Abstract
The marine environment is known as a rich source of chemical structures with numerous beneficial health effects. Among marine organisms, marine algae have been identified as an under-exploited plant resource, although they have long been recognized as valuable sources of structurally diverse bioactive compounds. Presently, several lines of studies have provided insight into biological activities and neuroprotective effects of marine algae including antioxidant, anti-neuroinflammatory, cholinesterase inhibitory activity and the inhibition of neuronal death. Hence, marine algae have great potential to be used for neuroprotection as part of pharmaceuticals, nutraceuticals and functional foods. This contribution presents an overview of marine algal neuroprotective effects and their potential application in neuroprotection.
1. Introduction
Ninety percent of the world’s living biomass is found in the oceans with marine species comprising approximately half of the total global biodiversity [1,2]. This wide diversity of organisms is being recognized as a reservoir of potent molecules which are elicited by marine organisms to help them survive in the hostile environment [2,3]. Among marine organisms, marine algae have been identified as an under-exploited plant resources [4,5]. The term marine algae, as used herein, generally refer to marine macroalgae or sometimes referred to as seaweeds.
Marine algae can be classified into three classes based on their pigmentation, namely brown, red, and green algae, which are referred to as Phaeophyceae, Rhodophyceae, and Chlorophyceae, respectively [6]. Since the 1940s, production of algal polysaccharides has attained commercial significance through their application as thickening and gelling agents for various industrial applications [7]. Moreover, marine algae are recognized as rich sources of structurally diverse biologically active compounds with great pharmaceutical and biomedical potential. Researchers have revealed that marine algal originated compounds exhibit various biological activities such as anticoagulant [8,9], anti-viral [10,11], antioxidant [12–14], anti-allergic [15], anti-cancer [16], anti-inflammatory [17], anti-obesity [18–20], etc. Furthermore, several scientific studies have provided insight into neuroprotective properties of marine algae. Many species of marine algae have long been used in food diets as well as traditional remedies in Eastern countries and more recently in Europe and America. Hence, marine algae have great potential to be used in neuroprotection [21].
In recent years, biological activities, nutritional value, and potential health benefits of marine algae have been intensively investigated and reviewed. This review, however, focuses specifically on the neuroprotective effects of marine algae and emphasizes their potential application as future pharmaceutical candidates to prevent neurodegenerative diseases.
2. Bioactivities and Neuroprotective Effects of Marine Algae
2.1. Antioxidant
Oxidative stress is the result of an imbalance between pro-oxidant and antioxidant homeostasis that leads to the generation of toxic reactive oxygen species (ROS) [22]. Compared to other parts of our body, the central nervous system (CNS) is more sensitive to oxidative stress due to its high oxygen consumption and lipid content. Increased oxidative stress in the CNS will further lead to lipid peroxidation, DNA and protein damage [23]. Oxidative stress in the CNS has been demonstrated to involve excitotoxicity and apoptosis, the two main causes of neuronal death. Furthermore, oxidative stress has also been implicated the progression of Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS) and other neurodegenerative diseases [24,25]. Antioxidants may have a positive effect in the CNS and seem to be a promising approach of neuroprotection therapy, as they can protect the CNS against free radical mediated oxidative damage [26]. However, our endogenous antioxidant defenses are not always completely effective and exposure to damaging environmental factors is increasing, therefore it seems reasonable to propose that exogenous antioxidants could be effective in diminishing the cumulative effects of oxidative damage. Presently, antioxidants constitute a major component of clinical and experimental drugs that are currently considered for prevention of neurodegenerative diseases and therapy [27].
Antioxidant activities of marine algae have been determined by various methods such as 1,1-diphenyl-2-picryl hydrazyl (DPPH) radical scavenging, 2,2′-azinobis-3-ethylbenzo thizoline-6-sulphonate (ABTS) radical scavenging, singlet oxygen quenching activity, lipid peroxide inhibition, superoxide and hydroxyl radical scavenging assays. Lim et al. demonstrated that Neorhodomela aculeate, which is also known as Rhodomela confervoides, was able to scavenge DPPH with an IC50 = 90 μg/mL and at a concentration of 20 μg/mL completely suppressed H2O2 induced lipid peroxidation in rat brain homogenate [28]. Furthermore, Fallarero et al. showed that Halimeda incrassata and Bryothamniom triquetrum are potent ROS scavengers in mouse hypothalamic (GT1–7) cells [29]. Novoa et al. reported that the antioxidant and ROS scavenging activity of B. triquetrum are related to their high phenolic contents [30]. Dieckol, a phenolic compound isolated from brown algae has been shown to scavenge ROS production in murine microglia (BV2) cells [31]. Wijesekara and Kim reported that most phenolic compounds which were purified from marine algae are responsible for marine algal antioxidant activities and protective effects against oxidative stress induced cell damage [32]. Phenolic compounds act as free radical scavengers, reducing agents and metal chelators, and thus effectively inhibit lipid oxidation. In addition, Yan et al. demonstrated that carotenoids have a strong radical scavenging activity and are found as a major antioxidant in marine algae [33,34]. Young and Lowe indicated that structure, physical form, location or site of action, potential interaction with another antioxidant, concentration and partial pressure to oxygen may affect the antioxidant activities of carotenoids in biological systems [35]. Fucoxanthin obtained from Padina tetrastromatic has shown higher potential to be used as an antioxidant than β-carotene in modulating antioxidant enzyme in plasma and liver of retinol deficient rats [36,37]. However, the exact mechanisms of action how fucoxanthin exerts antioxidative effect in rat induced by retinol deficiency are not yet completely understood. Moreover, the cytoprotective effect of fucoxanthin against ROS formation induced by H2O2 in monkey kidney fibroblast (Vero) cells has been observed [38]. Two hydroxyl groups present in the ring structure of fucoxanthin may correlate to the inhibition of ROS formation. Indeed, it has been reported that the number of hydroxyl groups on the ring structure is correlated with the effects of ROS suppression. Moreover, it has also been shown that some marine algal sulfated polysaccharides (SPs) can be used as potent antioxidants [39,40]. Antioxidant activity of marine algal SPs depends on their structural features such as degree of sulfating, molecular weight, type of the major sugar and glycosidic branching [41,42]. However, bioactivities of marine algal carotenoids and SPs against oxidative stress in the CNS have not been demonstrated yet.
