1. Introduction
Marine organisms are a major reservoir of bioactive natural products with potential biomedical application; several marine natural products are regarded as potential therapeutic agents for the treatment of multiple disease categories [
1]. Many bioactive marine natural products and their derivatives are produced by invertebrates, such as sponges, soft corals, tunicates, mollusks or bryozoans, and are evaluated advancedly in preclinical and even clinical trials [
1,
2]. Moreover, from 2005 to 2007, two of 13 natural products and natural products-derived drugs approved marketing worldwide are found from marine organisms [
3]. These facts attract us to pay more efforts on the research of bioactive natural products from other marine invertebrates.
Though seemingly defenseless, soft corals produce various chemical substances to discourage predation, instead of a protective calcium carbonate skeleton of hard corals as a defense mechanism. These chemicals are toxic to either predators or some neighboring hard corals, and some of these substances may have properties that can be beneficial to humans too. Because the geographic setting of Taiwan, between the West Pacific Ocean and East China Sea and at the crossroads of the Philippine-Japan I. Arc, has produced reefs of special biogeographical interest, three kinds of Formosan soft corals, including Cladiella australis (Alcyoniidae), Clavularia viridis (Clavulariidae) and Klyxum simplex (Alcyoniidae), were collected to be evaluated for biomedical research.
Oral cancer is a major global public health problem, causing high morbidity and mortality that have not improved in decades [
4]. Squamous cell carcinomas (SCCs) are the most common type of oral cancer. Although new surgery techniques and adjuvant measures, such as chemotherapy and radiotherapy, have progressed, patients with advanced oral SCCs still have a poor prognosis, with a 5-year survival rate of 65% [
5]. To develop new methods and improve existing protocols for diagnosis and treatment of SCCs become mandatory.
In the last decade, the manipulation of apoptosis (programmed cell death) has received considerable attention as a novel and promising strategy for cancer chemoprevention and therapy [
6,
7]. Cell apoptosis is characterized and involved by a series of typical morphological events, for example membrane blebbing, shrinkage of the cell, chromatin condensation, nuclear fragmentation, fragmentation into membrane-bound apoptotic bodies translocation of membrane phosphatidylserine and sub-G
1 fraction, and rapid phagocytosis by neighboring cells [
8]. Effective cancer therapeutic strategies often rely on preferential and efficient induction of apoptosis in tumor cells. The objective of this research was to evaluate the capability of ethyl acetate extracts of
C. australis,
C. viridis and
K. simplex to inhibit the growth and induce cell apoptosis on human oral SCCs cells.
2. Materials and Methods
2.1. Tissue extraction and sample preparation
The soft corals of C. australis, C. viridis and K. simplex were collected via scuba along the coast of Southern Taiwan, at a depth of 10–15 m and were stored in a freezer until extraction. A voucher specimen was deposited at the Department of Marine Biotechnology and Resources, National Sun Yat-Sen University, Kaohsiung, Taiwan. The tissues were freeze-dried and then exhaustively extracted with ethyl acetate. The ethyl acetate extracts were then filtered and concentrated under vacuum to provide a brownish semisolid crude extract. Organic extracts were dissolved freshly in dimethylsulfoxide (DMSO) and the final concentration of DMSO did not exceed 0.1% (v/v) throughout the experiments.
2.2. Cell culture and treatment
Human oral SCCs cells (moderate differentiation of SCC4, poor differentiation of SCC9 and well differentiation of SCC25) were cultured in Dulbecco’s Modified Eagle Medium (DMEM)/F12 medium supplemented with 0.4 μg/ml hydrocortisone. All culture cells were purchased from American Type Culture Collection (Manassas, VA) and maintained in medium supplemented with 10% fetal bovine serum (Hazelton Product, Denver, PA, USA) and 1% penicillin-streptomycin at 37°C in 5% CO2 humidified atmosphere. At various concentrations after the treatment, the cells were processed for the analyses of cell adhesion, cytotoxicity, morphology changes, cell cycle, and apoptosis.
