Abstract
Tetrodotoxin (TTX) is a highly specific blocker of voltage-gated sodium channels. The dissociation constant of block varies with different channel isoforms. Until recently, channel resistance was thought to be primarily imparted by amino acid substitutions at a single position in domain I. Recent work reveals a novel site for tetrodotoxin resistance in the P-region of domain IV.
1. Sodium channel structure and function
Voltage-gated sodium channels (Nav) are membrane-bound proteins that initiate action potentials in nerve and muscle cells and are critical elements of proper function in these tissues []. The channels open when the voltage across the cell membrane is depolarized by a few millivolts above the normally negative resting membrane potential. Channel activation allows sodium ions to enter the cell and further depolarize the membrane potential. The movement of sodium ions through the membrane comprises the rising phase of the action potential. Rapid inactivation of the channel stops the flow of sodium ions through the membrane. This step, along with the activation of voltage-gated potassium channels, allows the membrane to repolarize and ends the action potential. Action potentials act as electrical messages that travel along the axons of nerve cells and the surface of muscle fibers initiating the release of neurotransmitters by neurons and coordinating contractions in muscle. Thus, the loss of Nav activity paralyses nerve and muscle tissue.
Sodium channels are formed by a 260 kDa α-subunit that is associated with one β subunit (β1) in skeletal muscle cells and with two β subunits (β3 and β1 or β2) in the central nervous system []. The α subunit forms the pore and the protein contains components required for other aspects of channel function including voltage-dependent activation and fast inactivation (Figure 1). Although the kinetics and voltage dependence of channel gating are modified by the β subunits [, ], all the elements of channel function can be reconstituted when the α subunit is expressed alone in heterologous expression systems, e.g. Xenopus laevis oocytes [–]. An early model of the two-dimensional folding pattern of the α subunit predicted that the protein consists of four homologous domains (DI-IV) composed of six α-helical transmembrane segments (S1–S6) and that the regions of the protein between segments S5 and S6 of all four domains reenter the membrane to form the outer pore of the channel through which sodium ions enter the cell []. Further work on sodium channels has supported the general features of this model including the identification of the S4 segments as the voltage sensor []. A repeated motif of positively charged amino acids separated by two hydrophobic amino acids is found in the portions of the channel assigned to the S4 transmembrane segments []. When the positively charged residues in the S4 transmembrane regions are replaced with uncharged amino acids, the voltage-dependence of channel gating is altered as would be expected in the region of the channel that acts as a voltage sensor [, ]. Additionally, there is evidence that portions of the S4 segments move outward in response to the membrane depolarization. Residues in the S4 segments become more accessible to reaction with extracellular reagents and less accessible to reaction with intracellular reagents when the membrane is repeatedly depolarized [, ]. After channels open in response to membrane depolarization, they rapidly inactivate which stops the flow of sodium ions into the cell. This form of channel gating occurs when the linker between the third and fourth domains of the channel physically occludes the intracellular mouth of the channel pore [, –]. Channel opening, ion selectivity and inactivation are each controlled by separate regions of the α-subunit. The extent to which these regions interact is a subject of ongoing study.