Based on those findings, it can be suggested that among various organisms in the marine environment, marine algae prove to be one of the useful candidates that can protect the CNS against oxidative degradation. Hence, developing novel molecules derived from marine algae which promote antioxidant activity in the CNS may lead to the development of effective neuroprotective agents. Furthermore, it is also important to determine whether antioxidants derived from marine algae can be used as prophylactic neuroprotective agents in order to slow down the progression of neurodegenerative diseases in populations that are at high risk, such as the elderly. Additionally, antioxidant activities of marine algal carotenoids, SPs and other bioactive compounds in the CNS warrant further investigations.
2.2. Anti-Neuroinflammation
Inflammation has been found to be the pathophysiological mechanism underlying many chronic diseases such as cardiovascular disease, diabetes, certain cancers, arthritis, and neurodegenerative diseases [43]. Recent studies demonstrated that resulting production of inflammatory responses and neurotoxic factors in the CNS is sufficient to induce neurodegeneration in a rat model [44]. Several cell types have been demonstrated as contributors in inflammation-mediated neurodegeneration, yet microglia are implicated as critical components of the immunological insult to neurons [45]. Microglia are the immune cells in the CNS, they enters the system from the blood circulation early in an organism’s development and serve a role of immune surveillance [43]. Ramified or resting microglia constitute 5–20% of glial populations in the CNS [46]. Recent study demonstrated that activation of microglia and the resulting production of pro-inflammatory and neurotoxic factors are sufficient to induce neurodegeneration in a rat model. Furthermore, activation of microglia and excessive amounts of pro-inflammatory mediators release by microglia have been observed during the pathogenesis of PD, AD, MS, AIDS dementia complex, as well as post neuronal death in cerebral stroke and traumatic brain injury [44,47]. Therefore, a mechanism to regulate inflammatory response release by microglia may have important therapeutic potential for the treatment of neurodegenerative diseases.
Numerous studies has documented anti-inflammatory activities of marine algae in vitro and in vivo [48]. However, scientific analysis of anti-neuroinflammatory activity of marine algae has been poorly carried out and until now only few studies were reported. Ecklonia cava (Phaeophyceae; Laminareaceae), also known as “sea trumpet”, has been reported to possess anti-inflammatory activity [49–51]. E. cava was able to suppress the levels of pro-inflammatory mediators such as nitric oxide (NO), prostaglandine-E2 (PGE2) and pro-inflammatory cytokines (tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β)) in lipopolysaccharides (LPS)-stimulated BV2 cells by blocking nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs) activation [31,51]. Furthermore, N. aculeate decreased NO production and inhibiting inducible NO synthase (iNOS) expression in interferon-gamma (IFN-γ) stimulated BV2 cells [28]. A number of bromophenols have been previously isolated from N. aculeate and may be potential anti-neuroinflammatory candidates [52–55]. Another study conducted by Cui et al. [56] provide the first evidence that fucoidan isolated from Laminaria japonica has a potent inhibitory effect against LPS-induced NO production in BV2 cells. In their study, the average molecular weight of fucoidan was 7000 Dalton, consisting of 48% total sugar (including 28% fucose) and 29% sulfate. Fucoidan at a concentration of 125 μg/mL, significantly inhibited NO production to 75% [56]. NO is a cytotoxic, short lived highly diffusible signaling molecule [57]. A number of studies demonstrated that NO generated by iNOS causes injury and cell death of neuron and oligodendrocytes in the CNS, hence NO is implicated in pathogenesis of various neurodegenerative disease [57,58]. Anti-neuroinflammatory activity of another marine algae species, Ulva conglobata has been reported. U. conglobata methanolic extracts were able to suppress the expression of pro-inflammatory enzymes, iNOS and cyclooxygenase-2 (COX-2), which accounted for the large production of NO and PGE2, respectively [59,60]. Among other mediators released by microglia, NO and PGE2 are the main cytotoxic mediators participating in the innate response in the CNS [61,62]. Pro-inflammatory mediators have been found to be elevated in the brain of early AD [63]. For this reasons, agents that inhibit the production of pro-inflammatory mediators have been previously considered as potential candidates for the treatment of neurodegenerative diseases.
Epidemiological studies show that application of non-steroidal anti-inflammatory drugs (NSAIDs) reduces the risk and delays the onset of inflammation in the CNS which further participates in the pathogenesis of some neurodegenerative diseases. NSAIDs mainly act by inhibiting the production of pro-inflammatory mediators. Hence, attenuation of pro-inflammatory mediators in microglia by marine algae demonstrates its potential neuroprotective activity. Furthermore, marine algae as potential anti-neuroinflammatory agents have a great potential application in the pharmaceuticals area as well as the food industry. There are numerous advantages of marine algae use in pharmaceuticals and functional foods, such as relatively low production costs, low cytotoxicity, safety and wide acceptability. However, further studies are needed with clinical trials for marine algal anti-neuroinflammatory activity.
2.3. Cholinesterase Inhibitory Activity
Alzheimer’s diseases (AD) is an irreversible, progressive neurodegenerative disease, which resulting in memory loss, behavior disturbances, personality changes and a decline in cognitive abilities [64]. It was stated in the cholinergic hypothesis, that a serious loss of cholinergic function in the CNS contributes significantly to the cognitive symptoms associated with AD [65]. In accordance, neuropathological studies demonstrated that AD was associated with deficiency in the brain neurotransmitter acetylcholine (ACh) [66]. The inhibition of acetylcholinesterase (AChE) enzyme, which catalyzes the breakdown of ACh, may be one of the most realistic approaches to the symptomatic treatment of AD [67]. Presently, a variety of plants has been reported to possess AChE inhibitory activity. Huperzia serrata, a Chinese terrestrial herb has been demonstrated to be a potent AChE inhibitor [68]. In addition, Houghton et al. reported cholinesterase (ChE) inhibitory activity of Crinum jagus and Crinum glaucum, two Nigerian Crinum species [69]. A number of studies have recently shown AChE inhibitory activity of several marine algae species. A list of marine algae reported to have significant AChE inhibitory activity is presented in Table 1.

