Sulfated Polysaccharides in Marine Sponges: Extraction Methods and Anti-HIV Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Methods
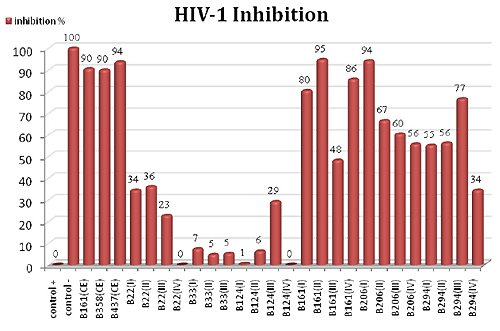
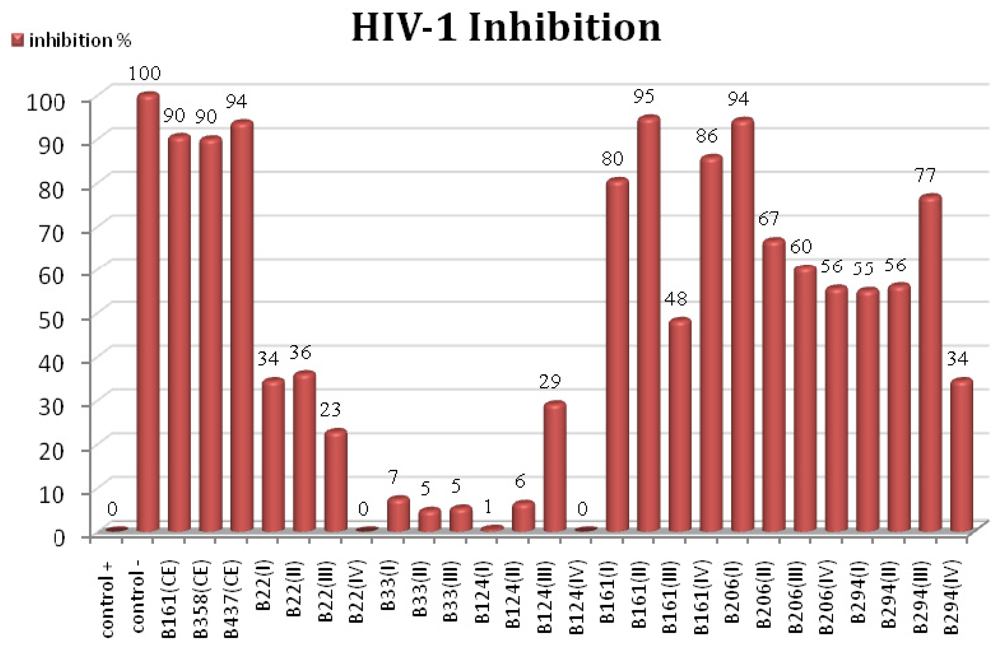
2.2. HIV-1 Inhibition Activity
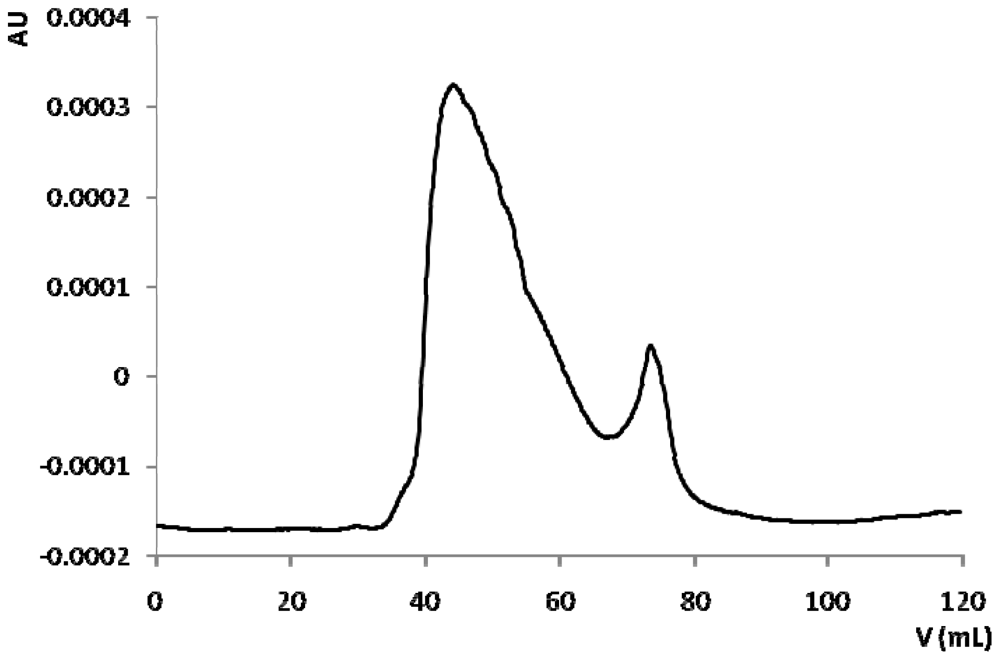
2.3. Bioassay Guided Fractionation
3. Experimental Section
3.1. Biological Material
3.2. Crude Extract Preparation
3.3. Polysaccharide Extraction
3.4. Quantification of Sulfated Polysaccharides
3.5. Chromatographic Fractionation
3.6. HIV-1 Inhibition Determination
4. Conclusions
Acknowledgements
- Samples Availability: Available from the authors.
References
- Hooper, JNA; van Soest, RWM. Systema Porifera: A Guide to the Classification of Sponges; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2002. [Google Scholar]
- Misevic, GN; Ripoll, C; Norris, J; Norris, V; Guerardel, Y; Maes, E; Strecker, G; Ballet, P; Karamanos, Y; Sumanovski, LT; Popescu, O; Misevic, N. Evolution of multicellularity in Porifera via self-assembly of glyconectin carbohydrates. In Porifera Research: Biodiversity, Innovation and Sustainability; Custódio, MR, Lôbo-Hajdu, G, Hajdu, E, Muricy, G, Eds.; Museu Nacional: Rio de Janeiro, Brazil, 2007. [Google Scholar]
- Li, C; Chen, J; Hua, T. Precambrian sponges with cellular structures. Science 1998, 279, 879–882. [Google Scholar]
- Hooper, JNA. Sponge Guide; Queensland Museum: Queensland, Austrialia, 1995. [Google Scholar]
- van Soest, RWM; Boury-Esnault, N; Hooper, JNA; Rützler, K; de Voogd, NJ; Alvarez, B; Hajdu, E; Pisera, AB; Vacelet, J; Manconi, R; Schoenberg, C; Janussen, D; Tabachnick, KR; Klautau, M. World Porifera Database. 2008. Available online: http://www.marinespecies.org/porifera accessed on 17 May 2010.
