Alkaloids in Marine Algae
Abstract
:1. Introduction
- Phenylethylamine alkaloids.
- Indole and halogenated indole alkaloids.
- Other alkaloids.
2. Phenylethylamine Group
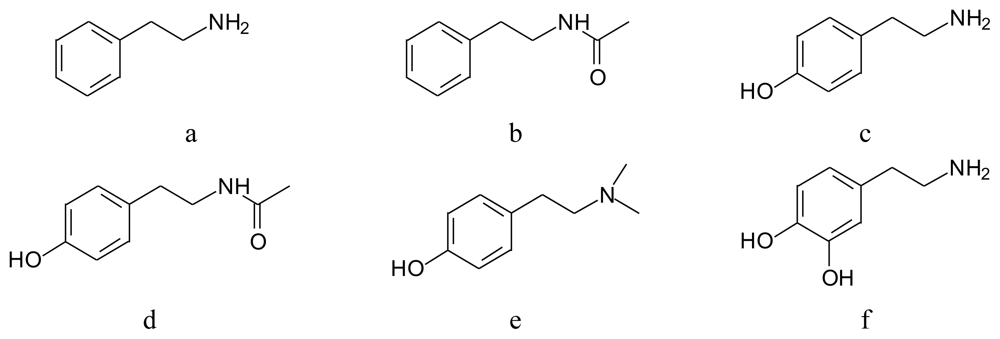
2.1. Phenylethylamine (PEA)
2.2. Tyramine (TYR, 4-hydroxyphenylethylamine; Figure 1c)
2.3. Hordenine (Anhaline) (HORD, 4-(2-dimethylaminoethyl) phenol; Figure 1e)
2.4. Dopamine (DOP, 3,4-dihydroxyphenethylamine; Figure 1f)
3. Indole Group
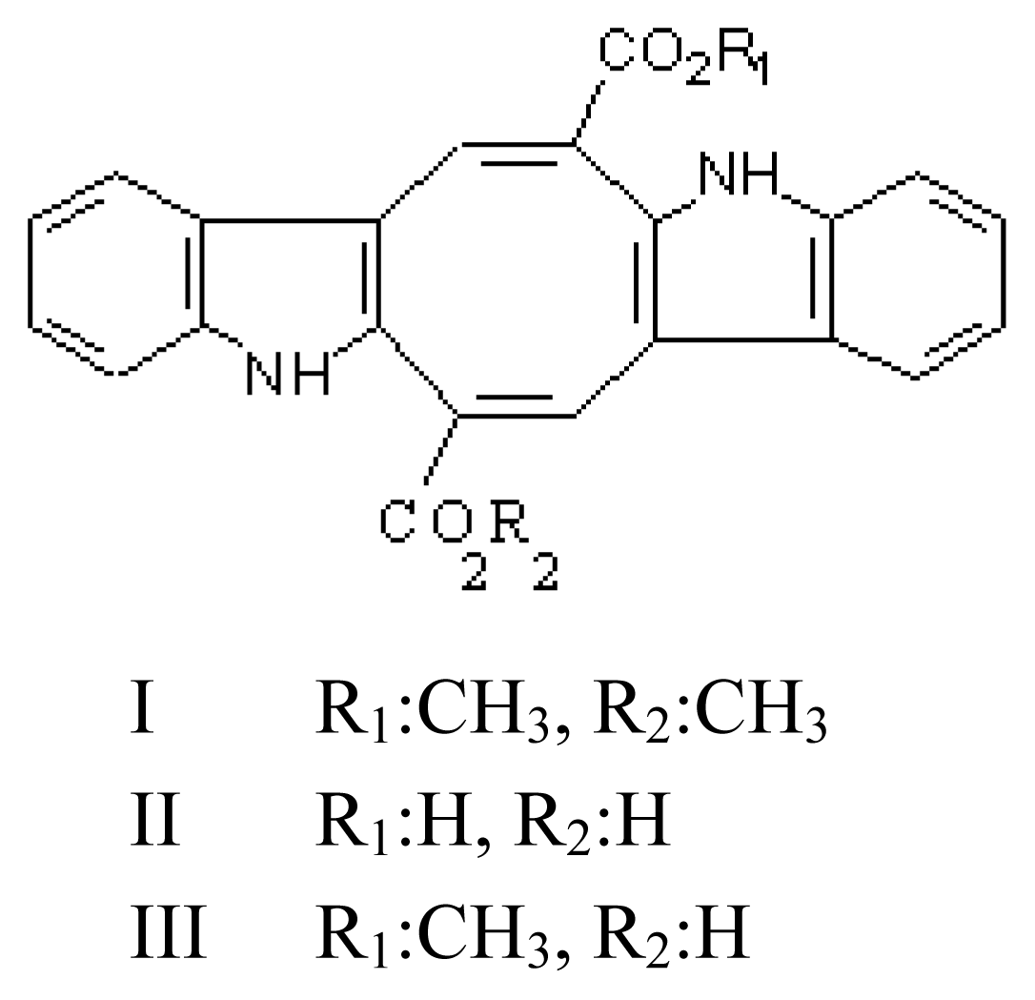
3.1. Caulerpin (CLP, dimethyl-6,13-dihydrodibenzo [b,i]phenazine-5,12-dicarboxylate methyl ester; Figure 2)
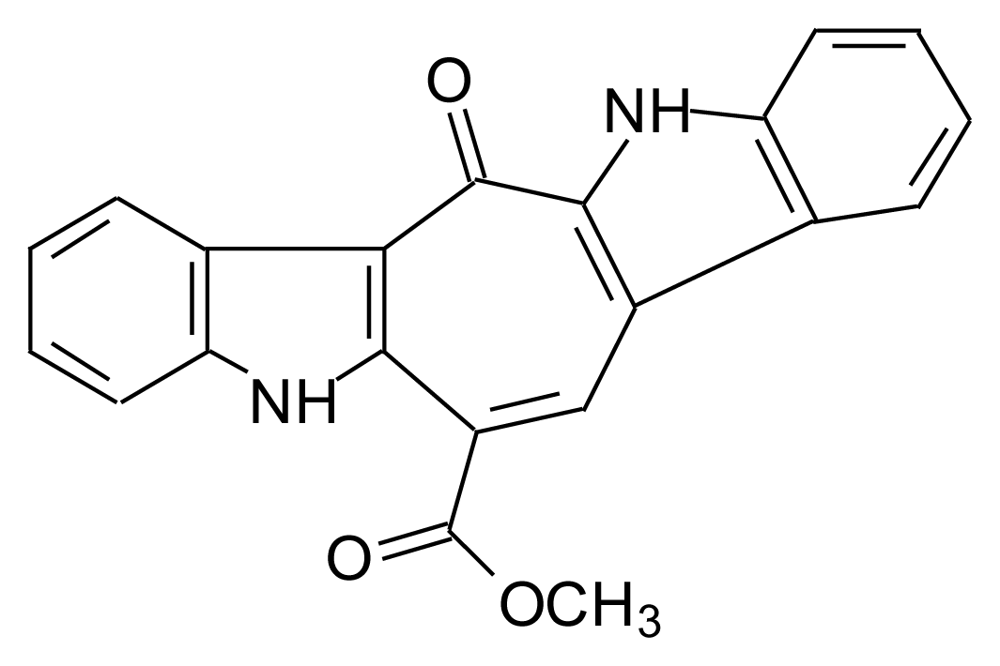