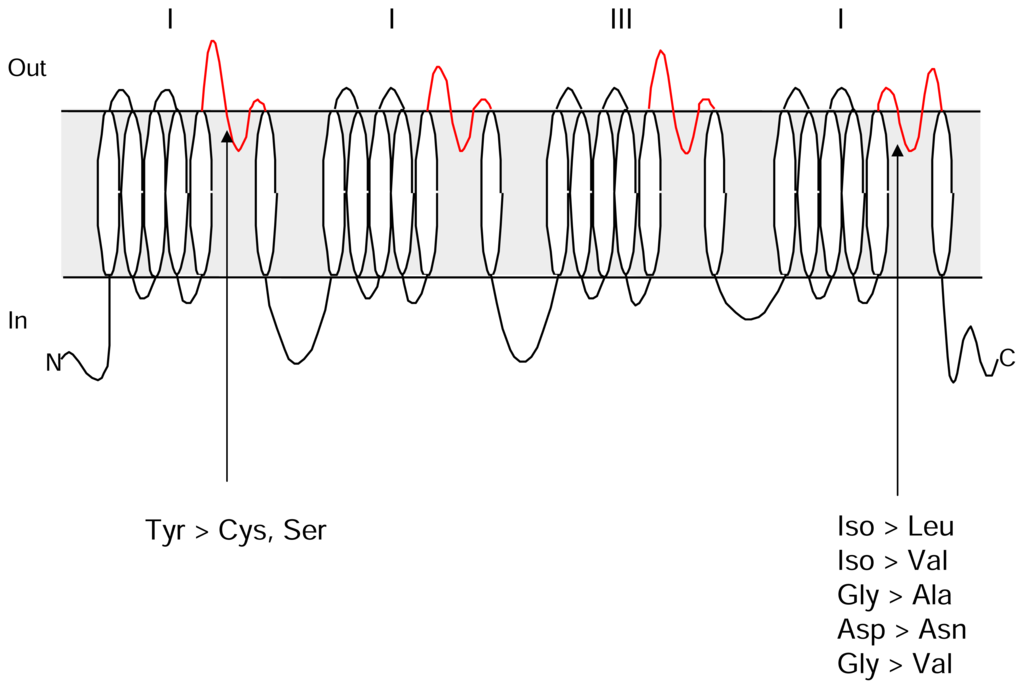
Figure 1.
Secondary structure of voltage gated sodium channels showing putative orientation of homologous domains and membrane spanning segments. Substitutions that impart TTX resistance in domains I and IV are shown.
2. Sodium channel gene family
Voltage-gated sodium channels are encoded by a multigene family [, ]. Different types of excitable tissue express different members of the sodium channel gene family and the tetrodotoxin (TTX) sensitivities of nerve or muscle cells are dependent on the type of channels expressed in the cell. There are ten members of the gene family in the three mammalian species where all members of the gene family have been identified []. In the weakly electric fish Sternopygus macrurus, six genes have been identified although there maybe as many as eight sodium channel isoforms in fish (personal communication by H. Zakon; []). The nine mammalian channel isoforms that have been identified and functionally expressed (NaV1.1 – NaV1.9) have greater than 50% identical amino acid sequence within each of the four domains and the linker between domains III and IV []. The tenth sodium channel isoform NaV has a more divergent sequence and may represent a distinct subfamily []. This channel isoform has never been functionally expressed in heterologous cells, but evidence from NaV expression patterns and NaV knockout mice indicate that the channel may not function as a voltage-gated channel but rather be important for sensing and regulating extracellular salt in the hypothalamus and visceral organs [, ].
The genes that encode NaV1.1 through NaV1.9 in humans and mice are found on four chromosomes and the chromosome segments containing sodium channel genes are paralogous []. A segment of chromosome 2 contains genes encoding NaV1.1, NaV1.2, NaV1.3 and NaV1.7 that have more than 90% amino acid sequence identity [, ].
The marine toxin TTX has long been recognized as a potent inhibitor of sodium currents in nerve and muscle. A more comprehensive review of TTX and its actions relative to other marine toxins is discussed elsewhere in this issue (Al-Sabi et al. []). TTX sensitivities of nerve or muscle cells are dependent on the type of channels expressed in the cell. Products from the cluster of genes located on chromosome 2 are all blocked by nanomolar concentrations of TTX and are expressed in neurons. A second cluster of genes containing NaV1.5, NaV1.8 and NaV1.9 is located on chromosome 3. These isoforms have 75% amino acid sequence identity as genes in the chromosome 2 cluster but are blocked by micromolar concentrations of TTX. The genes have more limited expression patterns, with NaV1.8 and NaV1.9 primarily expressed in neurons of the dorsal root ganglion and NaV1.5 primarily expressed in cardiac muscle cells. The two final sodium channel isoform genes, NaV1.4 and NaV1.6, are each located on separate chromosomes despite the fact that they have greater than 85% sequence identity with the genes clustered on chromosome 2 and are also blocked by nanomolar concentrations of TTX. One channel isoform, NaV1.6, is expressed in many types of neurons but NaV1.4 appears to be solely expressed in skeletal muscle fibers. Subtle differences in channel function may explain differences in the expression pattern of channel isoforms, but an understanding of the correspondence between channel function and expression is still at an early stage.