Table 1.
Acetylcholinesterase inhibitory activities of several marine algae.
Recently, Myung et al. reported that dieckol and phlorofucofluoroeckol possess memory enhancing and AChE inhibitory activity [74]. Furthermore, Yoon et al. screened ethanolic extracts of 27 Korean marine algae, for inhibitory activity on AChE, and found that extracts from Ecklonia stolonifera showed significant inhibitory activity [72]. Two sterols and eight phlorotannins were isolated from E. stolonifera. Eckol, dieckol, 2-phloroeckol and 7-phloroeckol demonstrated selective dose dependent inhibitory activities toward AChE; whereas, eckstolonol and phlorofucofuroeckol-A exhibited inhibitory activities toward both AChE and butyrylcholinesterase (BChE). However, phloroglucinol, which is a monomer, and triphlorethol-A, the opened-chain trimer of phloroglucinol, did not inhibit the cholinesterase (ChE) at the concentrations tested. The exact mechanisms underlying this phenomenon have not yet been identified. However, the possible relation between structure of phlorotannins and AChE inhibitory activity has been reported, it is suggested that phlorotannins as polymers of phloroglucinol have appropriately bulky structures, which is then able to mask the ChE and prevents the binding of the substrates. Moreover, as the phloroglucinol monomer and open-chain trimer of phloroglucinol were not able to inhibit the ChE activity, it may suggest that that degree of polymerization and closed-ring structure of phlorotannins play key roles in the inhibitory potential of phlorotannins toward the ChE [72]. In addition, Hypnea valentiae and Ulva reticulate, two marine algae species from Tamil Nadu, India, also reported to inhibit both AChE and BChE activity [71]. A good balance between AChE and BChE activity has been reported to result in higher efficacy for the treatment of AD [75]. BChE are considered to play a minor role in regulating brain AChE levels. Notably, AChE and BChE mixed inhibition have been found in tacrine and physostigmine, which are licensed drugs used in the treatment of AD.
Taken together, marine red, brown and green algae have potential to be use as functional neuroprotective agents due to their effectiveness in inhibiting ChE activity. Furthermore, some compounds derived from marine algae provided mixed type ChE (AChE and BChE) inhibitory activities, which have been considered to be more effective in the treatment of AD. Some AChE synthetic commercial drugs are known to produce side effects. Hence, researchers have a great interest to study natural herbs that can act as AChE inhibitors. Many kinds of marine algae, consumed for centuries in East Asia countries, are well tolerated and lack harmful side effects. Interestingly, several marine algae species have also been demonstrated as potential AChE inhibitors. Hence, AChE inhibitory activity of marine algae should be screened and further studies with clinical trials are also needed.
2.4. Inhibition of Neuronal Death
A common pathological hallmark of various neurodegenerative diseases is the loss of particular subsets of neurons [76]. Neurodegeneration of these neural subsets may be a consequence of various forms of neural cell death, including necrosis and apoptosis [77]. A study carried out by Jhamandas et al. successfully showed that fucoidan isolated from Fucus vesiculosus (Figure 1), was able to protect rat cholinergic neuronal death induced by Aβ1–42 [78]. Fucoidan pretreatment blocked the activation of caspase-9 and caspase-3. Caspase-9 and caspase-3 have been suggested to mediate the terminal stages of neuronal apoptosis [79]. Caspase-9 and caspase-3 are two of several central components of the machinery responsible for apoptosis. Therefore, the ability of fucoidan to block the activation of caspase-9 and caspase-3 suggest that inhibition of neuronal death by fucoidan mainly occurs through apoptotic inhibition. In neurodegenerative diseases, apoptosis might be pathogenic, and targeting this process might mitigate neurodegenerative diseases [80]. Furthermore, aqueous extracts of B. triquetrum has been demonstrated to protect GT1–7 cells death produced by severe (180 min) chemical hypoxia/aglycemia insult, which further reduced the cytotoxicity and early production of free radicals. The protection exerted by B. triquetrum extract seems to be linked to its ability to reduce free-radical generation [81]. The authors suggest that the protective effects of B. triquetrum extract are partially related to the presence of ferulic acid [81].
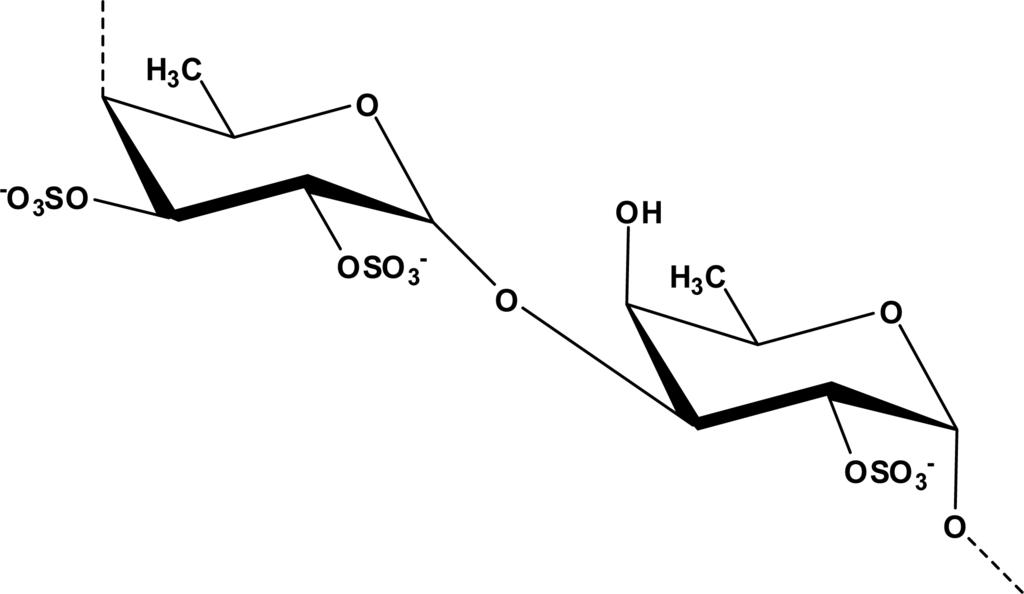
Figure 1.