3.2. Caulersin (CLS)
3.3. Martensia fragilis alkaloids
3.3.1. Fragilamide (FRG)
3.3.2. Martensines (MRT)
3.3.2.1. Martensine A
3.3.2.2. Martensine B
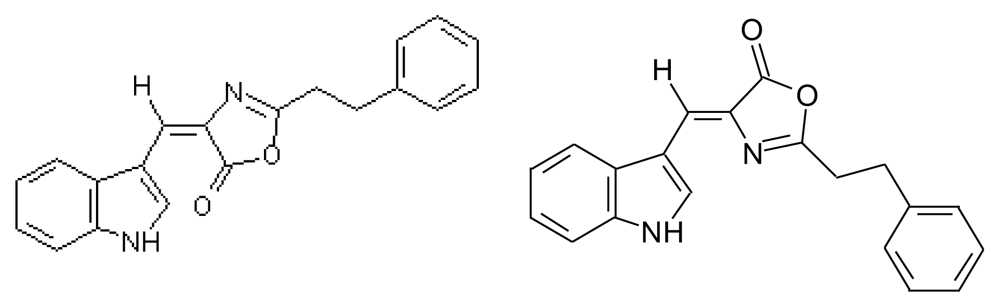
3.3.3. Martefragin A (MRF A)
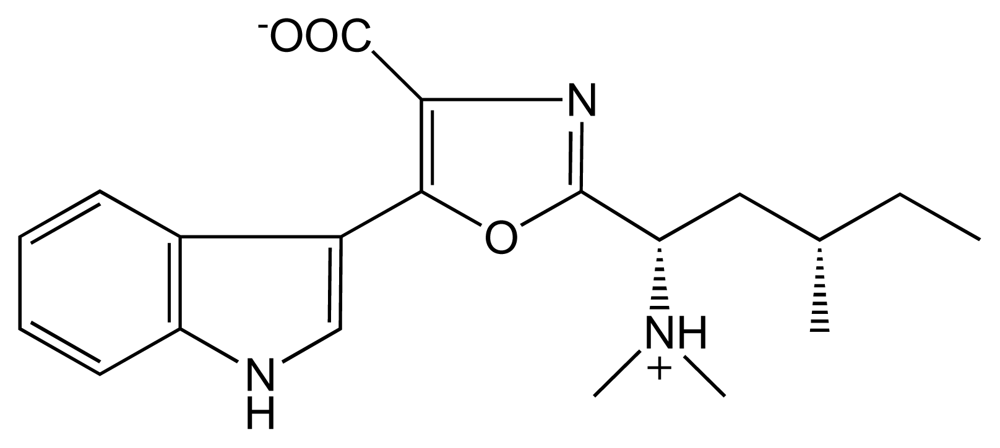
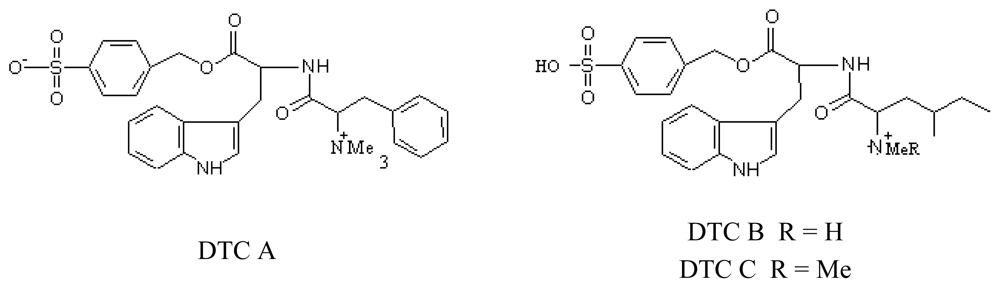
3.3.4. Denticins (DTC)
3.4. Almazolone (ALM)
4. Halogenated Indole Alkaloids (HLI)
4.1. Bromoindole
4.1.1. Bromoindoles and N-methylbromoindoles isolated from algae are given by the source
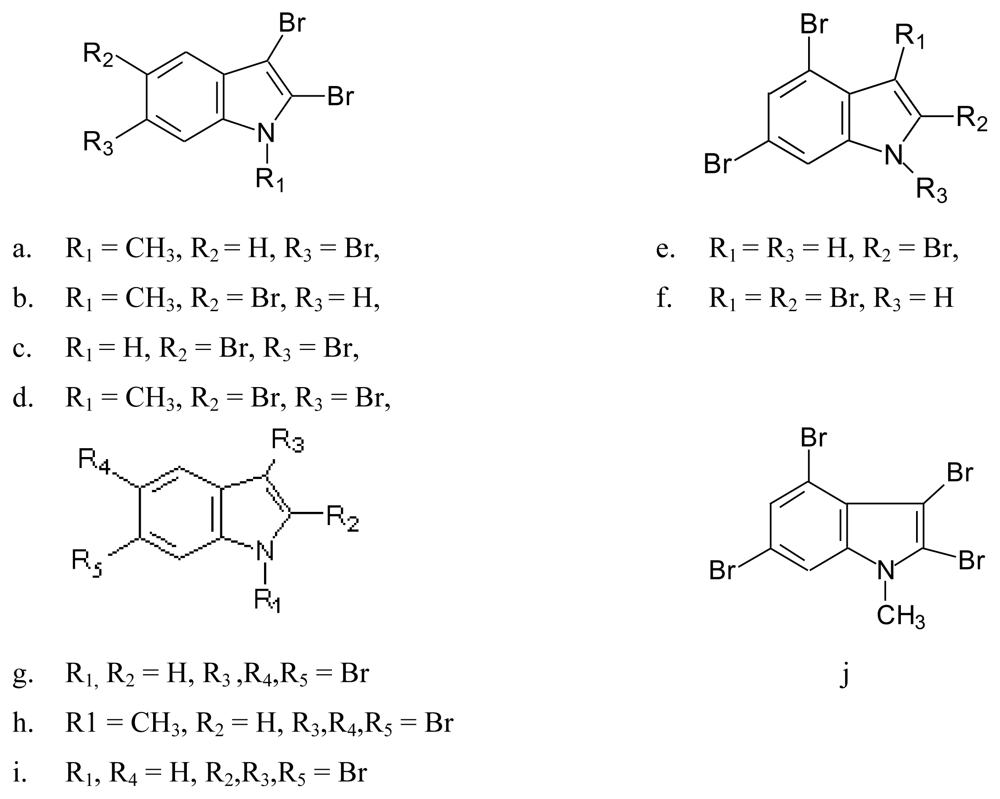
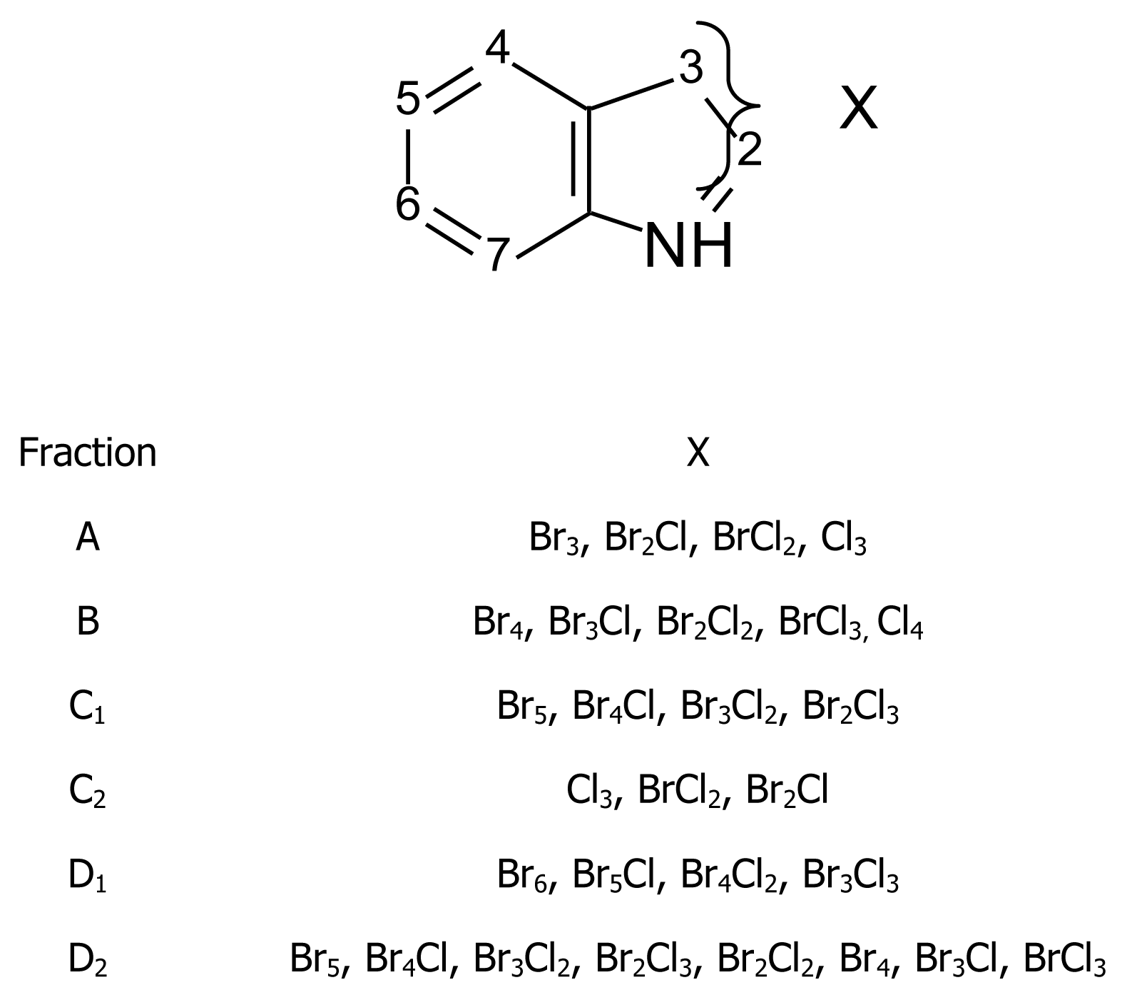
- Red alga Laurencia brongniartii collected from Caribbean Sea [70] and Okinawan Sea [71] (Figure 9): 2,3,6-tribromo-1-methyl indole (9a) [70], 2,3,5-tribromo-1-methyl indole (9b) [72], 2,3,5,6-tetrabromo-1H-indole (9c) [70], 2,3,5,6-tetrabromo-1-methyl indole (9d) [70], 2,4,6-tribromo-1H-indole (9e)[71] and 2,3,4,6-tetrabromo-1H-indole (9f) [71]. Compounds 9c and 9f were also identified in the red alga Laurencia similis collected from Pulau Gaya, Malaysia [72] and compound 9b was isolated from a red alga Laurencia decumbens collected from Weizhou Island (South China-Sea) [73].Pharmacological activity: Among compounds 9a–d only 9c showed antibacterial activity against Bacillus subtilis and Saccharomyces cerevisiae [70].