3. Sodium channel isoform expression patterns and function
Recent work has shed some light on the correspondence between channel isoform function and expression. Both NaV1.2 and NaV1.6 are expressed in neurons of the central nervous system but their expression patterns differ in the cell types and even the region within the same neuron in which they are found []. The nodes of Ranvier are the regions along the length of axons without myelin and glial cell wrapping that allow for the saltatory conduction of action potentials. The predominant isoform at the nodes of Ranvier in sensory and motor neurons of the adult central and peripheral nervous system is NaV1.6 []. However, in developing retinal ganglion cells both NaV1.2 and NaV1.6 are clustered at immature nodes of Ranvier, and only as myelination proceeds does NaV1.6 replace NaV1.2 [, ]. If NaV1.6 is required for proper function of mature axons, this suggests that NaV1.6 allows axons to transmit high frequency action potentials. Indeed, NaV1.2 and NaV1.6 respond differently to a rapid series of depolarizations, currents through NaV1.2 decrease and currents through NaV1.6 increase [, ]. Currents through NaV1.6 increase because repeated stimulations of the channels cause a use-dependent potentiation of channel opening where channels activate faster after repeated depolarizations. Currents may decrease through NaV1.2 after repeated stimulation because channels more rapidly enter a slow inactivated state and fewer channels are available to open with repeated stimulations. The slow inactivated state develops after prolonged membrane depolarization or repeated stimulation and sodium ions cannot pass through channels in this state [, ]. The expression of NaV1.6 in mature nodes of Ranvier may allow the rapid, repeated depolarization of the membrane and the transmission of high frequency action potentials along the length of the axon.
Cell maturation is accompanied by another change in sodium channel isoform expression in skeletal muscle tissue. Neonatal rats express both NaV1.5 and NaV1.4 in their skeletal muscle tissue. However, by postnatal day 35, mRNA encoding NaV1.5 is undetectable and mRNA encoding NaV1.4 has increased 10-fold [, ]. Intriguingly, after denervation NaV1.5 is expressed again and NaV1.4 transcript expression declines as if the muscle were reverting to an earlier developmental stage. Cardiac muscle tissue where NaV1.5 is primarily expressed has a very different pattern of activity than adult skeletal muscle where NaV1.4 is primarily expressed. Action potentials in the heart are repetitive and sustained with the membrane remaining depolarized for several hundred milliseconds []. In contrast, during the action potential in skeletal muscle fibers membrane depolarization lasts a few milliseconds and can occur at high frequency [, ]. If subjected to the prolonged depolarizations that occur during the cardiac action potential NaV1.4 channels enter the slow inactivated state and are no longer excitable unless the membrane is held at a negative potential for seconds []. However, NaV1.5 currents do not slow inactivate completely even after long depolarizations lasting seconds. These channels can function in the activity pattern found in heart muscle tissue and may also be able to function better in the altered activity pattern of immature and denervated muscle fibers that have depolarized resting membrane potentials and spontaneous action potentials []. The impairment of slow inactivation in NaV1.4 can lead to skeletal muscle dysfunction. Several mutations in NaV1.4 that are linked to the skeletal muscle diseases hyperkalemic periodic paralysis and paramyotonia congenital impair the ability of the channels to enter the slow inactivated state and reduce the use-dependent inhibition of sodium current after rapid stimulation [, ]. This defect accentuates the muscle membrane depolarization and hyperkalemia sensitivity that lead to muscle paralysis. Thus, an alteration of the sodium channel isoforms expressed in a tissue may impair the proper functioning of that tissue. Although a change in sodium channel expression pattern might allow certain tissue to resist the effects of TTX, the expression of an isoform with different kinetic and voltage-dependent properties might alter the functional properties of the tissue.
4. Tetrodotoxin binding
Tetrodotoxin blocks sodium channel activity by binding to the outer pore of the channel that is formed by S5–S6 linkers []. The portion of the linkers that interacts with TTX forms the pore α-helix, the selectivity filter and the outer vestibule of the pore [, ]. Sodium ions entering the cell pass through the outer vestibule of the pore and the narrow selectivity filter before they can enter the inner pore of the channel (Figure 2). Two rings of mostly negatively charged amino acids line the outer vestibule (shown in pink in Fig. 2), and the inner ring, composed of aspartate (DI), glutamate (DII), lysine (DIII), and alanine (DIV) (the DEKA filter shown as red, red, blue, and green space filling structures in Fig. 2) forms the selectivity filter [, ]. Structural support for the selectivity filter is provided by interactions of the amino acids between the negatively charged rings and those in the pore helices [, ]. The positively charged guandinium group and hydroxyls of TTX (Figure 3) interact with the side chains of the amino acids that form the negatively charged rings [, , ]. Changes in the amino acids of the S5–S6 linker from the pore helix to the outer negatively charged ring effect TTX binding either by altering the electrostatic interaction between TTX and an amino acid side chain directly or by altering the shape of this narrow portion of the pore where TTX binds [–, –].
