Antiviral Activities of Sulfated Polysaccharides Isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales)
Abstract
:1. Introduction
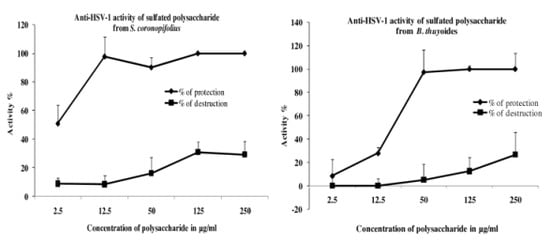
2. Results and Discussion
2.1. Chemical Composition from the Polysaccharides
2.2. Determination of the Molecular Weight of Polysaccharides
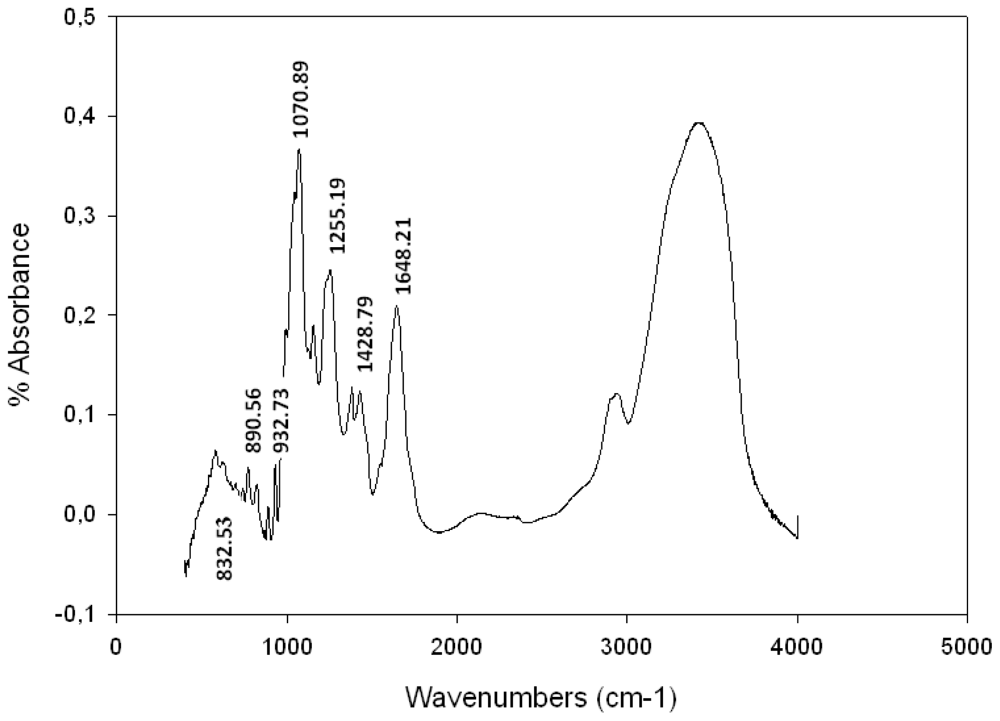
2.3. Spectroscopic Analysis
Fourier Transformed Infra Red (FTIR) Spectroscopy Analysis
3. Experimental Section
3.1. Extraction Techniques and Chemical Analysis Methods
3.2. Chemical Composition
3.3. Molecular Mass
3.4. FT-IR Spectroscopy
4. Conclusions
Acknowledgements
- Samples Availability: Available from the authors.