4.2. Sulfur-containing bromoalkaloids isolated from Laurencia brongniartii
- Six new indoles of which the two are sulfoxides from the Okinawan Sea [71].
- A bisindole collected in Okinawan waters [71].
4.3. Polyhalogenated indoles
4.4. Bromobisindole
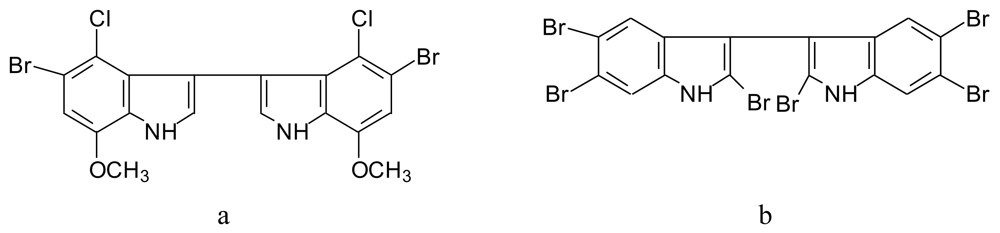
4.4.1. Polyhalogenated bisindoles (Figure 13)
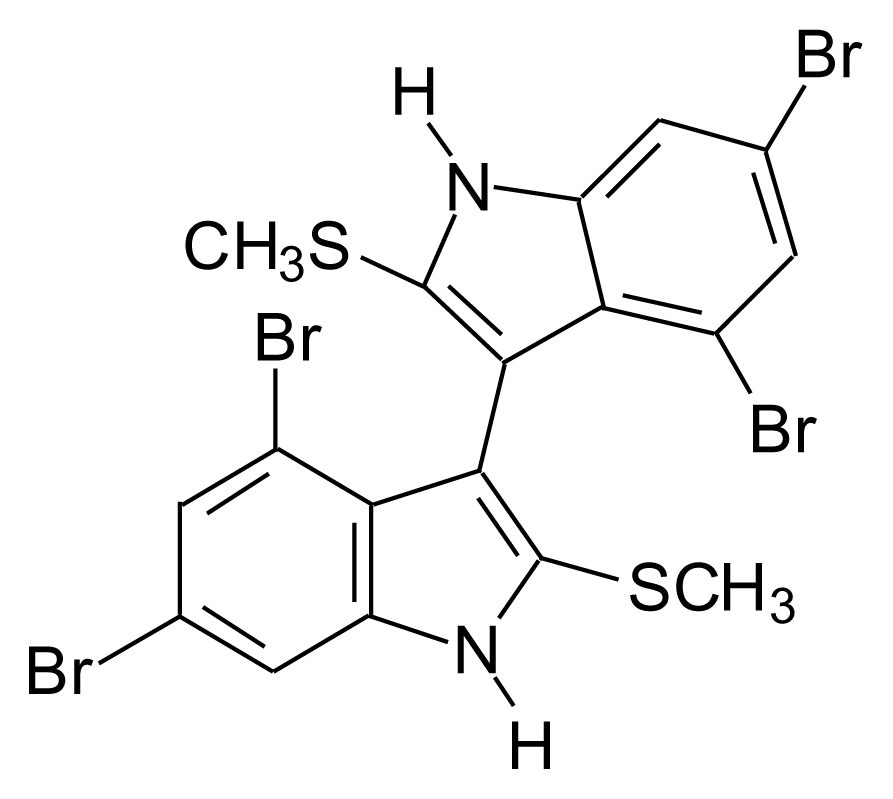
4.4.2. Thiomethyl-containing bromobisindoles
5. Other
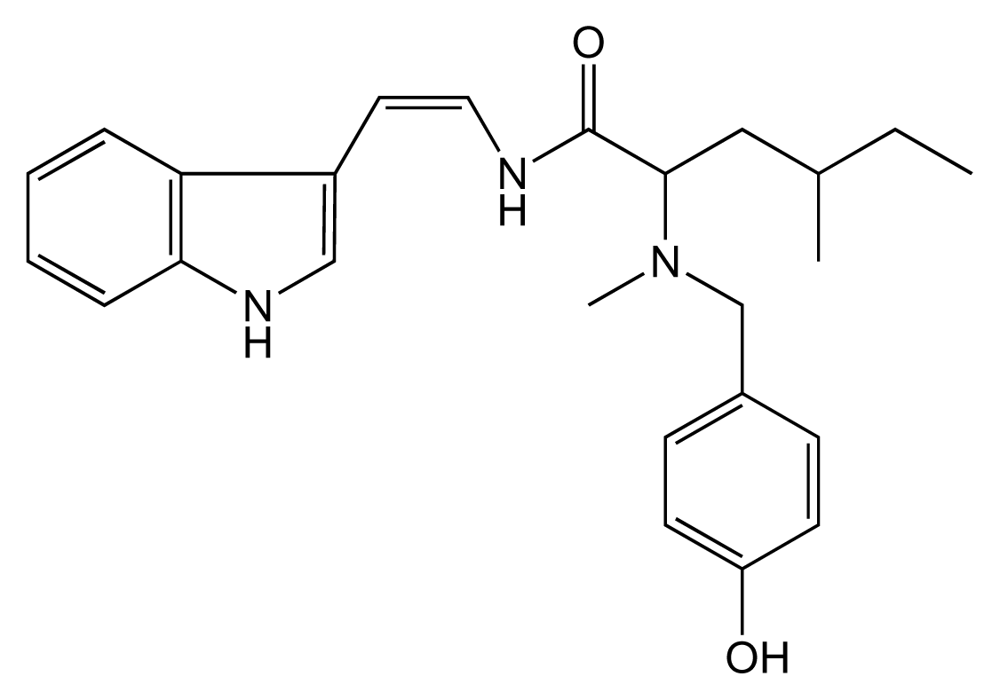
5.1. Lophocladines (LO, Figure 15)
6. Conclusions
Acknowledgements
- Samples Availability: Available from the authors.
References and Notes
- Pelletier, SW. Chemistry of the Alkaloids; Van Nostrand Reinhold: New York, NY, USA, 1970; p. 1. [Google Scholar]
- Trier, G. Die Alkaloide; Verlag von Gebrüder: Borntraeger, Berlin, Germany, 1931; pp. 1–10. [Google Scholar]
- Swan, GA. An Introduction to the Alkaloids; Black Well Scientific: Oxford and Edinburgh, UK, 1967; p. 1. [Google Scholar]
- Bentley, KW. The Alkaloids; Interscience: New York, NY, USA, 1957; p. 1. [Google Scholar]
- Numata, A; Takahashi, C; Ito, Y; Takada, T; Kawai, K; Usami, Y; Matsumura, E; Imachi, M; Ito, T; Hasegawo, T. Communesin, cytotoxic metabolites of a fungus isolated from a marine algae. Tetrahedron Lett 1993, 34, 2355–2358. [Google Scholar]
- Takahashi, C; Takai, Y; Kimura, Y; Numata, A; Shigematsu, N; Tanaka, H. Cytotoxic metabolites from a fungi adherent of a marine alga. Phytochemistry 1995, 38, 155–158. [Google Scholar]
- Kappelmeier, P. Die Konstitutions Erforschung der Wichtigten Opium Alkaloide; Verlag von Ferdinand Enke: Stuttgart, Germany, 1912; p. 4. [Google Scholar]
- Guven, KC; Bora, A; Sunam, G. Alkaloid content of marine algae. I. Hordenine from Phyllophora nervosa. Eczacılık Bul 1969, 11, 177–184. [Google Scholar]
- Guven, KC; Bora, A; Sunam, G. Hordenine from the alga Phyllophora nervosa. Phytochemistry 1970, (9), 1893. [Google Scholar]
- Boit, HG. Ergebnisse der Alkaloid-Chemic; Academie-Verlag: Berlin, Germany, 1961; p. 16. [Google Scholar]
- Steiner, M; Hartmann, T. Über Verkommen und Verbreitung flüchtiger Amine bei Meeresalgen. Planta 1968, 79, 113–121. [Google Scholar]
- Kneifel, H. Amine in Algae Marine Algae in Pharmaceutical. In Science; Hoppe, HA, Levring, T, Tanaka, Y, Eds.; Walter de Gruyter: Berlin, Germany, 1979; pp. 365–401. [Google Scholar]
- Smith, TA. Phenethylamine and related compounds in plant. Phytochemistry 1977, 16, 9–18. [Google Scholar]
- Percot, A; Yalçın, A; Aysel, V; Erdugan, H; Dural, B; Güven, KC. β-Phenylethylamine content in marine algae around Turkish coasts. Bot Mar 2009, 52, 87–90. [Google Scholar]
- Rolle, I; Hobucher, HE; Kneifel, H; Paschold, B; Riepe, W; Soeder, CJ. Amines in unicellular green algae. 2. Amines in Scenedesmus acutus. Anal Biochem 1977, 77, 103–109. [Google Scholar]
- Barroso, N; Rodriguez, M. Action of β-phenylethylamine and related amines on nigrostriatal dopamine neurotransmission. Eur J pharmacol 1996, 297, 195–203. [Google Scholar]