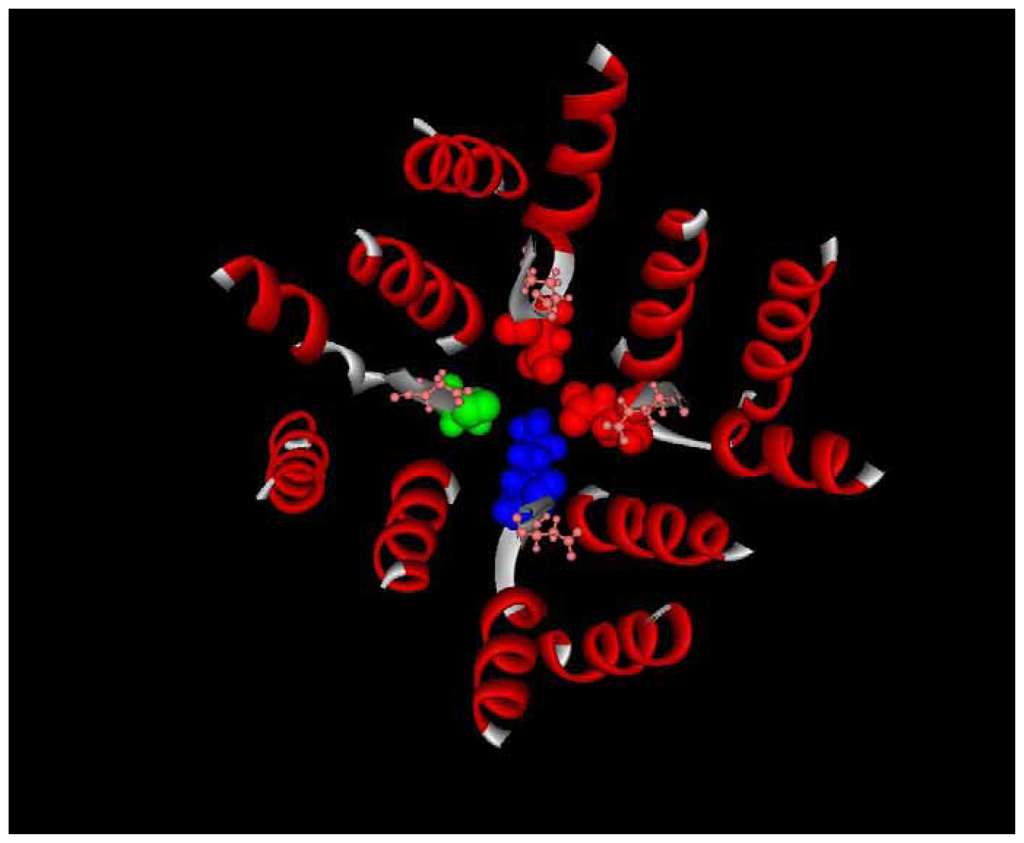
Figure 2.
Structure of sodium channel pore showing outer charged ring (pink sticks and balls) and DEKA selectivity filter (red, red, green, and blue space filling components).
