Two New Jaspamide Derivatives from the Marine Sponge Jaspis splendens
Abstract
:1. Introduction
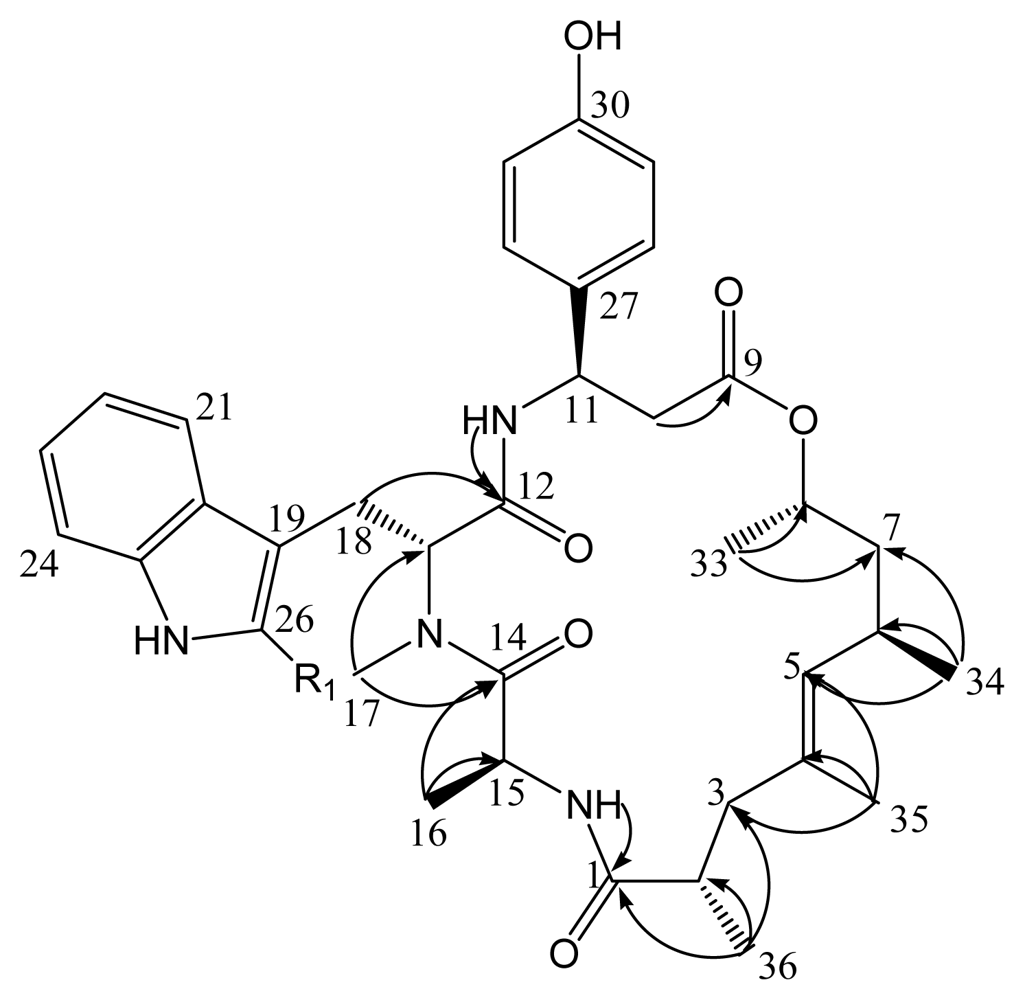
2. Results and Discussion
3. Experimental Section
General experimental procedures
Biological material
Extraction and isolation
Cell proliferation assay
Acknowledgements
- Samples Availability: Available from the authors.