- Saavedra, JM. β-Phenylethylamine: Is this biogenic amine related to neuropsychiatic disease. Mod Pharmacol Toxicol 1978, 12, 139–157. [Google Scholar]
- Percot, A; Guven, KC; Aysel, V; Erdugan, H; Gezgin, T. N-acetyltyramine from Phyllophora crispa (Hudson) P.S. Dixon and N-acetylphenylethylamine from Gelidium crinale (Hare ex Turner) Graillon. Acta Pharm Sci 2009, 51, 9–14. [Google Scholar]
- Kneifel, H; Meinicke, M; Soeder, ÇJ. Analysis of amines in algae by high performance liquid chromatography. J Phycol 1977, 13, 36. [Google Scholar]
- Tian-Shung, WU; Yanna-Lii, L; Yu-Yi, C. Constituents of the fresh leaves of Aristolochia cucurbitifolia. Chem Pharm Bull 1999, 47, 571–573. [Google Scholar]
- Von Studnitz, W; Hanson, A. Demonstration of urinary N-acetyltyramine in patients with neuroblastoma. Clin Chim Acta 1967, 16, 180–183. [Google Scholar]
- Heffter, A. Ueber Pellote. Ein Beitrag zur pharmakologischen Kenntnis der Cacteen. (Naunyn- Schmiedeberg’s). Arch Exp Path Pharm 1894, 34, 65–86. [Google Scholar]
- Leger, E. Hordenine: A new alkaloid extracted from the germ of barley. J Pharm Chim 1906, 23, 177–181. [Google Scholar]
- Kawauchi, H; Sasaki, T. Isolation and identification of hordenine, p-(2-dimethylamino) ethylphenol from Ahnfeltia paradoxa. Bull Jap Soc Sci Fish 1978, 44, 135–137. [Google Scholar]
- Barwell, C; Blunden, G. Hordenine from the red alga Gigartina stellata. J Nat Prod 1981, 44, 500–502. [Google Scholar]
- Percot, A; Yalcın, A; Erduğan, H; Guven, KC. Hordenine amount in Phyllophora nervosa (D.C. Grev) (Marine alga) collected from Şile (The Black Sea) and Dardanelle. Acta Pharm Sci 2007, 49, 127–132. [Google Scholar]
- Yalcın, A; Percot, A; Erdugan, H; Coban, B; Guven, KC. Hordenine in marine alga, Gelidium crinale (Hare ex Turner) Gaillon. Acta Pharm Sci 2007, 49, 213–218. [Google Scholar]
- Baslow, MH. Marine Pharmacology; The William & Wilkins Co: Baltimore, MD, USA, 1969; pp. 56–85. [Google Scholar]
- Schweitzer, A; Wright, S. Action of hordenine compounds on the central nervous system. J Physiol 1938, 92, 422–438. [Google Scholar]
- Hapke, HJ; Strathmann, W. Pharmacological effects of hordenine. Deutsche tierärztliche Wochenschrift 1995, 102, 228–232. [Google Scholar]
- Tocher, RD; Tocher, CA. Biosynthesis of 3-hdroxy tyramine in plants Enz. Dopa decarboxylaze XI International Botanical Congresse, University of Washington, Seattle, WA, USA, August 24–September 2, 1969.
- Sweetman, SC. Martindale. In The Complete Drug Reference; Pharmaceutical Press, Williams Clowes: Suffock, UK, 2005; p. 907. [Google Scholar]
- Aguilar-Santos, G; Doty, MS. Chemical studies on three species of the marine algal genus Caulerpa. In Drugs from the Sea; Freudenthal, HD, Ed.; Marine Technology Society: Washington, DC, USA, 1968; pp. 173–176. [Google Scholar]
