Trabectedin for Metastatic Soft Tissue Sarcoma: A Retrospective Single Center Analysis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients and Eligibility Criteria
2.2. Trabectedin
2.3. Imaging Studies and Restaging
2.4. Toxicity Assessment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Patient Characteristics
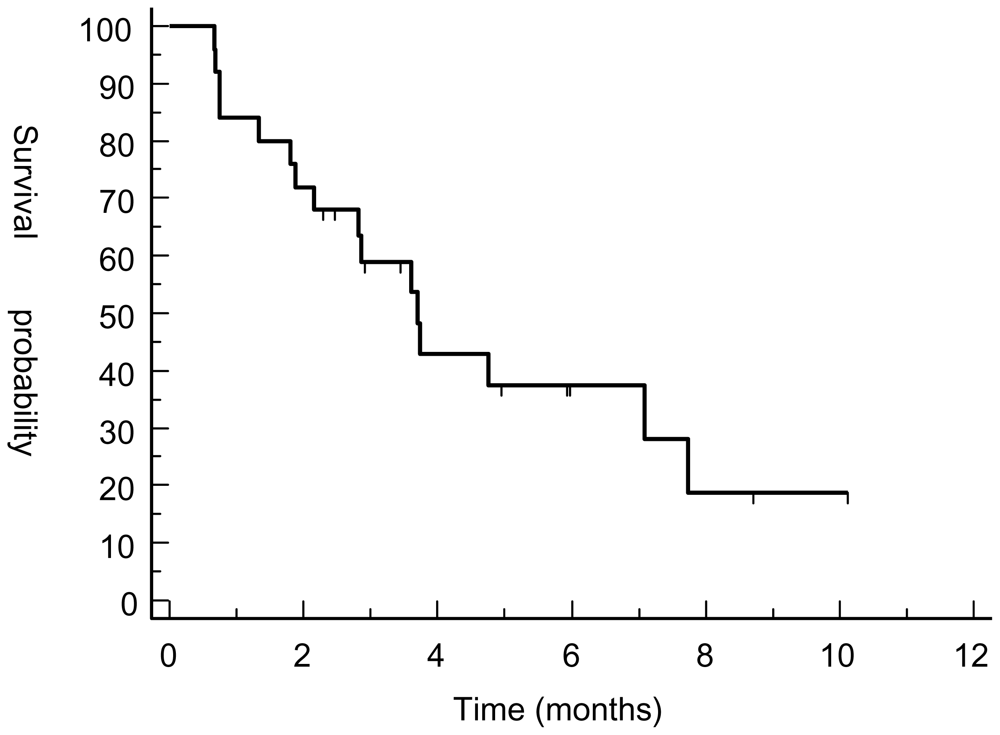
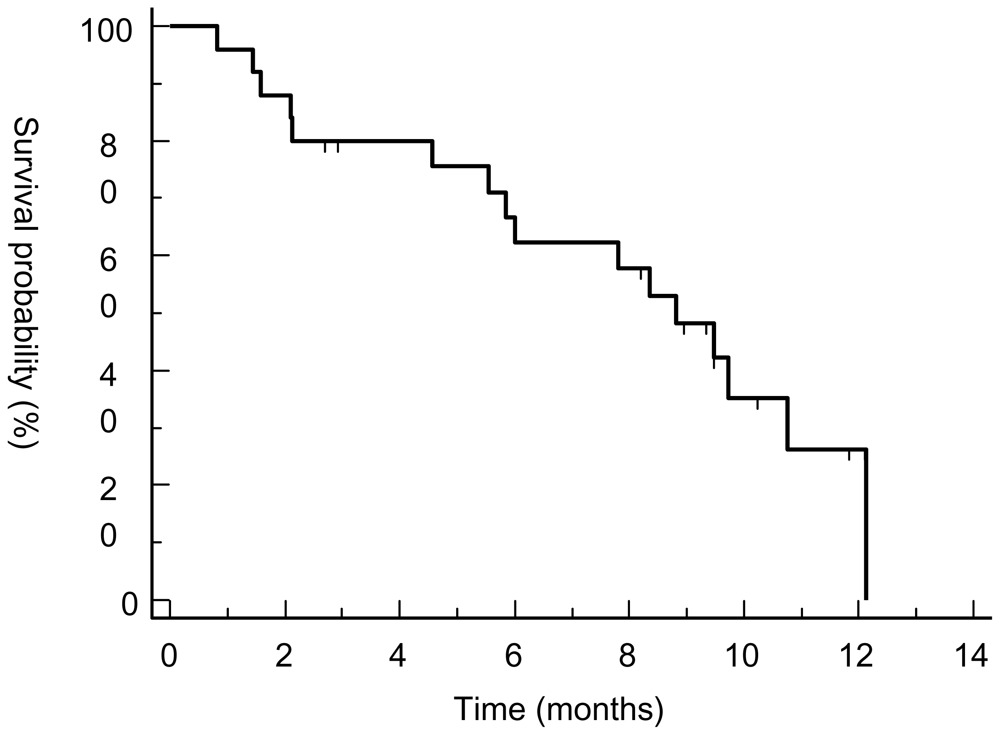
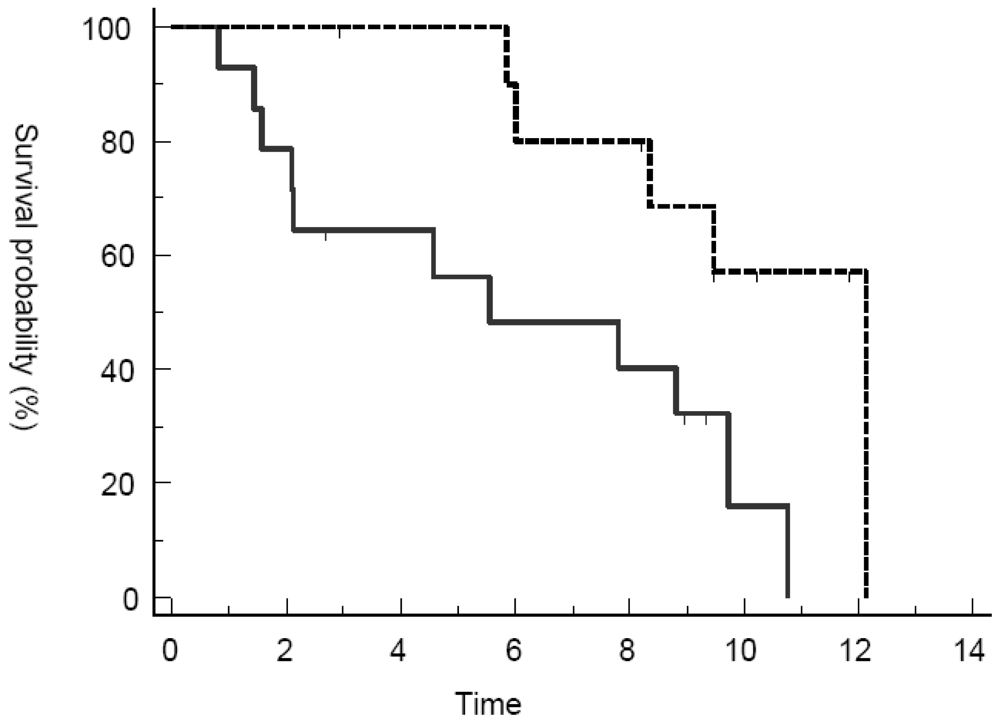
3.2. Response Assessment
3.3. Toxicity Assessment
3.4. Discussion
4. Conclusion
- Conflict of InterestThis retrospective analysis was financially supported by PharmaMar.
References
- Jemal, A; Siegel, R; Ward, E; Murray, T; Xu, J; Thun, MJ. Cancer statistics 2008. CA Cancer J. Clin 2008, 58, 71–96. [Google Scholar]
- Pisters, P. Pollock, RE, Ed.; Staging and Prognosis. In American Cancer Society Atlas of Clinical Oncology: Soft Tissue Sarcomas; BC Decker: Hamiliton, Ontario, Canada, 2002; pp. 80–88. [Google Scholar]
- Bramwell, VH; Anderson, D; Charette, ML. Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database Syst. Rev 2003, (3). Art. No.: CD003293. [Google Scholar] [CrossRef]
- Sleijfer, S; Seynaeve, C; Verweij, J. Using single-agent therapy in adult patients with advanced soft tissue sarcoma can still be considered standard care. Oncologist 2005, 10, 833–841. [Google Scholar]
- Carter, NJ; Keam, SJ. Trabectedin: a review of its use in soft tissue sarcoma and ovarian cancer. Drugs 2007, 67, 2257–2276. [Google Scholar]
- Garcia-Carbonero, R; Supko, JG; Maki, RG; Manola, J; Ryan, DP; Harmon, D; Puchalski, TA; Goss, G; Seiden, MV; Waxman, A; Quigley, MT; Lopez, T; Sancho, MA; Jimeno, J; Guzman, C; Demetri, GD. Ecteinascidin-743 (ET-743) for chemotherapy-naive patients with advanced soft tissue sarcomas: multicenter phase II and pharmacokinetic study. J. Clin. Oncol 2005, 23, 5484–5492. [Google Scholar]
- Le Cesne, A; Blay, JY; Judson, I; van Oosterom, A; Verweij, J; Radford, J; Lorigan, P; Rodenhuis, S; Ray-Coquard, I; Bonvalot, S; Collin, F; Jimeno, J; Di Paola, E; van Glabbeke, M; Nielsen, OS. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J. Clin. Oncol 2005, 23, 576–584. [Google Scholar]
- Yovine, A; Riofrio, M; Blay, JY; Brain, E; Alexandre, J; Kahatt, C; Taamma, A; Jimeno, J; Martin, C; Salhi, Y; Cvitkovic, E; Misset, JL. Phase II study of ecteinascidin-743 in advanced pretreated soft tissue sarcoma patients. J. Clin. Oncol 2004, 22, 890–899. [Google Scholar]
