Antiproliferative Activity of Violaxanthin Isolated from Bioguided Fractionation of Dunaliella tertiolecta Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antiproliferative Activity of Microalgae Extracts
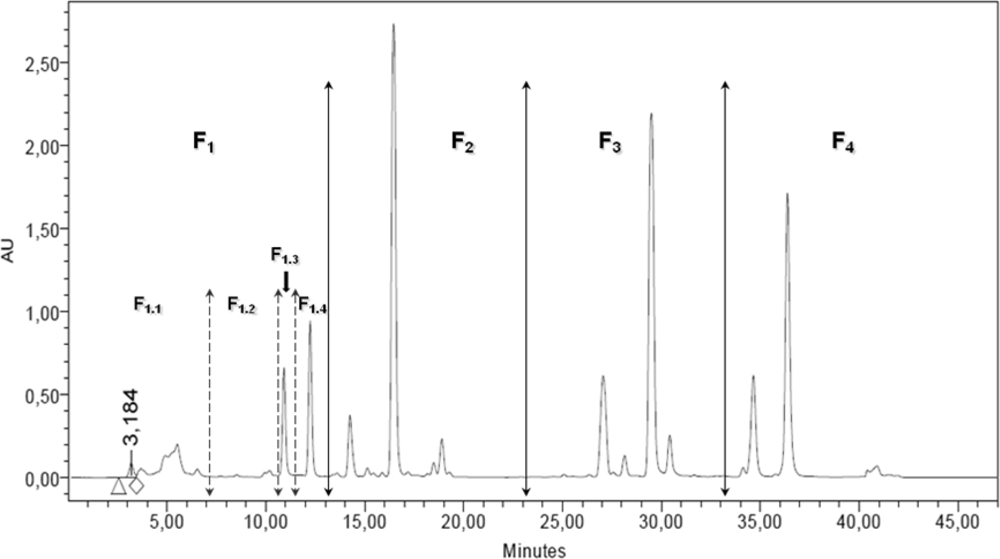
2.2. RP-HPLC Analysis, Fractionation and Sub-Fractionation of the DT DCM Extract
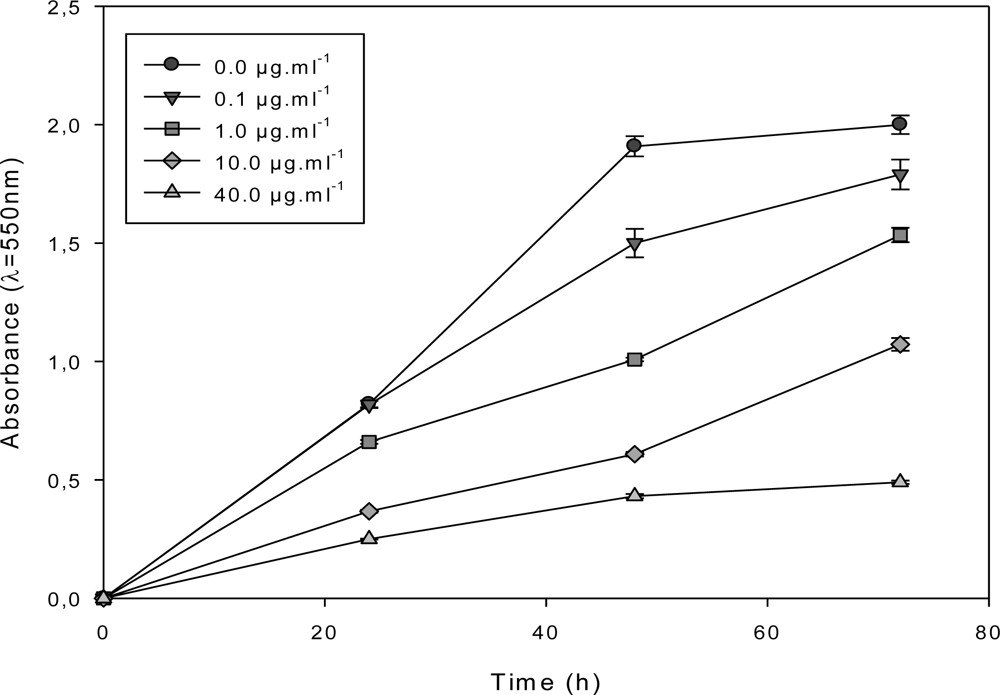
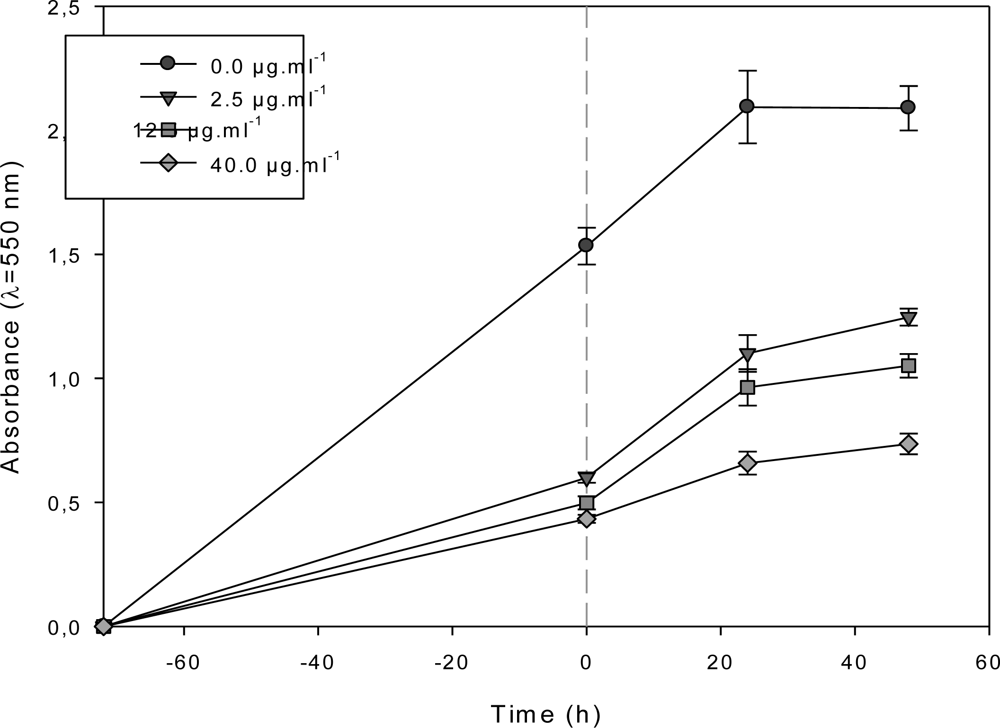