References
- Ioannou, E; Roussis, V. Natural products from seaweeds. In Plant-Derived Natural Products: Synthesis, Function, and Application; Springer: Berlin, Germany, 2009; pp. 51–81. [Google Scholar]
- De Sousa, APA; Torres, MR; Pessoa, C; De Moraes, MO; Filho, FDR; Alves, APNN; Costa-Lotufo, LV. In vivo growth-inhibition of Sarcoma 180 tumor by alginates from brown seaweed Sargassum vulgare. Carbohydr. Polym 2007, 69, 7–13. [Google Scholar]
- Cardozo, KHM; Guaratini, T; Barros, MP; Falcão, VR; Tonon, AP; Lopes, NP; Campos, S; Torres, MA; Souza, AO; Colepicolo, P; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. Part C 2007, 146, 60–78. [Google Scholar]
- Mayer, AMS; Rodriguez, AD; Berlinck, RGS; Hamann, MT. Marine pharmacology in 2005–6: Marine compounds with anthelmintic, antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the cardiovascular, immune and nervous systems, and other miscellaneous mechanisms of action. Biochim. Biophys. Acta 2009, 1790, 283–308. [Google Scholar]
- Wang, H; Ooi, EV; Ang, PO. Antiviral activities of extracts from Hong Kong seaweeds. J. Zhejiang. Univ. Sci. B 2008, 9, 969–976. [Google Scholar]
- Witvrouw, M; De Clercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol 1997, 29, 497–511. [Google Scholar]
- Haslin, C; Lahaye, M; Pellegrini, M; Chermann, JC. In vitro anti-HIV activity of sulfated cell-wall polysaccharides from gametic, carposporic and tetrasporic stages of the Mediterranean red alga Asparagopsis armata. Planta Med 2001, 67, 301. [Google Scholar]
- Bourgougnon, N. Anti-HIV compounds from red seaweeds. In Recent Advances in Marine Biotechnology, Volume 9: Biomaterials and Bioprocessing; Fingerman, M, Nagabhushanam, R, Eds.; Science Publishers: Enfield, NH, USA, 2003; pp. 16–206. [Google Scholar]
- Matsuhiro, B; Conte, AF; Damonte, EB; Kolender, AA; Matulewicz, MC; Mejías, EG; Pujol, CA; Zúñiga, EA. Structural analysis and antiviral activity of a sulfated galactan from the red seaweed Schizymenia binderi (Gigartinales, Rhodophyta). Carbohydr. Res 2005, 340, 2392–2402. [Google Scholar]
- Rodriguez, MC; Merino, ER; Pujol, CA; Damonte, EB; Cerezo, AS; Matulewicz, MC. Galactans from cystocarpic plants of the red seaweed Callophylis variegate (Kallymeniaceae, Gigartinales). Carbohydr. Res 2005, 340, 2742–2751. [Google Scholar]
- De SF-Tischer, PC; Talarico, LB; Noseda, MD; Guimaraes, SMPB; Damonte, EB; Duarte, MER. Chemical structure and antiviral activity of carrageenans from Meristiella gelidium against Herpes simplex and dengue virus. Carbohydr. Polym 2006, 63, 459–465. [Google Scholar]
- Chattopadhyay, K; Mateu, CG; Mandal, P; Pujol, CA; Damonte, EB; Ray, B. Galactan sulphate of Grateloupia indica: Isolation, structural features an antiviral activity. Phytochemistry 2007, 68, 1428–1435. [Google Scholar]
- Mandal, P; Pujol, CA; Carlucci, MJ; Chattopadhyay, K; Damonte, EB; Ray, B. Anti-herpetic activity of a sulfated xylomannan from Scinaia hatei. Phytochemistry 2008, 69, 2193–2199. [Google Scholar]
- Harden, EA; Falshaw, R; Carnachan, SM; Kern, ER; Prichard, MN. Virucidal activity of polysaccharide extracts from four algal species against Herpes simplex virus. Antiviral Res 2009, 83, 282–289. [Google Scholar]