- Grosso, F; Jones, RL; Demetri, GD; Judson, IR; Blay, JY; Le Cesne, A; Sanfilippo, R; Casieri, P; Collini, P; Dileo, P; Spreafico, C; Stacchiotti, S; Tamborini, E; Tercero, JC; Jimeno, J; D’Incalci, M; Gronchi, A; Fletcher, JA; Pilotti, S; Casali, PG. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol 2007, 8, 595–602. [Google Scholar]
- Demetri, GD; Chawla, SP; von Mehren, M; Ritch, P; Baker, LH; Blay, JY; Hande, KR; Keohan, ML; Samuels, BL; Schuetze, S; Lebedinsky, C; Elsayed, YA; Izquierdo, MA; Gómez, J; Park, YC; Le Cesne, A. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J. Clin. Oncol 2009, 27, 4188–4196. [Google Scholar]
- Eisenhauer, EA; Therasse, P; Bogaerts, J; Schwartz, LH; Sargent, D; Ford, R; Dancey, J; Arbuck, S; Gwyther, S; Mooney, M; Rubinstein, L; Shankar, L; Dodd, L; Kaplan, R; Lacombe, D; Verweij, J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar]
- Schuetze, SM; Baker, LH; Benjamin, RS; Canetta, R. Selection of response criteria for clinical trials of sarcoma treatment. Oncologist 2008, 13, 32–40. [Google Scholar]
- Jaffe, CC. Response assessment in clinical trials: implications for sarcoma clinical trial design. Oncologist 2008, 13, 14–18. [Google Scholar]
- van Glabbeke, M; Verweij, J; Judson, I; Nielson, OS. Progression-free rate as the principal end-point for phase II trials in soft-tissue sarcomas. Eur. J. Cancer 2002, 38, 543–549. [Google Scholar]
- Evilevitch, V; Weber, WA; Tap, WD; Allen-Auerbach, M; Chow, K; Nelson, SD; Eilber, FR; Eckardt, JJ; Elashoff, RM; Phelps, ME; Czernin, J; Eilber, FC. Reduction of glucose metabolic activity is more accurate than change in size at predicting histopathologic response to neoadjuvant therapy in high-grade soft-tissue sarcomas. Clin. Cancer Res 2008, 14, 715–720. [Google Scholar]
- Schuetze, SM; Rubin, BP; Vernon, C; Hawkins, DS; Bruckner, JD; Conrad, EU, 3rd; Eary, JF. Use of positron emission tomography in localized extremity soft tissue sarcoma treated with neoadjuvant chemotherapy. Cancer 2005, 103, 339–348. [Google Scholar]
- Dudeck, O; Zeile, M; Pink, D; Pech, M; Tunn, PU; Reichardt, P; Ludwig, WD; Hamm, B. Diffusion-weighted magnetic resonance imaging allows monitoring of anticancer treatment effects in patients with soft-tissue sarcomas. J. Magn. Reson. Imaging 2008, 27, 1109–1113. [Google Scholar]
