Oyster (Crassostrea gigas) Hydrolysates Produced on a Plant Scale Have Antitumor Activity and Immunostimulating Effects in BALB/c Mice
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Production of oyster hydrolysates on a laboratory scale and its scale-up to pilot and plant levels
2.3. Characterization of oyster hydrolysates
2.4. Animals
2.5. Analysis of the spleen and thymus indices of the mice
2.6. Assay of lymphocyte proliferation
2.7. Assay of NK cell activity
2.8. Assay of macrophage phagocytosis
2.9. Statistical analysis
3. Results
3.1. Production and characterization of the oyster hydrolysates generated on laboratory, pilot and plant scales
3.2. Antitumor activity of oyster hydrolysates on S180-bearing mice and their effects on thymus and spleen indices
3.3. Effects of oyster hydrolysates on cellular immunity in S180-bearing mice
3.4. Effects of oyster hydrolysates on macrophage phagocytosis in S180-bearing mice
4. Discussion
5. Conclusions
Acknowledgements
- Samples Availability: Available from the authors.
References
- In, MJ; Chae, HJ; Oh, NS. Process development for heme-enriched peptide by enzymatic hydrolysis of hemoglobin. Bioresource Technol 2002, 84, 63–68. [Google Scholar]
- Simpson, BK; Haard, NF. The use of proteolytic enzymes to extract carotenoproteins from shrimp wastes. J Appl Biochem 1985, 7, 212–222. [Google Scholar]
- Shahidi, F; Synowiecki, J; Balejko, J. Proteolytic hydrolysis of muscle proteins from Harp seal (Phoca groenladica). J Agric Food Chem 1994, 42, 2634–2638. [Google Scholar]
- Ferrer, J; Paez, G; Marmol, Z; Ramones, E; Garcia, H; Forster, CF. Acid hydrolysis of shrimp-shell wastes and the production of single cell protein from the hydrolysates. Bioresource Technol 1996, 57, 55–60. [Google Scholar]
- Gobbetti, M; Ferranti, P; Smacchi, E; Goffredi, F; Addeo, F. Production of angiotensin-I-converting enzyme inhibitory peptides in fermented milks started by Lactobacillus delbrueckii subsp. bulgaricus SS1 and Lactococcus lactis subsp. cremoris FT4. Appl Environ Microbiol 2000, 66, 3898–3904. [Google Scholar]
- Afonso, MD; Borquez, R. Review of the treatment of seafood processing wastewaters and recovery of proteins therein by membrane separation processes-prospects of the ultrafiltration of wastewaters from the fish meal industry. Desalination 2002, 142, 29–54. [Google Scholar]
- Egusa, S; Otani, H. Soybean protein fraction digested with neutral protease preparation, “Peptidase R”, produced by Rhizopus oryzae, stimulates innate cellular immune system in mouse. Int Immunopharmacol 2009, 9, 931–936. [Google Scholar]
