Integrated Human Surveillance Systems of West Nile Virus Infections in Italy: The 2012 Experience
Abstract
:1. Introduction
2. Methods
2.1. West Nile Neuroinvasive Disease Surveillance
- Possible: Patients with fever ≥38.5 °C and neurological symptoms (encephalitis, meningitis or Guillain-Barré syndrome or acute flaccid paralysis).
- Probable: A possible case + anti-WN IgM positive in blood and/or anti-WN IgG positive in blood.
- Confirmed: Possible/probable case and at least one of the following laboratory criteria: Viral isolation in blood or cerebrospinal fluid (CSF); anti-WN IgM positive in CSF; Polimerase Chain Reaction (PCR) positive in blood, CSF or urine; seroconversion (4 fold increase in anti-WN antibodies between different samples collected during the acute and convalescent stage of the disease); confirmed presence of anti-WN antibodies in blood by neutralization test.
2.2. West Nile Virus Surveillance in Blood Donors
2.3. West Nile Virus Surveillance in Solid Organs Donors
2.4. West Nile Fever Surveillance System in Veneto Region
2.5. Surveillance System Integration
3. Results
3.1. West Nile Neuroinvasive Disease Surveillance
| Region/Province | Age Groups | Total | ||||
|---|---|---|---|---|---|---|
| ≤14 | 15–44 | 45–64 | 65–74 | ≥75 | ||
| Basilicata | ||||||
| Matera | 1 | 1 | ||||
| Friuli V.G. | ||||||
| Gorizia | 1 | 1 | ||||
| Pordenone | 1 | 1 | 2 | |||
| Udine | 1 | 1 | ||||
| Sardegna | ||||||
| Oristano | 2 | 2 | ||||
| Veneto | ||||||
| Treviso | 1 | 1 | 2 | 2 | 6 | |
| Venice | 1 | 1 | 3 | 4 | 6 | 15 |
| Total | 1 | 3 | 7 | 6 | 11 | 28 |
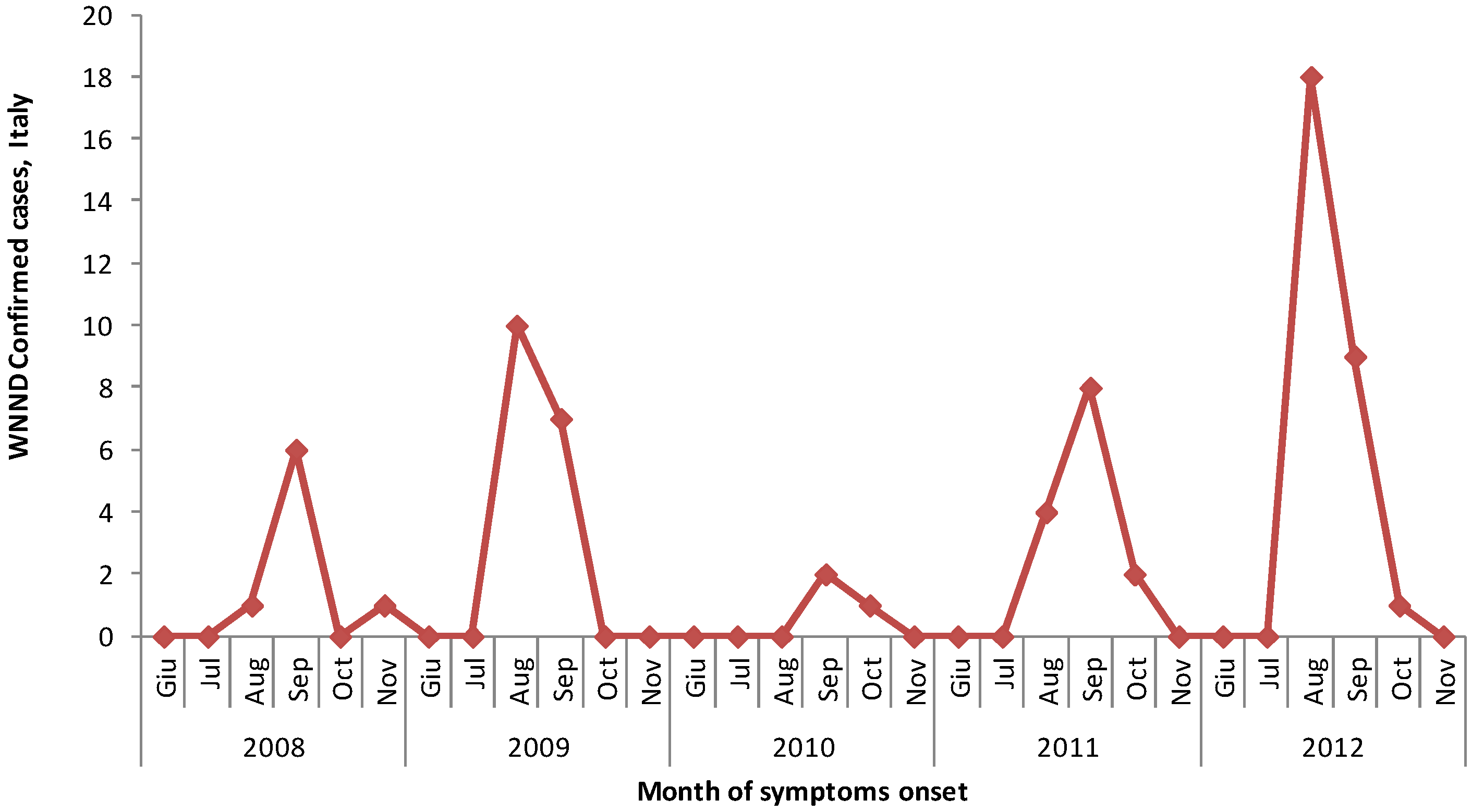
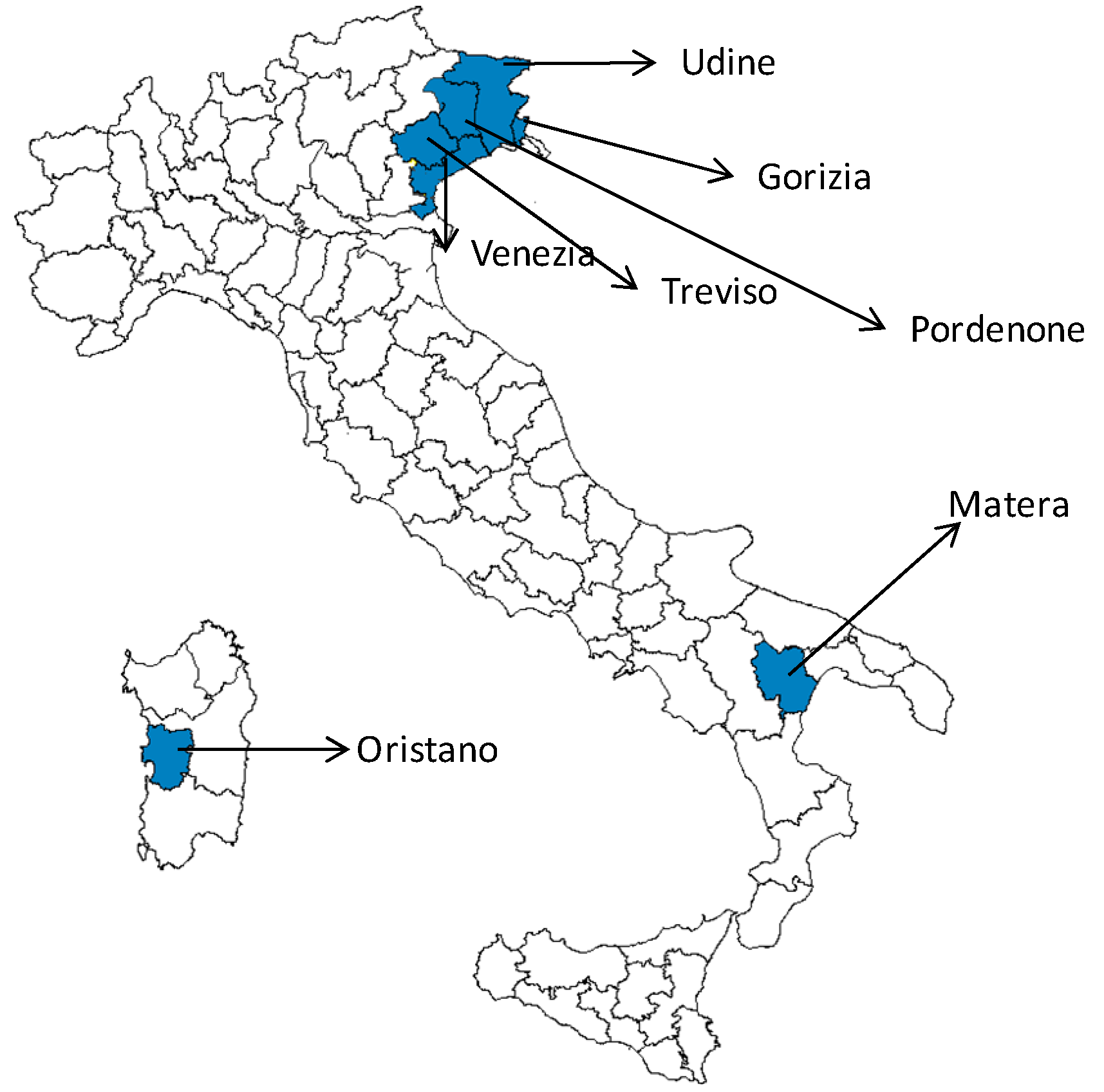
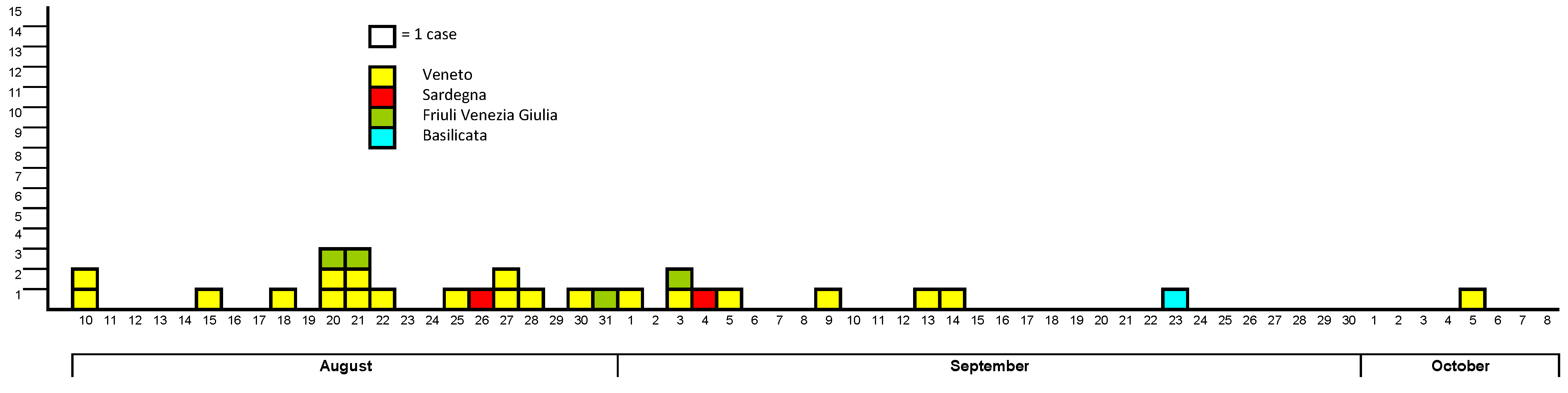
3.2. West Nile Virus Screening in Blood Donors
3.3. West Nile Virus Screening in Solid Organs Donors
3.4. West Nile Fever Surveillance System in Veneto region
