Polyhydroxylated Steroids from the Bamboo Coral Isis hippuris
Abstract
:1. Introduction
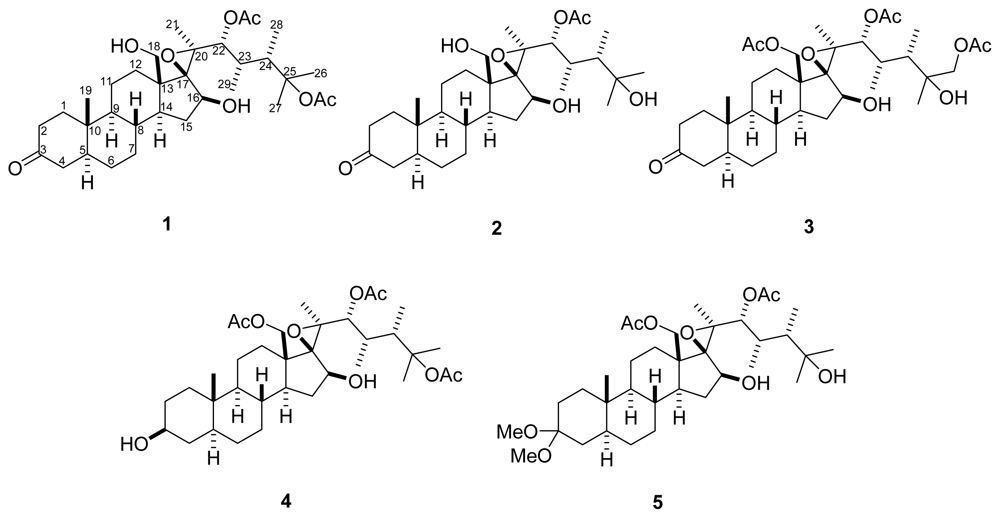
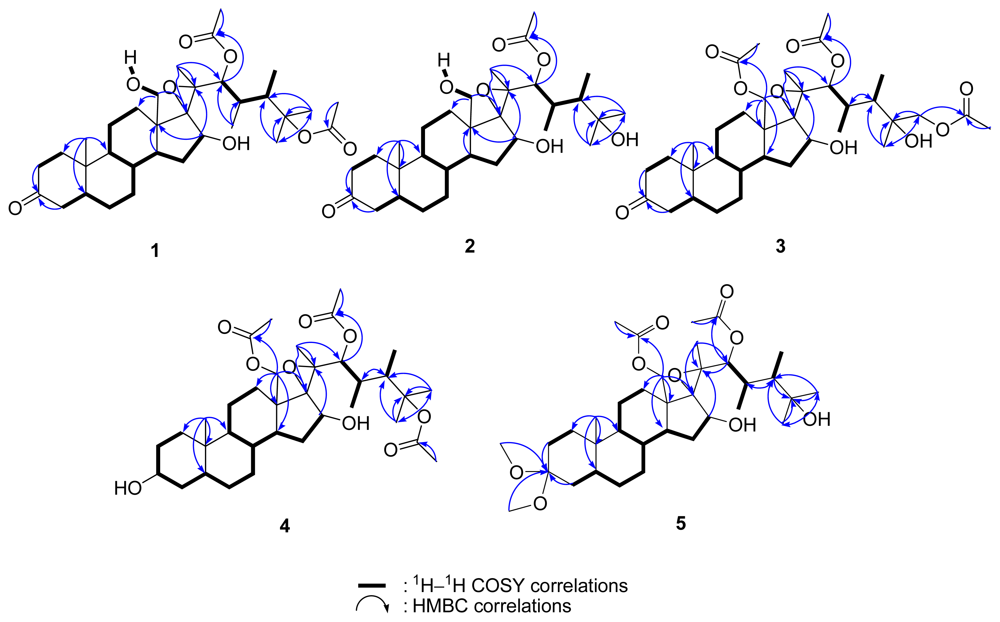
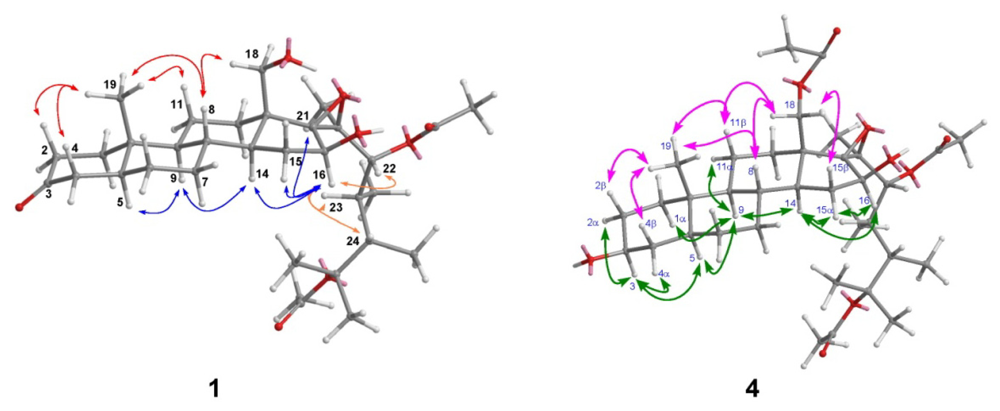
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
3.4. Cytotoxicity Assay
3.5. Anti-HCMV Assay
Acknowledgments
- Samples Availability: Not available.