- Kasper, B; Schmitt, T; Wuchter, P; Dimitrakopoulou-Strauss, A; Ho, AD; Egerer, G. The use of positron emission tomography in soft tissue sarcoma patients under therapy with trabectedin. Mar. Drugs 2009, 7, 331–340. [Google Scholar]
- Fayette, J; Boyle, H; Chabaud, S; Favier, B; Engel, C; Cassier, P; Thiesse, P; Méeus, P; Sunyach, MP; Vaz, G; Ray-Coquard, I; Ranchère, D; Decouvelaere, AV; Alberti, L; Pérol, D; Blay, JY. Efficacy of trabectedin for advanced sarcomas in clinical trials versus compassionate use programs: analysis of 92 patients treated in a single institution. Anticancer Drugs 2010, 21, 113–119. [Google Scholar]
- Roylance, R; Seddon, B; McTiernan, A; Sykes, K; Daniels, S; Whelan, J. Experience of the use of trabectedin (ET-743, Yondelis) in 21 patients with pre-treated advanced sarcoma from a single centre. Clin. Oncol. (R. Coll. Radiol.) 2007, 19, 572–576. [Google Scholar]
- Hartmann, JT; Oechsle, K; Huober, J; Jakob, A; Azemar, M; Horger, M; Kanz, L; Bokemeyer, C. An open label, non-comparative phase II study of gemcitabine as salvage treatment for patients with pretreated adult type soft tissue sarcoma. Invest. New Drugs 2006, 24, 249–253. [Google Scholar]
- Maki, RG; Wathen, JK; Patel, SR; Priebat, DA; Okuno, SH; Samuels, B; Fanucchi, M; Harmon, DC; Schuetze, SM; Reinke, D; Thall, PF; Benjamin, RS; Baker, LH; Hensley, ML. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002. J. Clin. Oncol 2007, 25, 2755–2763. [Google Scholar]
- Duffaud, F; Bui, BN; Penel, N; Cioffi, A; Isambert, N; Blay, JY; Cupissol, D; Jimenez, M; Rey, A; Pautier, P. A FNCLCC French Sarcoma Group—GETO multicenter randomized phase II study of gemcitabine (G) versus gemcitabine and docetaxel (G+D) in patients with metastatic or relapsed leiomyosarcoma (LMS). Proceedings of the American Society of Clinical Oncology Annual Meeting, Chicago, USA, 2007. J. Clin. Oncol 2007, 26. Abstract 10511. [Google Scholar]
- Minuzzo, M; Marchini, S; Broggini, M; Faircloth, G; D’Incalci, M; Mantovani, R. Interference of transcriptional activation by the antineoplastic drug ecteinascidin-743. Proc. Natl. Acad. Sci. USA 2000, 97, 6780–6784. [Google Scholar]
- Blay, JY; von Mehren, M; Samuels, BL; Fanucchi, MP; Ray-Coquard, I; Buckley, B; Gilles, L; Lebedinsky, C; Elsayed, YA; Le Cesne, A. Phase I combination study of trabectedin and doxorubicin in patients with soft-tissue sarcoma. Clin. Cancer Res 2008, 14, 6656–6662. [Google Scholar]