- Falshaw, R; Furneaux, RH; Stevenson, DE. Structural analysis of carrageenans from the red alga, Callophyllis hombroniana Mont. Kütz (Kallymeniaceae, Rhodophyta). Carbohydr. Res 2005, 340, 1149–1158. [Google Scholar]
- Kolender, AA; Matulewicz, MC. Sulfated polysaccharides from the red seaweed Georgiella confluens. Carbohydr. Res 2002, 337, 57–68. [Google Scholar]
- Baba, M; Snoeck, R; Pauwels, R; De Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped virus, including Herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother 1988, 32, 1742–1745. [Google Scholar]
- Bourgougnon, N; Chermann, JC; Lahaye, M; Kornprobst, JM. Anti-HIV activity and mode of action, in vitro, of the sulfated polysaccharide from Schizeymenia dubyi (Rhodophyta). Cell. Pharmacol 1996, 3, 104–108. [Google Scholar]
- Abourriche, A; Charrouf, M; Berrada, M; Bennamara, A; Chaib, N; Francisco, C. Antimicrobial activities and cytotoxicity of the brown alga Cystoseira tamariscifolia. Fitoterapia 1999, 70, 611–614. [Google Scholar]
- Etahiri, S; Bultel-Poncé, V; Elkouri, AE; Assobhei, O; Zaoui, D; Guyot, M. Antibacterial activity of marine algal extracts from the Atlantic coast of Morocco. Mar. Life 2003, 13, 3–9. [Google Scholar]
- Moujahidi, A; Bencharki, B; Hilali, L; Bagri, A; Najim, L. Activités antibactériennes et antifongique des extraits d’algues marines d’origine marocaine. Biol. Santé 2004, 4, 298–305. [Google Scholar]
- Souhaili, N; Lagzouli, M; Faid, M; Fellat-Zerrouck, K. Inhibition of growth and Mycotoxins formation in moulds by marine algae Cystoseira tamariscifolia. Afr. J. Biol 2004, 3, 71–75. [Google Scholar]
- Bourgougnon, N; Quemener, B; Lahaye, M; Chermann, JC; Rimbert, M; Cormaci, M; Furnari, G; Kornprobst, JM. Annual variation in composition and in vitro anti-HIV-1 activity of the sulfated glucuronogalactan from Schizymenia dubyi (Rhodophyta, Gigartina-les). J. Appl. Phys 1996, 8, 155–161. [Google Scholar]
- Bourgougnon, N; Quemener, B; Lahaye, M; Cormaci, M; Furnari, G; Kornprobst, JM. Chemical structural approach of water-soluble sulfated glucuronogalactan from Schizymenia dubyi (Rhodophyta, Gigartinales). J. Appl. Phys 1996, 8, 147–159. [Google Scholar]
- Bourgougnon, N; Lahaye, M; Chermann, JC; Kornprobst, JM. Composition and antiviral activities of a sulphated polysaccharide from Schizymenia dubyi (Rhodophyta, Gigartinales). Bioorg. Med. Chem. Lett 1993, 3, 1141–1146. [Google Scholar]
- Haslin, C; Lahaye, M; Pellegrini, M. Chemical composition structure of sulphated water-soluble cell-wall polysaccharides from the gametic, carposporic and tetrasporic stages of Asparagopsis armata Harvey (Rhodophyta, Bonnemaisoniaceae). Bot. Mar 2000, 43, 475–482. [Google Scholar]
- Whyte, JNC; Englar, JR. The agar component of the red seaweed Gelidium purpurascens. Phytochemistry 1981, 20, 237–240. [Google Scholar]
- Izumi, K. Chemical heterogeneity of the agar from Gracilaria verrucosa. J. Biochem 1972, 72, 135–140. [Google Scholar]
- Freile-Pelegrín, Y; Murano, E. Agars from three species of Gracilaria (Rhodophyta) from Yucatán Peninsula. Bioresour. Technol 2005, 96, 295–302. [Google Scholar]
- Rochas, C; Lahaye, M; Yaphe, W. Sulfate content of carrageenan and agar determined by Infrared spectroscopy. Bot. Mar 1986, 29, 335–340. [Google Scholar]