- Aguilar-Santos, G. Caulerpin, a new red pigment from green algae of the genus Caulerpa. J Chem Soc Perkin C: Org 1970, 6, 842–843. [Google Scholar]
- Maiti, BC; Thomson, RH; Mahendran, M. The structure of caulerpin, a pigment from Caulerpa algae. J Chem Res Synop 1978, 4, 126–127. [Google Scholar]
- Lu, Y; Lu, D; Zheng, QT; Lian, B; Su, JY; Cen, YZ. Crystal structure determination of caulerpin. Jiegou Huaxue 1994, 13, 472–476. [Google Scholar]
- Anjaneyulu, ASR; Prakash, CVS; Mallavadhani, UV. Two caulerpin analogues and a sesquiterpene from Caulerpa racemosa. Phytochemistry 1991, 30, 3041–3042. [Google Scholar]
- Santos, GA; Doty, MS. Constituents of the green alga Caulerpa lamourouxii. Lloydia 1971, 34, 88–90. [Google Scholar]
- McConnell, OJ; Hughes, PA; Targett, NM; Daley, J. Effects of secondary metabolites from marine algae on feeding by the sea urchin Lytechinus variegatus. J Chem Ecol 1982, 8, 1437–1453. [Google Scholar]
- Vest, SE; Dawes, CJ; Romeo, JT. Distribution of caulerpin and caulerpicin in eight species of the green alga Caulerpa (Caulerpales). Bot Mar 1983, 26, 313–316. [Google Scholar]
- Capon, RJ; Ghisalberti, EL; Jefferies, PR. Metabolites of the green algae, Caulerpa species. Phytochemistry 1983, 22, 1465–1467. [Google Scholar]
- Cheng, F; Zhou, Y; Wu, J; Zhou, K. Chemical constituents of Caulerpa peltata. Shizhen Guoyi Guoyao 2008, 19, 856–857. [Google Scholar]
- Vidal, JP; Laurent, D; Kabore, SA; Rechencq, E; Boucard, M; Girard, JP; Escale, R; Rossi, JC. Caulerpin, Caulerpicin, Caulerpa scalpelliformis: Comparative acute toxicity study. Bot Mar 1984, 27, 533–537. [Google Scholar]
- Mao, S.-C; Guo, Y.-W; Shen, X. Two novel aromatic valerenane-type sesquiterpenes from Chinese green alga Caulerpa taxifolia. Biorg Med Chem Lett 2006, 16, 2947–2950. [Google Scholar]
- Schroder, HC; Badria, FA; Ayyad, SN; Batel, R; Wiens, M; Hassanein, HMA; Kurelec, B; Müller, WEG. Inhibitory effects of extracts from the marine alga Caulerpa taxifolia and of toxin from Caulerpa racemosa on multixenobiotic resistance in the marine sponge Geodia cydonium. Environ Toxicol Pharmacol 1998, 5, 119–126. [Google Scholar]
- Anjaneyulu, ASR; Prakash, CVS; Mallavadhani, UV. Sterols and terpenes of the marine green algal species Caulerpa racemosa and Codium decorticatum. J Indian Chem Soc 1991, 68, 480. [Google Scholar]
- Yan, S; Su, J; Wang, Y; Zeng, L. Studies on chemical constituents of Halimeda incrassata. Redai Haiyang 1999, 18, 91–94. [Google Scholar]
- Xu, X; Su, J. The separation, identification and bioassay of caulerpin. Ziran Kexueban 1996, 35, 64–66. [Google Scholar]
- Xu, XH; Su, JY; Zeng, LM; Wang, MY. Chemical constituents of the alga Caloglossa leprieurii. Gadeng Xuexiao Huaxue Xuebao 1998, 19, 249–251. [Google Scholar]
- Govenkar, MB; Wahidulla, S. Constituents of Chondria armata. Phytochemistry 2000, 54, 979–981. [Google Scholar]