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Rossini, G.; Cavrini, F.; Pierro, A.; Macini, P.; Finarelli, A.; Po, C.; Peroni, G.; di Caro, A.; Capobianchi, M.; Nicoletti, L.; et al. First human case of West Nile virus neuroinvasive infection in Italy, September 2008—Case report. Euro Surveill. 2008, 13, 19002. [Google Scholar]
- Rizzo, C.; Vescio, F.; Declich, S.; Finarelli, A.C.; Macini, P.; Mattivi, A.; Rossini, G.; Piovesan, C.; Barzon, L.; Palù, G.; et al. West Nile virus transmission with human cases in Italy, August–September 2009. Euro Surveill. 2009, 14, 19353. [Google Scholar]
- Rizzo, C.; Salcuni, P.; Nicoletti, L.; Ciufolini, M.G.; Russo, F.; Masala, R.; Frongia, O.; Finarelli, A.C.; Gramegna, M.; Gallo, L.; et al. Epidemiological surveillance of West Nile neuroinvasive diseases in Italy, 2008 to 2011. Euro Surveill. 2012, 17, 20172. [Google Scholar]
- Sorveglianza della malattia di West Nile in Italia, 2010. (in Italian). Available online: http://www.normativasanitaria.it/normsan-pdf/0000/34923_1.pdf (accessed on 27 September 2013).