2.3. Measurement of cell adhesion
Cells (1.5 × 10
5 cells/well) were subcultured into 24-well plates and incubated. After 24 h of incubation, the medium was changed by adding medium containing 1% bovine serum albumin (BSA) and with or without serial concentrations of extracts for 18 h. Attached cell number was estimated using a DNA carmine-based colorimetric method [
9]. Briefly, cells were fixed with 100% methanol, dried, and stained with alcoholic/HCl carmine. Colorant was extracted with 0.01 N NaOH, and absorbance was determined at 540 nm. The cell number was estimated using a titration curve of cell density, and results were given as a percentage of the cell number with respect to control cells. For the titration curve, cells were plated at densities ranging form 1 × 10
3 to 7 × 10
5 cells/well in 24-well plates using serial dilutions of concentrated cell suspensions. After adhesion, some wells of each density were harvested with trypsin and cells were counted in a hemacytometer; meanwhile, parallel cultures were fixed and stained as described above [
9]. A relationship between the cell number and resultant absorbance after the colorant extraction, for each cell density, was accomplished and cell-density titration-curve construction, which measured cell adhesion.
2.4. Determination of cell viability
Cells (1.5 × 104 cells/well) were seeded in each 100 μl of 96-well multi-dishes for at least 24 h and then treatment with serial concentrations of extracts for 18 h. After replacing new medium, cellular cytotoxicity were determined by MTS [3-(4,5-di-methyl-thiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] assay (CellTiter 96™ AQ, Promega, Madison). The absorbance at 490 nm was measured by a multi-mode microplate reader (Synergy™2, BioTek Instruments, Inc., USA). Values are expressed as the percentage of mean cell viability relative to the untreated cultures. The IC50 and IC80 were calculated from the drug concentration that induced 50% and 80% of cell viability rate.
2.5. Assessment of cell morphological changes
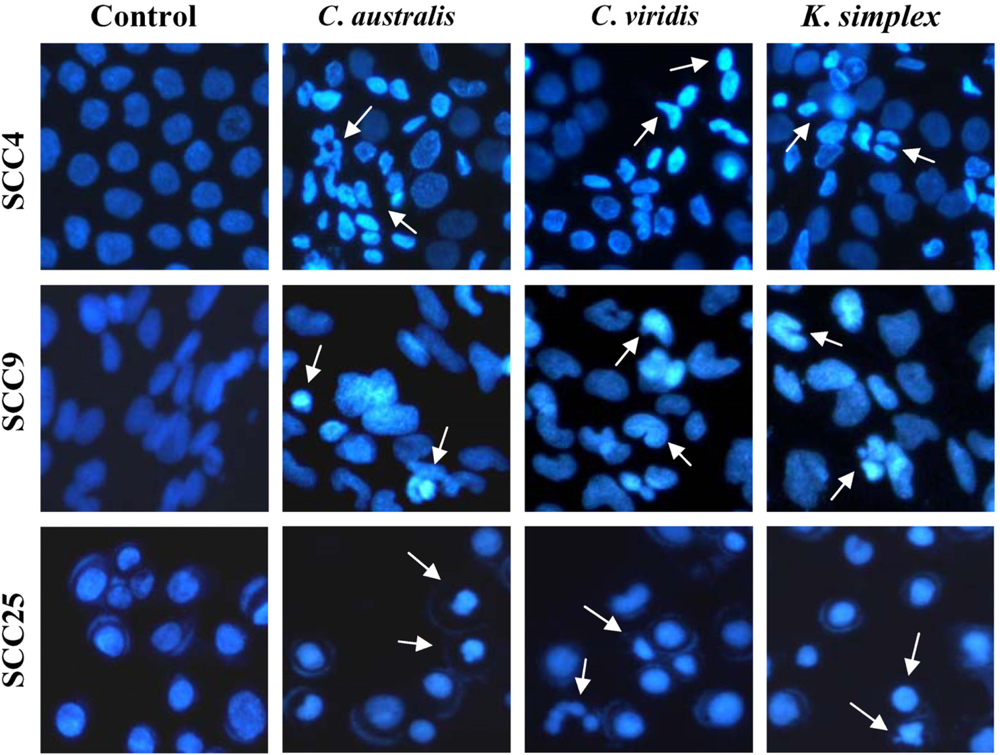
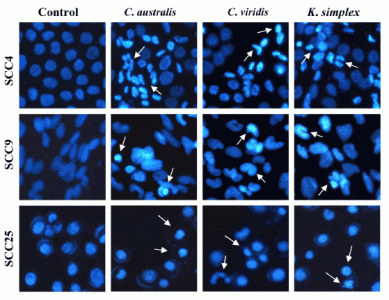
Cells (1.5 × 105 cells/well) were plated in 24-well plates then treated with IC50 concentrations of extracts for 18 h. After incubation, the medium was removed and cells were fixed in 4% paraformaldehyde and permeabilized in saponin (0.1% v/v in PBS-BSA). To assess specific apoptosis, Hoechst 33342 (1 μg/ml) (Promega, USA) was added to each well and further incubated at 37°C for 30 min in the dark. Living and apoptotic cells were visualized through blue filter of fluorescence inverted microscope (Nikon, TE2000-U, Japan) at 200× magnification.