- Vairappan, CS. Antibacterial activity of major secondary metabolities: found in four species of edible green macroalgae genus Caulerpa. Asian J Microbiol Biotech Environ Sci 2004, 6, 197–201. [Google Scholar]
- Higa, T; Kuniyoshi, M. Toxins associated with medicinal and edible seaweeds. J Toxicol Toxin Rev 2000, 19, 119–137. [Google Scholar]
- Rocha, FD; Soares, AR; Houghton, PJ; Pereira, RC; Kaplan, MAC; Teixeira, VL. Potential cytotoxic activity of some Brazilian seaweeds on human melanoma cells. Phytother Res 2007, 21, 170–175. [Google Scholar]
- Ayyad, SEN; Badria, FA. Caulerpine: An antitumor indole alkaloid from Caulerpa racemosa. Alexandria J Pharm Sci 1994, 8, 217–219. [Google Scholar]
- Xu, XH; Zhu, YD; Gao, W; Kong, CH. Agricultural lead compounds from Laurencia majuscula. Proceedings of International Forum on Green Chemical Science & Engineering and Process Systems Engineering, Tianjin, China, 8–10 October 2006; 1, pp. 538–541.
- Raub, MF; Cardellina, JH, II; Schwede, JG. The green algal pigment caulerpin as a plant growth regulator. Phytochemistry 1987, 26, 619–620. [Google Scholar]
- Huang, L; Cen, Y; Xu, S; Li, Y; Xu, S; Wu, Q. Research progress on caulerpin: plant growth regulator from algae. Tianran Chanwu Yanjiu Yu Kaifa 2001, 13, 74–78. [Google Scholar]
- Schwede, JG; Cardellina, JH; Grode, SH; James, TR, Jr; Blackman, AJ. Distribution of the pigment caulerpin in species of the green alga. Caulerpa Phytochemistry 1986, 26, 155–158. [Google Scholar]
- Su, JY; Zhu, Y; Zeng, LM; Xu, XH. A new bisindole from alga Caulerpa serrulata. J Nat Prod 1997, 60, 1043–1044. [Google Scholar]
- Fresneda, PM; Molina, P; Angeles, SM. The first synthesis of the bis (indole) marine alkaloid Caulersin. Synlett 1999, 10, 1652–1653. [Google Scholar]
- Wahlström, N; Stensland, B; Bergman, J. Synthesis of the marine alkaloid caulersin. Tetrahedron 2004, 60, 2147–2153. [Google Scholar]
- Miki, Y; Aoki, Y; Miyatake, H; Minematsu, T; Hibino, H. Synthesis of caulersin and its isomers by reaction of indole-2,3-dicarboxylic anhydrides with methyl indoleacetates. Tetrahedron Lett 2006, 47, 5215–5218. [Google Scholar]
- Bourderioux, A; Routier, S; Beneteau, V; Merour, J.-Y. Synthesis of benzo analogs of oxoarcyriaflavins and caulersine. Tetrahedron 2007, 63, 9465–9475. [Google Scholar]
- Kirkup, MP; Moore, RE. Indole alkaloids from the marine red alga Martensia fragilis. Tetrahedron Lett 1983, 24, 2087–2090. [Google Scholar]
- Takamatsu, S; Hodges, TW; Rajbhandari, I; Gerwick, WH; Hamann, MT; Nagle, DG. Marine natural products as novel antioxidant prototypes. J Nat Prod 2003, 66, 605–608. [Google Scholar]
- Takahashi, S; Matsunaga, T; Hasegawa, C; Saito, H; Fujita, D; Kiuchi, F; Tsuda, Y. Studies on bioactive substances in natural resources. VI. Isolation and structure elucidation of martefragin A. Toyama-ken Yakuji Kenkyusho Nenpo 1997, 24, 53–57. [Google Scholar]