References and Notes
- Blunt, JW; Copp, BR; Hu, W-P; Munro, MHG; Northcote, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2009, 26, 170–244. [Google Scholar]
- Hamann, MT; Scheuer, PJ. Kahalalide F: A bioactive depsipeptide from the sacoglossan mollusk Elysia rufescens and the green alga Bryopsis sp. J Am Chem Soc 1993, 115, 5825–5826. [Google Scholar]
- Hamada, T; Matsunaga, S; Yano, G; Fusetani, N. Polytheonamides A and B, highly cytotoxic, linear polypeptides with unprecedented structural features, from the marine sponge, Theonella swinhoei. J Am Chem Soc 2005, 127, 110–118. [Google Scholar]
- Nakao, Y; Masuda, A; Matsunaga, S; Fusetani, N. Pseudotheonamides, serine protease inhibitors from the marine sponge Theonella swinhoei. J Am Chem Soc 1999, 121, 2425–2431. [Google Scholar]
- Ford, PW; Gustafson, KR; McKee, TC; Shigematsu, N; Maurizi, LK; Pannell, LK; Williams, DE; Dilip de Silva, E; Lassota, P; Allen, TM; van Soest, R; Andersen, RJ; Boyd, MR. Papuamides A–D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J Am Chem Soc 1999, 121, 5899–5909. [Google Scholar]
- Reese, MT; Gulavita, NK; Nakao, Y; Hamann, MT; Yoshida, WY; Coval, SJ; Scheuer, PJ. Kulolide: A cytotoxic depsipeptide from a cephalaspidean mollusk, Philinopsis speciosa. J Am Chem Soc 1996, 118, 11081–11084. [Google Scholar]
- Renner, MK; Shen, YC; Cheng, XC; Jensen, PR; Frankmoelle, W; Kauffman, CA; Fenical, W; Emil, CJ. Cyclomarins A–C, new anti-inflammatory cyclic peptides produced by a marine bacterium Streptomyces sp. J Am Chem Soc 1999, 121, 11273–11276. [Google Scholar]
- Clark, WD; Corbett, T; Valeriote, F; Crews, P. Cylcocinamide A, an unusual cytotoxic halogenated hexapeptide from the marine sponge Psammocinia. J Am Chem Soc 1997, 119, 9285–9286. [Google Scholar]
- Nakao, Y; Yeung, BKS; Yoshida, WY; Scheuer, PJ; Kelly-Borges, M. Kapakahine B: A cyclic hexapeptide with an α-carboline ring system from the marine sponge Cribrochalina olemda. J Am Chem Soc 1995, 117, 8271–8272. [Google Scholar]
- Fusetani, N; Sugawara, T; Matsunaga, S; Hirota, H. Orbiculamide A: A novel cytotoxic cyclic peptide from a marine sponge Theonella sp. J Am Chem Soc 1991, 113, 7811–7812. [Google Scholar]
- Ashour, M; Edrada, RA; Ebel, R; Wray, V; Waetjen, W; Padmakumar, K; Mueller, WEG; Lin, WH; Proksch, P. Kahalalide derivatives from the Indian sacoglossan mollusk Elysia grandifolia. J Nat Prod 2006, 69, 1547–1553. [Google Scholar]
- Matsunaga, S; Fusetani, N; Hashimoto, K; Walchli, M. Theonellamide F: A novel antifungal bicyclic peptide from a marine sponge Theonella sp. J Am Chem Soc 1989, 111, 2582–2588. [Google Scholar]
- Ireland, C; Scheuer, PJ. Ulicyclamide and ulithiacyclamide, two new small peptides from a marine tunicate. J Am Chem Soc 1980, 102, 5688–5691. [Google Scholar]
- Zabriskie, TE; Klocke, JA; Ireland, CM; Marcus, AH; Molinski, TF; Faulkner, DJ; Xu, C; Clardy, JC. Jaspamide, a modified peptide from a Jaspis sponge, with insecticidal and antifungal activity. J Am Chem Soc 1986, 108, 3123–3124. [Google Scholar]