Chemical structure of fucoidan isolated from Fucus vesiculosus (Adapted from [39]).
2.5. Antineurotoxicity
Neurotoxins are a varied groups of compounds, whose highly specific effects on the nervous system of animals, including humans, is by interfering with nerve impulse transmission [82]. They are able to produce neuronal damage or neurodegeration when administered in vivo or in vitro [83]. As an example, β-amyloid (Aβ) peptides have been demonstrated to possess neurotoxic effect on neuron and glial cells although the precise mechanisms by which this occurs have yet to be elucidated [84]. Excessive accumulation of Aβ in the brain has been characterized as a major pathological hallmark of AD and recently, fucoidan has been reported to block Aβ neurotoxicity in neuronal cell [78]. Fucoidan treatment abolished the inhibitory effect of Aβ on the phosphorylation of protein kinase C (PKC) which has been demonstrated to stimulate the survival of neurons and prevents Aβ neurotoxicity. PKC causes GSK-3β inactivation and this inactivation in turn leads to the accumulation of cytoplasmic β-catenin and the subsequent translocation of β-catenin to the nucleus, causing TCF/LEF-1-dependent transcriptional activation of growth and differentiation related genes, which is required to stimulate neuronal survival [85]. In addition, Luo et al. showed that fucoidan isolated from L. japonica was able to protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity in animal model of Parkinsonism (C57/BL mice) and dopaminergic (MN9D) cells [86]. The mechanisms of protection provided by fucoidan may partly relate to its antioxidative activity. Furthermore, the results of those studies suggest potential application of fucoidan for PD prevention and or treatment. Moreover, the possible roles of alginates to protects human neuronal (NT2) cells against H2O2-induced neurotoxicity have previously been demonstrated [87]. H. incrassata and B. triquetrum at a concentration of 0.2 mg/mL has been shown to protect methyl mercury-induced neurotoxicity in GT1–7 cells [29]. Collectively, marine algae and its bioactive compounds can be used for the development of new generation therapeutic neuroprotective agents against neurotoxins in the CNS.
2.6. Other Neuroprotective Activities
Neurite outgrowth is a fundamental neuronal feature and plays an important role in neuronal development during embryogenesis and in the adult brain [88]. Sargassum macrocarpum and its two active component, sargaquinoic acid and sargachromechanol, have been shown to promote neurite outgrowth in rat pheochromocytoma (PC12) cells [89–91]. Structure and neurite outgrowth promoting relationship of sargaquinoic acid has been reported by Tsang et al. [92]. They reported that quinone is the structural moiety of the sargaquinoic acid molecule which is responsible for the neurite outgrowth-promoting activity. Notably, the hydroxyl group bonded to quinone had a significant effect on neuritogenic activity. In addition, pheophytin a, a chlorophyll-related compound and its analog, vitamin B12 derived from Sargassum fulvellum also has potential neurite outgrowth-promoting activity [93,94].
Phlorotannins derived from Eisenia bicyclis have been demonstrated to inhibit β-amyloid cleavage enzyme (BACE-1) activity [95]. BACE-1 represents candidate biomarkers of AD, since it initiates the formation of Aβ [96]. When considering that almost all currently available medications for AD are AChE inhibitors, suppression of BACE-1 by phlorotannins will enhance the medications and or therapy for AD patients.
In addition, Lee et al. demonstrated that fucoidan treatment resulted in an increase in cell proliferation of human neuroblastoma (SH-SY5Y) cell induced by Aβ [97]. Hence, it may suggest that fucoidan has potential neuroprotective effects.
3. Prospects of Marine Algae as Neuroprotective Agents
Neurodegenerative diseases are estimated to surpass cancer as the second most common cause of death among elderly by the 2040s [98,99]. For this reason, a great deal of attention has been expressed by scientists regarding safe and effective neuroprotective agents. Many categories of natural and synthetic neuroprotective agents have been reported. However, synthetic neuroprotective agents are believed to have certain side effects such as dry mouth, tiredness, drowsiness, sleepiness, anxiety or nervousness, difficulty to balance, etc. [100]. Hence, nowadays researchers have an interest in studying natural bioactive compounds that can act as neuroprotective agents. Marine algae represent one potential candidate neuroprotective agent. However, development of marine algae as neuroprotective agents still faces several challenges. The rationale for marine algal neuroprotective effects treatment in the CNS is based on established observations and experiments in vitro or in animal models only. Up to now, none of the marine algal neuroprotective effects have been examined in human subjects. Therefore, small clinical studies and further large-scale controlled studies are needed. Another important challenge in the development of marine algae as neuroprotective agents is that many drugs failed to provide real neuroprotection in practice. Potential reasons for this failure include inappropriate use of specific neuroprotection/s for a given disease or stage of disease progression or the use of suboptimal doses [101]. Hence, future studies are needed focusing on the synergistic benefits of consuming different marine algae species, recommended doses and timing of intake, and preparation methods for marine algal bioactive compounds in order to maximize the desired protective effect in the prevention of neurodegenerative diseases.
It has been reported that neurodegenerative diseases in East Asian countries were lower than in Europe (p < 0.0004) [102,103]. Many studies have indicated potential health benefits of marine algae consumption [7,104]. Thus, lower incidence of neurodegenerative diseases in East Asia may correlate to high fish and marine algae consumption by East Asian populations. More recently, there has been growing interest in marine algae and their constituents as functional foods and nutraceuticals with potential health benefit effects as sources of antioxidant to reduce the risk of neurodegenerative diseases. Marine algae are an important source of bioactive ingredients that can be applied to many aspects of processing healthier foods and developing functional neuroprotective foods.