References
- Kazlauskas, R; Murphy, PT; Quinn, RJ; Wells, RJ; Schonholzer, P. An unusual steroid from gorgonian Isis hippuris. Tetrahedron Lett 1977, 50, 4439–4442. [Google Scholar]
- Higa, T; Tanaka, J; Tsukitani, Y; Kikuchi, H. Hippuristanols, cytotoxic polyoxygenated steroids from the gorgonian Isis hippuris. Chem. Lett 1981, 11, 1647–1650. [Google Scholar]
- Gonzalez, N; Barral, MA; Rodriguez, J; Jimenez, C. New cytotoxic steroids from the gorgonian Isis hippuris. Tetrahedron 2001, 57, 3487–3497. [Google Scholar]
- Rao, CB; Ramana, KV; Rao, DV; Fahy, E; Faulkner, DJ. Metabolites of the gorgonian Isis hippuris from India. J. Nat. Prod 1988, 51, 954–958. [Google Scholar]
- Shen, YC; Prakash, CVS; Chang, YT; Hung, MC; Chen, SJ; Chen, HJ; Hsu, MC. Bioactive steroids from the Formosan gorgonian Isis hippuris. Chin. Pharm. J 2000, 52, 341–351. [Google Scholar]
- Sheu, JH; Chao, CH; Wang, GH; Hung, KC; Duh, CY; Chiang, MY; Wu, YC; Wu, CC. The first A-nor-hippuristanol and two novel 4,5-secosuberosanoids from the gorgonian Isis hippuris. Tetrahedron Lett 2004, 45, 6413–6416. [Google Scholar]
- Chao, CH; Huang, LF; Yang, YL; Su, JH; Wang, GH; Chiang, MY; Wu, YC; Dai, CF; Sheu, JH. Polyoxygenated steroids from the gorgonian Isis hippuris. J. Nat. Prod 2005, 68, 880–885. [Google Scholar]
- Qi, SH; Miao, L; Gao, CH; Xu, Y; Zhang, S; Qian, PY. New steroids and a new alkaloid from the gorgonian Isis minorbrachyblasta: structures, cytotoxicity, and antilarval activity. Helv. Chim. Acta 2010, 93, 511–516. [Google Scholar]
- Higa, T; Tanaka, J; Tachibana, K. 18-Oxygenated polyfunctional steroids from the horgonian Isis hippuris. Tetrahedron Lett 1981, 22, 2777–2780. [Google Scholar]
- Tanaka, J; Trianto, A; Musman, M; Issa, HH; Ohtani, II; Ichiba, T; Higa, T; Yoshida, WY; Scheuer, PJ. New polyoxygenated steroids exhibiting reversal of multidrug resistance from the gorgonian Isis hippuris. Tetrahedron 2002, 58, 6259–6266. [Google Scholar]
- Tanaka, J; Higa, T; Tachibana, K; Iwashita, T. Gorgost-5-ene-3β,7α,11α,12β-tetraol 12-monoacetate, a new marine sterol from the gorgonian Isis hippuris. Chem Lett 1982, 1295–1296. [Google Scholar]
- Shen, YC; Prakash, CVS; Chang, YT. Two new polyhydroxysteroids from the gorgonian Isis hippuris. Steroids 2001, 66, 721–725. [Google Scholar]
- Uddin, MH; Hanif, N; Trianto, A; Agarie, Y; Higa, T; Tanaka, J. Four new polyoxygenated gorgosterols from the gorgonian Isis hippuris. Nat. Prod. Res 2011, 25, 585–591. [Google Scholar]