2.3. Effect of the F1.4 Sub-Fraction on MCF-7 Growth
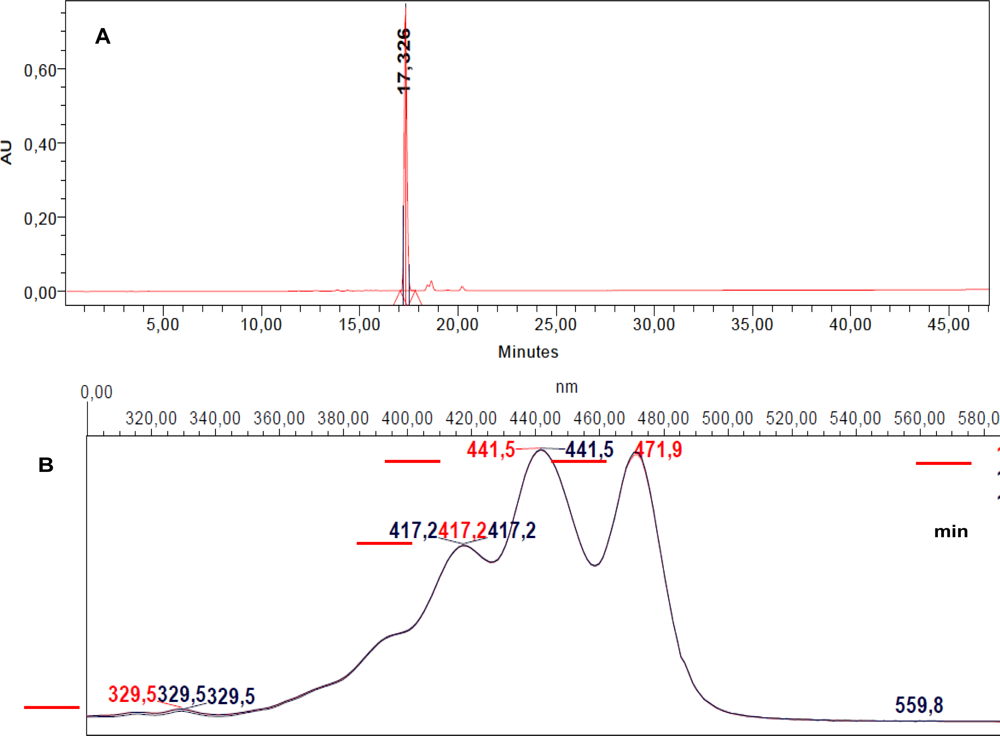
2.4. Characterization of the Antiproliferative Molecule Contained in F1.4
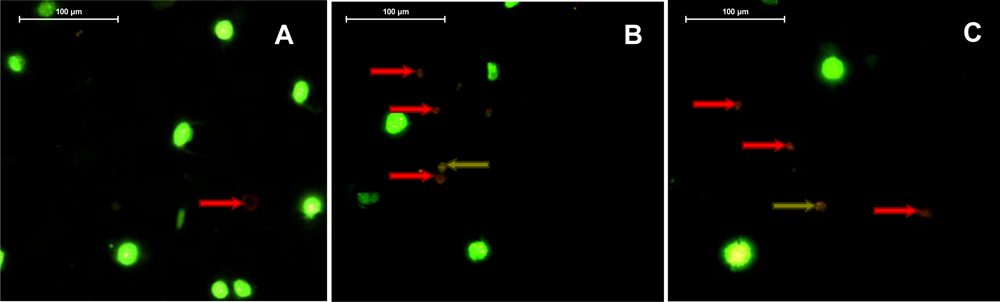
2.5. Violaxanthin Induces Morphological and Biochemical Changes Characteristic of Early Apoptosis in MCF-7
2.6. Violaxanthin Does Not Evoke MCF-7 DNA Fragmentation
3. Experimental Section
3.1. Microalgae
3.2. Microalgae Culture, Collection and Storage
3.3. Successive Extractions in Dichloromethane, Ethanol and Water
3.4. RP-HPLC Analysis and Fractionation
3.5. Cell Culture
3.6. Cell Viability Assay
3.7. Detection of Early and Late Steps of Apoptosis
3.7.1. Phosphatidylserines Translocation and DNA Staining
3.7.2. DNA Fragmentation
3.8. High Resolution Mass Spectrometry (HRMS)
3.9. Chemicals and Standards
4. Conclusions
Acknowledgments
- Samples Availability: Available from the authors.
