Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health
Abstract
:1. Introduction
2. Bioavailability, Metabolism, and Safety of Fucoxanthin
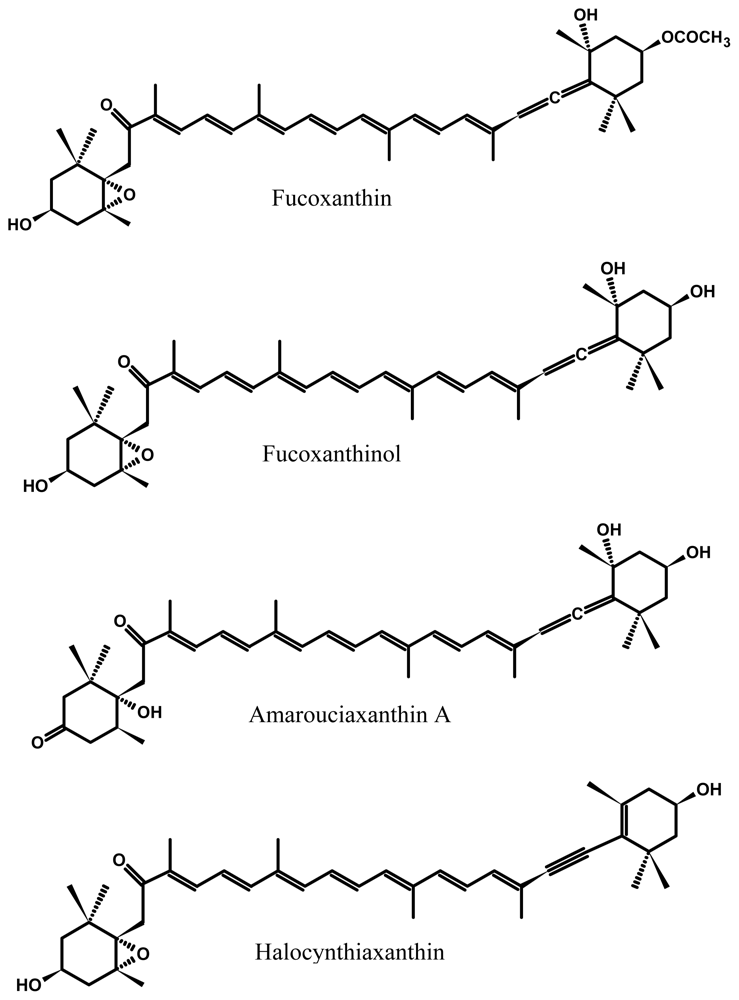
2.1. Bioavailability and Metabolism of Fucoxanthin
2.2. Safety of Fucoxanthin
3. Potential Health-Promoting Effects of Fucoxanthin
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Effects
3.3. Anticancer Activity
3.4. Anti-Obese Effect
3.5. Antidiabetic Activity
3.6. Hepatoprotective Effect
3.7. Skin-Protective Effect
3.8. Antiangiogenic Effect
3.9. Cerebrovascular Protective Effect
3.10. Bone-Protective Effect
3.11. Ocular Protective Effect
3.12. Antimalarial Effect
4. Conclusions
References
- Dembitsky, VM; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res 2007, 46, 328–375. [Google Scholar]
- Nomura, T; Kikuchi, M; Kubodera, A; Kawakami, Y. Proton-donative antioxidant activity of fucoxanthin with 1,1-diphenyl-2-picrylhydrazyl (DPPH). Biochem. Mol. Biol. Int 1997, 42, 361–370. [Google Scholar]
- Beppu, F; Niwano, Y; Tsukui, T; Hosokawa, M; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci 2009, 34, 501–510. [Google Scholar]
- Willstätter, R; Page, HJ. Chlorophyll. XXIV. The pigments of the brown algae. Justus Liebigs Ann. Chem 1914, 404, 237–271. [Google Scholar]
- Englert, G; Bjørnland, T; Liaaen-Jensen, S. 1D and 2D NMR study of some allenic carotenoids of the fucoxanthin series. Magn Reson Chem 1990, 28, 519–528. [Google Scholar]
- Sangeetha, RK; Bhaskar, N; Divakar, S; Baskaran, V. Bioavailability and metabolism of fucoxanthin in rats: structural characterization of metabolites by LC-MS (APCI). Mol. Cell. Biochem 2010, 333, 299–310. [Google Scholar]
- Bertrand, M. Carotenoid biosynthesis in diatoms. Photosynth. Res 2010, 106, 89–102. [Google Scholar]
- Airanthi, MWA; Hosokawa, M; Miyashita, K. Comparative antioxidant activity of edible Japanese brown seaweeds. J. Food Sci 2011, 76, C104–C111. [Google Scholar]
- Mise, T; Ueda, M; Yasumoto, T. Production of fucoxanthin-rich powder from Cladosiphon okamuranus. Adv. J. Food Sci. Technol 2011, 3, 73–76. [Google Scholar]
- Airanthi, MKWA; Sasaki, N; Iwasaki, S; Baba, N; Abe, M; Hosokawa, M; Miyashita, K. Effect of brown seaweed lipids on fatty acid composition and lipid hydroperoxide levels of mouse liver. J. Agric. Food Chem 2011, 59, 4156–4163. [Google Scholar]
- Strand, A; Herstad, O; Liaaen-Jensen, S. Fucoxanthin metabolites in egg yolks of laying hens. Comp. Biochem. Phys. A Mol. Integr. Physiol 1998, 119, 963–974. [Google Scholar]
- Zaragozá, MC; López, D; Sáiz, MP; Poquet, M; Pérez, J; Puig-Parellada, P; Mármol, F; Simonetti, P; Gardana, C; Lerat, Y; Burtin, P; Inisan, C; Rousseau, I; Besnard, M; Mitjavila, MT. Toxicity and antioxidant activity in vitro and in vivo of two Fucus vesiculosus extracts. J. Agric. Food Chem 2008, 56, 7773–7780. [Google Scholar]
- Nishino, H. Cancer prevention by carotenoids. Mutat. Res 1998, 402, 159–163. [Google Scholar]