- Sorveglianza dei casi umani delle malattie trasmesse da vettori con particolare riferimento alla chikungunya, dengue e West Nile disease—2011. (in Italian). Available online: http://www.normativasanitaria.it/normsan-pdf/0000/39170_1.pdf (accessed on 27 September 2013).
- Sorveglianza dei casi umani delle malattie trasmesse da vettori con particolare riferimento alla chikungunya, dengue e West Nile disease—Aggiornamento 2012. (in Italian). Available online: http://www.trovanorme.salute.gov.it/renderNormsanPdf?anno=0&codLeg=42895&parte=1%20&serie= (accessed on 27 September 2013).
- Pupella, S.; Pisani, G.; Cristiano, K.; Catalano, L.; Grazzini, G. West Nile virus in the transfusion setting with a special focus on Italian preventive measures adopted in 2008–2012 and their impact on blood safety. Blood Transfus. 2013, 11, 563–574. [Google Scholar]
- Indicazioni per la sorveglianza e la prevenzione della trasmissione dell’infezione da West Nile Virus (WNV) mediante la trasfusione di emocomponenti labili nella stagione estivo-autunnale 2012. (in Italian). Available online: http://www.centronazionalesangue.it/sites/default/files/prot._n._1037.cns_.2012_circolare_wnv_stagione_estivo-autunnale_2012.pdf (accessed on 27 September 2013).
- Nanni Costa, A.; National Transplantation Center, Rome, Italy. Personal communication, 2013.
- Nanni Costa, A.; Capobianchi, M.R.; Ippolito, G.; Palù, G.; Barzon, L.; Piccolo, G.; Andreetta, B.; Filippetti, M.; Fehily, D.; Lombardini, L.; et al. West Nile virus: The Italian national transplant network reaction to an alert in the north-eastern region, Italy 2011. Euro Surveill. 2011, 16, 19991. [Google Scholar]
- Barzon, L.; Pacenti, M.; Cusinato, R.; Cattai, M.; Franchin, E.; Pagni, S.; Martello, T.; Bressan, S.; Squarzon, L.; Cattelan, A.M.; et al. Human cases of West Nile virus infection in north-eastern Italy, 15 June to 15 November 2010. Euro Surveill. 2011, 16, 19949. [Google Scholar]
- The NUTS Classification (Nomenclature of Territorial Units for Statistics). Available online: http://epp.eurostat.ec.europa.eu/portal/page/portal/nuts_nomenclature/correspondence_tables/national_structures_eu (accessed on 15 November 2013).
- Commission Decision of 28 April 2008 Amending Decision 2002/253/EC Laying down Case Definitions for Reporting Communicable Diseases to the Community Network under Decision No 2119/98/EC of the European Parliament and of the Council. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:159:0046:0090:EN:PDF (accessed on 27 September 2013).
