Seasonal Variations of the Activity of Antioxidant Defense Enzymes in the Red Mullet (Mullus barbatus l.) from the Adriatic Sea
Abstract
:1. Introduction
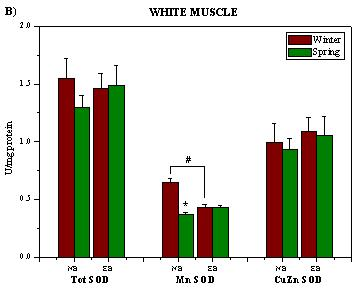
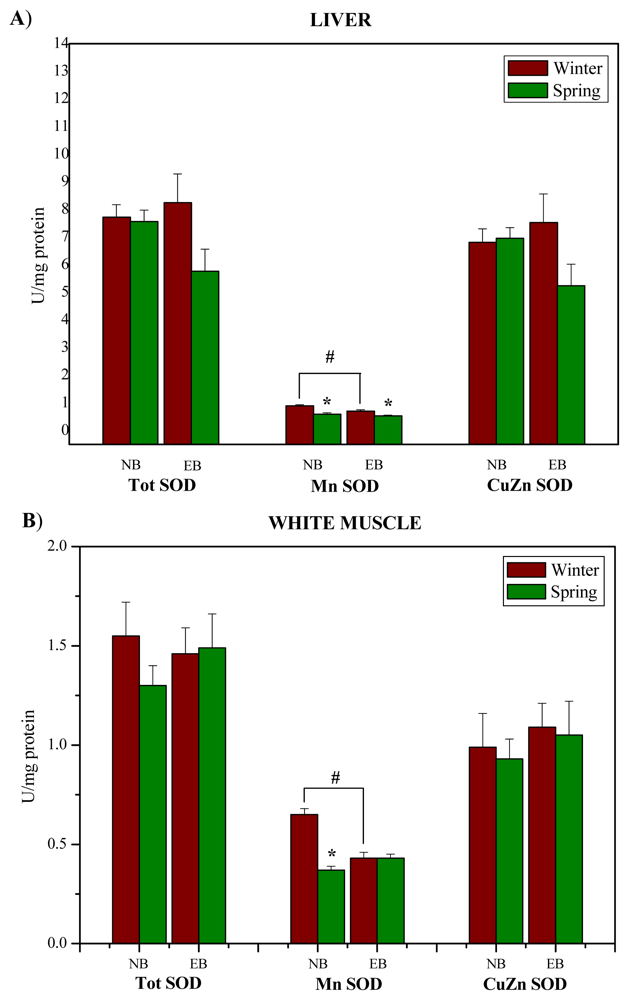
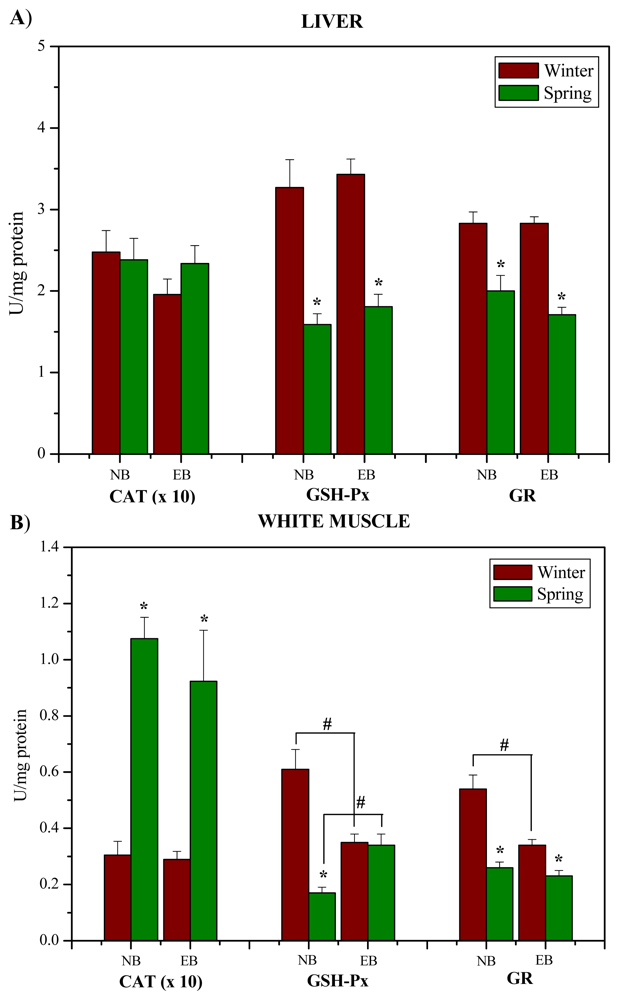
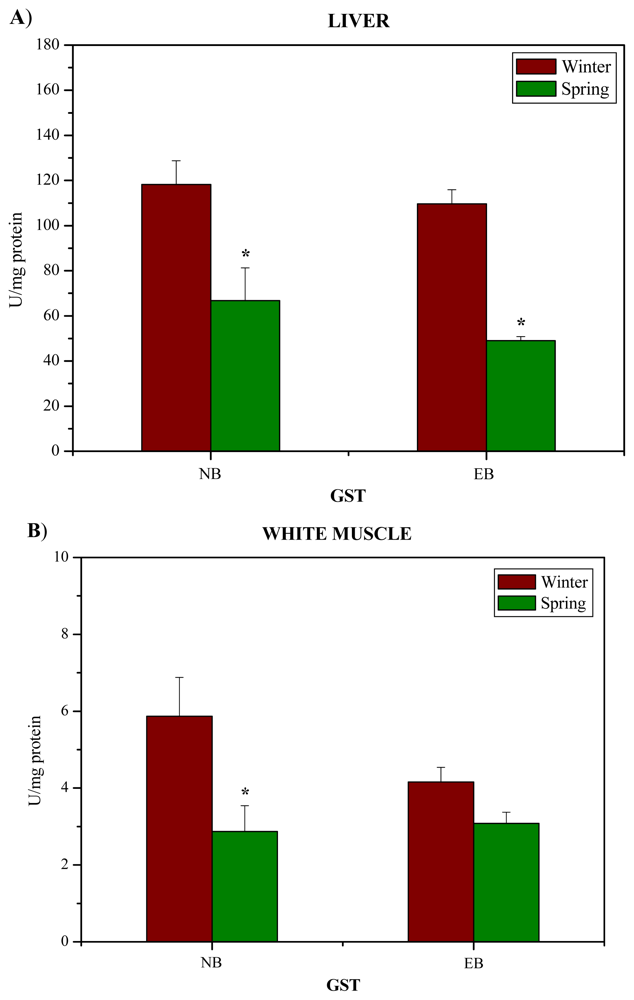
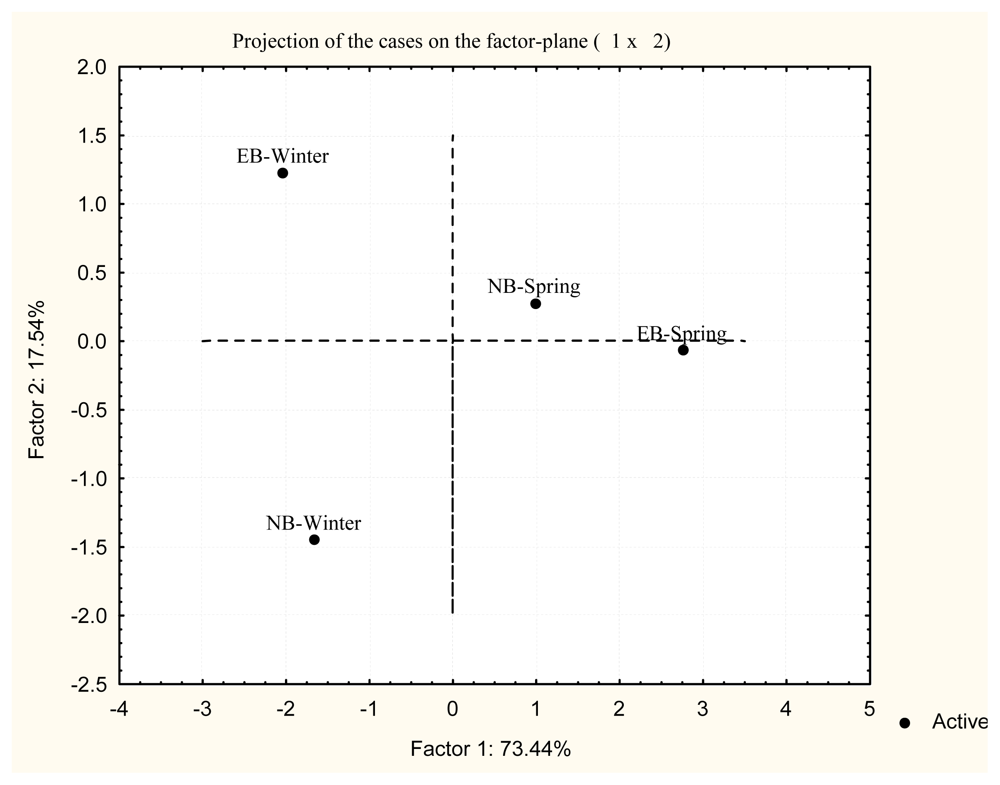
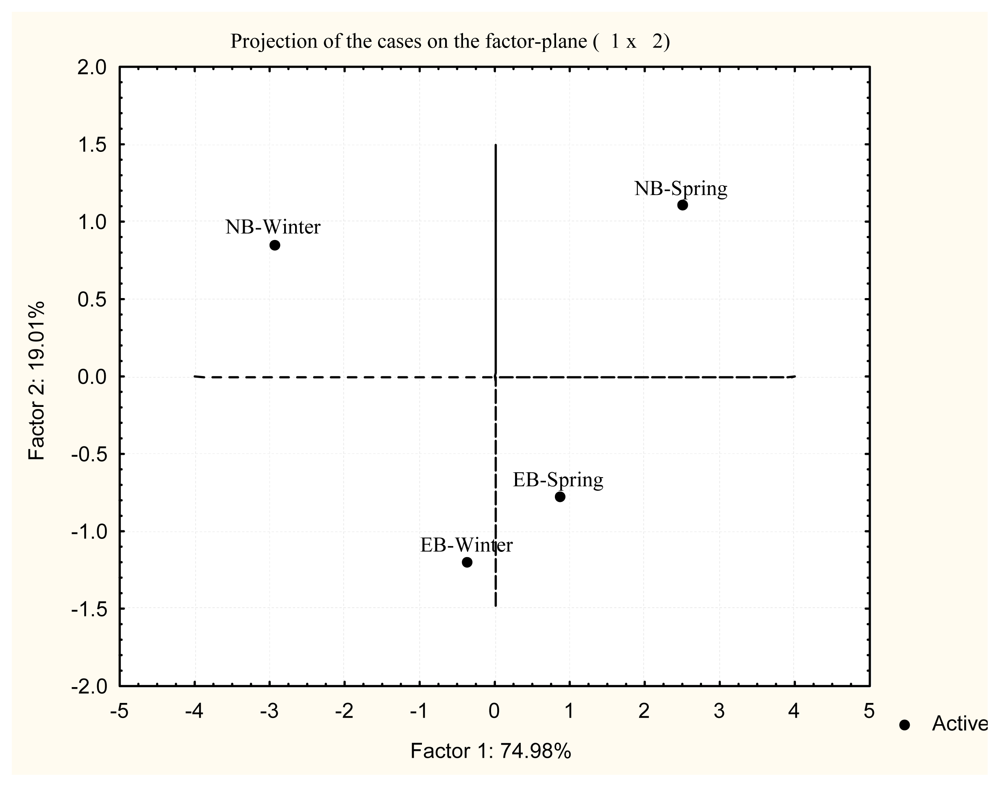