- Yan, X; Chuda, Y; Suzuki, M; Nagata, T. Fucoxanthin as the major antioxidant in Hijikia fusiformis, a common edible seaweed. Biosci. Biotechnol. Biochem 1999, 63, 605–607. [Google Scholar]
- Kim, KN; Heo, SJ; Kang, SM; Ahn, G; Jeon, YJ. Fucoxanthin induces apoptosis in human leukemia HL-60 cells through a ROS-mediated Bcl-xL pathway. Toxicol. Vitro 2010, 24, 1648–1654. [Google Scholar]
- Das, SK; Hashimoto, T; Kanazawa, K. Growth inhibition of human hepatic carcinoma HepG2 cells by fucoxanthin is associated with down-regulation of cyclin D. Biochim. Biophys. Acta 2008, 1780, 743–749. [Google Scholar]
- Zhang, ZY; Zhang, PJ; Hamada, M; Takahashi, S; Xing, GQ; Liu, JQ; Sugiura, N. Potential chemoprevention effect of dietary fucoxanthin on urinary bladder cancer EJ-1 cell line. Oncol. Rep 2008, 20, 1099–1103. [Google Scholar]
- Miyata, M; Koyama, T; Kamitani, T; Toda, T; Yazawa, K. Anti-obesity effect on rodents of the traditional Japanese food, tororokombu, shaved Laminaria. Biosci. Biotechnol. Biochem 2009, 73, 2326–2328. [Google Scholar]
- Heo, SJ; Yoon, WJ; Kim, KN; Ahn, GN; Kang, SM; Kang, DH; Affan, A; Oh, C; Jung, WK; Jeon, YJ. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol 2010, 48, 2045–2051. [Google Scholar]
- Murakami, C; Takemura, M; Sugiyama, Y; Kamisuki, S; Asahara, H; Kawasaki, M; Ishidoh, T; Linn, S; Yoshida, S; Sugawara, F; Yoshida, H; Sakaguchi, K; Mizushina, Y. Vitamin A-related compounds, all-trans retinal and retinoic acids, selectively inhibit activities of mammalian replicative DNA polymerases. Biochim. Biophys. Acta 2002, 1574, 85–92. [Google Scholar]
- Afolayan, AF; Bolton, JJ; Lategan, CA; Smith, PJ; Beukes, DR. Fucoxanthin, tetraprenylated toluquinone and toluhydroquinone metabolites from Sargassum heterophyllum inhibit the in vitro growth of the malaria parasite Plasmodium falciparum. Z. Naturforsch. C 2008, 63, 848–852. [Google Scholar]
- Heo, SJ; Ko, SC; Kang, SM; Kang, HS; Kang, HS; Kim, JP; Kim, SH; Lee, KW; Cho, MG; Jeon, YJ. Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2-induced cell damage. Eur. Food Res. Technol 2008, 228, 145–151. [Google Scholar]
- Hosokawa, M; Wanezaki, S; Miyauchi, K; Kurihara, H; Kohno, H; Kawabata, J; Kawabata, J; Odashima, S; Takahashi, K. Apoptosis-inducing effect of fucoxanthin on human leukemia cell line HL-60. Food Sci. Technol. Res 1999, 5, 243–246. [Google Scholar]
- Ikeda, K; Kitamura, A; Machida, H; Watanabe, M; Negishi, H; Hiraoka, J; Nakano, T. Effect of Undaria pinnatifida (wakame) on the development of cerebrovascular diseases in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol 2003, 30, 44–48. [Google Scholar]
- Hosokawa, M; Kudo, M; Maeda, H; Kohno, H; Tanaka, T; Miyashita, K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim. Biophys. Acta 2004, 1675, 113–119. [Google Scholar]
