Excavatoids O and P, New 12-Hydroxybriaranes from the Octocoral Briareum excavatum
Abstract
:1. Introduction
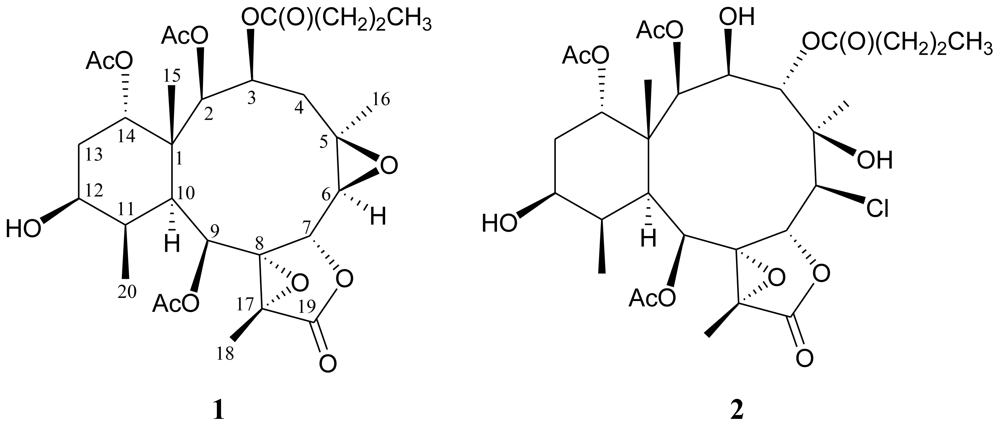
2. Results and Discussion
3. Experimental
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Human Neutrophil Elastase Release
Acknowledgements
- Samples Availability: Not available.
References and Notes
- Sung, P-J; Sheu, J-H; Xu, J-P. Survey of briarane-type diterpenoids of marine origin. Heterocycles 2002, 57, 535–579. [Google Scholar]
- Sung, P-J; Chang, P-C; Fang, L-S; Sheu, J-H; Chen, W-C; Chen, Y-P; Lin, M-R. Survey of briarane-type diterpenoids-Part II. Heterocycles 2005, 65, 195–204. [Google Scholar]
- Sung, P-J; Chang, P-C; Fang, L-S; Sheu, J-H; Chen, W-C; Chen, Y-P; Lin, M-R. Survey of briarane-type diterpenoids-Part III. Heterocycles 2008, 75, 2627–2648. [Google Scholar]
- Elastatinal was used as a positive control in anti-inflammatory activity testing. This compound displayed inhibitory effects on elastase release by human neutrophils (IC50 = 31.0 μM).
- Bayer, FM. Key to the genera of octocorallia exclusive of Pennatulacea (Coelenterata: anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash 1981, 94, 902–947. [Google Scholar]
- Benayahu, Y; Jeng, M-S; Perkol-Finkel, S; Dai, C-F. Soft corals (Octocorallia: Alcyonacea) from southern Taiwan. II. Species diversity and distribution patterns. Zool. Stud 2004, 43, 548–560. [Google Scholar]
- Fabricius, K; Alderslade, P. Soft Corals and Sea Fans—A Comprehensive Guide to the Tropical Shallow-Water Genera of the Central-West Pacific, the Indian Ocean and the Red Sea, 1st ed; Australian Institute of Marine Science: Queensland, Australia, 2001. [Google Scholar]
- Hwang, T-L; Li, G-L; Lan, Y-H; Chia, Y-C; Hsieh, P-W; Wu, Y-H; Wu, Y-C. Potent inhibitors of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic. Biol. Med 2009, 46, 520–528. [Google Scholar]
- Hwang, T-L; Su, Y-C; Chang, H-L; Leu, Y-L; Chung, P-J; Kuo, L-M; Chang, Y-J. Suppression of superoxide anion and elastase release by C18 unsaturated fatty acids in human neutrophils. J. Lipid Res 2009, 50, 1395–1408. [Google Scholar]


| 1 | 2 | |||
|---|---|---|---|---|
| Position | 1Ha | 13Cb | 1Hc | 13Cd |
| 1 | 43.3 (s)f | 44.0 (s) | ||
| 2 | 5.81 d (2.0)e | 69.3 (d) | 4.62 s | 88.2 (d) |
| 3 | 5.13 br s | 69.9 (d) | 5.07 d (11.6) | 68.8 (d) |
| 4 | 2.25 m (2H) | 33.7 (t) | 5.82 s | 70.9 (d) |
| 5 | 62.1 (s) | 77.8 (s) | ||
| 6 | 3.11 d (8.8) | 63.0 (d) | 4.29 s | 65.9 (d) |
| 7 | 4.69 d (8.8) | 78.3 (d) | 5.26 s | 75.7 (d) |
| 8 | 72.6 (s) | 67.5 (s) | ||
| 9 | 5.76 s | 68.5 (d) | 5.36 d (8.4) | 66.0 (d) |
| 10 | 2.18 br s | 45.3 (d) | 3.52 dd (8.4, 4.4) | 39.5 (d) |
| 11 | 2.32 br s | 34.5 (d) | 2.54 m | 37.3 (d) |
| 12 | 3.96 br s | 69.3 (d) | 4.13 m | 66.9 (d) |
| 13 | 1.92 m (2H) | 34.8 (t) | 1.83 m (α) | 30.3 (t) |
| 1.96 m (β ) | ||||
| 14 | 5.16 br s | 73.0 (d) | 4.84 br s | 80.8 (d) |
| 15 | 1.52 s | 18.2 (q) | 0.86 s | 18.2 (q) |
| 16 | 1.35 s | 21.1 (q) | 1.55 s | 22.3 (q) |
| 17 | 63.3 (s) | 60.6 (s) | ||
| 18 | 1.57 s | 11.1 (q) | 1.63 s | 10.0 (q) |