In addition, the wide diversity of marine algae and numerous undiscovered unique metabolites present in marine algae are interesting sources to increase numbers of novel drugs against neurodegenerative diseases. However, large-scale human studies are required to identify the prophylactic and therapeutic neuroprotective effect of marine algae.
4. Conclusions
In conclusion, marine algae are a valuable source of neuroprotective agents and could be introduced for the preparation of novel functional ingredients in pharmaceuticals and functional foods as a good approach for the treatment and or prevention of neurodegenerative disease. Marine algae can be suggested as an alternative source to synthetic ingredients that can contribute to neuroprotection by being a part of pharmaceuticals and functional foods. Furthermore, the wide range of biological activities associated with natural compounds derived from marine algae such as phlorotannins, alginates, fucoidan, sargaquinoic acid, SPs and carotenoids increase the potential to expand the neuroprotective effects and health beneficial value of marine algae in the pharmaceutical industry. Until now, most of the biological and neuroprotective activities of marine algae and its natural compounds have been observed in vitro or in mouse model systems. Therefore, further research studies are needed in order to investigate marine algae neuroprotective activities in human subjects and further in large-scale controlled studies.
Acknowledgments
This study was supported by a grant from the Marine Bioprocess Research Center of the Marine Bio 21 Project funded by the Ministry of Land, Transport and Maritime, Republic of Korea.
- Samples Availability: Available from the authors.
References
- Kim, S; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J Funct Foods 2010, 2, 1–9. [Google Scholar]
- Swing, J. What future for the oceans. Foreign Aff 2003, 82, 139–152. [Google Scholar]
- Alonso, D; Castro, A; Martinez, A. Marine compounds for the therapeutic treatment of neurological disorders. Expert Opin Ther Patents 2005, 15, 1377–1386. [Google Scholar]
- Heo, SJ; Hwang, JY; Choi, JI; Han, JS; Kim, HJ; Jeon, YJ. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent [alpha]-glucosidase and [alpha]-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur J Pharmacol 2009, 615, 252–256. [Google Scholar]
- Pangestuti, R; dan Limantara, L. Rumput Laut, Zamrud Tak Tergali Dari Laut. BioS 2010, 2, 2–10. [Google Scholar]
- Khan, S; Kong, C; Kim, J; Kim, S. Protective effect of Amphiroa dilatata on ROS induced oxidative damage and MMP expressions in HT1080 cells. Biotech Bioproc Eng 2010, 15, 191–198. [Google Scholar]
- Burtin, P. Nutritional value of seaweeds. EJEAFChe 2003, 2, 498–503. [Google Scholar]
- Matsubara, K; Matsuura, Y; Hori, K; Miyazawa, K. An anticoagulant proteoglycan from the marine green alga, Codium pugniformis. J Appl Phycol 2000, 12, 9–14. [Google Scholar]
- Athukorala, Y; Lee, K; Kim, S; Jeon, Y. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour Technol 2007, 98, 1711–1716. [Google Scholar]
- Artan, M; Li, Y; Karadeniz, F; Lee, S; Kim, M; Kim, S. Anti-HIV-1 activity of phloroglucinol derivative, 6, 6′-bieckol, from Ecklonia cava. Bioorgan Med Chem 2008, 16, 7921–7926. [Google Scholar]
- Huheihel, M; Ishanu, V; Tal, J; Arad, S. Activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J Biochem Biophys Meth 2002, 50, 189–200. [Google Scholar]
- Heo, SJ; Park, EJ; Lee, KW; Jeon, YJ. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour Technol 2005, 96, 1613–1623. [Google Scholar]
- Park, P; Heo, S; Park, E; Kim, S; Byun, H; Jeon, B; Jeon, Y. Reactive oxygen scavenging effect of enzymatic extracts from Sargassum thunbergii. J Agr Food Chem 2005, 53, 6666–6672. [Google Scholar]
- Zou, Y; Qian, Z; Li, Y; Kim, M; Lee, S; Kim, S. Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J Agr Food Chem 2008, 56, 7001–7009. [Google Scholar]
- Li, Y; Lee, S; Le, Q; Kim, M; Kim, S. Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and Fc RI. J Agr Food Chem 2008, 56, 12073–12080. [Google Scholar]
- Kong, CS; Kim, JA; Yoon, NY; Kim, SK. Induction of apoptosis by phloroglucinol derivative from Ecklonia cava in MCF-7 human breast cancer cells. Food Chem Toxicol 2009, 47, 1653–1658. [Google Scholar]
- Kim, M; Rajapakse, N; Kim, S. Anti inflammatory effect of Ishige okamurae ethanolic extract via inhibition of NF B transcription factor in RAW 264.7 cells. Phytother Res 2009, 23, 628–634. [Google Scholar]
- Maeda, H; Hosokawa, M; Sashima, T; Miyashita, K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay Mice. J Agr Food Chem 2007, 55, 7701–7706. [Google Scholar]
- Tsukui, T; Konno, K; Hosokawa, M; Maeda, H; Sashima, T; Miyashita, K. Fucoxanthin and fucoxanthinol enhance the amount of docosahexaenoic acid in the liver of KKAy obese/diabetic mice. J Agr Food Chem 2007, 55, 5025–5029. [Google Scholar]
- Kong, C; Kim, J; Ahn, B; Vo, T; Yoon, N; Kim, S. 1-(3,5-Dihydroxyphenoxy)-7-(2,4,6-trihydroxyphenoxy)-2,4,9-trihydroxydibenzo-1,4-dioxin inhibits adipocyte differentiation of 3T3-L1 fibroblasts. Mar Biotechnol 2010, 12, 299–307. [Google Scholar]