- Sessa, C; Perotti, A; Noberasco, C; De Braud, F; Gallerani, E; Cresta, S; Zucchetti, M; Viganò, L; Locatelli, A; Jimeno, J; Feilchenfeldt, JW; D’Incalci, M; Capri, G; Ielmini, N; Gianni, L. Phase I clinical and pharmacokinetic study of trabectedin and doxorubicin in advanced soft tissue sarcoma and breast cancer. Eur. J. Cancer 2008, 45, 1153–1161. [Google Scholar]
- Forni, C; Minuzzo, M; Virdis, E; Tamborini, E; Simone, M; Tavecchio, M; Erba, E; Grosso, F; Gronchi, A; Aman, P; Casali, P; D’Incalci, M; Pilotti, S; Mantovani, R. Trabectedin (ET-743) promotes differentiation in myxoid liposarcoma tumors. Mol. Cancer Ther 2009, 8, 449–457. [Google Scholar]
- Theman, TA; Hartzell, TL; Sinha, I; Polson, K; Morgan, J; Demetri, GD; Orgill, DP; George, S. Recognition of a new chemotherapeutic vesicant: trabectedin (ecteinascidin-743) extravasation with skin and soft tissue damage. J. Clin. Oncol 2009, 27, 198–200. [Google Scholar]

| Patient | Age | Gender | Histology | Grading | Primary tumor | Metastases | Previous CTX lines | #Cycles | Staging | PFS (in days) | OS (in days) | Complications | Alive | COD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 001 | 71 | M | Spindle cell sarcoma | G3 | Trunk | S | 2 | 2 | PD | 21 | 64 | None | No | Progression |
| 002 | 64 | M | Leiomyosarcoma | G3 | Extremity | P | 2 | 2 | PD | 23 | 48 | None | No | Progression |
| 003 | 36 | F | Liposarcoma | G2 | Abdomen | S, P | 2 | 3 | SD | 145 | 255 | None | No | Progression |
| 004 | 16 | F | Synovial sarcoma | G3 | Trunk | S, P | 6 | 1 | PD | 23 | 44 | None | No | Progression |
| 005 | 53 | F | Liposarcoma | G3 | Retroperitoneum | S | 3 | 3 | PR | 236 | 289 | None | No | Progression |
| 006 | 55 | M | Pleomorphic Sarcoma | G3 | Extremity | P, S | 3 | 3 | PD | 87 | 139 | None | No | Progression |
| 007 | 56 | M | Pleomorphic Sarcoma | G3 | Abdomen | P | 2 | 5 | PD | 110 | 269 | None | No | Progression |
| 008 | 29 | F | Leiomyosarcoma | G3 | Abdomen | S, P | 4 | 3 | PD | 86 | 169 | N/V, liver tox | No | Progression |
| 009 | 46 | M | Myxoid Liposarcoma | G1 | Extremity | S, B | 2 | 5 | SD | 114 | 183 | None | No | Progression |
| 010 | 56 | F | Leiomyosarcoma | G3 | Abdomen | S, P | 2 | 7 | SD | 216 | 370 | None | No | Progression |
| 011 | 58 | M | Liposarcoma | G3 | Retroperitoneum | S | 3 | 3 | PD | 55 | 238 | None | No | Progression |