- Crews, P; Manes, LV; Boehler, M. Jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis sp. Tetrahedron Lett 1986, 27, 2797–2800. [Google Scholar]
- Scott, VR; Boehme, R; Matthews, TR. New class of antifungal agents: Jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis species. Antimicrob Agents Chemother 1988, 32, 1154–1157. [Google Scholar]
- Inman, W; Crews, P. Novel marine sponge derived amino acids, 8. conformational analysis of jasplakinolide. J Am Chem Soc 1989, 111, 2822–2829. [Google Scholar]
- Zampella, A; Giannini, C; Debitus, C; Roussakis, C; D’Auria, MV. New jaspamide derivatives from the marine sponge Jaspis splendans collected in Vanuatu. J Nat Prod 1999, 62, 332–334. [Google Scholar]
- Gala, F; D’Auria, MV; De Marino, S; Zollo, F; Smith, CD; Copper, JE; Zampella, A. New jaspamide derivatives with antimicrofilament activity from the sponge Jaspis splendans. Tetrahedron 2007, 63, 5212–5219. [Google Scholar]
- Gala, F; D’Auria, MV; De Marino, S; Sepe, V; Zollo, F; Smith, CD; Copper, JE; Zampella, A. Jaspamides H–L, new actin-targeting depsipeptides from the sponge Jaspis splendans. Tetrahedron 2008, 64, 7127–7130. [Google Scholar]
- Gala, F; D’Auria, MV; De Marino, S; Sepe, V; Zollo, F; Smith, CD; Keller, SN; Zampella, A. Jaspamides M–P: New tryptophan modified jaspamide derivatives from the sponge Jaspis splendans. Tetrahedron 2009, 65, 51–56. [Google Scholar]
- Ravi, BN; Wells, RJ; Croft, KD. Malabaricane triterpenes from a Fijian collection of the sponge Jaspis stellifera. J Org Chem 1981, 46, 1998–2001. [Google Scholar]
- Tsuda, M; Ishibashi, M; Agemi, K; Sasaki, T; Kobayashi, J. Stelliferins A–F, new antineoplastic isomalabaricane triterpenes from the Okinawan marine sponge Jaspis stellifera. Tetrahedron 1991, 47, 2181–2194. [Google Scholar]
- Kobayashi, J; Yuasa, K; Kobayashi, T; Sasaki, T; Tsuda, M. Jaspiferals A–G, new cytotoxic isomalabaricane-type nortriterpenes from Okinawan marine sponge Jaspis stellifera. Tetrahedron 1996, 52, 5745–5750. [Google Scholar]
- Zampella, A; D’Auria, MV; Debitus, C; Menou, J-L. New isomalabaricane derivatives from a new species of Jaspis sponge collected at the Vanuatu islands. J Nat Prod 2000, 63, 943–946. [Google Scholar]
- Meragelman, KM; McKee, TC; Boyd, MR. New cytotoxic isomalabaricane triterpenes from the sponge Jaspis species. J Nat Prod 2001, 64, 389–392. [Google Scholar]
- Tang, SA; Deng, ZW; Li, J; Fu, HZ; Pei, YH; Zhang, S; Lin, WH. A new isomalabaricane triterpenoid from sponge Jaspis sp. Chin Chem Lett 2005, 16, 353–355. [Google Scholar]
- Tang, S; Pei, Y; Fu, H; Deng, Z; Li, J; Proksch, P; Lin, W. Jaspolides A–F, six new isomalabaricane-type terpenoids from the sponge Jaspis sp. Chem Pharm Bull 2006, 54, 4–8. [Google Scholar]
- Tang, S; Deng, Z; Proksch, P; Lin, W. Jaspolides G and H, unique bisisomalabaricane from the Chinese marine sponge Jaspis sp. Tetrahedron Lett 2007, 48, 5443–5447. [Google Scholar]