2. Results and Discussion
3. Materials and Methods
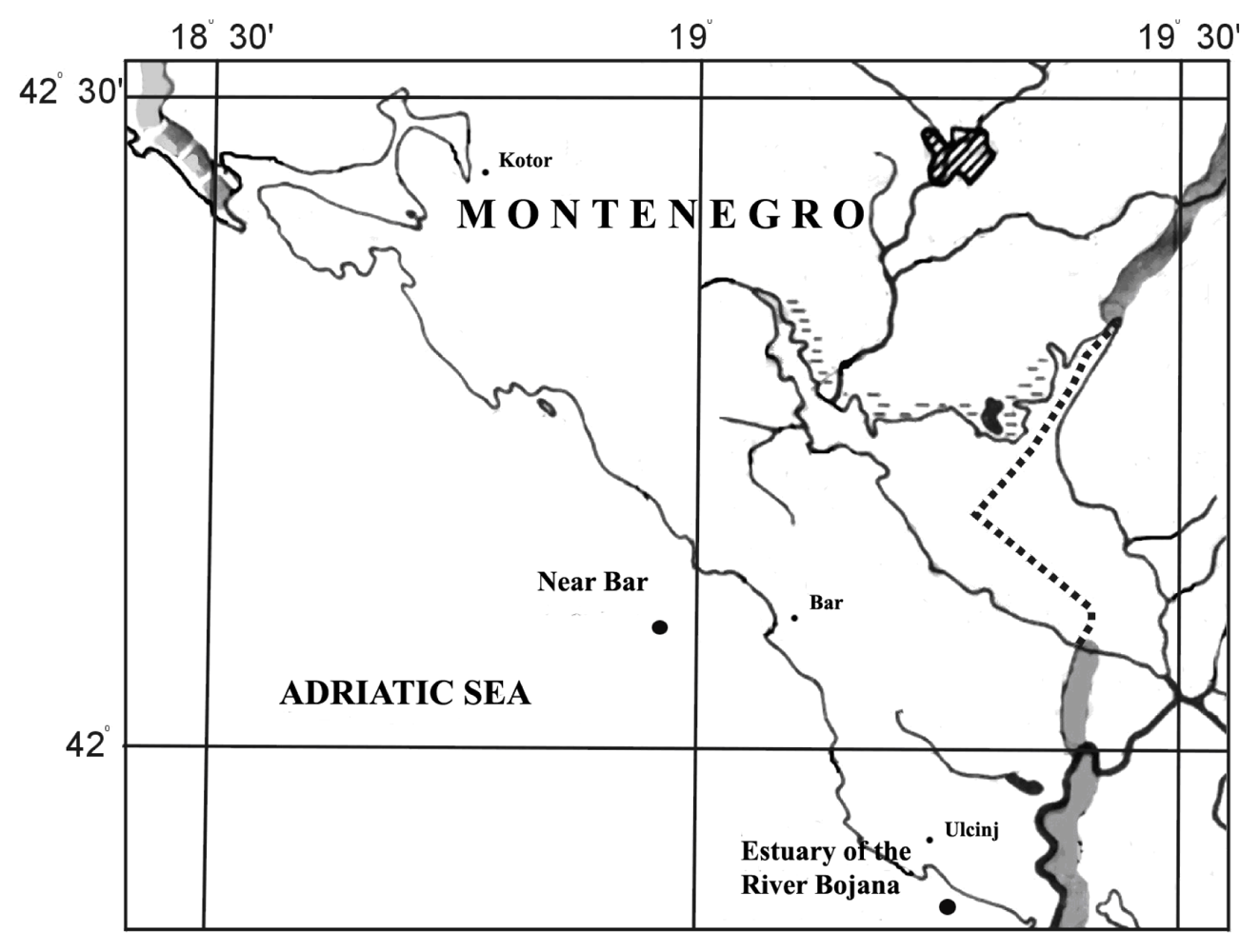
3.1. Site description and sample collection
3.2. Measurements of environmental parameters
3.3. Tissue preparation
3.4. Protein concentration measurements
3.5. Determination of antioxidant defense enzyme activities
3.6. Statistical analysis
4. Conclusions
Acknowledgment
- Samples Availability: Available from the authors.