- Fenoradosoa, TA; Delattre, C; Laroche, C; Wadouachi, A; Dulong, V; Picton, L; Andriamadio, P; Michaud, P. Highly sulphated galactan from Halymenia durvillei (Halymeniales, Rhodophyta), a red seaweed of Madagascar marine coasts. Int. J. Biol. Macromol 2009, 45, 140–145. [Google Scholar]
- Duarte, MER; Noseda, MD; Cardoso, MA; Tulio, S; Cerezo, AS. The structure of a galactan sulfate from the red seaweed Bostrychia montagnei. Carbohydr. Res 2002, 337, 1137–1144. [Google Scholar]
- Sekkal, M; Legrand, P; Huvenne, JP; Verdus, MC. The use of FTIR microspectormetry as a tool for the identification in situ of polygalactanes in red seaweeds. J. Mol. Struct 1993, 294, 227–230. [Google Scholar]
- Ananthi, S; Raghavendran, HRB; Sunil, AG; Gayathri, V; Ramakrishnan, GV; asanthi, HR. In vitro antioxidant and in vivo anti-inflammatory potential of crude polysaccharide from Turbinaria ornata (brown alga). Food Chem. Toxicol 2010, 48, 187–192. [Google Scholar]
- Lloyd, AG; Dodgson, KS. Infrared studies on sulphate esters. II. Monosaccharide sulfates. Biochim. Biophys. Acta 1961, 46, 116–120. [Google Scholar]
- Lloyd, AG; Dodgson, KS; Price, RG; Rose, FA. Infrared studies on sulphate esters. I. Polysaccharide sulfates. Biochim. Biophys. Acta 1961, 46, 108–115. [Google Scholar]
- Pereira, L; Amado, AM; Critchley, AT; van De Velde, F; Ribeiro-Claro, PJA. Identification of selected seaweed polysaccharides (phycocolloids) by vibrational spectroscopy (FTIR-ATR and FT-Raman). Food Hydrocolloids 2009, 23, 1903–1909. [Google Scholar]
- McCandless, EL; West, JA; Guiry, MD. Carrageenan patterns in the phyllophoraceae. Biochem. Syst. Biol 1982, 10, 275–284. [Google Scholar]
- Sekkal, M; Legrand, P. A spectroscopic investigation of the carrageenans and agar in the 1500–100 cm−1 spectral range. Spectrochim. Acta 1993, 49, 209–221. [Google Scholar]
- Liao, ML; Chiovitti, A; Munro, SLA; Craik, DJ; Kraft, AB. Sulfated galactans from Australian specimens of the red alga Phacelocarpus peperocarpos (Gigartinales, Rhodophyta). Carbohydr. Res 1996, 296, 237–247. [Google Scholar]
- Bi, F; Arman, M; Mahmood-ul-Hassan; Iqbal, S. Chemical and thermodynamic studies of k-carrageenan isolated from Hypnea musciformis (Red algae) of Krachi coast. Trends Appl. Sci. Res 2007, 2, 395–403. [Google Scholar]
- Melo, MRS; Feitosa, JPA; Freitas, ALP; de Paula, RCM. Isolation and characterization of soluble sulfated polysaccharide from the red seaweed Gracilaria cornea. Carbohydr. Polym 2002, 49, 491–498. [Google Scholar]
- Bodard, M; Christiaen, D. Spectroscopic infrarouge de films d’agar de Gracilaria verrucosa (Huds) Papenfuss. Bot. Mar 1983, 26, 425–427. [Google Scholar]
- Usov, AI; Bilan, MI; Shashkov, AS. Polysaccharide of algae. Characterizaton of hybrid structure of substituted agarose from Polysiphonia morrowii (Rhodophyta, Rhodomelaceae) using β-agarase and 13C-NMR Spectroscopy. Carbohydr. Res 1997, 303, 93–102. [Google Scholar]
- Usov, AI; Ivanova, EG; Shashkov, AS. Polysaccharides of algae. XXXIII. Isolation and 13C-NMR spectral study of some new gel-forming polysaccharides from Japan sea red seaweeds. Bot. Mar 1983, 26, 285–294. [Google Scholar]
- Miller, IJ; Furneaux, RH. The structural determination of the agaroid polysaccharides from four New Zealand algae in the order ceramiales by means of 13C NMR spectroscopy. Bot. Mar 1997, 40, 333–339. [Google Scholar]