- Kobayashi, J; Murata, O; Shigemori, H; Sasaki, T. Jaspisamides A–C, new cytotoxic macrolides from the Okinawan marine sponge Jaspis sp. J Nat Prod 1993, 56, 787–791. [Google Scholar]
- Rodriguez, J; Nieto, RM; Crews, P. New structures and bioactivity patterns of bengazole alkaloids from a choristid marine sponge. J Nat Prod 1993, 56, 2034–2040. [Google Scholar]
- Searle, PA; Richter, RK; Molinski, TF. Bengazoles C–G from the sponge Jaspis sp. synthesis of the side chain and determination of absolute configuration. J Org Chem 1996, 61, 4073–4079. [Google Scholar]
- D’Auria, MV; Giannini, C; Minale, L; Zampella, A; Debitus, C; Frostin, M. Bengamides and related amino acid derivatives from the new Caledonian marine sponge Jaspis carteri. J Nat Prod 1997, 60, 814–816. [Google Scholar]
- Groweiss, A; Newcomer, JJ; O’Keefe, BR; Blackman, A; Boyd, MR. Cytotoxic metabolites from an Australian collection of the sponge Jaspis species. J Nat Prod 1999, 62, 1691–1693. [Google Scholar]
- Thale, Z; Kinder, FR; Bair, KW; Bontempo, J; Czuchta, AM; Versace, RW; Philips, PE; Sanders, ML; Wattanasin, S; Crews, P. Bengamides revisited: New structures and antitumor studies. J Org Chem 2001, 66, 1733–1741. [Google Scholar]
- Park, Y; Liu, Y; Hong, J; Lee, C-O; Cho, H; Kim, D-K; Im, KS; Jung, JH. New bromotyrosine derivatives from an association of two sponges, Jaspis wondoensis and Poecillastra wondoensis. J Nat Prod 2003, 66, 1495–1498. [Google Scholar]
- Shinde, PB; Lee, YM; Dang, HT; Hong, J; Lee, C-O; Jung, JH. Cytotoxic bromotyrosine derivatives from a two-sponge association of Jaspis sp. and Poecillastra sp. Bioorg Med Chem Lett 2008, 18, 6414–6418. [Google Scholar]
- Ohta, S; Kobayashi, H; Ikegami, S. Isojaspisin: A novel styryl sulfate from a marine sponge, Jaspis sp., that inhibits hatching of sea urchin embryos. Tetrahedron Lett 1994, 35, 4579–4580. [Google Scholar]
- Tsukamoto, S; Kato, H; Hirota, H; Fusetani, N. Narains: N,N-Dimethylguanidinium styryl sulfates, metamorphosis inducers of ascidian larvae from a marine sponge Jaspis sp. Tetrahedron Lett 1994, 35, 5873–5874. [Google Scholar]
- Ohta, S; Kobayashi, H; Ikegami, S. Jaspisin, a novel styryl sulfate from the marine sponge, Jaspis species. Biosci Biotech Biochem 1994, 58, 1752–1753. [Google Scholar]
- Tsukamoto, S; Kato, H; Hirota, H; Fusetani, N. 3,4-Dihydroxystyrene dimmers, inducers of larval metamorphosis in ascidians, from a marine sponge Jaspis sp. Tetrahedron 1994, 50, 13583–13592. [Google Scholar]
- Chang, YH; Shin, D; Na, Z; Lee, H-S; Kim, D-D; Oh, K-B; Shin, J. Dihydroxystyrene metabolites from an association of the sponge Poecillastra wondoensis and Jaspis sp. J Nat Prod 2008, 71, 779–783. [Google Scholar]
- Ebada, SS; Edrada, RA; Lin, W; Proksch, P. Methods for isolation, purification and structural elucidation of bioactive secondary metabolites from marine invertebrates. Nat Prot 2008, 3, 1820–1831. [Google Scholar]
- Kahn, M; Nakanishi, H; Su, T; Lee, JH; Johnson, M. Design and synthesis of nonpeptide mimetics of jaspamide. Int J Pept Protein Res 1991, 38, 324–334. [Google Scholar]