- Rützler, K. Sponges on coral reefs: a community shaped by competitive cooperation. In Sponge Science in the New Millennium; Pansini, M, Pronzato, R, Bavestrello, G, Manconi, R, Eds.; Bollettino dei Musei e degli Instituti Biologici dell’Universitá di Genova: Rapallo, Italy, 2004; Volume 68, pp. 85–129. [Google Scholar]
- Bergmann, W; Burke, DC. Contributions to the study of marine products. XXXIX. The nucleosides of sponges. III. Spongothymidine and Spongouridine. J Org Chem 1955, 20, 1501–1507. [Google Scholar]
- Bergmann, W; Feeney, RJ. Contributions to the study of marine products. XXXII. The nucleosides of sponges. I. J Org Chem 1951, 16, 981–987. [Google Scholar]
- Gochfeld, DJ; Sayed, KAE; Yousaf, M; Hu, JF; Bartyzel, P; Dunbar, DC; Wilkins, SP; Zjawiony, JK; Schinazi, RF; Wirtz, SS; Tharnish, PM; Hamman, MT. Marine natural products as lead anti-HIV agents. Mini Rev Med Chem 2003, 3, 401–424. [Google Scholar]
- Beutler, JA; McKee, TC; Fuller, RW; Tischler, M; Cardellina, JH; Snader, KM; McCloud, TG; Boyd, MR. Frequent ocurrence of HIV-inhibitory sulphated polysaccharides in marine invertebrates. Antivir Chem Chemother 1993, 4, 167–172. [Google Scholar]
- Roussis, V; Tziveleka, LA; Vagias, C. Natural products with anti-HIV activity from marine organisms. Curr Top Med Chem 2003, 3, 1512–1535. [Google Scholar]
- O’Keefe, BR; Erim, T; Beutler, JA; Cardellina, JH; Gulakowski, RJ; Krepps, BL; McMahon, JB; Sowder, RC; Johnson, DG; Buckheit, RW; Halliday, S; Boyd, MR. Isolation and characterization of adociavirin, a novel HIV-inhibitoy protein from the sponge Adocia sp. FEBS Lett 1998, 431, 85–90. [Google Scholar]
- Rajaganapathi, J; Kathiresan, K; Singh, TP. Purification of anti-HIV protein from purple fluid of the sea hare Bursatella leachii de Blainville. Mar Biotechnol 2002, 4, 447–453. [Google Scholar]
- O’Keefe, BR; Beutler, JA; Cardellina, JH; Gulakowski, RJ; Krepps, BL; McMahon, JB; Sowder, RC; Henderson, LE; Pannell, LK; Pomponi, SA; Boyd, MR. Isolation and characterization of niphatevirin, a human-immunodeficiency-virus-inhibitory glycoprotein from the marine sponge Niphates erecta. Eur J Biochem 1997, 245, 47–53. [Google Scholar]
- Goud, TV; Reddy, NS; Swamy, NR; Ram, TS; Venkateswarlu, Y. Anti-HIV active petrosins from the marine sponge Petrosia similis. Biol Pharm Bull 2003, 26, 1498–1501. [Google Scholar]
- Ahn, M; Yoon, K; Min, S; Lee, JS; Kim, JH; Kim, TG; Kim, SH; Kim, N; Huh, H; Kim, J. Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava . Biol Pharm Bull 2004, 27, 544–547. [Google Scholar]
- Asres, K; Seyoum, A; Veeresham, C; Bucar, F; Gibbons, S. Naturally derived anti-HIV agents. Phytother Res 2005, 19, 557–581. [Google Scholar]
- Clerq, ED. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med Res Rev 2000, 20, 323–349. [Google Scholar]
- Jung, M; Lee, S; Kim, H; Kim, H. Recent studies on natural products as anti-HIV agents. Curr Med Chem 2000, 7, 649–661. [Google Scholar]
- Taylor, MW; Radax, R; Steger, D; Wagner, M. Sponge-associated microorganisms: evolution, ecology and biotechnological potential. Microbiol Mol Biol Rev 2007, 71, 295–347. [Google Scholar]
- Smit, AJ. Medicinal and pharmaceutical uses of seaweed natural products: A review. J Appl Phycol 2004, 16, 245–262. [Google Scholar]
- Food and Drug Administration. Antiretroviral Drugs Used in the Treatment of HIV Infection; U.S. Department of Health and Human Services: Washington, DC, USA, 2010. Available online: http://www.fda.gov/ForConsumers/byAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm118915.htm accessed on 6 January 2010.