- Maeda, H; Hosokawa, M; Sashima, T; Funayama, K; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun 2005, 332, 392–397. [Google Scholar]
- Sachindra, NM; Sato, E; Maeda, H; Hosokawa, M; Niwano, Y; Kohno, M; Miyashita, K. Radical scavenging and singlet oxygen quenching activity of marine carotenoid fucoxanthin and its metabolites. J. Agric. Food Chem 2007, 55, 8516–8522. [Google Scholar]
- Asai, A; Yonekura, L; Nagao, A. Low bioavailability of dietary epoxyxanthophylls in humans. Br. J. Nutr 2008, 100, 273–277. [Google Scholar]
- Khan, MNA; Lee, MC; Kang, JY; Park, NG; Fujii, H; Hong, YK. Effects of the brown seaweed Undaria pinnatifida on erythematous inflammation assessed using digital photo analysis. Phytother. Res 2008, 22, 634–639. [Google Scholar]
- Liu, CL; Huang, YS; Hosokawa, M; Miyashita, K; Hu, ML. Inhibition of proliferation of a hepatoma cell line by fucoxanthin in relation to cell cycle arrest and enhanced gap junctional intercellular communication. Chem. Biol. Interact 2009, 182, 165–172. [Google Scholar]
- Nakazawa, Y; Sashima, T; Hosokawa, M; Miyashita, K. Comparative evaluation of growth inhibitory effect of stereoisomers of fucoxanthin in human cancer cell lines. J. Funct. Foods 2009, 1, 88–97. [Google Scholar]
- Okada, T; Mizuno, Y; Sibayama, S; Hosokawa, M; Miyashita, K. Antiobesity effects of Undaria lipid capsules prepared with scallop phospholipids. J. Food Sci 2011, 76, H2–H6. [Google Scholar]
- Iio, K; Okada, Y; Ishikura, M. Single and 13-week oral toxicity study of fucoxanthin oil from microalgae in rats. J. Food Hyg. Soc. Jpn 2011, 52, 183–189. [Google Scholar]
- Iio, K; Okada, Y; Ishikura, M. Bacterial reverse mutation test and micronucleus test of fucoxanthin oil from microalgae. Shokuhin Eiseigaku Zasshi 2011, 52, 190–193. [Google Scholar]
- Rijstenbil, JW. Effects of UVB radiation and salt stress on growth, pigments and antioxidative defence of the marine diatom Cylindrotheca closterium. Mar. Ecol. Prog. Ser 2003, 254, 37–48. [Google Scholar]
- Moreau, D; Tomasoni, C; Jacquot, C; Kaas, R; Le Guedes, R; Cadoret, JP; Muller-Feuga, A; Kontiza, I; Vagias, C; Roussis, V; Roussakis, C. Cultivated microalgae and the carotenoid fucoxanthin from Odontella aurita as potent anti-proliferative agents in bronchopulmonary and epithelial cell lines. Environ. Toxicol. Pharmcol 2006, 22, 97–103. [Google Scholar]
- Sugawara, T; Kushiro, M; Zhang, H; Nara, E; Ono, H; Nagao, A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J. Nutr 2001, 131, 2921–2927. [Google Scholar]
- Yonekura, L; Kobayashi, M; Terasaki, M; Nagao, A. Keto-carotenoids are the major metabolites of dietary lutein and fucoxanthin in mouse tissues. J. Nutr 2010, 140, 1824–1831. [Google Scholar]
- Hashimoto, T; Ozaki, Y; Taminato, M; Das, SK; Mizuno, M; Yoshimura, K; Maoka, T; Kanazawa, K. The distribution and accumulation of fucoxanthin and its metabolites after oral administration in mice. Br. J. Nutr 2009, 102, 242–248. [Google Scholar]