- Miller, IJ. Evaluation of the structures of polysaccharides from two New Zealand members of the Rhodomelaceae by 13C NMR spectroscopy. Bot. Mar 2003, 46, 386–391. [Google Scholar]
- Prado, HJ; Ciancia, M; Matulewicz, MC. Agarans from the red seaweed Polysiphonia nigrescens (Rhodomelaceae, Ceramiales). Carbohydr. Res 2008, 343, 711–718. [Google Scholar]
- Batey, JF; Turvey, JR. The galactan sulphate of the red alga Polysiphonia lanosa. Carbohydr. Res 1975, 43, 133–143. [Google Scholar]
- Van de Velde, F. Structure and function of hybrid carrageenans. Food Hydrocolloids 2008, 22, 727–734. [Google Scholar]
- Gonzalez, ME; Alarcon, B; Carrasco, L. Polysaccharides as antiviral agents: Antiviral activity of carrageenan. Antimicrob. Agents Chemother 1987, 31, 1388–1393. [Google Scholar]
- Talarico, LB; Zibetti, RGM; Faria, PCS; Scolaro, LA; Duarte, MER; Noseda, MD; Pujol, CA; Damonte, EB. Anti-herpes simplex virus activity of sulfated galactans from the red seaweeds Gymnogongrus griffithsiae and Cryptonemia crenulata. Int. J. Biol. Macromol 2004, 34, 63–71. [Google Scholar]
- Kornprobst, JM. Substances Naturelles D’origine Marine: Généralités, Micro-Organismes, Algues; Editions Tec & Doc: Paris, France, 2005; Volume 1. [Google Scholar]
- Damonte, EB; Matulewicz, MC; Cerezo, AS. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem 2004, 11, 2399–2419. [Google Scholar]
- Damonte, EB; Matulewicz, MC; Cerezo, AS; Coto, CE. Herpes simplex virus inhibitory sulfated xylogalactans from the red seaweed Nothogenia fastigiata. Chemotherapy 1996, 42, 57–64. [Google Scholar]
- Adhikari, U; Mateu, CG; Chattopadhyay, K; Pujol, CA; Damonte, EB; Ray, B. Structure and antiviral of sulfated fucans from Stoechospermum marginatum. Phytochemistry 2006, 67, 2474–2482. [Google Scholar]
- Bourgougnon, N. Activité antivirale et antiprolifératrice in vitro du polysaccharide sulphate de Schizymenia dubyi (Rhodophytes, Gigartinales). PhD Thesis, Université de Nantes, Nantes, France, 1994. [Google Scholar]
- Bergé, J-P; Bourgougnon, N; Alban, S; Pojer, F; Chermann, J-C; Billaudel, S; Robert, J-M; Durand, P; Franz, G. Antiviral and anticoagulant activities of a water soluble compound extracted from the marine diatom Haslea ostrearia. Planta Med 1999, 65, 604–609. [Google Scholar]
- Ghosh, T; Chattopadhyay, K; Marschall, M; Karmakar, P; Mandal, P; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar]
- Carlucci, MJ; Ciancia, M; Matulewicz, MC; Cerezo, AS; Damonte, EB. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antiviral Res 1999, 43, 93–102. [Google Scholar]
- Witvrouw, M; Este, JA; Mateu, MEQ; Reymen, D; Andrei, G; Snoeck, R; Ikeda, S; Pauwels, R; Bianchini, NV; Desmyter, J; De Clercq, E. Activity of a sulfated polysaccharide extracted from the red seaweed Aghardhiella tenera against human immunodeficiency virus and other enveloped viruses. Antiviral Chem. Chemother 1994, 5, 297–303. [Google Scholar]
- McClure, MO; Moore, JP; Blanc, DF; Scotting, P; Cook, GMW; Keynes, RJ; Weber, JN; Davies, D; Weiss, RA. Investigations into the mechanism by which sulfated polysaccharides inhibit HIV-infection in vitro. AIDS Res. Hum. Retroviruses 1992, 8, 19–26. [Google Scholar]