References
- Weber, DN; Eisch, S; Spieler, RE; Petering, DH. Metal redistribution in largemouth bass (Micropterus salmoides) in response to restrainment stress and dietary cadmium: role of metallothionein and other metal-binding proteins. Comp Biochem Physiol C 1992, 101, 255–262. [Google Scholar]
- Bodin, N; Burgeot, T; Stanisiére, JY; Bockuené, G; Menard, D; Minier, C; Boutet, I; Amat, A; Cherel, Y; Budzinski, H. Seasonal variations of a battery of biomarkers and physiological indices for the mussel Mytilus galloprovincialis transplanted into the northwest Mediterranean Sea. Comp Biochem Physiol C 2004, 138, 411–427. [Google Scholar]
- Carney Almroth, B; Sturve, J; Stephensen, E; Fredrik, Holth; Förlin, L. Protein carbonyls and antioxidative defenses in corkwing wrasse (Symphodus melops) from a heavy metal polluted and PAH polluted site. Mar Environ Res 2008, 66, 271–277. [Google Scholar]
- Winston, GW; Di Giulio, RT. Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol 1991, 19, 137–161. [Google Scholar]
- Bocchetti, R; Virno Lamberti, C; Pisanelli, B; Razzetti, EM; Maggi, C; Catalano, B; Sesta, G; Martuccio, G; Gabellini, M; Regoli, F. Seasonal variations of exposure biomarkers, oxidative stress responses and cell damage in the clams, Tapes philippinarum, and mussels, Mytilus galloprovincialis, from Adriatic Sea. Mar Environ Res 2008, 66, 24–26. [Google Scholar]
- Wilhelm Filho, D; De Giulivi, C; Boveris, A. Antioxidant defences in marine fish I. Teleosts. Comp Biochem Physiol C 1993, 106, 409–413. [Google Scholar]
- Wilhelm Filho, D; Torres, MA; Marcon, JL; Fraga, CG; Boveris, A. Comparative antioxidant defences in vertebrates–emphasis on fish and mammals. Trends Comp Biochem Physiol 2000, 7, 33–45. [Google Scholar]
- Van der Oost, R; Beyer, J; Vermeulen, N. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environ Toxicol Pharmacol 2003, 13, 57–149. [Google Scholar]
- Pavlović, SZ; Belić, D; Blagojević, DP; Radojičić, RM; Žikić, RV; Saičić, ZS; Lajšić, GG; Spasić, MB. Seasonal variations of cytosolic antioxidant enzyme activities in liver and white muscle of thinlip gray mullet (Liza ramada Risso) from the Adriatic Sea. Cryo Lett 2004, 25, 273–285. [Google Scholar]
- Pavlović, SZ; Borković, SS; Kovačević, TB; Ognjanović, BI; Žikić, RV; Štajn, AŠ; Saičić, ZS. Antioxidant defense enzyme activities in the liver of red mullet (Mullus barbatus L) from the Adriatic Sea: The effects of locality and season. Fresen Environ Bull 2008, 17, 558–563. [Google Scholar]
- Valavanidis, A; Vlahogianni, T; Dassenakis, M; Scoullos, M. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 2006, 64, 178–189. [Google Scholar]
- Fernando Gonzáles, J; Reimschuessel, R; Shaikh, B; Kane, SA. Kinetics of hepatic phase I and II biotransformation reactions in eight finfish species. Mar Environ Res 2009, 67, 183–188. [Google Scholar]
- Sheehan, D; Power, A. Effects of seasonality on xenobiotic and antioxidant defence mechanisms of bivalve molluscs. Comp Biochem Physiol C 1999, 123, 193–199. [Google Scholar]
- Borković, SS; Šaponjić,, JS; Pavlović,, SZ; Blagojević,, DP; Milošević,, SM; Kovačević,, TB; Radojičić,, RM; Spasić,, MB; Žikić,, RV; Saičić,, ZS. The activity of antioxidant defense enzymes in mussels (Mytilus galloprovincialis) from the Adriatic Sea. Comp Biochem Physiol C 2005, 141, 366–374. [Google Scholar]