- Yang, MP; Eoum, HY; Na, KJ; Araki, S; El-Abasy, M; Motobu, M; Hirota, Y. Enhanced phagocytic activity of neutrophils caused by administration of egg white derivatives (EWD) in cats injected with cyclophosphamide (CPA). J Vet Med Sci 2001, 63, 269–274. [Google Scholar]
- Meisel, H. Overview on milk protein-derived peptides. Int Dairy J 1998, 8, 363–373. [Google Scholar]
- Gildberg, A; Bogwald, J; Johansen, A; Stenberg, E. Isolation of acid peptide fractions from a fish protein hydrolysate with strong stimulatory effect on Atlantic salmon (Salmo salar) head kidney leucocytes. Comp Biochem Physiol 1996, 11, 97–101. [Google Scholar]
- Yang, R; Zhang, Z; Pei, X; Han, X; Wang, J; Wang, L; Long, Z; Shen, X; Li, Y. Immunomodulatory effects of marine oligopeptide preparation from Chum Salmon (Oncorhynchus keta) in mice. Food Chem 2009, 113, 464–470. [Google Scholar]
- Morris, HJ; Carrillo, O; Almarales, A; Bermudez, RC; Lebeque, Y; Fontaine, R; Llaurado, G; Beltran, Y. Immunostimulant activity of an enzymatic protein hydrolysate from green microalga Chlorella vulgaris on undernourished mice. Enzyme Microb Technol 2007, 40, 456–460. [Google Scholar]
- Plaza, M; Herrero, M; Cifuentes, A; Ibánez, E. Innovative natural functional ingredients from microalgae. J Agric Food Chem 2009, 57, 7159–7170. [Google Scholar]
- Je, JY; Park, JY; Jung, WK; Park, PJ; Kim, SK. Isolation of angiotensin I converting enzyme (ACE) inhibitor from fermented oyster sauce Crassostrea gigas. Food Chem 2005, 90, 809–814. [Google Scholar]
- Liu, ZY; Zeng, MY; Dong, SY; Xu, J; Song, HX; Zhao, YH. Effect of an antifungal peptide from oyster enzymatic hydrolysates for control of gray mold (Botrytis cinerea) on harvested strawberries. Postharvest Biol Technol 2007, 46, 95–98. [Google Scholar]
- Li, P; Li, QF; Huang, DC; Li, XQ; Liu, M. The research of isolation bioactive peptides from Saccostrea cucullata and biological effects on the human gastric adenocarcinoma BGC-823 cells (in Chinese). J Xiamen University (Natural Science) 2002, 41, 618–622. [Google Scholar]
- He, HL; Chen, XL; Li, JW; Zhang, YZ; Gao, PJ. Taste improvement of refrigerated meat treated with cold adapted protease. Food Chem 2004, 84, 307–311. [Google Scholar]
- Mullally, MM; Meisel, H; FitzGerald, RJ. Angiotensin-I-converting enzyme inhibitory activities of gastric and pancreatic proteinase digests of whey proteins. Int Dairy J 1997, 7, 299–303. [Google Scholar]