- McIlroy, RJ. The Chemistry of Polysaccharides; Jarrald & Sons: Norwich, UK, 1948. [Google Scholar]
- Müller, WE; Müller, I. Sponge cell aggregation. Mol Cell Biochem 1980, 29, 131–143. [Google Scholar]
- Parish, CR; Jakobsen, KB; Coombe, DR; Bacic, A. Isolation and characterization of cell adhesion molecules from the marine sponge, Ophlitaspongia tenuis. Biochim Biophys Acta 1991, 1073, 56–64. [Google Scholar]
- Bucior, I; Burger, MM. Carbohydrate-carbohydrate interaction as a major force initiating cell-cell recognition. Glycoconj J 2004, 21, 111–123. [Google Scholar]
- Witvrouw, M; DeClercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen Pharmacol 1997, 29, 497–511. [Google Scholar]
- Rider, CC; Coombe, DR; Harrop, HA; Hounsel, EF; Bauer, C; Feeney, J; Mulloy, B; Mahmood, N; Hay, A; Parish, CR. Anti-HIV-1 Activity of Chemically Modified Heparins: Correlation between Binding to the V3 Loop of gp120 and Inhibition of Cellular HIV-1 Infection in vitro. Biochemistry 1994, 33, 6974–6980. [Google Scholar]
- Schols, D; Pauwels, R; Desmyter, J; Clercq, ED. Dextran sulphate and other polyanionic anti-HIV compounds specifically interact with the viral gp120 glycoprotein expressed by T-cells persistently infected with HIV-1. Virology 1990, 175, 556–561. [Google Scholar]
- Tözsér, J. Stages of HIV replication and targets for therapeutic intervention. Curr Top Med Chem 2003, 3, 1447–1457. [Google Scholar]
- Trinchero, J; Ponce, NMA; Cordoba, OL; Flores, ML; Pampuro, S; Stortz, CA; Salomon, H; Turk, G. Antiretroviral activity of fucoidans extracted from the brown seaweed Adenocystis utricularis. Phytother Res 2009, 23, 707–712. [Google Scholar]
- Wang, SC; Bligh, SWA; Shi, SS; Wang, Z; Hu, ZB; Crowder, J; Brandford-White, C; Vella, C. Structural features and anti-HIV-1 activity of novel polysaccharides from red algae Grateloupia longifolia and Grateloupia filicina. Int J Biol Macromol 2007, 41, 369–375. [Google Scholar]
- Damonte, EB; Matulewicz, M; Cerezo, SS. Sulfated seaweed polysaccharides as antiviral agents. Curr Med Chem 2004, 11, 2399–2419. [Google Scholar]
- Haslin, C; Lahaye, M; Pellegrini, M; Chermann, JC. In vitro anti-HIV activity of sulfated cell-wall polysaccharides from gametic, carposporic and tetrasporic stages of the Mediterranean red alga Asparagopsis armata. Planta Med 2001, 67, 301–305. [Google Scholar]
- Schaeffer, DJ; Krylov, V. Anti-HIV activity of extracts and compounds from algae and cyanobacteria. Ecotoxicol Environ Saf 2000, 45, 208–227. [Google Scholar]
- Bourgougnon, N; Lahaye, M; Quemener, B; Chermann, JC; Rimbert, M; Cormaci, M; Furnari, G; Kornprobst, JM. Annual variation in composition and in vitro anti-HIV-1 activity of the sulfated glucuronogalactan from Schizymenia dubyi (Rodophyta, Gigartinales). J Appl Phycol 1996, 8, 155–161. [Google Scholar]
- Hayashi, K; Hamada, J; Hayashi, T. A screening strategy for selection of anti-HSV-1 and anti-HIV extracts from algae. Phytother Res 1996, 10, 233–237. [Google Scholar]