- Lesser, MP; Kruse, VA. Seasonal temperature compensation in the horse mussel, Modiolus modiolus: Metabolic enzymes, oxidative stress and heat shock proteins. Comp Biochem Physiol A 2004, 137, 495–504. [Google Scholar]
- Manduzio, H; Monsinjon, T; Galap, C; Leboulenger, F; Rocher, B. Seasonal variations in antioxidant defences in blue mussels Mytilus edulis collected from a polluted area: Major contributions in gills of an inducible isoform of Cu/Zn-superoxide dismutase and glutathione-S-transferase. Aquat Toxicol 2004, 70, 83–93. [Google Scholar]
- Wilhelm Filho, D; Tribes, T; Gaspari, C; Claudio, FD; Torres, MA; Magalhaes, ARM. Seasonal changes in antioxidant defenses of the digestive gland of the brown mussel (Perna perna). Aquaculture 2001, 203, 149–158. [Google Scholar]
- Porte, C; Escartin, E; Garcia de la Parra, LM; Biosca, X; Albaiges, J. Assessment of coastal pollution by combined determination of chemical and biochemical markers in Mullus barbatus. Mar Ecol Prog Ser 2002, 235, 205–216. [Google Scholar]
- Regoli, F; Pellegrini, D; Winston, GW; Gorbi, S; Giuliani, S; Virno-Lamberti, C; Bompadre, S. Application of biomarkers for assessing the biological impact of dredged materials in the Mediterranean: the relationship between antioxidant responses and susceptibility to oxidative stress in the red mullet (Mullus barbatus). Mar Poll Bull 2002, 44, 912–922. [Google Scholar]
- Stjepčević, J. Ecology of mussel (Mytilus galloprovincialis LAMK.) and oyster (Ostrea edulis L.) in cultures of Boka Kotorska bay. Studia Marina 1974, 7, 3–164. [Google Scholar]
- Livingstone, DR. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Poll Bull 2001, 42, 656–666. [Google Scholar]
- Regoli, F; Principato, G. Glutathione, glutathione-dependent and antioxidant enzymes in mussels, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: implications for the use of biochemical biomarkers. Aquat Toxicol 1995, 31, 143–164. [Google Scholar]
- Yakota, T; Oishi, T. Seasonal change in the locomotor activity rhythm of the medaka Oryzias latipes. Int J Biometereol 1992, 36, 39–44. [Google Scholar]
- Viarengo, A; Canesi, L; Pertica, M; Livingstone, DR. Seasonal variation in the antioxidant defence system and lipid peroxidation of the digestive gland of mussels. Comp Biochem Physiol C 1991, 100, 187–190. [Google Scholar]
- Cancio, I; Ibabe, A; Cajaraville, MP. Seasonal variation of peroxisomal enzyme activities and peroxisomal structure in mussels Mytilus galloprovincialis and its relationship with the lipid content. Comp Biochem Physiol C 1999, 123, 135–144. [Google Scholar]
- Ronisz, D; Larsson, DGJ; Forlin, L. Seasonal variations in the activities of selected hepatic biotransformation and antioxidant enzymes in eelpout (Zoarces viviparus). Comp Biochem Physiol C 1999, 124, 271–279. [Google Scholar]
- Mathieu, A; Lemaire, P; Carriere, S; Drai, P; Giudicelli, J; Lafaurie, M. Seasonal and sexlinked variations in hepatic and extrahepatic biotransformation activities in striped mullet (Mullus barbatus). Ecotoxicol Environ Saf 1991, 22, 45–57. [Google Scholar]