- Kapel, R; Chabeau, A; Lesage, J; Riviere, G; Ravallec-Ple, R; Lecouturier, D; Wartelle, M; Guillochon, D; Dhulster, P. Production, in continuous enzymatic membrane reactor, of an anti-hypertensive hydrolysates from an industrial alfalfa white protein concentrate exhibiting ACE inhibitory and opioid activities. Food Chem 2006, 98, 120–126. [Google Scholar]
- He, HL; Chen, XL; Sun, CY; Zhang, YZ; Zhou, BC. Preparation and functional evaluation of oligopeptide-enriched hydrolysates from shrimp (Acetes chinensis) treated with crude protease from Bacillus sp. SM98011. Bioresource Technol 2006, 97, 385–390. [Google Scholar]
- Denizot, F; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Method 1986, 89, 271–277. [Google Scholar]
- Yuan, H; Song, J; Li, X; Li, N; Dai, J. Immunomodulation and antitumor activity of κ-carrageenan oligosaccharides. Cancer Lett 2006, 243, 228–234. [Google Scholar]
- Wang, JB; Yu, HL; Shen, XY; Long, Z; Yan, SF; Kodama, N; Aoki, H; Li, Y. Effect of Chinese soft-shell turtle egg powder on immune functions in mice. Food Agr Immunol 2003, 15, 207–216. [Google Scholar]
- Achour, A; Lachgar, A; Astgen, A; Chams, V; Bizzini, B; Tapiero, H; Zagury, D. Potentialization of IL-2 effects on immune cells by oyster extract (JCOE) in normal and HIV infected individuals. Biomed Pharmacotherapy 1997, 51, 427–429. [Google Scholar]
- Tapiero, H; Tew, KD. Increased glutathione expression in cells induced by Crassostrea gigas extract (JCOE). Biomed Pharmacotherapy 1996, 50, 149–153. [Google Scholar]
- Tapiero, H; Gaté, L; Dhalluin, S; Nguyen, BG; Soupramanien, V; Kouyate, J; Tew, KD. The antioxidant effects of Crassostrea gigas extract (JCOE) in human volunteers. In vivo 1998, 12, 305–309. [Google Scholar]
- Lampidis, TJ; Kolonias, D; Tapiero, H. Effects of Crassoatrea gigas extract (JCOE) on cardiac cell function in vitro: Antiarrhythmic activity. Cell Pharmacol 1996, 4, 241–247. [Google Scholar]
- Lee, TG; Maruyama, S. Isolation of HIV-1 protease-inhibiting peptides from thermolysin hydrolysates of oyster proteins. Biochem Biophys Res Commun 1998, 253, 604–608. [Google Scholar]
- Zheng, R; Jie, S; Hanchuan, D; Moucheng, W. Characterization and immunomodulating activities of polysaccharide from Lentinus edodes. Int Immunopharmacol 2005, 5, 811–820. [Google Scholar]
- Han, SB; Lee, CW; Jeon, YJ; Hongc, ND; Yooa, ID; Yang, KH. The inhibitory effect of polysaccharides isolated from Phellinus linteus on tumor growth and metastasis. Immunopharmacology 1999, 41, 157–164. [Google Scholar]