- Zarros, A. In which cases is neuroprotection useful. Adv Altern Think Neurosci 2009, 1, 3–5. [Google Scholar]
- Barnham, KJ; Masters, CL; Bush, AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov 2004, 3, 205–214. [Google Scholar]
- Akyol, Ö; Herken, H; Uz, E; FadIllIolu, E; Ünal, S; Söüt, S; Özyurt, H; Sava, H. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients* 1:: The possible role of oxidant/antioxidant imbalance. Progr Neuro-Psychopharmacol Biol Psychiatr 2002, 26, 995–1005. [Google Scholar]
- Migliore, L; Coppedè, F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat Res-Gen Tox En 2009, 674, 73–84. [Google Scholar]
- Behl, C; Moosmann, B. Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Rad Biol Med 2002, 33, 182–191. [Google Scholar]
- Andersen, J. Oxidative stress in neurodegeneration: Cause or consequence. Nat Rev Neurosci 2004, 5, S18–S25. [Google Scholar]
- Moosmann, B; Behl, C. Antioxidants as treatment for neurodegenerative disorders. Expert Opin Investig Drugs 2002, 11, 1407–1435. [Google Scholar]
- Lim, C; Jin, D; Sung, J; Lee, J; Choi, H; Ha, I; Han, J. Antioxidant and anti-inflammatory activities of the methanolic extract of Neorhodomela aculeate in hippocampal and microglial cells. Biol Pharm Bull 2006, 29, 1212–1216. [Google Scholar]
- Fallarero, A; Loikkanen, JJ; Männistö, PT; Castañeda, O; Vidal, A. Effects of aqueous extracts of Halimeda incrassata (Ellis) Lamouroux and Bryothamnion triquetrum (S.G.Gmelim) Howe on hydrogen peroxide and methyl mercury-induced oxidative stress in GT1–7 mouse hypothalamic immortalized cells. Phytomedicine 2003, 10, 39–47. [Google Scholar]
- Vidal Novoa, A; Motidome, M; Mancini Filho, J; Fallarero Linares, A; Tanae, M; Torres, L; Lapa, A. Actividad antioxidante y ácidos fenólicos del alga marina Bryothamnion triquetrum (SG Gmelim) Howe; Antioxidant activity related to phenolic acids in the aqueous extract of the marine seaweed Bryothamnin triquetrum (SG Gmelim) Howe. Rev Bras Ciênc Farm(Impr) 2001, 37, 373–382. [Google Scholar]
- Jung, W; Heo, S; Jeon, Y; Lee, C; Park, Y; Byun, H; Choi, Y; Park, S; Choi, I. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. J Agr Food Chem 2009, 57, 4439–4446. [Google Scholar]
- Wijesekara, I; Yoon, N; Kim, S. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. BioFactors 2010, 306, 408–414. [Google Scholar]
- Yan, X; Chuda, Y; Suzuki, M; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci Biotech Biochem 1999, 63, 605–607. [Google Scholar]
- Nomura, T; Kikuchi, M; Kubodera, A; Kawakami, Y. Proton-donative antioxidant activity of fucoxanthin with 1, 1-diphenyl-2-picrylhydrazyl (DPPH). IUBMB Life 1997, 42, 361–370. [Google Scholar]
- Young, AJ; Lowe, GM. Antioxidant and prooxidant properties of carotenoids. Arch Biochem Biophys 2001, 385, 20–27. [Google Scholar]
- Sangeetha, R; Bhaskar, N; Baskaran, V. Comparative effects of β-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol Cell Biochem 2009, 331, 59–67. [Google Scholar]
- Ravi Kumar, S; Narayan, B; Vallikannan, B. Fucoxanthin restrains oxidative stress induced by retinol deficiency through modulation of Na+ Ka+-ATPase and antioxidant enzyme activities in rats. Eur J Nutr 2008, 47, 432–441. [Google Scholar]
- Heo, S; Ko, S; Kang, S; Kang, H; Kim, J; Kim, S; Lee, K; Cho, M; Jeon, Y. Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2-induced cell damage. Eur Food Res Tech A 2008, 228, 145–151. [Google Scholar]
- Wijesekara, I; Pangestuti, R; Kim, S. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohyd Polym 2010, 84, 14–21. [Google Scholar]
- Jiao, G; Yu, G; Zhang, J; Ewart, H. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs 2011, 9, 196–223. [Google Scholar]
- Qi, H; Zhang, Q; Zhao, T; Chen, R; Zhang, H; Niu, X; Li, Z. Antioxidant activity of different sulfate content derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta) in vitro. Int J Biol Macromol 2005, 37, 195–199. [Google Scholar]
- Zhang, Q; Li, N; Zhou, G; Lu, X; Xu, Z; Li, Z. In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodephyta) in aging mice. Pharmacol Res 2003, 48, 151–155. [Google Scholar]
- Allen, N; Barres, B. Neuroscience: Glia—More than just brain glue. Nature 2009, 457, 675–677. [Google Scholar]
- Liu, BIN; Gao, HM; Wang, JY; Jeohn, GH; Cooper, CL; Hong, JS. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci 2002, 962, 318–331. [Google Scholar]
- Block, M; Zecca, L; Hong, J. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat Rev Neurosci 2007, 8, 57–69. [Google Scholar]
- Kim, S; de Vellis, J. Microglia in health and disease. J Neurosci Res 2005, 81, 302–313. [Google Scholar]
- Lull, ME; Block, ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [Green Version]
- Abad, M; Bedoya, L; Bermejo, P. Natural marine anti-inflammatory products. Mini Rev Med Chem 2008, 8, 740–754. [Google Scholar]
- Maegawa, M; Yokohama, Y; Aruga, Y. Critical light conditions for young Ecklonia cava and Eisenia bicyclis with reference to photosynthesis. Hydrobiologia 1987, 151, 447–455. [Google Scholar]
- Serisawa, Y; Yokohama, Y; Aruga, Y; Tanaka, J. Photosynthesis and respiration in bladelets of Ecklonia cava Kjellman (Laminariales, Phaeophyta) in two localities with different temperature conditions. Phycol Res 2001, 49, 1–11. [Google Scholar]