- Sugawara, T; Baskaran, V; Tsuzuki, W; Nagao, A. Brown algae fucoxanthin is hydrolyzed to fucoxanthinol during absorption by Caco-2 human intestinal cells and mice. J. Nutr 2002, 132, 946–951. [Google Scholar]
- Das, SK; Ren, RD; Hashimoto, T; Kanazawa, K. Fucoxanthin induces apoptosis in osteoclast-like cells differentiated from RAW264.7 cells. J. Agric. Food Chem 2010, 58, 6090–6095. [Google Scholar]
- Asai, A; Sugawara, T; Ono, H; Nagao, A. Biotransformation of fucoxanthinol into amarouciaxanthin A in mice and HepG2 cells: formation and cytotoxicity of fucoxanthin metabolites. Drug Metab. Dispos 2004, 32, 205–211. [Google Scholar]
- Tsukui, T; Baba, N; Hosokawa, M; Sashima, T; Miyashita, K. Enhancement of hepatic docosahexaenoic acid and arachidonic acid contents in C57BL/6J mice by dietary fucoxanthin. Fish. Sci 2009, 75, 261–263. [Google Scholar]
- Sangeetha, RK; Bhaskar, N; Baskaran, V. Comparative effects of β-carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol. Cell. Biochem 2009, 331, 59–67. [Google Scholar]
- Murakami, A; Nakashima, M; Koshiba, T; Maoka, T; Nishino, H; Yano, M; Sumida, T; Kim, OK; Koshimizu, K; Ohigashi, H. Modifying effects of carotenoids on superoxide and nitric oxide generation from stimulated leucocytes. Cancer Lett 2000, 149, 115–123. [Google Scholar]
- Maoka, T. Carotenoids in marine animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar]
- Maoka, T; Fujiwara, Y; Hashimoto, K; Akimoto, N. Carotenoids in three species of corbicula clams, Corbicula japonica, Corbicula sandai, and Corbicula sp. (Chinese freshwater corbicula clam). J. Agric. Food Chem 2005, 53, 8357–8364. [Google Scholar]
- Nishino, H; Tsushima, M; Matsuno, T; Tanaka, Y; Okuzumi, J; Murakoshi, M; Satomi, Y; Takayasu, J; Tokuda, H; Nishino, A. Anti-neoplastic effect of halocynthiaxanthin, a metabolite of fucoxanthin. Anticancer Drugs 1992, 3, 493–497. [Google Scholar]
- Konishi, I; Hosokawa, M; Sashima, T; Kobayashi, H; Miyashita, K. Halocynthiaxanthin and fucoxanthinol isolated from Halocynthia roretzi induce apoptosis in human leukemia, breast and colon cancer cells. Comp. Biochem. Phys. C 2006, 142, 53–59. [Google Scholar]
- Maeda, H; Hosokawa, M; Sashima, T; Miyashita, K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J. Agric. Food Chem 2007, 55, 7701–7706. [Google Scholar]
- Maeda, H; Hosokawa, M; Sashima, T; Funayama, K; Miyashita, K. Effect of medium-chain triacylglycerols on anti-obesity effect of fucoxanthin. J. Oleo Sci 2007, 56, 615–621. [Google Scholar]
- Abidov, M; Ramazanov, Z; Seifulla, R; Grachev, S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab 2010, 12, 72–81. [Google Scholar]
- Ishikawa, C; Tafuku, S; Kadekaru, T; Sawada, S; Tomita, M; Okudaira, T; Nakazato, T; Toda, T; Uchihara, JN; Taira, N; Ohshiro, K; Yasumoto, T; Ohta, T; Mori, N. Antiadult T-cell leukemia effects of brown algae fucoxanthin and its deacetylated product fucoxanthinol. Int. J. Cancer 2008, 123, 2702–2712. [Google Scholar]