- Carmichael, J; DeGraff, WG; Gazdar, AF; Minna, JD; Mitchell, JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of radiosensitivity. Cancer Res 1987, 47, 943–946. [Google Scholar]

| Position | Jaspamide Q (2) | Jaspamide R (3) |
|---|---|---|
| δH mult. (J Hz) | δH mult. (J Hz) | |
| 2 | 2.51 (1H, m) | 2.49 (1H, m) |
| 3 | A) 2.37 (1H, dd, 11.5, 15.8 Hz) | A) 2.37 (1H, dd, 11.5, 15.8 Hz) |
| B) 1.90 (1H, d, 15.8 Hz) | B) 1.90 (1H. d, 15.8 Hz) | |
| 5 | 4.79 (1H, d, 6.7 Hz) | 4.74 (1H, d, 6.7 Hz) |
| 6 | 2.23 (1H, m) | 2.23 (1H, m) |
| 7 | A) 1.32 (1H, m), B) 1.10 (1H, m) | A) 1.32 (1H, m), B) 1.10 (1H, m) |
| 8 | 4.64 (1H, m) | 4.60 (1H, m) |
| 10 | A) 2.67 (1H, dd, 4.7, 15.0 Hz) | A) 2.67 (1H, dd, 4.7, 15.0 Hz) |
| B) 2.61 (1H, dd, 5.7, 14.8 Hz) | B) 2.61 (1H, dd, 5.7, 14.8 Hz) | |
| 11 | 5.25 (1H, dd, 5.3, 8.6 Hz) | 5.25 (1H, dd, 5.3, 8.6 Hz) |
| 13 | 5.63 (1H, dd, 6.3, 10.0 Hz) | 5.73 (1H, dd, 6.3, 10.0 Hz) |
| 15 | 4.77 (1H, m) | 4.72 (1H, m) |
| 16 | 1.04 (3H, d, 6.6 Hz) | 0.81 (3H, d, 6.7 Hz) |
| 17 | 2.97 (3H, s) | 2.98 (3H, s) |
| 18 | A) 3.43 (1H, dd, 6.3, 15.5 Hz) | A) 3.33 (1H, dd, 6.3, 15.5 Hz) |
| B) 3.17 (1H, dd, 10.4, 15.2 Hz) | B) 3.18 (1H, dd, 10.4, 15.2 Hz) | |
| 21 | 7.61 (1H, d, 8.0 Hz) | 7.41 (1H, br s) |
| 22 | 7.11 (1H, t, 8.0 Hz) | |
| 23 | 7.18 (1H, t, 8.0 Hz) | 7.20 (1H, d, 8.2 Hz) |
| 24 | 7.35 (1H, d, 8.0 Hz) | 7.42 (1H, d, 8.2 Hz) |
| 26 | 6.87 (1H, br s) | |
| 28 | 6.90 (1H, d, 8.3 Hz) | 6.98 (1H, d, 8.4 Hz) |
| 29 | 6.69 (1H, d, 8.3 Hz) | 6.71 (1H, d, 8.4 Hz) |
| 31 | 6.69 (1H, d, 8.3 Hz) | 6.71 (1H, d, 8.4 Hz) |
| 32 | 6.90 (1H, d, 8.3 Hz) | 6.98 (1H, d, 8.4 Hz) |
| 33 | 1.06 (3H, d, 6.3 Hz) | 1.05 (3H, d, 6.1 Hz) |
| 34 | 0.83 (3H, d, 6.5 Hz) | 0.81 (3H, d, 6.7 Hz) |
| 35 | 1.59 (3H, s) | 1.57 (3H, s) |
| 36 | 1.15 (3H, d, 6.8 Hz) | 1.13 (3H, d, 6.8 Hz) |
| NH-Tyr | 7.46 (1H, d, 8.6 Hz) | 7.53 (1H, d, 8.6 Hz) |
| NH-Trp | 8.24 (1H, br s) | 8.42 (1H, br s) |
| NH-Ala | 6.73 (1H, d, 6.4 Hz) | 6.65 (1H, d, 6.5 Hz) |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ebada, S.S.; Wray, V.; De Voogd, N.J.; Deng, Z.; Lin, W.; Proksch, P. Two New Jaspamide Derivatives from the Marine Sponge Jaspis splendens. Mar. Drugs 2009, 7, 435-444. https://doi.org/10.3390/md7030435
Ebada SS, Wray V, De Voogd NJ, Deng Z, Lin W, Proksch P. Two New Jaspamide Derivatives from the Marine Sponge Jaspis splendens. Marine Drugs. 2009; 7(3):435-444. https://doi.org/10.3390/md7030435
Chicago/Turabian StyleEbada, Sherif S., Victor Wray, Nicole J. De Voogd, Zhiwei Deng, Wenhan Lin, and Peter Proksch. 2009. "Two New Jaspamide Derivatives from the Marine Sponge Jaspis splendens" Marine Drugs 7, no. 3: 435-444. https://doi.org/10.3390/md7030435
APA StyleEbada, S. S., Wray, V., De Voogd, N. J., Deng, Z., Lin, W., & Proksch, P. (2009). Two New Jaspamide Derivatives from the Marine Sponge Jaspis splendens. Marine Drugs, 7(3), 435-444. https://doi.org/10.3390/md7030435