References
- Kornprobst, J-M. Substances Naturelles D’origine Marine: Chimiodiversité, Pharmacodiversité, Biotechnologies; Lavoisier: Paris, France, 2005; Volume 1. [Google Scholar]
- Folmer, F; Jaspars, M; Dicato, M; Diederich, M. Photosynthetic marine organisms as a source of anticancer compounds. Phytochem Rev 2010, 9, 557–579. [Google Scholar]
- Nishino, H; Murakoshi, M; Tokuda, H; Satomi, Y. Cancer prevention by carotenoids. Arch Biochem Biophys 2009, 483, 165–168. [Google Scholar]
- Nishino, H; Tokuda, H; Murakoshi, M; Satomi, Y; Masuda, M; Onozuka, M; Yamaguchi, S; Takayasu, J; Tsuruta, J; Okuda, M; et al. Cancer prevention by natural carotenoids. BioFactors 2000, 13, 89–94. [Google Scholar]
- Del Campo, JA; Moreno, J; Rodríguez, H; Vargas, MA; Rivas, J; Guerrero, MG. Carotenoid content of chlorophycean microalgae: Factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J Biotechnol 2000, 76, 51–59. [Google Scholar]
- Dufossé, L; Galaup, P; Yaron, A; Arad, SM; Blanc, P; Chidambara Murthy, KN; Ravishankar, GA. Microorganisms and microalgae as sources of pigments for food use: A scientific oddity or an industrial reality. Trends Food Sci Technol 2005, 16, 389–406. [Google Scholar]
- Milledge, JJ. Commercial application of microalgae other than as biofuels: A brief review. Rev Environ Sci Biotechnol 2010, 10, 31–41. [Google Scholar]
- Cui, Y; Lu, Z; Bai, L; Shi, Z; Zhao, WE; Zhao, B. β-Carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor γ expression and reactive oxygen species production in MCF-7 cancer cells. Eur J Cancer 2007, 43, 2590–2601. [Google Scholar]
- Kotake-Nara, E; Asai, A; Nagao, A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett 2005, 220, 75–84. [Google Scholar]
- Das, SK; Hashimoto, T; Kanazawa, K. Growth inhibition of human hepatic carcinoma HepG2 cells by fucoxanthin is associated with down-regulation of cyclin D. Biochim Biophys Acta Gen Subj 2008, 1780, 743–749. [Google Scholar]
- Das, SK; Hashimoto, T; Shimizu, K; Yoshida, T; Sakai, T; Sowa, Y; Komoto, A; Kanazawa, K. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1. Biochim Biophys Acta Gen Subj 2005, 1726, 328–335. [Google Scholar]
- Hosokawa, M; Kudo, M; Maeda, H; Kohno, H; Tanaka, T; Miyashita, K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim Biophys Acta Gen Subj 2004, 1675, 113–119. [Google Scholar]
- Hosokawa, M; Wanezaki, S; Miyauchi, K; Kurihara, H; Kohno, H; Kawabata, J; Odashima, S; Takahashi, K. Apoptosis-inducing effect of fucoxanthin on human leukemia cell line HL-60. Food Sci Technol Res 1999, 5, 243–246. [Google Scholar]
- Moreau, D; Tomasoni, C; Jacquot, C; Kaas, R; Le Guedes, R; Cadoret, JP; Muller-Feuga, A; Kontiza, I; Vagias, C; Roussis, V; et al. Cultivated microalgae and the carotenoid fucoxanthin from Odontella aurita as potent anti-proliferative agents in bronchopulmonary and epithelial cell lines. Environ Toxicol Pharm 2006, 22, 97–103. [Google Scholar]
- Nakazawa, Y; Sashima, T; Hosokawa, M; Miyashita, K. Comparative evaluation of growth inhibitory effect of stereoisomers of fucoxanthin in human cancer cell lines. J Funct Foods 2009, 1, 88–97. [Google Scholar]