| 012 | 37 | M | Leiomyosarcoma | G3 | Abdomen | S, P, L | 1 | 6 | SD | 113 | 178 | None | No | Progression |
| 013 | 57 | M | Synovial sarcoma | G3 | Extremity | P, L | 3 | 1 | PD | 20 | 25 | None | No | Progression |
| 014 | 70 | F | Leiomyosarcoma | G3 | Abdomen | P | 2 | 3 | PD | 66 | 297 | N/V | No | Progression |
| 015 | 49 | F | Leiomyosarcoma | G2 | Abdomen | S, P, L | 1 | 2 | PD | 57 | 328 | N/V | No | Progression |
| 016 | 49 | F | Leiomyosarcoma | G1 | Abdomen | P | 1 | 8 | SD | 182 | 240 | hem tox | Yes | |
| 017 | 64 | M | Leiomyosarcoma | G2 | Extremity | P, B | 4 | 6 | SD | 309 | 309 | hem tox | Yes | |
| 018 | 49 | F | Leiomyosarcoma | G3 | Abdomen | S | 2 | 12 | SD | 266 | 266 | None | Yes | |
| 019 | 53 | M | Leiomyosarcoma | G2 | Extremity | S, B | 2 | 3 | PD | 75 | 237 | hem tox | Yes | |
| 020 | 49 | M | Synovial sarcoma | G3 | Trunk | P, L | 1 | 1 | PD | 41 | 65 | liver tox | No | Progression |
| 021 | 31 | F | Pleomorphic Sarcoma | G3 | Abdomen | L, B | 2 | 3 | PD | 70 | 212 | N/V | Yes | |
| 022 | 71 | M | Liposarcoma | G3 | Extremity | S, P | 3 | 8 | SD | 181 | 205 | hem tox | Yes | |
| 023 | 58 | M | Rhabdomyosarcoma | G3 | Head/Neck | L | 1 | 5 | SD | 105 | 105 | None | Yes | |
| 024 | 24 | F | Fibromyxoid sarcoma | G1 | Extremity | S, P | 1 | 5 | SD | 151 | 188 | None | Yes | |
| 025 | 64 | M | Rhabdomyosarcoma | G3 | Extremity | P, L | 1 | 3 | PD | 89 | 89 | None | Yes |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Schmitt, T.; Keller, E.; Dietrich, S.; Wuchter, P.; Ho, A.D.; Egerer, G. Trabectedin for Metastatic Soft Tissue Sarcoma: A Retrospective Single Center Analysis. Mar. Drugs 2010, 8, 2647-2658. https://doi.org/10.3390/md8102647
Schmitt T, Keller E, Dietrich S, Wuchter P, Ho AD, Egerer G. Trabectedin for Metastatic Soft Tissue Sarcoma: A Retrospective Single Center Analysis. Marine Drugs. 2010; 8(10):2647-2658. https://doi.org/10.3390/md8102647
Chicago/Turabian StyleSchmitt, Thomas, Eva Keller, Sascha Dietrich, Patrick Wuchter, Anthony D. Ho, and Gerlinde Egerer. 2010. "Trabectedin for Metastatic Soft Tissue Sarcoma: A Retrospective Single Center Analysis" Marine Drugs 8, no. 10: 2647-2658. https://doi.org/10.3390/md8102647
APA StyleSchmitt, T., Keller, E., Dietrich, S., Wuchter, P., Ho, A. D., & Egerer, G. (2010). Trabectedin for Metastatic Soft Tissue Sarcoma: A Retrospective Single Center Analysis. Marine Drugs, 8(10), 2647-2658. https://doi.org/10.3390/md8102647