- Jung, WK; Ahn, YW; Lee, SH; Choi, YH; Kim, SK; Yea, SS; Choi, I; Park, SG; Seo, SK; Lee, SW; Choi, IW. Ecklonia cava ethanolic extracts inhibit lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in BV2 microglia via the MAP kinase and NF-[kappa]B pathways. Food Chem Toxicol 2009, 47, 410–417. [Google Scholar]
- Zhao, J; Fan, X; Wang, S; Li, S; Shang, S; Yang, Y; Xu, N; Lü, Y; Shi, J. Bromophenol derivatives from the red alga Rhodomela confervoides. J Nat Prod 2004, 67, 1032–1035. [Google Scholar]
- Xu, N; Fan, X; Yan, X; Li, X; Niu, R; Tseng, CK. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry 2003, 62, 1221–1224. [Google Scholar]
- Fan, X; Xu, NJ; Shi, JG. Bromophenols from the red alga Rhodomela confervoides. J Nat Prod 2003, 66, 455–458. [Google Scholar]
- Ma, M; Zhao, J; Wang, S; Li, S; Yang, Y; Shi, J; Fan, X; He, L. Bromophenols coupled with methyl γ-ureidobutyrate and bromophenol sulfates from the red alga Rhodomela confervoides. J Nat Prod 2006, 69, 206–210. [Google Scholar]
- Cui, Y; Zhang, L; Zhang, T; Luo, D; Jia, Y; Guo, Z; Zhang, Q; Wang, X; Wang, XM. Inhibitory effect of fucoidan on nitric oxide production in lipopolysaccharide activated primary microglia. Clin Exp Pharmacol Physiol 2010, 37, 422–428. [Google Scholar]
- Heales, S; Bolaños, J; Stewart, V; Brookes, P; Land, J; Clark, J. Nitric oxide, mitochondria and neurological disease. Biochim Biophys Acta 1999, 1410, 215–228. [Google Scholar]
- Lee, J; Grabb, M; Zipfel, G; Choi, D. Brain tissue responses to ischemia. J Clin Invest 2000, 106, 723–731. [Google Scholar]
- Jin, D; Lim, C; Sung, J; Choi, H; Ha, I; Han, J. Ulva conglobata, a marine algae, has neuroprotective and anti-inflammatory effects in murine hippocampal and microglial cells. Neurosci Lett 2006, 402, 154–158. [Google Scholar]
- Salvemini, D; Manning, P; Zweifel, B; Seibert, K; Connor, J; Currie, M; Needleman, P; Masferrer, J. Dual inhibition of nitric oxide and prostaglandin production contributes to the antiinflammatory properties of nitric oxide synthase inhibitors. J Clin Invest 1995, 96, 301–308. [Google Scholar]
- Vane, J; Botting, R. New insights into the mode of action of anti-inflammatory drugs. Inflamm Res 1995, 44, 1–10. [Google Scholar]
- Boscá, L; Zeini, M; Través, P; Hortelano, S. Nitric oxide and cell viability in inflammatory cells: A role for NO in macrophage function and fate. Toxicology 2005, 208, 249–258. [Google Scholar]
- Blasko, I; Stampfer-Kountchev, M; Robatscher, P; Veerhuis, R; Eikelenboom, P; Grubeck-Loebenstein, B. How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: The role of microglia and astrocytes. Aging Cell 2004, 3, 169–176. [Google Scholar]
- Pietrini, P; Alexander, G; Furey, M; Hampel, H; Guazzelli, M. The neurometabolic landscape of cognitive decline: In vivo studies with positron emission tomography in Alzheimer’s disease. Int J Psychophysiol 2000, 37, 87–98. [Google Scholar]
- Bartus, RT. On neurodegenerative diseases, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol 2000, 163, 495–529. [Google Scholar]
- Tabet, N. Acetylcholinesterase inhibitors for Alzheimer’s disease: Anti-inflammatories in acetylcholine clothing! Age Ageing 2006, 35, 336–338. [Google Scholar]
- Pangestuti, R; Kim, SK. Neuroprotective properties of chitosan and its derivatives. Mar Drugs 2010, 8, 2117–2128. [Google Scholar]
- Cheng, DH; Ren, H; Tang, XC. Huperzine A, a novel promising acetylcholinesterase inhibitor. Neuroreport 1996, 8, 97–101. [Google Scholar]
- Houghton, PJ; Agbedahunsi, JM; Adegbulugbe, A. Choline esterase inhibitory properties of alkaloids from two Nigerian Crinum species. Phytochemistry 2004, 65, 2893–2896. [Google Scholar]
- Stirk, W; Reinecke, D; van Staden, J. Seasonal variation in antifungal, antibacterial and acetylcholinesterase activity in seven South African seaweeds. J Appl Phycol 2007, 19, 271–276. [Google Scholar]
- Yoon, N; Chung, H; Kim, H; Choi, J. Acetyl and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from. Ecklonia stolonifera Fish Sci 2008, 74, 200–207. [Google Scholar]
- Yoon, NY; Lee, SH; Yong, L; Kim, SK. Phlorotannins from Ishige okamurae and their acetyl- and butyrylcholinesterase inhibitory effects. J Funct Foods 2009, 1, 331–335. [Google Scholar]
- Suganthy, N; Karutha Pandian, S; Pandima Devi, K. Neuroprotective effect of seaweeds inhabiting South Indian coastal area (Hare Island, Gulf of Mannar marine biosphere reserve): Cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci Lett 2010, 468, 216–219. [Google Scholar]
- Myung, C; Shin, H; Bao, H; Yeo, S; Lee, B; Kang, J. Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: Possible involvement of the inhibition of acetylcholinesterase. Arch Pharm Res 2005, 28, 691–698. [Google Scholar]
- Greig, N; Lahiri, D; Sambamurti, K. Butyrylcholinesterase: An important new target in Alzheimer’s disease therapy. Int Psychogeriatr 2002, 14, 77–91. [Google Scholar]
- Mattson, MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 2000, 1, 120–130. [Google Scholar]
- Bains, JS; Shaw, CA. Neurodegenerative disorders in humans: The role of glutathione in oxidative stress-mediated neuronal death. Brain Res Rev 1997, 25, 335–358. [Google Scholar]