- Yamamoto, K; Ishikawa, C; Katano, H; Yasumoto, T; Mori, N. Fucoxanthin and its deacetylated product, fucoxanthinol, induce apoptosis of primary effusion lymphomas. Cancer Lett 2011, 300, 225–234. [Google Scholar]
- Kadekaru, T; Toyama, H; Yasumoto, T. Safety evaluation of fucoxanthin purified from Undaria pinnatifida. J. Jpn. Soc. Food Sci 2008, 55, 304–308. [Google Scholar]
- Beppu, F; Niwano, Y; Sato, E; Kohno, M; Tsukui, T; Hosokawa, M; Miyashita, K. In vitro and in vivo evaluation of mutagenicity of fucoxanthin (FX) and its metabolite fucoxanthinol (FXOH). J. Toxicol. Sci 2009, 34, 693–698. [Google Scholar]
- Woo, MN; Jeon, SM; Kim, HJ; Lee, MK; Shin, SK; Shin, YC; Park, YB; Choi, MS. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact 2010, 186, 316–322. [Google Scholar]
- Burton, GW; Ingold, KU. β-Carotene: an unusual type of lipid antioxidant. Science 1984, 224, 569–573. [Google Scholar]
- Le Tutour, B; Benslimane, F; Gouleau, MP; Gouygou, JP; Saadan, B; Quemeneur, F. Antioxidant and prooxidant activities of the brown algae, Laminaria digitata, Himanthalia elongata, Fucus vesiculosus, Fucus serratus and Ascophyllum nodosum. J. Appl. Phycol 1998, 10, 121–129. [Google Scholar]
- Sangeetha, RK; Bhaskar, N; Baskaran, V. Fucoxanthin restrains oxidative stress induced by retinol deficiency through modulation of Na+K+-ATPase and antioxidant enzyme activities in rats. Eur. J. Nutr 2008, 47, 432–441. [Google Scholar]
- Li, TL; King, JM; Min, DB. Quenching mechanisms and kinetics of carotenoids in riboflavin photosensitized singlet oxygen oxidation of vitamin D2. J. Food Biochem 2000, 24, 477–492. [Google Scholar]
- Liu, CL; Chiu, YT; Hu, ML. Fucoxanthin enhances HO-1 and NQO1 expression in murine hepatic BNL CL.2 cells through activation of the Nrf2/ARE system partially by its pro-oxidant activity. J Agric Food Chem 2011. [Google Scholar] [CrossRef]
- Choi, SK; Park, YS; Choi, DK; Chang, HI. Effects of astaxanthin on the production of NO and the epression of COX-2 and iNOS in LPS-simulated BV2 microglial cells. J. Microbiol. Biotechnol 2008, 18, 1990–1996. [Google Scholar]
- Lee, SJ; Bai, SK; Lee, KS; Namkoong, S; Na, HJ; Ha, KS; Han, JA; Yim, SV; Chang, K; Kwon, YG; Lee, SK; Kim, YM. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Mol. Cells 2003, 16, 97–105. [Google Scholar]
- Kim, KN; Heo, SJ; Yoon, WJ; Kang, SM; Ahn, G; Yi, TH; Jeon, YJ. Fucoxanthin inhibits the inflammatory response by suppressing the activation of NF-κB and MAPKs in lipopolysaccharide-induced RAW 264.7 macrophages. Eur. J. Pharmacol 2010, 649, 369–375. [Google Scholar]
- Sakai, S; Sugawara, T; Matsubara, K; Hirata, T. Inhibitory effect of carotenoids on the degranulation of mast cells via suppression of antigen-induced aggregation of high affinity IgE receptor. J. Biol. Chem 2009, 284, 28172–28179. [Google Scholar]