- Bagnarelli, P.; Marinelli, K.; Trotta, D.; Monachetti, A.; Tavio, M.; del Gobbo, R.; Capobianchi, M.; Menzo, S.; Nicoletti, L.; Magurano, F.; et al. Human case of autochthonous West Nile virus lineage 2 infection in Italy, September 2011. Euro Surveill. 2011, 16, 20002. [Google Scholar]
- Murray, K.; Walker, C.; Herrington, E.; Lewis, J.A.; McCormick, J.; Beasley, D.W.C.; Tesh, R.B.; Fisher-Hoch, S. Persistent infection with West Nile virus years after initial infection. J. Infect. Dis. 2010, 201, 2–4. [Google Scholar] [CrossRef]
- Sambri, V.; Capobianchi, M.R.; Cavrini, F.; Charrel, R.; Donoso-Mantke, O.; Escadafal, C.; Franco, L.; Gaibani, P.; Gould, E.A.; Niedrig, M.; et al. Diagnosis of West Nile virus human infections: Overview and proposal of diagnostic protocols considering the results of external quality assessment studies. Viruses 2013, 5, 2329–2348. [Google Scholar] [CrossRef]
- Barzon, L.; Pacenti, M.; Franchin, E.; Pagni, S.; Martello, T.; Cattai, M.; Cusinato, R.; Palù, G. Excretion of West Nile virus in urine during acute infection. J. Infect. Dis. 2013, 208, 1086–1092. [Google Scholar] [CrossRef]
- Busch, M.P.; Kleinman, S.H.; Tobler, L.H.; Kamel, H.T.; Norris, P.J.; Walsh, I.; Matud, J.L.; Prince, H.E.; Lanciotti, R.S.; Wright, D.J.; et al. Virus and antibody dynamics in acute West Nile virus infection. J. Infect. Dis. 2008, 198, 984–993. [Google Scholar] [CrossRef]
- Biggerstaff, B.J.; Petersen, L.R. Estimated risk of West Nile virus transmission through blood transfusion during an epidemic in Queens, New York City. Transfusion 2002, 42, 1019–1026. [Google Scholar] [CrossRef]
- Indicazioni in merito alla trasmissione del Virus West Nile (WNV) mediante trapianto d’organo, tessuti e cellule nella stagione estivo-autunnale 2012. (in Italian). Available online: http://www.trapianti.salute.gov.it/imgs/C_17_primopianoCNT_311_listaFile_itemName_0_file.pdf (accessed on 27 September 2013).
- Barzon, L.; Pacenti, M.; Franchin, E.; Martello, T.; Lavezzo, E.; Squarzon, L.; Toppo, S.; Fiorin, F.; Marchiori, G.; Scotton, G.P.; et al. Clinical and virological findings in the ongoing outbreak of West Nile virus Livenza strain in northern Italy, July to September 2012. Euro Surveill. 2011, 16, 19883. [Google Scholar]
- West Nile Disease in Italy in 2012. Available online: http://sorveglianza.izs.it/emergenze/west_nile/pdf/Bollettino_riassuntivo_2012ENG_DEF.pdf (accessed on 15 November 2013).
- Sambri, V.; Capobianchi, M.; Charrel, R.; Fyodorova, M.; Gaibani, P.; Gould, E.; Niedrig, M.; Papa, A.; Pierro, A.; Rossini, G.; et al. West Nile virus in Europe: Emergence, epidemiology, diagnosis, treatment, and prevention. Clin. Microbiol. Infect. 2013, 19, 699–704. [Google Scholar] [CrossRef]
- Calzolari, M.; Gaibani, P.; Bellini, R.; Defilippo, F.; Pierro, A.; Albieri, A.; Maioli, G.; Luppi, A.; Rossini, G.; Balzani, A.; et al. Mosquito, bird and human surveillance of West Nile and Usutu viruses in Emilia-Romagna Region (Italy) in 2010. PLoS One 2012, 7. [Google Scholar] [CrossRef]
- West Nile Disease in Italy in 2011. Available online: http://sorveglianza.izs.it/emergenze/west_nile/pdf/Bollettino_riassuntivi_WND%202011_15_03_2013_en_rev.pdf (accessed on 15 November 2013).
- Kramer, L.D.; Li, J.; Shi, P.-Y. West Nile virus. Lancet Neurol. 2007, 6, 171–181. [Google Scholar] [CrossRef]
- Papa, A. West Nile virus infections in humans—Focus on Greece. J. Clin. Virol. 2013, 28, 351–353. [Google Scholar] [CrossRef]
- Kopel, E.; Amitai, Z.; Bin, H.; Shulman, L.M.; Mendelson, E.; Sheffer, R. Surveillance of West Nile virus disease, Tel Aviv district, Israel, 2005 to 2010. Euro Surveill. 2011, 16, 19894. [Google Scholar]
- West Nile Virus Disease Cases and Deaths Reported to CDC by Year and Clinical Presentation, 1999–2012. Available online: http://www.cdc.gov/westnile/resources/pdfs/cummulative/99_2012_CasesAndDeathsClinicalPresentationHumanCases.pdf (accessed on 27 September 2013).