- Šaponjić, JS; Borković, SS; Kovačević, TB; Pavlović, SZ; Labus-Blagojević, SD; Blagojević, DP; Saičić, ZS; Radojičić, RM; Žikić, RV; Spasić, MB. The activity of antioxidant defense enzymes in the Mediterranean sea shrimp (Parapenaeus longirostris): Relation to the presence of PCBs and PAHs in the south Adriatic Sea. Period Biol 2006, 108, 117–125. [Google Scholar]
- Fessard, V; Livingstone, DR. Development of western analysis of oxidized proteins as a biomarker of oxidative damage in liver of fish. Mar Environ Res 1998, 46, 407–410. [Google Scholar]
- Ferreira, M; Moradas-Ferreira, P; Reis-Henriques, MA. Oxidative stress biomarkers in two resident species, mullet (Mugil cephalus) and flounder (Platichtys flesus), from a polluted site in River Douro Estuary, Portugal. Aquat Toxicol 2005, 71, 39–48. [Google Scholar]
- Pascual, P; Pedrajas, RJ; Toribio, F; López-Barea, J; Peinado, J. Effect of food deprivation on oxidative stress biomarkers in fish (Sparus aurata). Chem Biol Interact 2003, 145, 191–199. [Google Scholar]
- Guderley, H. Metabolic responses to low temperature in fish muscle. Biol Rev 2004, 79, 409–427. [Google Scholar]
- Bozcaarmutlu, A; Sapmaz, C; Aygun, Z; Arinç, E. Assessment of pollution in the West Black Sea Coast of Turkey using biomarker responses in fish. Mar Environ Res 2009, 67, 167–176. [Google Scholar]
- Zhou, J; Wang, WN; Wang, AL; He, WY; Zhou, QT; Liu, Y; Xu, J. Glutathione S-transferase in the white shrimp Litopenaeus vannemei: Characterization and regulation under pH stress. Comp Biochem Physiol C 2009, 150, 224–230. [Google Scholar]
- Stegeman, JJ; Brouwer, M; Richard, TDG; Förlin, L; Fowler, BA; Sanders, BM; van Veld, PA. Molecular responses to environmental contamination: Enzyme and protein systems as indicators of chemical exposure and effect. In Biomarkers: Biochemical, Physiological and Histological Markers of Anthropogenic Stress; Huggart, RJ, Kimerly, RA, Mehrle, PM, Bergman, HL, Eds.; Lewis Publishers: Chelsea, MI, USA, 1992; pp. 235–335. [Google Scholar]
- Holmstrup, M; Bayley, M; Sjursen, H; Hojer, R; Bossen, S; Friis, K. Interactions between environmental pollution and cold tolerance of soil invertebrates: A neglected field of research. Cryo Lett 2000, 21, 309–314. [Google Scholar]
- Petrović, S; Semenčić, L; Ozretić, B; Ozretić, M. Seasonal variations of physiological and cellular biomarkers and their use in the biomonitoring of north adriatic coastal waters (Croatia). Mar Poll Bull 2004, 49, 713–720. [Google Scholar]
- Žikić, RV; Ognjanović, BI; Marković, SD; Pavlović, SZ; Mihajlović, RP; Saičić, ZS; Štajn, AŠ. Lipid peroxidation and the concentration of antioxidant compounds (vitamin E and vitamin C) in the liver and white muscle of red mullet (Mullus barbatus L.) from the Adriatic Sea. Period Biol 2006, 108, 139–143. [Google Scholar]
- Lionetto, MG; Caricato, R; Giordano, ME; Pascariello, MF; Marinosci, L; Schettino, T. Integrated use of biomarkers (acetylcholineesterase and antioxidant enzyme activities) in Mytilus galloprovincialis and Mullus barbatus in an Italian coastal marine area. Mar Poll Bull 2003, 46, 324–330. [Google Scholar]
- Rossi, MA; Cecchini, G; Dianzani, MM. Glutathione peroxidase, glutathione reductase and glutathione transferase in two different hepatomas and in normal liver. IRCS Med Sci Biochem 1983, 11, 805. [Google Scholar]