- Nishida, A; Fuwa, M; Fujikaw, Y. First total synthesis of Martefragin A, a potent inhibitor of lipid peroxidation. Tetrahedron Lett 1998, 39, 5983–5986. [Google Scholar]
- Murakami, H; Kato, T; Mimura, A; Takahara, Y. New indole derivatives from Martensia denticulata seaweed. Biosci Biotechnol Biochem 1994, 58, 535–538. [Google Scholar]
- Guella, G; N’Diaye, I; Fofana, M; Mancini, I. Isolation synthesis and photochemical properties of almazolone, a new indole alkaloid from a red alga of Senegal. Tetrahedron 2006, 62, 1165–1170. [Google Scholar]
- Carter, GT; Rinehart, KL, Jr; Li, LH; Kuentzel, SL; Connor, JL. Brominated indoles from Laurencia brongniartii. Tetrahedron Lett 1978, 46, 4479–4482. [Google Scholar]
- Tanaka, J; Higa, T; Bernardinelli, G; Jefford, CW. Sulfur-containing polybromoindoles from the red alga Laurencia brongniartii. Tetrahedron 1989, 45, 7301–7310. [Google Scholar]
- Masuda, M; Kawaguchi, S; Takahashi, Y; Okamoto, K; Suzuki, M. Halogenated secondary metabolites of Laurencia similis (Rhodomelaceae, Rhodophyta). Bot Mar 1999, 42, 199–202. [Google Scholar]
- Ji, NY; Li, XM; Cui, CM; Wang, BG. Terpenes and polybromoindoles from the marine red alga Laurencia decumbens (Rhodomelacea). Helv Chim Acta 2007, 90, 1731–1736. [Google Scholar]
- Ji, N-Y; Li, X-M; Ding, L-P; Wang, B-G. Aristolane sesquiterpenes and highly brominated indoles from the marine red alga Laurencia similis (Rhodomelaceae). Helv Chim Acta 2007, 90, 385–391. [Google Scholar]
- Brennan, MR; Erickson, KL. Polyhalogenated indoles from the marine alga Rhodophyllis membranacea Harvey. Tetrahedron Lett 1978, 19, 1637–1640. [Google Scholar]
- Shi, DY; Han, LJ; Sun, J; Li, S; Wang, SJ; Yang, YC; Fan, X; Shi, JG. A new halogenated biindole and a new apo-carotenone from green alga Chaetomorpha basiretorsa Setchell. Chin Chem Lett 2005, 16, 777–780. [Google Scholar]
- Su, H; Yuan, ZH; Li, J; Guo, SJ; Deng, LP; Han, LJ; Zhu, XB; Shi, DY. Two new bromoindoles from red alga Laurencia similis. Chin Chem Lett 2009, 20, 456–458. [Google Scholar]
- Gross, H; Goeger, DE; Hills, P; Mooberry, SL; Ballantine, DL; Murray, TF; Valeriote, FA; Gerwick, WH. Lophocladines Bioactive alkaloids from red alga Lophocladia sp. J Nat Prod 2006, 69, 640–644. [Google Scholar]

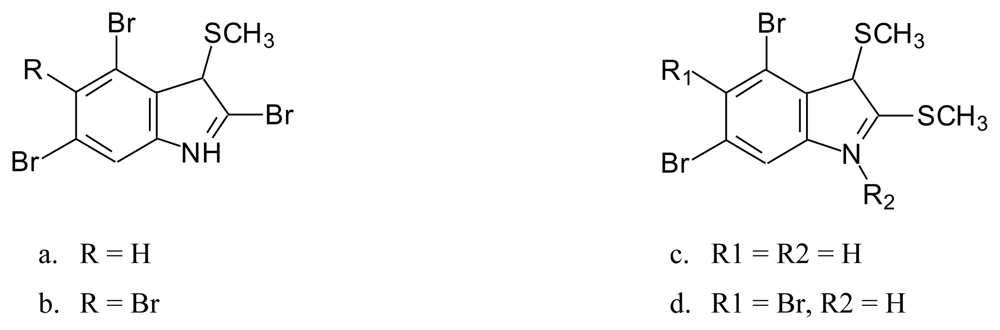

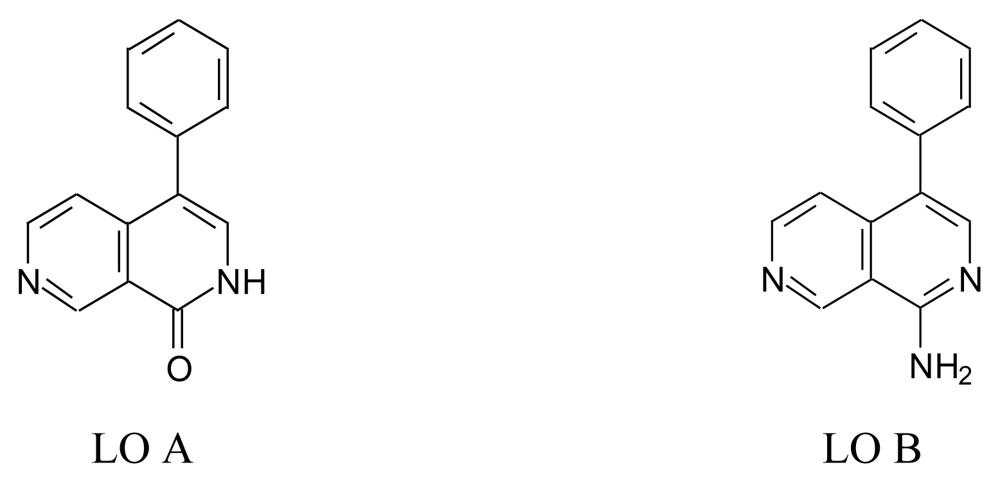
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Güven, K.C.; Percot, A.; Sezik, E. Alkaloids in Marine Algae. Mar. Drugs 2010, 8, 269-284. https://doi.org/10.3390/md8020269
Güven KC, Percot A, Sezik E. Alkaloids in Marine Algae. Marine Drugs. 2010; 8(2):269-284. https://doi.org/10.3390/md8020269
Chicago/Turabian StyleGüven, Kasım Cemal, Aline Percot, and Ekrem Sezik. 2010. "Alkaloids in Marine Algae" Marine Drugs 8, no. 2: 269-284. https://doi.org/10.3390/md8020269
APA StyleGüven, K. C., Percot, A., & Sezik, E. (2010). Alkaloids in Marine Algae. Marine Drugs, 8(2), 269-284. https://doi.org/10.3390/md8020269