| 19 | 170.8 (s) | 170.0 (s) | ||
| 20 | 1.19 d (7.2) | 16.3 (q) | 1.11 d (7.6) | 9.0 (q) |
| OH-3 | 3.18 d (11.6) | |||
| OH-5 | 2.36 s | |||
| OH-12 | n.o.g | 2.17 br s | ||
| 2-OAc | 169.5 (s) | 172.0 (s) | ||
| 2.12 s | 21.1 (q) | 2.03 s | 21.1 (q) | |
| 9-OAc | 169.3 (s) | 170.4 (s) | ||
| 2.18 s | 21.2 (q) | 2.43 s | 21.4 (q) | |
| 14-OAc | 170.1 (s) | 170.3 (s) | ||
| 1.96 s | 21.1 (q) | 2.17 s | 21.3 (q) | |
| 3-OCOPr | 173.6 (s) | |||
| 2.23 m (2H) | 35.6 (t) | |||
| 1.64 m (2H) | 17.8 (t) | |||
| 0.95 t (7.2) | 13.6 (q) | |||
| 4-OCOPr | 173.9 (s) | |||
| 2.33 t (7.6) (2H) | 36.3 (t) | |||
| 1.66 m (2H) | 18.4 (t) | |||
| 0.98 t (7.6) | 13.7 (q) | |||
| 1 | 2 | |||
|---|---|---|---|---|
| Position | 1H-1H COSY | HMBC | 1H-1H COSY | HMBC |
| H-2 | H-3 | C-1, -4, -14, -15, acetate carbonyl | H-3 | C-1, -3, -4, -10, -14, acetate carbonyl |
| H-3 | H-2, H2-4 | C-4 | H-2, H-4, OH-3 | C-1, -5 |
| H-4 | H-3 | C-3, -5, -6 | H-3 | C-2, -5, -16, n-butyrate carbonyl |
| H-6 | H-7 | n.o. | H-7 | C-4, -5, -7, -8, -16 |
| H-7 | H-6 | C-5, -6, -17, -19 | H-6 | C-5, -6, -9, -19 |
| H-9 | H-10 | C-1, -7, -8, -10, -11, acetate carbonyl | H-10 | C-7, -8, -10, -11, -17, acetate carbonyl |
| H-10 | H-9, H-11 | C-1, -2, -11, -12, -14 | H-9, H-11 | C-1, -8, -9, -11, -12, -15, -20 |
| H-11 | H-10, H-12, H3-20 | C-10, -20 | H-10, H-12, H3-20 | C-1, -10, -12, -20 |
| H-12 | H-11, H2-13 | n.o. | H-11, H2-13 | C-20 |
| H-13 | H-12, H-14 | C-1, -14 | H-12, H-14 | C-12 |
| H-14 | H2-13 | C-1, -2, -12, -13, acetate carbonyl | H2-13 | C-10, -12 |
| H-15 | C-1, -2, -10, -14 | C-1, -2, -10, -14 | ||
| H-16 | C-4, -5, -6 | C-4, -5, -6 | ||
| H-18 | C-8, -17, -19 | C-8, -17, -19 | ||
| H-20 | H-11 | C-10, -11, -12 | H-11 | C-10, -11, -12 |
| OH-3 | H-3 | C-3 | ||
| OH-5 | C-4, -5, -16 | |||
| OH-12 | n.o.a | n.o. | H-12 | C-11 |
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sung, P.-J.; Li, G.-Y.; Su, Y.-D.; Lin, M.-R.; Chang, Y.-C.; Kung, T.-H.; Lin, C.-S.; Chen, Y.-H.; Su, J.-H.; Lu, M.-C.; et al. Excavatoids O and P, New 12-Hydroxybriaranes from the Octocoral Briareum excavatum. Mar. Drugs 2010, 8, 2639-2646. https://doi.org/10.3390/md8102639
Sung P-J, Li G-Y, Su Y-D, Lin M-R, Chang Y-C, Kung T-H, Lin C-S, Chen Y-H, Su J-H, Lu M-C, et al. Excavatoids O and P, New 12-Hydroxybriaranes from the Octocoral Briareum excavatum. Marine Drugs. 2010; 8(10):2639-2646. https://doi.org/10.3390/md8102639
Chicago/Turabian StyleSung, Ping-Jyun, Gung-Ying Li, Yin-Di Su, Mei-Ru Lin, Yu-Chia Chang, Ting-Hsuan Kung, Chan-Shing Lin, Yung-Husan Chen, Jui-Hsin Su, Mei-Chin Lu, and et al. 2010. "Excavatoids O and P, New 12-Hydroxybriaranes from the Octocoral Briareum excavatum" Marine Drugs 8, no. 10: 2639-2646. https://doi.org/10.3390/md8102639
APA StyleSung, P.-J., Li, G.-Y., Su, Y.-D., Lin, M.-R., Chang, Y.-C., Kung, T.-H., Lin, C.-S., Chen, Y.-H., Su, J.-H., Lu, M.-C., Kuo, J., Weng, C.-F., & Hwang, T.-L. (2010). Excavatoids O and P, New 12-Hydroxybriaranes from the Octocoral Briareum excavatum. Marine Drugs, 8(10), 2639-2646. https://doi.org/10.3390/md8102639