- Vlieghe, P; Clerc, T; Pannecouque, C; Witvrouw, M; De Clercq, E; Salles, JP; Kraus, JL. Synthesis of new covalently bound kappa-carrageenan-AZT conjugates with improved anti-HIV activities. J. Med. Chem 2002, 45, 1275–1283. [Google Scholar]
- Dubois, M; Gilles, KA; Hamilton, JK; Rebers, PA; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem 1956, 28, 350–356. [Google Scholar]
- Blumenkrantz, N; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem 1973, 54, 484–489. [Google Scholar]
- Filisetti-Cozzi, TMCC; Carpita, NC. Measurement of uronic acids without interference from neutral sugars. Anal. Biochem 1991, 197, 157–162. [Google Scholar]
- Kamerling, JP; Gerwig, GJ; Vliegenthart, JFG; Clamp, JR. Characterisation by gas-liquid chromatography mass spectrometry of permethylsilylglycosides obtained in the methanolysis of glycoproteins and glycolipids. Biochem. J 1975, 151, 491–495. [Google Scholar]
- Montreuil, J; Bouquelet, S; Debray, H; Fournet, B; Spick, G; Strecker, G. Glycoproteins. In Carbohydrate Analysis: A Practical Approach; Chaplin, MF, Kennedy, JF, Eds.; IRL Press: Oxford, UK, 1986; pp. 143–204. [Google Scholar]
- Yaphe, W; Arsenault, GP. Improved resorcinol reagent for determination of fructose, and 3,6-anhydrogalactose in polysaccharides. Anal. Biochem 1965, 13, 143–148. [Google Scholar]
- Craigie, JS; Wen, ZC; van der Meer, JP. Interspecific, intraspecific and nutritionally-determined variations in the composition of agars from Gracilaria spp. Bot. Mar 1984, 27, 55–61. [Google Scholar]
- Smith, PK; Krohn, RI; Hermanson, GT; Mallia, AK; Gartner, FH; Provenzano, MD; Fujimoto, EK; Goeke, NM; Olson, BJ; Klenk, DC. Measurement of protein using bicinchoninic acid. Anal. Biochem 1985, 150, 76–85. [Google Scholar]
- Sloneker, JH; Orentas, DG. Pyruvic acid, a unique component of an exocellular bacterial polysaccharide. Nature 1962, 194, 478–479. [Google Scholar]
- Rigouin, C; Ladrat, CD; Sinquin, C; Colliec-Jouault, S; Dion, M. Assessment of bio-chemical methods to detect enzymatic depolymerization of polysaccharides. Carbohydr. Polym 2009, 76, 279–284. [Google Scholar]
- Le Contel, C; Galéa, P; Silvy, F; Hirsch, I; Chermann, JC. Identification of the β2m-derived epitope responsible for neutralization of HIV isolates. Cell. Pharmacol. AIDS Sci 1996, 3, 68–73. [Google Scholar]
- Langlois, M; Allard, JP; Nugier, F; Aymard, M. A rapid and automated colorimetric assay for evaluating in the sensitivity of herpes simplex strains to antiviral drugs. J. Biol. Stand 1986, 14, 201–211. [Google Scholar]
- Olicard, C; Renault, T; Torhy, C; Benmansour, A; Bourgougnon, N. Putative antiviral activity in hemolymph from adult Pacific oysters, Crassostrea gigas. Antiviral Res 2005, 66, 147–152. [Google Scholar]


| Water-extracted polysaccharides
| ||
|---|---|---|
| S. coronopifolius | B. thuyoides | |
| Neutral sugars | ||
| Galactose | 33.1 | 25.4 |
| Xylose | 1.80 | 2.80 |
| Glucose | 1.70 | 3.00 |
| Rhamnose | 0 | 0.30 |
| 3,6-anhydrogalactose | 11.00 | 16.00 |
| Glucuronic acid | 6.70 | 0 |
| Galacturonic acid | 1.0 | 3.2 |
| Sulfate | 24.00 | 7.60 |
| Proteins | 0 | 6.00 |
| Pyruvic acid | 0.34 | 0 |
| Ash | 13.24 | 13.05 |
| M | EC50 PSC | EC50 PBT | EC50 ACV | |
|---|---|---|---|---|