2.6. Assessment of cell cycle distribution and apoptotic cells
Cells (1.5 × 105 cells/well) were seeded in 24-well plates and incubated with or without IC50 concentration of extracts for 18 h. Cells were then fixed in 70% ethanol/PBS, pelleted and resuspended in buffer containing 200 μg/ml RNase A and 0.01 mg/ml propidium iodide (PI). The cells were incubated in the dark for 15 min at room temperature and then analyzed by FACScan flow cytometer (Becton Dickinson, San Jose, CA). The cell distribution in each phase (sub-G1, G0/G1, S and G2/M phases) of the cell cycle was determined using Windows Multiple Document Interface software (WinMDI), including subG1-peak of apoptotic cells.
2.7. Determination of the activation of caspase-3 expressions
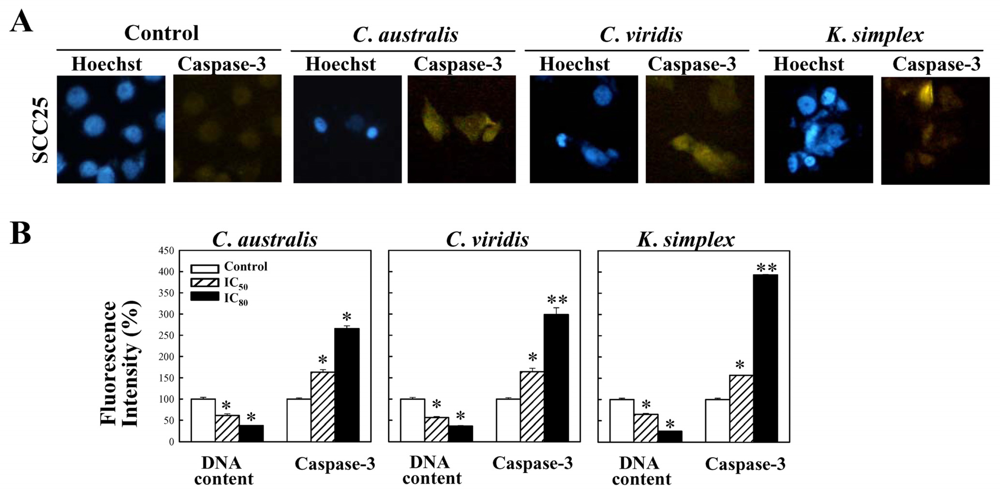
Cells were treated with IC80 concentration of extracts for 18 h and the expressions of caspase-3 were studied. Cells were stained with mouse anti-cleaved caspasae-3 monoclonal antibodies (1:300) (Santa Cruz, CA) in 1× PBS containing 0.5% BSA (PBS-BSA) and 0.1% sodium azide (Sigma–Aldrich) for 45 min at 4°C. Cells were then washed twice with cold PBS and incubated with FITC-conjugated anti-mouse IgG (1:500) (Santa Cruz, CA) at 4°C for 30 min. Cells were washed with cold PBS and fixed in 4% paraformaldehyde. The cell nuclei were stained with 0.1 μg/ml of Hoechst 33342 (Promega, USA) and inspected using a fluorescent microscope (Nikon, TE2000-U, Japan).