- Li, X; Jiao, LL; Zhang, X; Tian, WM; Chen, S; Zhang, LP. Anti-tumor and immunomodulating activities of proteoglycans from mycelium of Phellinus nigricans and culture medium. Int Immunopharmacol 2008, 8, 909–915. [Google Scholar]
- Neutra, M; Krachenbuhl, J. M cells as a pathway for antigen uptake and processing. In Essentials of Mucosal Immunology; Kagnoff, M, Kiyono, H, Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 29–36. [Google Scholar]
- Wang, WP; Iigo, M; Sato, J; Sekine, K; Adachi, I; Tsuda, H. Activation of intestinal mucosal immunity in tumor-bearing mice by lactoferrin. Jpn J Cancer Res 2000, 91, 1022–1027. [Google Scholar]
- Engelhard, VH. How cells process antigens. Sci Am 1994, 271, 62–72. [Google Scholar]
- Fearon, DT; Locksley, RM. The instructive role of innate immunity in the acquired immune response. Science 1996, 272, 50–52. [Google Scholar]
- Compton, R; Williams, D; Browder, W. The beneficial effect of enhanced macrophage function on the healing of bowel anastomoses. Am Surg 1996, 62, 14–18. [Google Scholar]
- Duarte, J; Vinderola, G; Ritz, B; Perdigón, G; Matar, C. Immunomodulating capacity of commercial fish protein hydrolysate for diet supplementation. Immunobiology 2006, 211, 341–350. [Google Scholar]
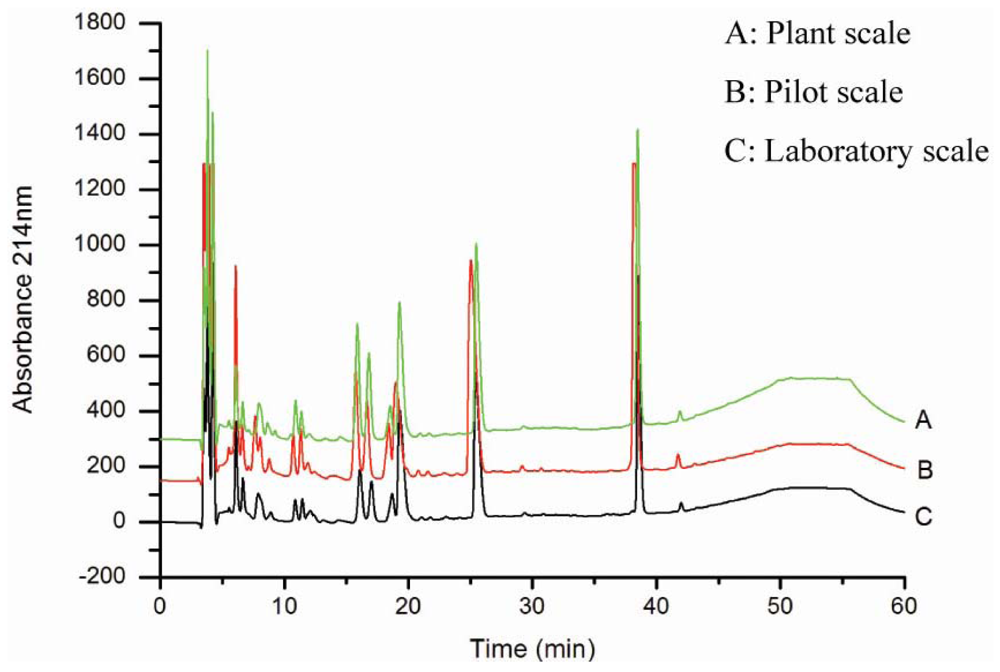
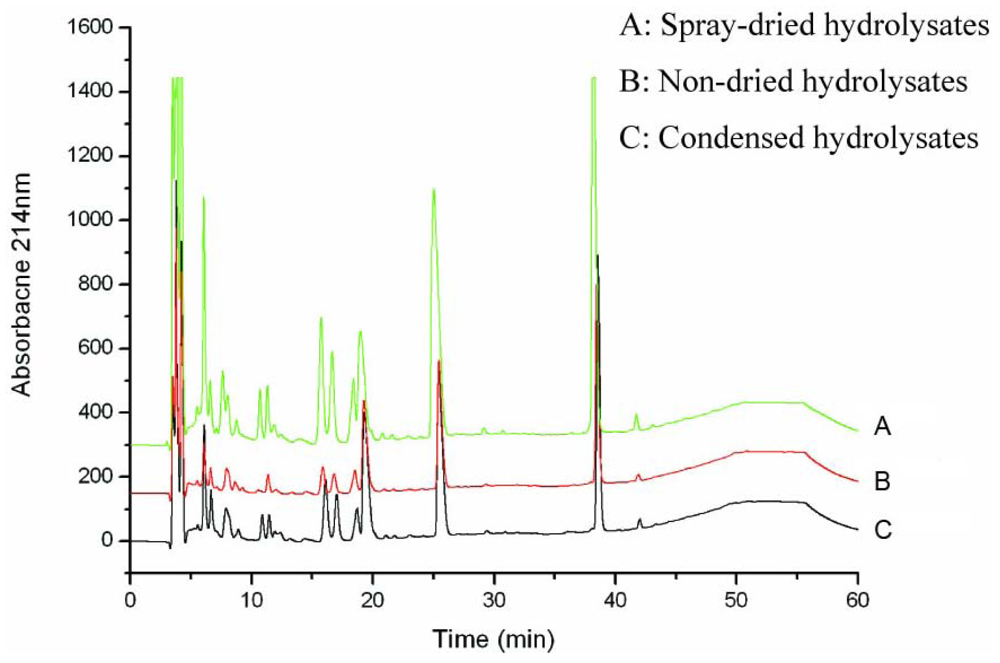
| Amino acid | Lyophilized hydrolysates at laboratory scale (g/100 g) | Spray dried hydrolysates at pilot scale (g/100 g) | Spray dried hydrolysates at plant scale (g/100 g) |
|---|---|---|---|
| Asp | 6.26 | 6.39 | 6.63 |
| Ser | 2.69 | 2.75 | 2.98 |
| Glu | 9.15 | 9.24 | 9.27 |
| Gly | 3.85 | 3.66 | 3.51 |
| His | 1.71 | 1.65 | 1.77 |
| Arg | 7.98 | 8.40 | 8.12 |
| Thr | 2.82 | 2.72 | 2.76 |
| Ala | 3.70 | 3.67 | 3.86 |
| Pro | 2.74 | 2.76 | 2.97 |
| Cys | 0.23 | 0.22 | 0.21 |
| Tyr | 0.65 | 0.65 | 0.76 |
| Val | 1.52 | 1.55 | 1.64 |
| Met | 3.82 | 3.82 | 3.94 |
| Lys | 4.24 | 4.21 | 4.42 |
| Ile | 3.91 | 3.96 | 4.17 |
| Leu | 6.17 | 6.30 | 6.53 |
| Phe | 6.15 | 5.85 | 6.28 |
| Total | 67.59 | 67.80 | 69.82 |
| Groups | Tumor weight (g) | Inhibition rate (%) | Thymus index (mg/g) | Spleen index (mg/g) |
|---|---|---|---|---|
| Normal control | - | - | 0.6480 ± 0.1143* | 6.0961 ± 0.3930*** |
| CTX | 0.449 ± 0.118*** | 82.5 | 0.5953 ± 0.0565** | 5.6334 ± 1.0100** |
| S180 control | 2.567 ± 0.077 | - | 0.8154 ± 0.0740 | 8.8186 ± 0.3967 |
| a 0.25 mg/g | 2.393 ± 0.111 | 6.8 | 0.6969 ± 0.1410 | 9.4700 ± 1.1800 |
| a 0.5 mg/g | 1.781 ± 0.226** | 30.6 | 0.8665 ± 0.0100 | 9.4267 ± 0.7975 |