| Effect before infection | >250 | >250 | >5 | |
| Virucidal assays | 53.2 | 73.9 | - | |
| Virus adsorption assay | Treatment I | 35.4 | 41.1 | 0.8 |
| Treatment II | 26.2 | 20.7 | 0.2 | |
| Treatment III | 3.6 | 13.1 | 0.1 | |
| Effect of time of polysaccharide addition | 0 h | <2.5 | 10.7 | 0.5 |
| 1 h after infection | <2.5 | 22.7 | 1.8 | |
| 2 h after infection | 2.5 | 32.4 | 2.7 | |
| 3 h after infection | >250 | >250 | 3.2 | |
| 5 h after infection | >250 | >250 | 4.1 |
| Concentration | D3 | D4 | D5 | D6 | D7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SC 1 mg/mL | 100 μg/mL | - | - | - | - | - | - | - | - | - | - |
| 50 μg/mL | - | - | - | - | - | - | - | - | - | - | |
| 25 μg/mL | - | - | - | - | - | - | - | - | - | - | |
| 12.5 μg/mL | - | - | - | - | - | - | - | - | - | - | |
| Control HIV-1 NDK 7.5 × 10−4 | (+) | (+) | + | + | ++ | ++ | T | T | T | T | |
| (+)/+ | (+) | +/++ | + | ++ | ++/T | T | T | T | T | ||
| SC 1 mg/mL | 100 μg/mL | - | - | - | - | - | - | - | - | - | - |
| 50 μg/mL | - | - | - | - | - | - | - | - | - | - | |
| 25 μg/mL | - | - | - | - | - | - | - | - | - | - | |
| 12.5 μg/mL | - | - | - | - | - | - | - | - | - | - | |
| Control HIV-1 NDK 5 × 10−4 | (+) | (+) | ++ | ++ | ++/T | ++/T | T | T | T | T | |
| - | - | + | +/++ | T | ++/T | T | ++/T | T | T | ||
| Control cells MT4 | - | - | - | - | - | - | - | - | - | - | |
| Concentration | D3 | D4 | D5 | D6 | D7 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BT 1 mg/mL | 100 μg/mL | - | - | - | - | - | - | - | - | - | - |
| 50 μg/mL | - | - | - | (+) | - | (+)/+ | - | + | - | + | |
| 25 μg/mL | - | - | (+) | - | + | (+) | ++ | ++ | T | T | |
| 12.5 μg/mL | - | - | (+)/+ | + | ++/T | ++/T | T | T | T | T | |
| Control HIV-1 NDK 7.5 × 10−4 | (+) | (+) | + | + | ++ | ++ | T | T | T | T | |
| (+)/+ | (+) | +/++ | + | ++ | ++/T | T | T | T | T | ||
| BT 1 mg/mL | 100 μg/mL | - | - | - | - | - | - | - | - | - | - |
| 50 μg/mL | - | - | - | - | - | (+) | - | (+)/+ | - | + | |
| 25 μg/mL | - | - | + | (+)/+ | ++ | ++ | T | T | T | T | |
| 12.5 μg/mL | (+) | - | ++ | +/++ | ++/T | ++/T | T | T | T | T | |
| Control HIV-1 NDK 5 × 10−4 | (+) | (+) | ++ | ++ | ++/T | ++/T | T | T | T | T | |
| - | - | + | +/++ | T | ++/T | T | ++/T | T | T | ||
| Control cells MT4 | - | - | - | - | - | - | - | - | - | - | |
| Time of action | Concentration | D3 | D4 | D5 | D6 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before and during (a) | SC | 12.5 μg/mL | (+) | (+) | ++ | ++ | T | T | T | T |
| AZT | 0.4 μM | (+) | (+) | + | + | T | T | T | T | |
| During (b) | SC | 12.5 μg/mL | + | + | +/++ | +/++ | T | T | T | T |
| AZT | 0.4 μM | (+) | (+) | +/++ | +/++ | T | T | T | T | |
| After (c) | SC | 12.5 μg/mL | - | - | - | - | - | - | - | - |
| AZT | 0.4 μM | - | - | - | - | - | - | - | - | |
| All the time (d) | SC | 12.5 μg/mL | - | - | - | - | - | - | - | - |
| AZT | 0.4 μM | - | - | - | - | - | - | - | - | |
| Control HIV-1 NDK 1 × 10−4 | ++ | ++ | ++ | ++/++T | T | T | T | T | ||
| ++ | + | ++ | ++ | T | T | T | T | |||
| Before and during (a) | SC | 12.5 μg/mL | (+)/+ | + | +/++ | ++ | T | T | T | T |
| AZT | 0.4 μg/mL | (+) | - | + | (+)/+ | ++T | ++T | T | T | |
| During (b) | SC | 12.5 μg/mL | + | + | ++ | ++ | T | T | T | T |