- Chao, CH; Huang, LF; Wu, SL; Su, JH; Huang, HC; Sheu, JH. Steroids from the gorgonian Isis hippuris. J. Nat. Prod 2005, 68, 1366–1370. [Google Scholar]
- Sheu, JH; Chen, SP; Sung, PJ; Chiang, MY; Dai, CF. Hippuristerone A, a novel polyoxygenated steroid from the gorgonian Isis hippuris. Tetrahedron Lett 2000, 41, 7885–7888. [Google Scholar]
- Sheu, JH; Huang, LF; Chen, SP; Yang, YL; Sung, PJ; Wang, GH; Su, JH; Chao, CH; Hu, WP; Wang, JJ. Hippuristerones E–I, new polyoxygenated steroids from the gorgonian coral Isis hippuris. J. Nat. Prod 2003, 66, 917–921. [Google Scholar]
- Bordeleau, ME; Mori, A; Oberer, M; Lindqvist, L; Chard, LS; Higa, T; Belsham, GJ; Wagner, G; Tanaka, J; Pelletier, J. Functional characterization of IRESes by an inhibitor of the RNA helicase eIF4A. Nat. Chem. Biol 2006, 2, 213–220. [Google Scholar]
- Lindqvist, L; Oberer, M; Reibarkh, M; Cencic, R; Bordeleau, ME; Vogt, E; Marintchev, A; Tanaka, J; Fagotto, F; Altmann, M; et al. Selective pharmacological targeting of a DEAD Box RNA helicase. PLoS One 2008, 3, e1583. [Google Scholar]
- Geran, RI; Greenberg, NH; MacDonald, MM; Schumacher, AM; Abbott, BJ. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep 1972, 3, 1–91. [Google Scholar]
- Hou, RS; Duh, CY; Chiang, MY; Lin, CN. Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa. J. Nat. Prod 1995, 58, 1126–1130. [Google Scholar]
- Stevens, M; Balzarini, J; Tabarrini, O; Andrei, G; Snoeck, R; Cecchetti, V; Fravolini, A; De Clercq, E; Pannecouque, C. Cell-dependent interference of a series of new 6-aminoquinolone derivatives with viral (HIV/CMV) transactivation. J. Antimicrob. Chemother 2005, 56, 847–855. [Google Scholar]

| C# | 1, a δC, type | 2, a δC, type | 3, a δC, type | 4, b δC, type | 5, c δC, type |
|---|---|---|---|---|---|
| 1 | 38.3, CH2 | 38.3, CH2 | 38.2, CH2 | 36.7, CH2 | 35.6, CH2 |
| 2 | 38.1, CH2 | 38.1, CH2 | 38.0, CH2 | 31.4, CH2 | 29.2, CH2 |
| 3 | 211.7, qC | 211.7, qC | 211.5, qC | 71.2, CH | 100.7, qC |
| 4 | 44.5, CH2 | 44.5, CH2 | 44.5, CH2 | 38.0, CH2 | 36.2, CH2 |
| 5 | 46.5, CH | 46.5, CH | 46.4, CH | 44.7, CH | 43.0, CH |
| 6 | 28.6, CH2 | 28.5, CH2 | 28.5, CH2 | 28.3, CH2 | 28.8, CH2 |
| 7 | 31.7, CH2 | 31.7, CH2 | 31.5, CH2 | 31.9, CH2 | 32.7, CH2 |
| 8 | 34.5, CH | 34.4, CH | 34.4, CH | 34.5, CH | 35.1, CH |
| 9 | 53.1, CH | 53.6, CH | 53.4, CH | 54.0, CH | 55.1, CH |