2.8. Quantitative analysis of the activation of caspase-3 expressions
Cells were treated with IC50 and IC80 concentration of extracts for 18 h and the expressions of caspase-3 were studied. Cells were stained with mouse anti-cleaved caspasae-3 monoclonal antibodies (1:300) (Santa Cruz, CA) in 1× PBS containing 0.5% BSA (PBS-BSA) and 0.1% sodium azide (Sigma–Aldrich) for 45 min at 4°C. Cells were then washed twice with cold PBS and incubated with FITC-conjugated anti-mouse IgG (1:500) (Santa Cruz, CA) at 4°C for 30 min. Cells were washed with cold PBS and fixed in 4% paraformaldehyde. The cell nuclei were stained with 0.1 μg/ml of Hoechst 33342 (Promega, USA). For percentage of fluorescent staining analysis, caspase-3 expressions (Ex, 495 nm; Em, 525 nm) and the cell nuclei (Ex, 346 nm; Em, 460 nm) were measured from three independent experiments by Multi-Detection Microplate Reader (Synergy™2, BioTek Instruments, Inc., USA) and calculated using Gene5™ software.
2.9. Statistical analysis
To evaluate the statistical significance of the difference of all the values, statistical analysis was performed on the means of the triplicates of at least three independent experiments using a two-tailed Student’s t-test. P values less than 0.05 was considered significant for all tests.
4. Discussion
Coral reef is a basic and important biomass in ocean. The ecological factors in coral reef, such as species competition and limiting resources in space and light, lead soft corals to develop a delicate chemical balance for self protection, range from chemical defense against predation to intra-specific cues for larval settlement. Meanwhile, some of these secondary metabolites have also shown potential as new medicines for the treatment of a variety of human diseases. During the past decades, intense attention has been focused on the anti-tumor property of marine natural products and their derivatives, such as pachymatismin from the marine sponge (
Pachymatisma johnstonii), didemnin B from a Caribbean tunicate (
Trididemnum solidum) and bryostatins from the bryozoan (
Bugula neritina) [
10]. Soft corals (coelenterata, octocorallia, alcyonaceae) are a rich source of steroids and terpenoids [
2,
11], in which most isolated diterpenes are cembranolides [
12]. In addition, marine eicosanoids, such as clavulones, halogenated clavulones and prostanoids, have attracted much interest because of their novel structures and significant antitumor, antileukemic effect and antiviral activities [
13]. These prostanoids have been found in the different species of marine organism such as
C. viridis,
Telesto riisei, and
Gracilaria sp. [
14]. Additionally, the
C. viridis is rich in bioactive prostanoids with different structural types such as preclavulone lactones, clavirins, tricycloclavulone, and clavubicyclone [
14,
15]. The prostanoids have been suggested to display anti-tumor activities in several types of human tumors [
14–
16]. Recently, several new marine prostanoids isolated from
C. viridis among these prostanoids, bromovulone III showed inducing of Fas clustering [
17], enhancing of endoplasmic reticulum stress [
10] and promising anti-tumor activity and apoptosis in human hormone-resistant prostate cancers and human hepatocellular carcinoma cells [
10,
17]. Previous reports have shown that eunicellin-based diterpenoids, cladiellane diterpenes and australins A-D from the ethanol extract of
C. australis has cytotoxic activity against human breast cell lines (MCF-7, MDA-MB-231) and liver cell lines (HepaG2/DMEM-12) [
18]. However, none thorough cytotoxicity research has been performed on
K. simplex and the mechanisms underlying the anti-oral cancer efficiency of
C. australis,
C. viridis and
K. simplex extracts and their secondary metabolites are poorly understood. Additional research is required to confirm the inhibitory effects and action mechanisms of