- Rashid, ZM; Lahaye, E; Defer, D; Douzenel, P; Perrin, B; Bourgougnon, N; Sire, O. Isolation of a sulphated polysaccharide from a recently discovered sponge species (Celtodoryx girardae) and determination of its anti-herpetic activity. Int J Biol Macromol 2009, 44, 286–293. [Google Scholar]
- Cimino, P; Bifulco, G; Casapullo, A; Bruno, I; Gomez-Paloma, L; Riccio, R. Isolation and NMR characterization of rosacelose, a novel sulfated polysaccharide from the sponge Mixylla rosacea. Carbohydr Res 2001, 334, 39–47. [Google Scholar]
- Zierer, MS; Vieira, RP; Mulloy, B; Mourão, PAS. A novel acidic glycogen extracted from the marine sponge Aplysina fulva (porifera-demospongiae). Carbohydr Res 1995, 274, 233–244. [Google Scholar]
- Zierer, MS; Mourão, PAS. A wide diversity of sulfated polysaccharides are synthesized by different species of marine sponges. Carbohydr Res 2000, 328, 209–216. [Google Scholar]
- Vilanova, E; Zilberberg, C; Kochem, M; Custódio, MR; Mourão, PAS. A novel biochemical method to distinguish cryptic species of Chondrilla (Chondrosida, Demospongiae) based on its sulfated polysaccharides. In Porifera Research: Biodiversity, Innovation and Sustainability; Custódio, MR, Lôbo-Hajdo, G, Hajdu, E, Muricy, G, Eds.; Museu Nacional: Rio de Janeiro, Italy, 2007. [Google Scholar]
- Ghosh, T; Chattopadhyay, K; Marschall, M; Karmakar, P; Mandal, P; Ray, B. Focus on antivirally active sulfated polysaccharides: from structure-activity to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar]
- Hayashi, K; Hayashi, T; Kojima, I. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: in vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Res Hum Retroviruses 1996, 12, 1463–1471. [Google Scholar]
- Jagodzinski, PP; Trzeciak, W. The V3 region of gp120 is responsible for anti-HIV activity of heparin sulphate. Acta Biochim Pol 1998, 45, 799–804. [Google Scholar]
- Harrop, HA; Rider, CC. Heparin and its derivatives bind to HIV-1 recombinant envelope glycoproteins, rather than to recombinant HIV-1 receptor, CD4. Glycobiology 1998, 8, 131–137. [Google Scholar]
- Coombe, DR; Jakobsen, KB; Parish, CR. A role for sulphated polysaccharide recognition in sponge cell aggregation. Exp Cell Res 1987, 170, 381–401. [Google Scholar]
- Vieira, RP; Mulloy, B; Mourão, PAS. Structure of a Fucose-branched Chondroitin Sulfate from Sea Cucumber. J Biol Chem 1991, 266, 13530–13536. [Google Scholar]
- Albano, RM; Mourão, PAS. Isolation, fractionation, and preliminary characterization of a novel class of sulfated glycans from the tunic of Styela plicata (Chordata Tunicata). J Biol Chem 1986, 261, 758–765. [Google Scholar]
- The Division of AIDS, DAIDS Virology Manual for HIV Laboratories. In Publication NIH-97-3828; U.S. Department of Health and Human Services: Washington DC, USA, 1997.
- UNAIDS. 2009 AIDS Epidemic Update; Joint United Nations Programme on HIV/AIDS (UNAIDS) and World Health Organization: Geneve, Switzerland, 2009. Available online: http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf accessed on 2 September 2010.
