaxmi; Scarfi, M.R. Induction of adaptive response in human blood lymphocytes exposed to 900 MHz radiofrequency fields: Influence of cell cycle. Int. J. Radiat. Biol. 2011, 87, 993–999. [Google Scholar]
- Zeni, O.; Sannino, A.; Romeo, S.; Massa, R.; Sarti, M.; Reddy, A.B.; Prihoda, T.J.; Vijayalaxmi; Scarfi, M.R. Induction of an adaptive response in human blood lymphocytes exposed to radiofrequency fields: Influence of the universal mobile telecommunication system (UMTS) signal and the specific absorption rate. Mutat. Res. 2012, 747, 29–35. [Google Scholar] [CrossRef]
- Sannino, A.; Zeni, O.; Romeo, S.; Massa, R.; Gialanella, G.; Grossi, G.; Manti, L.; Vijayalaxmi; Scarfi, M.R. Adaptive response in human blood lymphocytes exposed to non-ionizing radiofrequency fields: Resistance to ionizing radiation-induced damage. J. Radiat. Res. 2013, 210–217. [Google Scholar]
- Vijayalaxmi; Cao, Y.; Scarfi, M.R. Adaptive response in mammalian cells exposed to non-ionizing radiofrequency fields: A review and gaps in knowledge. Mutat. Res. 2014. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cao, Y.; Tong, J. Adaptive Response in Animals Exposed to Non-Ionizing Radiofrequency Fields: Some Underlying Mechanisms. Int. J. Environ. Res. Public Health 2014, 11, 4441-4448. https://doi.org/10.3390/ijerph110404441
Cao Y, Tong J. Adaptive Response in Animals Exposed to Non-Ionizing Radiofrequency Fields: Some Underlying Mechanisms. International Journal of Environmental Research and Public Health. 2014; 11(4):4441-4448. https://doi.org/10.3390/ijerph110404441
Chicago/Turabian StyleCao, Yi, and Jian Tong. 2014. "Adaptive Response in Animals Exposed to Non-Ionizing Radiofrequency Fields: Some Underlying Mechanisms" International Journal of Environmental Research and Public Health 11, no. 4: 4441-4448. https://doi.org/10.3390/ijerph110404441
APA StyleCao, Y., & Tong, J. (2014). Adaptive Response in Animals Exposed to Non-Ionizing Radiofrequency Fields: Some Underlying Mechanisms. International Journal of Environmental Research and Public Health, 11(4), 4441-4448. https://doi.org/10.3390/ijerph110404441