- Jeffrey, SW; Mantoura, RFC; Wright, SW. Phytoplankton Pigments in Oceanography; UNESCO Publishing: Paris, France, 1997; pp. 458–553. [Google Scholar]
- Jeffrey, SW; Mantoura, RFC; Wright, SW. Phytoplankton Pigments in Oceanography; UNESCO Publishing: Paris, France, 1997; p. 292. [Google Scholar]
- Jeffrey, SW; Mantoura, RFC; Wright, SW. Phytoplankton Pigments in Oceanography; UNESCO Publishing: Paris, France, 1997; p. 331. [Google Scholar]
- Cha, KH; Koo, SY; Lee, D. Antiproliferative effects of carotenoids extracted from Chlorella ellipsoidea and Chlorella vulgaris on human colon cancer cells. J Agric Food Chem 2008, 56, 10521–10526. [Google Scholar]
- Barua, AB. Intestinal absorption of epoxy-β-carotenes by humans. Biochem J 1999, 339, 359–362. [Google Scholar]
- Hashimoto, T; Ozaki, Y; Taminato, M; Das, S; Mizuno, M; Yoshimura, K; Maoka, T; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br J Nutr 2009, 102, 242–248. [Google Scholar]
- Beppu, F; Niwano, Y; Sato, E; Kohno, M; Tsukui, T; Hosokawa, M; Miyashita, K. In vitro and in vivo evaluation of mutagenicity of fucoxanthin (FX) and its metabolite fucoxanthinol (FXOH). J Toxicol Sci 2009, 34, 693–698. [Google Scholar]



| Extraction solvent | Cell line | |||
|---|---|---|---|---|
| MCF-7 | MDA-MB-231 | A549 | LNCaP | |
| Water | ≫ | ≫ | ≫ | ≫ |
| EtOH | 61.5 | ≫ | ≫ | ≫ |
| DCM | 56.1 | ≫ | ≫ | 60.9 |
| DT DCM fractions | F1 | F2 | F3 | F4 |
| GI50 (μg·mL−1) | 14.3 | ≫ | ≫ | ≫ |
| DT DCM sub-fractions | F1.1 | F1.2 | F1.3 | F1.4 |
| GI50 (μg·mL−1) | > | 20.5 | 18.9 | 11.7 |
| SEM GI50 (μg·mL−1) | 2.2 | 8.85 | 0.2 |
| Lead compound F1.4 | |
|---|---|
| Molecular formula | [M + Na]+ (C40H56O4Na) |
| Theoretical molecular weight | 623.40763 |
| z | 1 |
| Theoretical m/z value | 623.40708 |
| Experimental m/z value [M + Na] | 623.4068 (0 ppm) |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.-B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V.; et al. Antiproliferative Activity of Violaxanthin Isolated from Bioguided Fractionation of Dunaliella tertiolecta Extracts. Mar. Drugs 2011, 9, 819-831. https://doi.org/10.3390/md9050819
Pasquet V, Morisset P, Ihammouine S, Chepied A, Aumailley L, Berard J-B, Serive B, Kaas R, Lanneluc I, Thiery V, et al. Antiproliferative Activity of Violaxanthin Isolated from Bioguided Fractionation of Dunaliella tertiolecta Extracts. Marine Drugs. 2011; 9(5):819-831. https://doi.org/10.3390/md9050819
Chicago/Turabian StylePasquet, Virginie, Perrine Morisset, Said Ihammouine, Amandine Chepied, Lucie Aumailley, Jean-Baptiste Berard, Benoit Serive, Raymond Kaas, Isabelle Lanneluc, Valerie Thiery, and et al. 2011. "Antiproliferative Activity of Violaxanthin Isolated from Bioguided Fractionation of Dunaliella tertiolecta Extracts" Marine Drugs 9, no. 5: 819-831. https://doi.org/10.3390/md9050819
APA StylePasquet, V., Morisset, P., Ihammouine, S., Chepied, A., Aumailley, L., Berard, J.-B., Serive, B., Kaas, R., Lanneluc, I., Thiery, V., Lafferriere, M., Piot, J.-M., Patrice, T., Cadoret, J.-P., & Picot, L. (2011). Antiproliferative Activity of Violaxanthin Isolated from Bioguided Fractionation of Dunaliella tertiolecta Extracts. Marine Drugs, 9(5), 819-831. https://doi.org/10.3390/md9050819