- Jhamandas, JH; Wie, MB; Harris, K; MacTavish, D; Kar, S. Fucoidan inhibits cellular and neurotoxic effects of β-amyloid (Aβ) in rat cholinergic basal forebrain neurons. Eur J Neurosci 2005, 21, 2649–2659. [Google Scholar]
- Cowan, CM; Thai, J; Krajewski, S; Reed, JC; Nicholson, DW; Kaufmann, SH; Roskams, AJ. Caspases 3 and 9 send a pro-apoptotic signal from synapse to cell body in olfactory receptor neurons. J Neurosci 2001, 21, 7099–7109. [Google Scholar]
- Vila, M; Przedborski, S. Targeting programmed cell death in neurodegenerative diseases. Nat Rev Neurosci 2003, 4, 365–375. [Google Scholar]
- Fallarero, A; Peltoketo, A; Loikkanen, J; Tammela, P; Vidal, A; Vuorela, P. Effects of the aqueous extract of Bryothamnion triquetrum on chemical hypoxia and aglycemia-induced damage in GT1–7 mouse hypothalamic immortalized cells. Phytomedicine 2006, 13, 240–245. [Google Scholar]
- Patockaa, J; Stredab, L. Brief review of natural nonprotein neurotoxins. ASA Newslett 2002, 89, 16–24. [Google Scholar]
- Segura-Aguilar, J; Kostrzewa, R. Neurotoxins and neurotoxic species implicated in neurodegeneration. Neurotox Res 2004, 6, 615–630. [Google Scholar]
- Butterfield, DA. Amyloid β-peptide (1–42)-induced oxidative stress and neurotoxicity: Implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Rad Res 2002, 36, 1307–1313. [Google Scholar]
- Garrido, J; Godoy, J; Alvarez, A; Bronfman, M; Inestrosa, N. Protein kinase C inhibits amyloid {beta} peptide neurotoxicity by acting on members of the Wnt pathway. FASEB J 2002, 16, 1982–1984. [Google Scholar]
- Luo, D; Zhang, Q; Wang, H; Cui, Y; Sun, Z; Yang, J; Zheng, Y; Jia, J; Yu, F; Wang, X. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur J Pharmacol 2009, 617, 33–40. [Google Scholar]
- Eftekharzadeh, B; Khodagholi, F; Abdi, A; Maghsoudi, N. Alginate protects NT2 neurons against H2O2-induced neurotoxicity. Carbohyd Polym 2010, 79, 1063–1072. [Google Scholar]
- Khodosevich, K; Monyer, H. Signaling involved in neurite outgrowth of postnatally born subventricular zone neurons in vitro. BMC Neurosci 2010, 11, 18:1–18:11. [Google Scholar]
- Tsang, C; Ina, A; Goto, T; Kamei, Y. Sargachromenol, a novel nerve growth factor-potentiating substance isolated from Sargassum macrocarpum, promotes neurite outgrowth and survival via distinct signaling pathways in PC12D cells. Neuroscience 2005, 132, 633–643. [Google Scholar]
- Tsang, C; Kamei, Y. Sargaquinoic acid supports the survival of neuronal PC12D cells in a nerve growth factor-independent manner. Eur J Pharmacol 2004, 488, 11–18. [Google Scholar]
- Kamei, Y; Sagara, A. Neurite outgrowth promoting activity of marine algae from Japan against rat adrenal medulla pheochromocytoma cell line, PC12D. Cytotechnology 2002, 40, 99–106. [Google Scholar]
- Tsang, C; Sagara, A; Kamei, Y. Structure-activity relationship of a neurite outgrowth-promoting substance purified from the brown alga, Sargassum macrocarpum, and its analogues on PC12D cells. J Appl Phycol 2001, 13, 349–357. [Google Scholar]
- Ina, A; Hayashi, K; Nozaki, H; Kamei, Y. Pheophytin a, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int J Dev Neurosci 2007, 25, 63–68. [Google Scholar]
- Ina, A; Kamei, Y. Vitamin B 12, a chlorophyll-related analog to pheophytin a from marine brown algae, promotes neurite outgrowth and stimulates differentiation in PC12 cells. Cytotechnology 2006, 52, 181–187. [Google Scholar]
- Jung, H; Oh, S; Choi, J. Molecular docking studies of phlorotannins from Eisenia bicyclis with BACE1 inhibitory activity. Bioorgan Med Chem Lett 2010, 20, 3211–3215. [Google Scholar]
- Tang, K; Hynan, L; Baskin, F; Rosenberg, R. Platelet amyloid precursor protein processing: A bio-marker for Alzheimer’s disease. J Neurol Sci 2006, 240, 53–58. [Google Scholar]
- Lee, HR; Do, H; Lee, SR; Sohn, ES; Pyo, S; Son, E. Effects of fucoidan on neuronal cell proliferation-association with NO production through the iNOS pathway. J Food Sci Nutr 2007, 12, 74–78. [Google Scholar]
- Bjarkam, CR; Sørensen, JC; Sunde, NÅ; Geneser, FA; Østergaard, K. New strategies for the treatment of Parkinson’s disease hold considerable promise for the future management of neurodegenerative disorders. Biogerontology 2001, 2, 193–207. [Google Scholar]
- Ansari, J; Siraj, A; Inamdar, N. Pharmacotherapeutic approaches of Parkinson’s disease. Int J Pharmacol 2010, 6, 584–590. [Google Scholar]
- Narang, S; Gibson, D; Wasan, AD; Ross, EL; Michna, E; Nedeljkovic, SS; Jamison, RN. Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J Pain 2008, 9, 254–264. [Google Scholar]
- Gilgun-Sherki, Y; Melamed, E; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975. [Google Scholar]
- Mishra, S; Palanivelu, K. The effect of curcumin (turmeric) on Alzheimer’s disease: An overview. Ann Indian Acad Neurol 2008, 11, 13–19. [Google Scholar]
- Jorm, AF; Jolley, D. The incidence of dementia: A meta-analysis. Neurology 1998, 51, 728–733. [Google Scholar]
- Smit, AJ. Medicinal and pharmaceutical uses of seaweed natural products: A review. J Appl Phycol 2004, 16, 245–262. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).