| 10 | 35.7, qC | 35.7, qC | 35.6, CH | 35.5, qC | 36.4, qC |
| 11 | 21.0, CH2 | 21.0, CH2 | 21.5, CH2 | 21.4, CH2 | 22.0, CH2 |
| 12 | 30.6, CH2 | 31.0, CH2 | 32.4, CH2 | 32.2, CH2 | 33.3, CH2 |
| 13 | 46.8, qC | 46.7, qC | 45.6, qC | 45.6, qC | 46.5, qC |
| 14 | 47.7, CH | 48.7, CH | 49.2, CH | 48.7, CH | 50.3, CH |
| 15 | 33.3, CH2 | 33.3, CH2 | 33.5, CH2 | 33.4, CH2 | 34.4, CH2 |
| 16 | 70.0, CH | 70.1, CH | 70.3, CH | 70.1, CH | 71.1, CH |
| 17 | 80.0, qC | 79.7, qC | 77.2, qC | 77.7,qC | 78.6, qC |
| 18 | 61.9, CH2 | 61.9, CH2 | 63.5, CH2 | 63.5, CH2 | 64.3, CH2 |
| 19 | 11.3, CH3 | 11.4, CH3 | 11.4, CH3 | 12.2, CH3 | 12.0, CH3 |
| 20 | 67.1, qC | 67.5, qC | 66.7, qC | 66.4, qC | 67.3, qC |
| 21 | 16.1, CH3 | 15.9, CH3 | 16.1, CH3 | 16.2, CH3 | 17.1, CH3 |
| 22 | 77.2, CH | 77.2, CH | 77.2, CH | 77.2, CH | 78.1, CH |
| 23 | 33.5, CH | 32.9, CH | 32.5, CH | 33.6, CH | 33.6, CH |
| 24 | 39.9, CH | 41.7, CH | 38.8, CH | 40.1, CH | 42.2, CH |
| 25 | 85.5, qC | 73.7, qC | 74.2, qC | 85.6, qC | 73.5, qC |
| 26 | 23.2, CH3 | 30.9, CH3 | 71.0, CH2 | 22.8, CH3 | 31.2, CH3 |
| 27 | 25.1, CH3 | 25.8, CH3 | 20.3, CH3 | 25.1, CH3 | 25.9, CH3 |
| 28 | 10.4, CH3 | 11.4, CH3 | 10.9, CH3 | 10.5, CH3 | 11.7, CH3 |
| 29 | 11.9, CH3 | 12.1, CH3 | 12.3, CH3 | 11.9, CH3 | 12.6, CH3 |
| OAc | 20.9, CH3 | 20.9, CH3 | 21.2, CH3 | 21.2, CH3 | 21.1, CH3 |
| 171.6, qC | 171.6, qC | 171.1, qC | 171.0, qC | 170.6, qC | |
| 22.6, CH3 | 21.1, CH3 | 21.0, CH3 | 20.9, CH3 | ||
| 169.8, qC | 171.3, qC | 171.2, qC | 171.4, qC | ||
| 21.1, CH3 | 22.7, CH3 | ||||
| 170.8, qC | 169.9, qC | ||||
| OMe | 47.6, CH3 | ||||
| 47.5, CH3 |
| H# | 1, δH (J in Hz) a | 2, δH (J in Hz) a | 3, δH (J in Hz) a | 4, δH (J in Hz) b | 5, δH (J in Hz) c |
|---|---|---|---|---|---|
| 1 | α: 1.39 m | α: 1.35 m | α: 1.32 m | α: 1.02 m | α: 1.33 m |
| β: 2.02 m | β: 2.00 m | β: 1.97 m | β: 1.69 m | β: 1.06 m | |
| 2 | α: 2.32 m | α: 2.31 m | α: 2.31 m | α: 1.82 m | α: 1.86 m |
| β: 2.38 m | β: 2.39 m | β: 2.37 m | β: 1.41 m | β: 1.41 m | |
| 3 | 3.60 m | ||||
| 4 | α: 2.12 dd ovl | α: 2.09 dd ovl | α: 2.12 dd ovl | α: 1.58 m | α: 1.86 dd (13.6, 3.6) |
| β: 2.28 t (13.6) | β: 2.27 t (13.6) | β: 2.26 t (13.6) | β: 1.29 m | β: 1.41 dd ovl | |
| 5 | 1.56 m | 1.54 m | 1.55 m | 1.54 m | 1.34 m |
| 6 | 1.38 m | 1.38 m | 1.39 m | 1.34 m | 1.08 m |
| 7 | 1.79 m | 1.78 m | 1.82 m | 1.78 m | 1.54 m |
| 0.93 m | 0.92 m | 0.95 m | 0.91 m | 0.67 m | |