| a 1 mg/g | 1.335 ± 0.066*** | 48.0 | 1.0014 ± 0.0898* | 10.7345 ± 0.7030** |
| Crude b (1 mg/g) | 1.922 ± 0.216** | 25.1 | 0.9397 ± 0.1937** | 10.2135 ± 0.6570* |
| Groups | NK activity (%) | Lymphocyte proliferation A570 |
|---|---|---|
| Normal control | 42.22 ± 12.60 | 0.6800 ± 0.0735* |
| CTX | 20.70 ± 6.76* | 0.1589 ± 0.0868** |
| S180 control | 29.37 ± 8.83 | 0.5070 ± 0.0558 |
| a 0.25 mg/g | 43.64 ± 9.70* | 0.7084 ± 0.1807 |
| a 0.5 mg/g | 51.65 ± 9.69** | 0.8219 ± 0.0256*** |
| a 1 mg/g | 58.35 ± 12.50*** | 0.8640 ± 0.0456*** |
| Crude b (1 mg/g) | 44.30 ± 12.10* | 0.7536 ± 0.1196* |
| Groups | Phagocytic rate (%) | Phagocytic index |
|---|---|---|
| Normal control | 50.0 ± 3.6 | 1.077 ± 0.045 |
| CTX | 37.3 ± 1.5* | 0.650 ± 0.036* |
| S180 control | 46.0 ± 1.0 | 1.130 ± 0.061 |
| a 0.25 mg/g | 45.7 ± 1.5 | 1.073 ± 0.059 |
| a 0.5 mg/g | 54.3 ± 2.1* | 0.970 ± 0.070 |
| a 1 mg/g | 61.0 ± 3.6* | 1.173 ± 0.061 |
| Crude b (1 mg/g) | 43.0 ± 2.7 | 0.953 ± 0.050 |
Abbreviations
| NK | natural killer |
| ACE | angiotensin-converting enzyme |
| Con A | Concanavalin A |
| MTT | 3-(4,5-dimethylththiazoyl-2-yl]-2,5-diphenyltetrazolium bromide |
| FBS | fetal bovine serum |
| RP-HPLC | reversed phase high-performance liquid chromatography |
| CTX | cyclophosphamide |
| DMSO | dimethyl sulfoxide |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Y.-K.; He, H.-L.; Wang, G.-F.; Wu, H.; Zhou, B.-C.; Chen, X.-L.; Zhang, Y.-Z. Oyster (Crassostrea gigas) Hydrolysates Produced on a Plant Scale Have Antitumor Activity and Immunostimulating Effects in BALB/c Mice. Mar. Drugs 2010, 8, 255-268. https://doi.org/10.3390/md8020255
Wang Y-K, He H-L, Wang G-F, Wu H, Zhou B-C, Chen X-L, Zhang Y-Z. Oyster (Crassostrea gigas) Hydrolysates Produced on a Plant Scale Have Antitumor Activity and Immunostimulating Effects in BALB/c Mice. Marine Drugs. 2010; 8(2):255-268. https://doi.org/10.3390/md8020255
Chicago/Turabian StyleWang, Yu-Kai, Hai-Lun He, Guo-Fan Wang, Hao Wu, Bai-Cheng Zhou, Xiu-Lan Chen, and Yu-Zhong Zhang. 2010. "Oyster (Crassostrea gigas) Hydrolysates Produced on a Plant Scale Have Antitumor Activity and Immunostimulating Effects in BALB/c Mice" Marine Drugs 8, no. 2: 255-268. https://doi.org/10.3390/md8020255
APA StyleWang, Y.-K., He, H.-L., Wang, G.-F., Wu, H., Zhou, B.-C., Chen, X.-L., & Zhang, Y.-Z. (2010). Oyster (Crassostrea gigas) Hydrolysates Produced on a Plant Scale Have Antitumor Activity and Immunostimulating Effects in BALB/c Mice. Marine Drugs, 8(2), 255-268. https://doi.org/10.3390/md8020255