- Papa, A. West Nile virus infections in Greece: An update. Expert Rev. Anti-infect. Ther. 2012, 10, 743–750. [Google Scholar] [CrossRef]
- Magurano, F.; Remoli, M.E.; Baggieri, M.; Fortuna, C.; Marchi, A.; Fiorentini, C.; Bucci, P.; Benedetti, E.; Ciufolini, M.G.; Rizzo, C.; et al. Circulation of West Nile virus lineage 1 and 2 during an outbreak in Italy. Clin. Microbiol. Infect. 2012, 18, E545–E547. [Google Scholar]
- Rossini, G.; Landini, M.P.; Gelsomino, F.; Sambri, V.; Varani, S. Innate host responses to West Nile virus: Implications for central nervous system immunopathology. World J. Virol. 2013, 12, 49–56. [Google Scholar]
- Kong, K.-F.; Delroux, K.; Wang, X.; Qian, F.; Arjona, A.; Malawista, S.E.; Fikrig, E.; Montgomery, R.R. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J. Virol. 2008, 82, 7613–7623. [Google Scholar] [CrossRef]
- Tsai, T.F.; Popovici, F.; Cernescu, C.; Campbell, G.L.; Nedelcu, N.I. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998, 352, 767–771. [Google Scholar] [CrossRef]
- Barzon, L.; Pacenti, M.; Franchin, E.; Lavezzo, E.; Masi, G.; Squarzon, L.; Pagni, S.; Toppo, S.; Russo, F.; Cattai, M.; et al. Whole genome sequencing and phylogenetic analysis of West Nile virus lineage 1 and lineage 2 from human cases of infection, Italy, August 2013. Euro Surveill. 2013, 18, 20591. [Google Scholar]
- Zinsstag, J.; Schelling, E.; Waltner-Toews, D.; Tanner, M. From “one medicine” to “one health” and systemic approaches to health and well-being. Prev. Vet. Med. 2011, 101, 148–156. [Google Scholar] [CrossRef]
- Napoli, C.; Salcuni, P.; Pompa, M.G.; Declich, S.; Rizzo, C. Estimated imported infections of Chikungunya and Dengue in Italy, 2008 to 2011. J. Travel. Med. 2012, 19, 294–297. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Napoli, C.; Bella, A.; Declich, S.; Grazzini, G.; Lombardini, L.; Costa, A.N.; Nicoletti, L.; Pompa, M.G.; Pupella, S.; Russo, F.; et al. Integrated Human Surveillance Systems of West Nile Virus Infections in Italy: The 2012 Experience. Int. J. Environ. Res. Public Health 2013, 10, 7180-7192. https://doi.org/10.3390/ijerph10127180
Napoli C, Bella A, Declich S, Grazzini G, Lombardini L, Costa AN, Nicoletti L, Pompa MG, Pupella S, Russo F, et al. Integrated Human Surveillance Systems of West Nile Virus Infections in Italy: The 2012 Experience. International Journal of Environmental Research and Public Health. 2013; 10(12):7180-7192. https://doi.org/10.3390/ijerph10127180
Chicago/Turabian StyleNapoli, Christian, Antonino Bella, Silvia Declich, Giuliano Grazzini, Letizia Lombardini, Alessandro Nanni Costa, Loredana Nicoletti, Maria Grazia Pompa, Simonetta Pupella, Francesca Russo, and et al. 2013. "Integrated Human Surveillance Systems of West Nile Virus Infections in Italy: The 2012 Experience" International Journal of Environmental Research and Public Health 10, no. 12: 7180-7192. https://doi.org/10.3390/ijerph10127180
APA StyleNapoli, C., Bella, A., Declich, S., Grazzini, G., Lombardini, L., Costa, A. N., Nicoletti, L., Pompa, M. G., Pupella, S., Russo, F., & Rizzo, C. (2013). Integrated Human Surveillance Systems of West Nile Virus Infections in Italy: The 2012 Experience. International Journal of Environmental Research and Public Health, 10(12), 7180-7192. https://doi.org/10.3390/ijerph10127180