- Sakai, S; Sugawara, T; Hirata, T. Inhibitory effect of dietary carotenoids on dinitrofluorobenzene-induced contact hypersensitivity in mice. Biosci. Biotechnol. Biochem 2011, 75, 1013–1015. [Google Scholar]
- Khan, MNA; Suk-Choi, J; Lee, MC; Kim, E; Nam, TJ; Fujii, H; Hong, YK. Anti-inflammatory activities of methanol extracts from various seaweed species. J. Environ. Biol 2008, 29, 465–469. [Google Scholar]
- Khan, MNA; Yoon, SJ; Choi, JS; Park, NG; Lee, HH; Cho, JY; Hong, YK. Anti-edema effects of brown seaweed (Undaria pinnatifida) extract on phorbol 12-myristate 13-acetate-induced mouse ear inflammation. Am. J. Chin. Med 2009, 37, 373–381. [Google Scholar]
- Kotake-Nara, E; Kushiro, M; Zhang, H; Sugawara, T; Miyashita, K; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr 2001, 131, 3303–3306. [Google Scholar]
- Kotake-Nara, E; Sugawara, T; Nagao, A. Antiproliferative effect of neoxanthin and fucoxanthin on cultured cells. Fish. Sci 2005, 71, 459–461. [Google Scholar]
- Kotake-Nara, E; Terasaki, M; Nagao, A. Characterization of apoptosis induced by fucoxanthin in human promyelocytic leukemia cells. Biosci. Biotechnol. Biochem 2005, 69, 224–227. [Google Scholar]
- Ganesan, P; Noda, K; Manabe, Y; Ohkubo, T; Tanaka, Y; Maoka, T; Sugawara, T; Hirata, T. Siphonaxanthin, a marine carotenoid from green algae, effectively induces apoptosis in human leukemia (HL-60) cells. Biochim. Biophys. Acta 2011, 1810, 497–503. [Google Scholar]
- Das, SK; Hashimoto, T; Shimizu, K; Yoshida, T; Sakai, T; Sowa, Y; Komoto, A; Kanazawa, K. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1. Biochim. Biophys. Acta 2005, 1726, 328–335. [Google Scholar]
- Kotake-Nara, E; Asai, A; Nagao, A. Neoxanthin and fucoxanthin induce apoptosis in PC-3 human prostate cancer cells. Cancer Lett 2005, 220, 75–84. [Google Scholar]
- Satomi, Y; Nishino, H. Fucoxanthin, a natural carotenoid, induces G1 arrest and GADD45 gene expression in human cancer cells. Vivo 2007, 21, 305–309. [Google Scholar]
- Satomi, Y; Nishino, H. Implication of mitogen-activated protein kinase in the induction of G1 cell cycle arrest and gadd45 expression by the carotenoid fucoxanthin in human cancer cells. Biochim. Biophys. Acta 2009, 1790, 260–266. [Google Scholar]
- Yu, RX; Hu, XM; Xu, SQ; Jiang, ZJ; Yang, W. Effects of fucoxanthin on proliferation and apoptosis in human gastric adenocarcinoma MGC-803 cells via JAK/STAT signal pathway. Eur. J. Pharmacol 2011, 657, 10–19. [Google Scholar]
- Okuzumi, J; Nishino, H; Murakoshi, M; Iwashima, A; Tanaka, Y; Yamane, T; Fujita, Y; Takahashi, T. Inhibitory effects of fucoxanthin, a natural carotenoid, on N-myc expression and cell cycle progression in human malignant tumor cells. Cancer Lett 1990, 55, 75–81. [Google Scholar]
- Das, SK; Hashimoto, T; Baba, M; Nishino, H; Komoto, A; Kanazawa, K. Japanese kelp (kombu) extract suppressed the formation of aberrant crypt foci in azoxymethane challenged mouse colon. J. Clin. Biochem. Nutr 2006, 38, 119–125. [Google Scholar]