| AZT | 0.4 μg/mL | (+) | (+) | + | +/++ | T | ++T | T | T | |
| After (c) | SC | 12.5 μg/mL | - | - | - | - | - | - | - | - |
| AZT | 0.4 μg/mL | - | - | - | - | - | - | - | - | |
| All the time (d) | SC | 12.5 μg/mL | - | - | - | - | - | - | - | - |
| AZT | 0.4 μg/mL | - | - | - | - | - | - | - | - | |
| Control HIV-1 NDK 7.5 ×10−5 | + | + | + | + | T | T | T | T | ||
| ++ | ++ | + | +/++ | T | T | T | T | |||
| MT4 | - | - | - | - | - | - | - | - | ||
| Time of action | Concentration | D3 | D4 | D5 | D6 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before and during (a) | BT | 75 μg/mL | (+) | (+) | ++ | ++ | T | T | T | T |
| AZT | 0.4 μM | (+) | (+) | + | + | T | T | T | T | |
| During (b) | BT | 75 μg/mL | (+) | + | +/++ | +/++ | T | T | T | T |
| AZT | 0.4 μM | (+) | (+) | +/++ | +/++ | T | T | T | T | |
| After (c) | BT | 75 μg/mL | - | - | - | - | - | - | - | - |
| AZT | 0.4 μM | - | - | - | - | - | - | - | - | |
| All the time (d) | BT | 75 μg/mL | - | - | - | - | - | + | - | + |
| AZT | 0.4 μM | - | - | - | - | - | - | - | - | |
| Control HIV-1 NDK 1 × 10−4 | ++ | ++ | ++ | ++/++T | T | T | T | T | ||
| ++ | + | ++ | ++ | T | T | T | T | |||
| Before and during (a) | BT | 75 μg/mL | + | (+)/+ | ++ | ++ | ++T | T | T | T |
| AZT | 0.4 μg/mL | (+) | - | + | (+)/+ | ++T | ++T | T | T | |
| During (b) | BT | 75 μg/mL | + | + | ++ | ++ | T | T | T | T |
| AZT | 0.4 μg/mL | (+) | (+) | + | +/++ | T | ++T | T | T | |
| After (c) | BT | 75 μg/mL | - | - | - | - | - | - | - | - |
| AZT | 0.4 μg/mL | - | - | - | - | - | - | - | - | |
| All the time (d) | BT | 75 μg/mL | (+) | - | - | + | - | + | - | + |
| AZT | 0.4 μg/mL | - | - | - | - | - | - | - | - | |
| Control HIV-1 NDK 7.5 × 10−5 | + | + | + | + | T | T | T | T | ||
| ++ | ++ | + | +/++ | T | T | T | T | |||
| MT4 | - | - | - | - | - | - | - | - | ||
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bouhlal, R.; Haslin, C.; Chermann, J.-C.; Colliec-Jouault, S.; Sinquin, C.; Simon, G.; Cerantola, S.; Riadi, H.; Bourgougnon, N. Antiviral Activities of Sulfated Polysaccharides Isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Mar. Drugs 2011, 9, 1187-1209. https://doi.org/10.3390/md9071187
Bouhlal R, Haslin C, Chermann J-C, Colliec-Jouault S, Sinquin C, Simon G, Cerantola S, Riadi H, Bourgougnon N. Antiviral Activities of Sulfated Polysaccharides Isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Marine Drugs. 2011; 9(7):1187-1209. https://doi.org/10.3390/md9071187
Chicago/Turabian StyleBouhlal, Rhimou, Camille Haslin, Jean-Claude Chermann, Sylvia Colliec-Jouault, Corinne Sinquin, Gaelle Simon, Stephane Cerantola, Hassane Riadi, and Nathalie Bourgougnon. 2011. "Antiviral Activities of Sulfated Polysaccharides Isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales)" Marine Drugs 9, no. 7: 1187-1209. https://doi.org/10.3390/md9071187
APA StyleBouhlal, R., Haslin, C., Chermann, J.-C., Colliec-Jouault, S., Sinquin, C., Simon, G., Cerantola, S., Riadi, H., & Bourgougnon, N. (2011). Antiviral Activities of Sulfated Polysaccharides Isolated from Sphaerococcus coronopifolius (Rhodophytha, Gigartinales) and Boergeseniella thuyoides (Rhodophyta, Ceramiales). Marine Drugs, 9(7), 1187-1209. https://doi.org/10.3390/md9071187