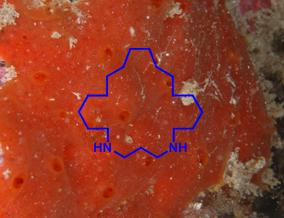
1,5-Diazacyclohenicosane, a New Cytotoxic Metabolite from the Marine Sponge Mycale sp.
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. Animal material
3.3. Extraction and isolation
3.4. Biological activity
Acknowledgements
- Samples Availability: Samples of compound 1 are available from the authors.
References and Notes
- Blunt, JW; Copp, BR; Munro, MHG; Northcote, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2009, 26, 170–244, and previous papers in this series. [Google Scholar]
- Faulkner, DJ. Marine natural products. Nat Prod Rep 2002, 19, 1–49, and previous papers in this series. [Google Scholar]
- Perry, NB; Blunt, JW; Munro, MHG; Pannel, LK. Mycalamide A, an antiviral compound from a New Zealand sponge of the genus Mycale. J Am Chem Soc 1988, 110, 4850–4851. [Google Scholar]
- Perry, NB; Blunt, JW; Munro, MHG; Thompson, AM. Antiviral and antitumor agents from a New Zealand sponge, Mycale sp. 2. Structures and solution conformations of mycalamides A and B. J Org Chem 1990, 55, 223–227. [Google Scholar]
- Fusetani, N; Yasumuro, K; Matsunaga, S; Hashimoto, K. Mycalolides A-C, hybrid macrolides of ulapualides and halichondramide, from a sponge of the genus Mycale. Tetrahedron Lett 1989, 30, 2809–2812. [Google Scholar]
- Matsunaga, S; Sugawara, T; Fusetani, N. New mycalolides from the marine sponge Mycale magellanica and their interconversion. J Nat Prod 1998, 61, 1164–1167. [Google Scholar]
- Phuwapraisirisan, P; Matsunaga, S; van Soest, RWM; Fusetani, N. Isolation of a new mycalolide from the marine sponge Mycale izuensis. J Nat Prod 2002, 65, 942–943. [Google Scholar]
- Northcote, PT; Blunt, JW; Munro, MHG. Pateamine: A potent cytotoxin from the New Zealand marine sponge, Mycale sp. Tetrahedron Lett 1991, 32, 6411–6414. [Google Scholar]
- West, LM; Northcote, PT; Battershill, CN. Peloruside A: A potent cytotoxic macrolide isolated from the New Zealand marine sponge Mycale sp. J Org Chem 2000, 65, 445–449. [Google Scholar]
- Nakao, Y; Yoshida, S; Matsunaga, S; Shindoh, N; Terada, Y; Nagai, K; Yamashita, JK; Ganesan, A; van Soest, RWM; Fusetani, N. Azumamides A-E: Histone deacetylase inhibitory cyclic tetrapeptides from the marine sponge Mycale izuensis. Angew Chem Int Ed 2006, 45, 7553–7557. [Google Scholar]
- Williams, DE; Lassota, P; Andersen, RJ. Motuporamines A-C, cytotoxic alkaloids isolated from the marine sponge Xestospongia exigua (Kirkpatrick). J Org Chem 1998, 63, 4838–4841. [Google Scholar]