- Takada, Y; Noguchit, T; Kayiyama, M. Superoxide dismutase in various tissues from rabbits bearing the Vx-2 carcinoma in the maxillary sinus. Cancer Res 1982, 42, 4233–4235. [Google Scholar]
- Lowry, OH; Rosebrough, NL; Farr, AL; Randall, RI. Protein measurement with Folin phenol reagent. J Biol Chem 1951, 193, 265–275. [Google Scholar]
- Misra, HP; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and simple assay for superoxide dismutase. J Biol Chem 1972, 247, 3170–3175. [Google Scholar]
- Claiborne, A. Handbook of Methods for Oxygen Radical Research; Greenwald, RA, Ed.; CRC Press Inc: Boca Raton, USA, 1984. [Google Scholar]
- Tamura, M; Oschino, N; Chance, B. Some characteristics of hydrogen and alkyl-hydroperoxides metabolizing systems in cardiac tissue. J Biochem 1982, 92, 1019–1031. [Google Scholar]
- Glatzle, D; Vulliemuier, JP; Weber, F; Decker, K. Glutathione reductase test with whole blood a convenient procedure for the assesment of the riboflavin status in humans. Experientia 1974, 30, 665–667. [Google Scholar]
- Habig, WH; Pubst, MJ; Jakoby, WB. Glutathione S-transferase. J Biol Chem 1974, 249, 7130–7139. [Google Scholar]
- Darlington, RB; Weinberg, S; Walberg, H. Canonical variate analysis and related techniques. Rev Educational Res 1973, 43, 433–454. [Google Scholar]
- Dinneen, LC; Blakesley, BC. A generator for the sampling distribution of the Mann Whitney U statistic. Appl Stat 1973, 22, 269–273. [Google Scholar]
| Location | Season | Temperature(°C) | Salinity (‰) | O2 concentration (mg/L) | O2 saturation |
|---|---|---|---|---|---|
| NB | Winter | 11.60 | 32.85 | 8.30 | 91.0 |
| Spring | 19.37 | 37.97 | 7.13 | 101.3 | |
| EB | Winter | 11.67 | 37.67 | 8.37 | 94.3 |
| Spring | 18.57 | 37.20 | 7.63 | 103.0 | |
| Location | Season | LIVER | WHITE MUSCLE |
|---|---|---|---|
| NB | Winter (n=5) | 315.12 ± 17.58 | 122.02 ± 9.17 |
| Spring (n=5) | 306.70 ± 11.63 | 162.42 ± 8.19* | |
| EB | Winter (n=5) | 394.90 ± 13.17 | 167.76 ± 9.46 |
| Spring (n=5) | 339.34 ± 3.31* | 151.20 ± 8.67 | |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pavlović, S.Z.; Mitić, S.S.B.; Radovanović, T.B.; Perendija, B.R.; Despotović, S.G.; Gavrić, J.P.; Saičić, Z.S. Seasonal Variations of the Activity of Antioxidant Defense Enzymes in the Red Mullet (Mullus barbatus l.) from the Adriatic Sea. Mar. Drugs 2010, 8, 413-428. https://doi.org/10.3390/md8030413
Pavlović SZ, Mitić SSB, Radovanović TB, Perendija BR, Despotović SG, Gavrić JP, Saičić ZS. Seasonal Variations of the Activity of Antioxidant Defense Enzymes in the Red Mullet (Mullus barbatus l.) from the Adriatic Sea. Marine Drugs. 2010; 8(3):413-428. https://doi.org/10.3390/md8030413
Chicago/Turabian StylePavlović, Sladjan Z., Slavica S. Borković Mitić, Tijana B. Radovanović, Branka R. Perendija, Svetlana G. Despotović, Jelena P. Gavrić, and Zorica S. Saičić. 2010. "Seasonal Variations of the Activity of Antioxidant Defense Enzymes in the Red Mullet (Mullus barbatus l.) from the Adriatic Sea" Marine Drugs 8, no. 3: 413-428. https://doi.org/10.3390/md8030413
APA StylePavlović, S. Z., Mitić, S. S. B., Radovanović, T. B., Perendija, B. R., Despotović, S. G., Gavrić, J. P., & Saičić, Z. S. (2010). Seasonal Variations of the Activity of Antioxidant Defense Enzymes in the Red Mullet (Mullus barbatus l.) from the Adriatic Sea. Marine Drugs, 8(3), 413-428. https://doi.org/10.3390/md8030413