- Kim, JM; Araki, S; Kim, DJ; Park, CB; Takasuka, N; Baba-Toriyama, H; Ota, T; Nir, Z; Khachik, F; Shimidzu, N; Tanaka, Y; Osawa, T; Uraji, T; Murakoshi, M; Nishino, H; Tsuda, H. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis 1998, 19, 81–85. [Google Scholar]
- Okuzumi, J; Takahashi, T; Yamane, T; Kitao, Y; Inagake, M; Ohya, K; Tanaka, Y. Inhibitory effects of fucoxanthin, a natural carotenoid, on N-ethyl-N′-nitro-N-nitrosoguanidine-induced mouse duodenal carcinogenesis. Cancer Lett 1993, 68, 159–168. [Google Scholar]
- Miyashita, K; Nishikawa, S; Beppu, F; Tsukui, T; Abe, M; Hosokawa, M. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J. Sci. Food Agric 2011, 91, 1166–1174. [Google Scholar]
- Maeda, H; Hosokawa, M; Sashima, T; Takahashi, N; Kawada, T; Miyashita, K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Med 2006, 18, 147–152. [Google Scholar]
- Park, HJ; Lee, MK; Park, YB; Shin, YC; Choi, MS. Beneficial effects of Undaria pinnatifida ethanol extract on diet-induced-insulin resistance in C57BL/6J mice. Food Chem. Toxicol 2011, 49, 727–733. [Google Scholar]
- Maeda, H; Tsukui, T; Sashima, T; Hosokawa, M; Miyashita, K. Seaweed carotenoid, fucoxanthin, as a multi-functional nutrient. Asia Pac. J. Clin. Nutr 2008, 17, 196–199. [Google Scholar]
- Woo, MN; Jeon, SM; Shin, YC; Lee, MK; Kang, MA; Choi, MS. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice. Mol. Nutr. Food Res 2009, 53, 1603–1611. [Google Scholar]
- Jeon, SM; Kim, HJ; Woo, MN; Lee, MK; Shin, YC; Park, YB; Choi, MS. Fucoxanthin-rich seaweed extract suppresses body weight gain and improves lipid metabolism in high-fat-fed C57BL/6J mice. Biotechnol. J 2010, 5, 961–969. [Google Scholar]
- Maeda, H; Hosokawa, M; Sashima, T; Murakami-Funayama, K; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep 2009, 2, 897–902. [Google Scholar]
- Hosokawa, M; Miyashita, T; Nishikawa, S; Emi, S; Tsukui, T; Beppu, F; Okada, T; Miyashita, K. Fucoxanthin regulates adipocytokine mRNA expression in white adipose tissue of diabetic/obese KK-Ay mice. Arch. Biochem. Biophys 2010, 504, 17–25. [Google Scholar]
- Matsumoto, M; Hosokawa, M; Matsukawa, N; Hagio, M; Shinoki, A; Nishimukai, M; Miyashita, K; Yajima, T; Hara, H. Suppressive effects of the marine carotenoids, fucoxanthin and fucoxanthinol on triglyceride absorption in lymph duct-cannulated rats. Eur. J. Nutr 2010, 49, 243–249. [Google Scholar]
- Miyashita, K; Maeda, H; Okada, T; Abe, M; Hosokawa, M. Anti-obesity and anti-diabetic effects of allenic carotenoid, fucoxanthin. Agro. Food Ind. Hi Tech 2010, 21, 24–27. [Google Scholar]
- Sugawara, T; Matsubara, K; Akagi, R; Mori, M; Hirata, T. Antiangiogenic activity of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. J. Agric. Food Chem 2006, 54, 9805–9810. [Google Scholar]