| 8 | 1.58 m | 1.58 m | 1.72 m | 1.67 m | 1.45 m |
| 9 | 0.85 m | 0.74 m | 0.81 m | 0.75 m | 0.70 m |
| 11 | α: 1.66 m | α: 1.65 m | α: 1.63 m | α: 1.60 m | α: 1.48 m |
| β: 1.48 m | β: 1.44 m | β: 1.33 m | β: 1.23 m | β: 1.23 m | |
| 12 | α: 1.28 m | α 1.28 m | α: 1.34 m | α: 1.38 m | α: 1.44 m |
| β: 2.44 m | β: 2.44 m | β: 2.16 m | β: 2.17 m | β: 2.28 m | |
| 14 | 1.36 m | 1.18 m | 1.23 m | 1.31 m | 1.28 m |
| 15 | α: 2.23 m | α: 2.24 m | α: 2.21 m | α: 2.21 m | α: 2.22 m |
| β: 1.44 m | β: 1.46 m | β: 1.48 m | β: 1.46 m | β: 1.59 m | |
| 16 | 4.10 t (7.2) | 4.13 t (7.6) | 4.06 t (7.6) | 4.04 dd (8.0, 7.5) | 4.38 t (7.5) |
| 18 | 3.75 t (10.4) | 3.74 t (11.2) | 4.23 d (11.6) | 4.27 d (11.5) | 4.55 d (11.5) |
| 3.94 d (11.6) | 3.94 dd (11.6, 2.4) | 4.30 d (11.6) | 4.20 d (11.5) | 4.49 d (11.5) | |
| 19 | 1.02 s | 1.02 s | 1.02 s | 0.82 s | 0.64 s |
| 21 | 1.64 s | 1.66 s | 1.60 s | 1.59 s | 1.84 s |
| 22 | 4.62 d (10.8) | 4.60 d (10.8) | 4.66 d (10.8) | 4.67 d (11.0) | 5.04 d (10.5) |
| 23 | 2.28 m | 2.47 m | 2.50 m | 2.29 m | 2.43 m |
| 24 | 1.97 q (8.0) | 1.47 q (6.8) | 1.64 q (6.8) | 1.92 q (7.0) | 1.55 q (7.5) |
| 26 | 1.56 s | 1.24 s | 3.89 d (11.6) | 1.56 s | 0.88 s |
| 4.04 d (11.6) | |||||
| 27 | 1.43 s | 1.21 s | 1.18 s | 1.46 s | 0.78 s |
| 28 | 0.90 d (8.0) | 0.90 d (6.8) | 0.88 d (6.8) | 0.91 d (7.0) | 0.65 d (7.5) |
| 29 | 0.88 d (6.4) | 0.86 d (6.4) | 0.88 d (6.8) | 0.87 d (7.0) | 0.80 d (7.0) |
| OAc | 2.14 s, 1.99 s | 2.14 s | 2.07 s, 2.13 s, 2.13 s | 2.06 s, 2.00 s, 2.13 s | 1.76 s, 1.69 s |
| OMe | 3.12 s, 3.02s | ||||
| OH-16 | 3.36 s | 3.43 s | 3.27 br s | 3.19 br s | 3.83 br s |
| OH-18 | 2.44 d ovl | 3.46 d ovl |
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, W.-H.; Wang, S.-K.; Duh, C.-Y. Polyhydroxylated Steroids from the Bamboo Coral Isis hippuris. Mar. Drugs 2011, 9, 1829-1839. https://doi.org/10.3390/md9101829
Chen W-H, Wang S-K, Duh C-Y. Polyhydroxylated Steroids from the Bamboo Coral Isis hippuris. Marine Drugs. 2011; 9(10):1829-1839. https://doi.org/10.3390/md9101829
Chicago/Turabian StyleChen, Wei-Hua, Shang-Kwei Wang, and Chang-Yih Duh. 2011. "Polyhydroxylated Steroids from the Bamboo Coral Isis hippuris" Marine Drugs 9, no. 10: 1829-1839. https://doi.org/10.3390/md9101829
APA StyleChen, W.-H., Wang, S.-K., & Duh, C.-Y. (2011). Polyhydroxylated Steroids from the Bamboo Coral Isis hippuris. Marine Drugs, 9(10), 1829-1839. https://doi.org/10.3390/md9101829