- Faulkner, DJ. Marine natural products. Nat Prod Rep 2001, 18, 1–49. [Google Scholar]
- Karim, QA; Karim, SSA; Frohlich, JA; Grobler, AC; Baxter, C; Mansoor, LE; Kharsany, ABM; Sibeko, S; Mlisana, KP; Omar, Z; Gengiah, TN; Maarschalk, S; Arulappan, N; Mlotshwa, M; Morris, L; Taylor, D. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Science 2010, 329, 1168–1174. [Google Scholar]
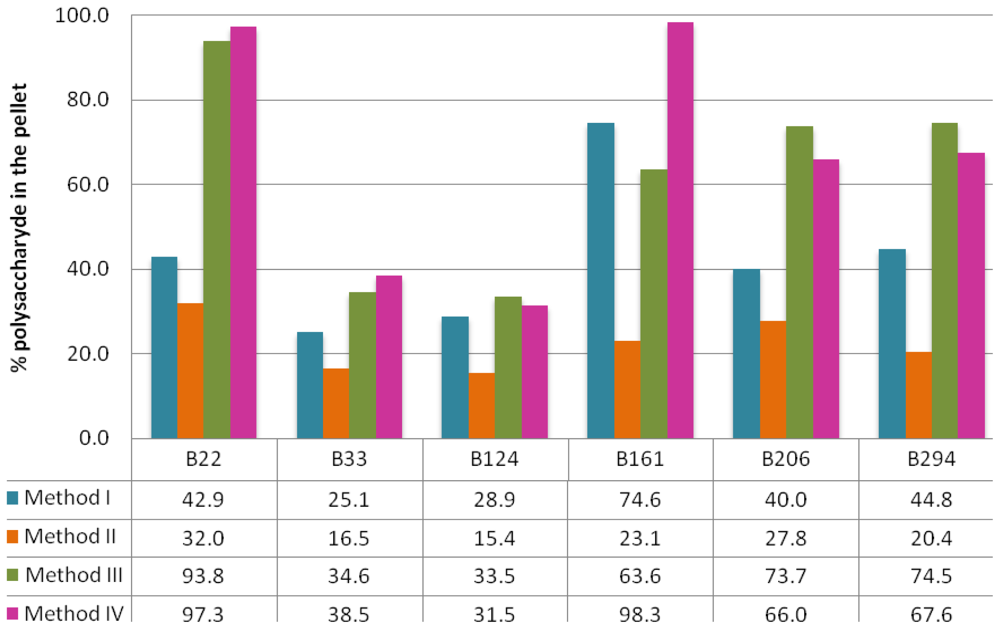
| Method | I | II | III | IV |
|---|---|---|---|---|
| Sample | ||||
| B22 | 98.09 | 20.87 | 35.47 | 24.07 |
| B33 | 9.43 | 13.80 | 5.46 | 0.52 |
| B124 | 13.37 | 8.39 | 6.22 | 4.72 |
| B161 | 299.20 | 84.27 | 89.44 | 35.59 |
| B206 | 139.46 | 28.36 | 81.08 | 20.80 |
| B294 | 135.15 | 32.27 | 52.02 | 20.76 |
| Method | I | II | III | IV |
|---|---|---|---|---|
| Sample | ||||
| B22 | 42.11 | 6.67 | 33.27 | 23.41 |
| B33 | 2.36 | 2.28 | 1.89 | 0.20 |
| B124 | 3.86 | 1.29 | 2.09 | 1.49 |
| B161 | 223.34 | 19.46 | 56.84 | 34.98 |
| B206 | 55.72 | 7.90 | 59.72 | 13.72 |
| B294 | 60.57 | 6.59 | 38.76 | 14.03 |
| Sample | Species |
|---|---|
| B161 | Erylus discophorus |
| B358 | |
| B437 | |
| B206 | |
| B294 | |
| B22 | Stelletta sp. |
| B33 | Cliona celata |
| B124 | |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Esteves, A.I.S.; Nicolai, M.; Humanes, M.; Goncalves, J. Sulfated Polysaccharides in Marine Sponges: Extraction Methods and Anti-HIV Activity. Mar. Drugs 2011, 9, 139-153. https://doi.org/10.3390/md9010139
Esteves AIS, Nicolai M, Humanes M, Goncalves J. Sulfated Polysaccharides in Marine Sponges: Extraction Methods and Anti-HIV Activity. Marine Drugs. 2011; 9(1):139-153. https://doi.org/10.3390/md9010139
Chicago/Turabian StyleEsteves, Ana I. S., Marisa Nicolai, Madalena Humanes, and Joao Goncalves. 2011. "Sulfated Polysaccharides in Marine Sponges: Extraction Methods and Anti-HIV Activity" Marine Drugs 9, no. 1: 139-153. https://doi.org/10.3390/md9010139
APA StyleEsteves, A. I. S., Nicolai, M., Humanes, M., & Goncalves, J. (2011). Sulfated Polysaccharides in Marine Sponges: Extraction Methods and Anti-HIV Activity. Marine Drugs, 9(1), 139-153. https://doi.org/10.3390/md9010139