- Yim, MJ; Hosokawa, M; Mizushina, Y; Yoshida, H; Saito, Y; Miyashita, K. Suppressive effects of amarouciaxanthin A on 3T3-L1 adipocyte differentiation through down-regulation of PPARγ and C/EBPα mRNA expression. J. Agric. Food Chem 2011, 59, 1646–1652. [Google Scholar]
- Kang, SI; Ko, HC; Shin, HS; Kim, HM; Hong, YS; Lee, NH; Kim, SJ. Fucoxanthin exerts differing effects on 3T3-L1 cells according to differentiation stage and inhibits glucose uptake in mature adipocytes. Biochem. Biophys. Res. Commun 2011, 409, 769–774. [Google Scholar]
- Okada, T; Nakai, M; Maeda, H; Hosokawa, M; Sashima, T; Miyashita, K. Suppressive effect of neoxanthin on the differentiation of 3T3-L1 adipose cells. J. Oleo Sci 2008, 57, 345–351. [Google Scholar]
- Tsukui, T; Konno, K; Hosokawa, M; Maeda, H; Sashima, T; Miyashita, K. Fucoxanthin and fucoxanthinol enhance the amount of docosahexaenoic acid in the liver of KKAy obese/diabetic mice. J. Agric. Food Chem 2007, 55, 5025–5029. [Google Scholar]
- Liu, CL; Liang, AL; Hu, ML. Protective effects of fucoxanthin against ferric nitrilotriacetate-induced oxidative stress in murine hepatic BNL CL.2 cells. Toxicol. Vitro 2011, 25, 1314–1319. [Google Scholar]
- Heo, SJ; Jeon, YJ. Protective effect of fucoxanthin isolated from Sargassum siliquastrum on UV-B induced cell damage. J. Photochem. Photobiol. B 2009, 95, 101–107. [Google Scholar]
- Urikura, I; Sugawara, T; Hirata, T. Protective effect of fucoxanthin against UVB-induced skin photoaging in hairless mice. Biosci. Biotechnol. Biochem 2011, 75, 757–760. [Google Scholar]
- Shimoda, H; Tanaka, J; Shan, SJ; Maoka, T. Anti-pigmentary activity of fucoxanthin and its influence on skin mRNA expression of melanogenic molecules. J. Pharm. Pharmacol 2010, 62, 1137–1145. [Google Scholar]
- Van Tenten, Y; Schuitmaker, HJ; De Wolf, A; Willekens, B; Vrensen, GFJM; Tassignon, MJ. The effect of photodynamic therapy with bacteriochlorin a on lens epithelial cells in a capsular bag model. Exp. Eye Res 2001, 72, 41–48. [Google Scholar]
- Shiratori, K; Ohgami, K; Ilieva, I; Jin, XH; Koyama, Y; Miyashita, K; Yoshida, K; Kase, S; Ohno, S. Effects of fucoxanthin on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp. Eye Res 2005, 81, 422–428. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Mar. Drugs 2011, 9, 1806-1828. https://doi.org/10.3390/md9101806
Peng J, Yuan J-P, Wu C-F, Wang J-H. Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Marine Drugs. 2011; 9(10):1806-1828. https://doi.org/10.3390/md9101806
Chicago/Turabian StylePeng, Juan, Jian-Ping Yuan, Chou-Fei Wu, and Jiang-Hai Wang. 2011. "Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health" Marine Drugs 9, no. 10: 1806-1828. https://doi.org/10.3390/md9101806
APA StylePeng, J., Yuan, J.-P., Wu, C.-F., & Wang, J.-H. (2011). Fucoxanthin, a Marine Carotenoid Present in Brown Seaweeds and Diatoms: Metabolism and Bioactivities Relevant to Human Health. Marine Drugs, 9(10), 1806-1828. https://doi.org/10.3390/md9101806