- Williams, DE; Craig, KS; Patrick, B; McHardy, LM; van Soest, R; Roberge, M; Andersen, RJ. Motuporamines, anti-invasion and anti-angiogenic alkaloids from the marine sponge Xestospongia exigua (Kirkpatrick): Isolation, structure elucidation, analogue synthesis, and conformational analysis. J Org Chem 2002, 67, 245–258. [Google Scholar]
- Koren-Goldshlager, G; Kashman, Y; Schleyer, M. Haliclorensin, a novel diamino alkaloid from the marine sponge Haliclona tulearensis. J Nat Prod 1998, 61, 282–284. [Google Scholar]
- Heinrich, MR; Kashman, Y; Spiteller, P; Steglich, W. Revision of the structure of haliclorensin to (S)-7-methyl-1,5-diazacyclotetradecane and confirmation of the new structure by synthesis. Tetrahedron 2001, 57, 9973–9978. [Google Scholar]
- Skehan, P; Storeng, R; Scudiero, D; Monks, A; McMahon, J; Vistica, D; Warren, JT; Bokesch, H; Kenney, S; Boyd, MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 1990, 82, 1107–1112. [Google Scholar]
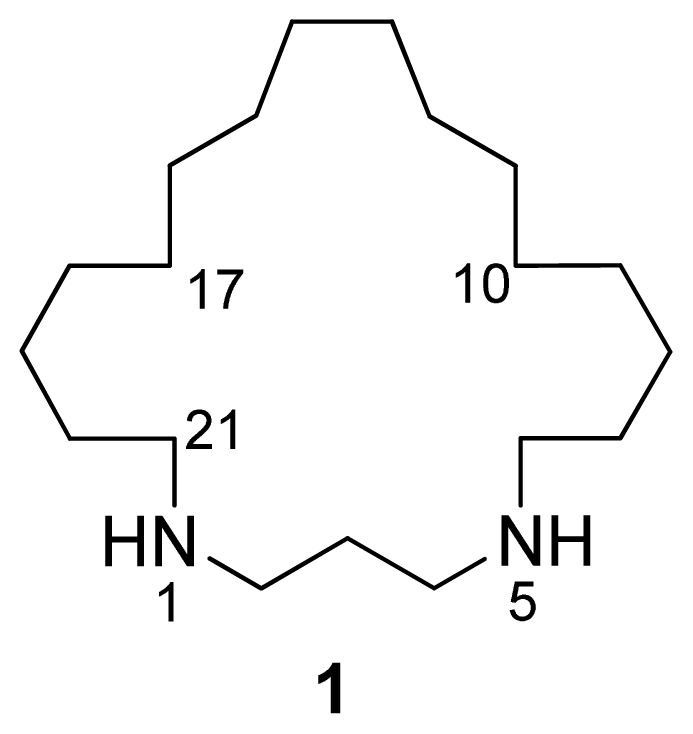
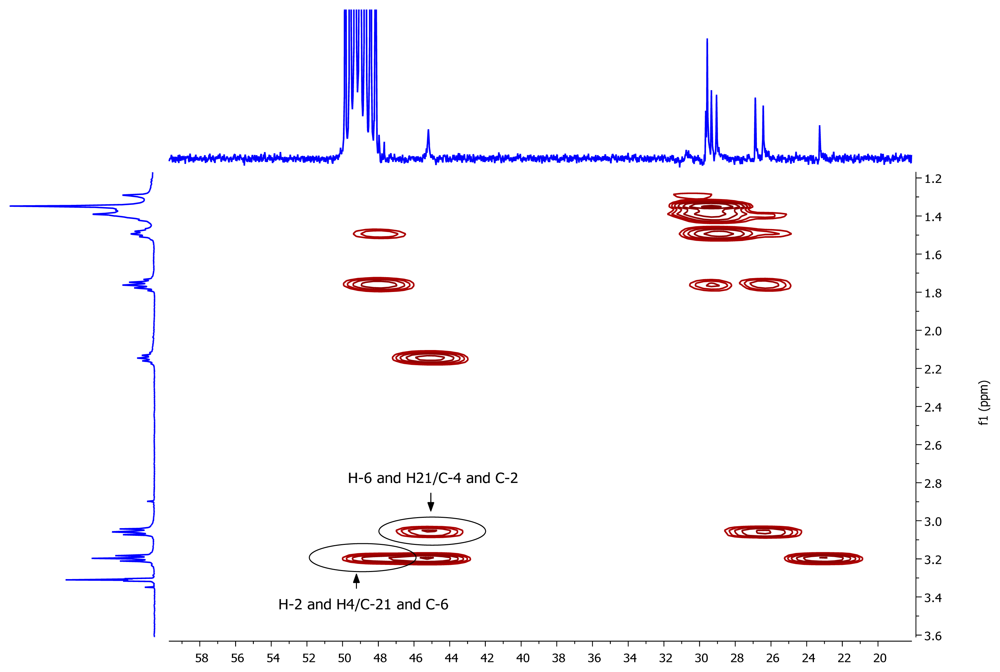
| Nº | δC, mult. | δH, mult. (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 2 | 45.2, CH2 | 3.20, t (7.2) | 3 | 3, 4, 21 |
| 3 | 23.3, CH2 | 2.15, quint (7.2) | 2, 4 | 2, 4 |
| 4 | 45.2, CH2 | 3.20, t (7.2) | 3 | 2, 3, 6 |
| 6 | 48.2, CH2 | 3.06, m | 7 | 4, 7, 8 |
| 7 | 26.4, CH2 | 1.76, m | 6, 8 | 6, 8, 9 |
| 8 | 26.9, CH2 | 1.49, m | 7, 9 | 6,7, 9, 10 |
| 9 | 29.3, CH2 | 1.39, m | ||
| 10 | 29.1, CH2 | 1.35, m | ||
| 11 | 29.6, CH2* | 1.35, m | ||
| 12 | 29.6, CH2* | 1.35, m | ||
| 13 | 29.7, CH2* | 1.35, m | ||
| 14 | 29.7, CH2* | 1.35, m | ||
| 15 | 29.6, CH2* | 1.35, m | ||
| 16 | 29.6, CH2* | 1.35, m | ||
| 17 | 29.1, CH2 | 1.35, m | ||
| 18 | 29.3, CH2 | 1.39, m | ||
| 19 | 26.9, CH2 | 1.49, m | 18, 20 | 17, 18, 20, 21 |
| 20 | 26.4, CH2 | 1.76, m | 19, 21 | 18, 19, 21 |
| 21 | 48.2, CH2 | 3.06, m | 20 | 2, 19, 20 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Coello, L.; Martín, M.J.; Reyes, F. 1,5-Diazacyclohenicosane, a New Cytotoxic Metabolite from the Marine Sponge Mycale sp. Mar. Drugs 2009, 7, 445-450. https://doi.org/10.3390/md7030445
Coello L, Martín MJ, Reyes F. 1,5-Diazacyclohenicosane, a New Cytotoxic Metabolite from the Marine Sponge Mycale sp. Marine Drugs. 2009; 7(3):445-450. https://doi.org/10.3390/md7030445
Chicago/Turabian StyleCoello, Laura, María Jesús Martín, and Fernando Reyes. 2009. "1,5-Diazacyclohenicosane, a New Cytotoxic Metabolite from the Marine Sponge Mycale sp." Marine Drugs 7, no. 3: 445-450. https://doi.org/10.3390/md7030445
APA StyleCoello, L., Martín, M. J., & Reyes, F. (2009). 1,5-Diazacyclohenicosane, a New Cytotoxic Metabolite from the Marine Sponge Mycale sp. Marine Drugs, 7(3), 445-450. https://doi.org/10.3390/md7030445