C. australis,
C. viridis and
K. simplex extracts on different cancer cells and possible clinical use. This study shows the action mechanism of
C. australis,
C. viridis and
K. simplex organic extracts in the human oral SCCs cells.
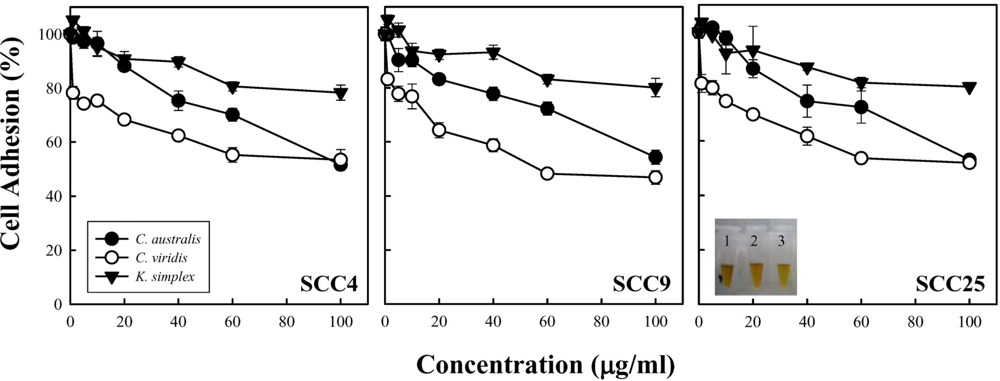
Cell density and cell proliferation is usually evaluated during
in vitro studies to pharmacological effects of specific compounds [
9]. In this study, cell adhesion and MTS assay revealed that
C. australis,
C. viridis and
K. simplex extracts impeded SCC4, SCC9 and SCC25 cells growth in a concentration-dependent manner. The
C. viridis was the most potent inhibitor of cells adhesion and exhibited the activity in inhibiting cell growth; that is, better than those of
C. australis and
K. simplex. Nevertheless, the intrinsic structure and properties of these three soft corals are still not to be clarified. Additionally, the relation yield of compounds purified form soft corals is too few to carry out apoptosis experiments. This study is a preliminary test for cytotoxic activity of soft corals and very few correlated researches could be found. At least, these results could provide the useful information to determine whether it is worthy to further isolate the natural product or not.
Although 5-fluorouracil, 5% imiquimod cream, and 3% diclofenac gel are available as agents for topical skin cancer field therapies. Nevertheless, previous reports have shown that a successful treatment of topical 5-fluorouracil cream is inevitably accompanied by pain, pruritus, burning, erythema, erosion, and scar, not only in diseased skin but also occurred in peripheral normal skin [
19]. In addition, previous reports have demonstrated that clinical drugs such as 5-fluorouracil and imiquimod incubated with cancer cell lines exhibited obvious cytotoxicity after treatment extension to 72 h. The concentrations of 5-fluorouracil that induced cell death by 50% (IC
50) were approximately 46.3 μg/ml for SCC25 cells (data not shown). Therefore, no significantly cytotoxic difference between these extracts and 5-fluorouracil could be found at the same and short interaction time for 18 h. Maybe the more deeply research is necessary in the near future to realize the true effect of these compounds on oral cancer cells.
Cancer is characterized by deregulated cell proliferation combined with suppressed apoptosis [
20]. Most chemotherapeutic drugs exert their cytotoxic action by inhibiting cancer cell growth and inducing apoptosis [
21]. Previous studies have demonstrated that
Sinularia sp. extracts exhibited potent cytotoxicity against A431 cells [
22] and NAKATA cells [
23], and induced apoptotic DNA fragmentation and condensation of chromatin in A431 cells obtained from SCC [
24]. In this study, morphologic alterations, nuclear chromatin condensation and formation of apoptotic bodies demonstrate that
C. australis,
C. viridis and
K. simplex extracts cause apoptosis of SCCs cells.
Cells contain various pathways designed for protection from the genomic instability or toxicity that cell cycle play a pivotal role of this response. During tumorigenesis, tumor cells frequently loose checkpoint controls, which facilitate tumor development. Therefore, one important approach for cancer chemotherapy is to regulate cell-cycle progression. Previous reports have shown that clavulone and the halogenated analogues are anti-tumor prostanoids isolated from the
C. viridis. It has been reported that clavulones show the antitumor activities against human leukemia HL-60 cells through the arrest of the cell cycle progression from G
1 to S phase [
25]. In this study, the cell cycle distribution indicates that
C. australis,
C. viridis and
K. simplex extracts sensitized cells in the G
0/G
1 and S-G
2/M phases with a concomitant significant increase in the sub-G
1 phase. Additionally, the caspases family can be divided into major subgroups based on their substrate-specificity, sequence homology and biochemical functions; particularly, caspase-3 plays a pivotal role in the terminal phase of apoptosis [
26]. This study has revealed that
C. australis,
C. viridis and
K. simplex extracts may accelerate caspase-3 activation, thus resulting in apoptosis of SCC25 cells.
This study first presented evidence that human oral SCCs cells are sensitive to ethyl acetate extracts from C. australis, C. viridis and K. simplex. In conclusion, the present evidence suggests that C. australis, C. viridis and K. simplex extracts exerts its cytotoxicity effect through inhibition of the cell cycle and induction of apoptosis underwent activation of caspase-3 in SCCs cells. But the exact pathway such as what is the receptor involved in the caspase-3 activation is still unclear in these studies. Apparently, further investigations are needed for future studies.