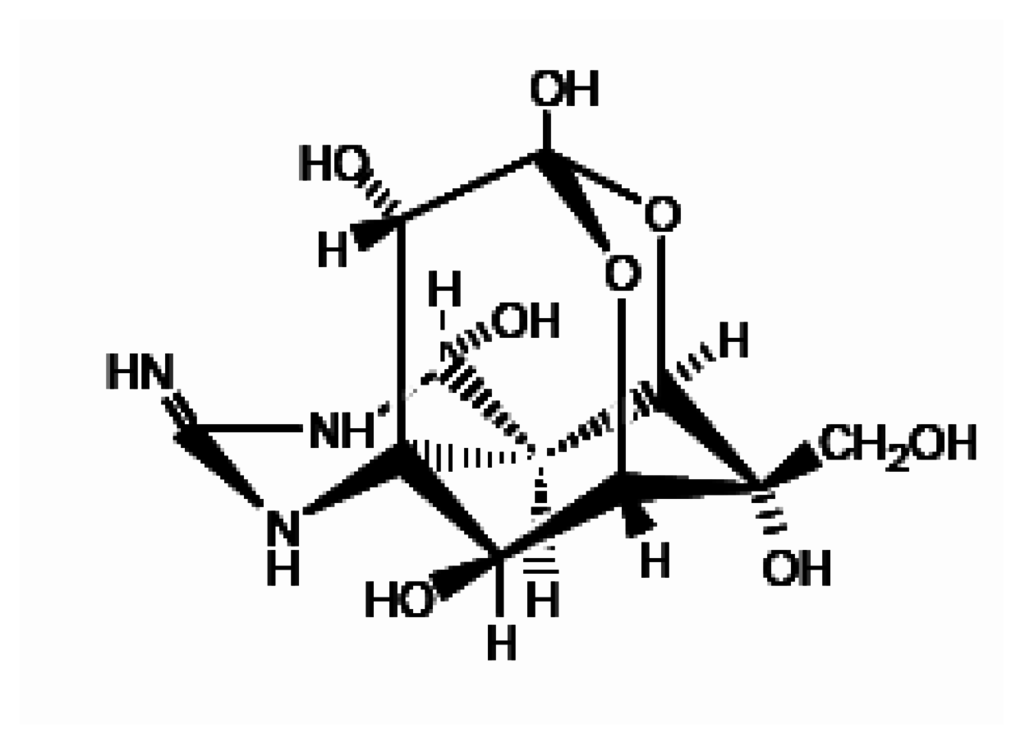
Figure 3.
Molecular structure of tetrodotoxin.
The binding affinity of the channel for TTX is also altered by changes in membrane potential [, ]. The percentage of channels blocked by TTX increases with repeated stimulation of the channel. One model of use-dependent block interprets the increased block that accompanies repeated membrane depolarizations as indicative that the binding site for TTX becomes accessible and the probability of TTX binding increases when the channel activates in response to membrane depolarization [, ]. Another model of TTX use-dependent block suggests that toxin binding in the pore can be affected by a cation (Na+ or Ca2+) bound in the closed pore [, ]. When the channel opens the ion passes into the interior of the cell and allows TTX to bind to the outer pore. The determination of which model correctly describes use-dependent block will probably best be made with data from the crystal structure of the pore in the open and closed state. These data should show whether the conformation of the outer pore is different when the channel is in the closed and open state.
In addition to the block of ion permeation by TTX, external application of toxin appears to affect sodium channel gating. The steady state voltage dependence of gating charge immobilization – the restriction of voltage sensor movement during inactivation [] – is hyperpolarized by TTX in crayfish giant axons []. The effect of TTX on charge immobilization is reduced, but not entirely eliminated, by internal perfusion of N-bromoacetamide (NBA). This result suggests that TTX alters the voltage dependence of both fast and slow inactivation, or that NBA alters channel structure in such a way that the effect of TTX on inactivation is reduced.
Although a number of amino acid changes have been identified that affect TTX binding to the channel through mutational analysis, naturally occurring differences in TTX sensitivity among members of the sodium channel gene family arise from differences at a single amino acid position in the outer pore (Figure 1). Tetrodotoxin-sensitive members of the gene family can be distinguished by the presence of an aromatic amino acid at the domain I position above the selectivity filter and TTX-resistant members have a cysteine or a serine at the same position [–]. In tetrodotoxic animals, TTX resistance appears to be derived from the substitution of non-aromatic amino acids at this critical position in domain I in the TTX-sensitive members of the sodium channel gene family [, ]. A hydrophobic portion of TTX is thought to interact with the aromatic amino acid above the selectivity filter in domain I [, ]. The type of amino acid at this position also affects the ability of the channel to conduct sodium ions through the membrane. The TTX-resistant channel NaV1.5 that is primarily expressed in heart muscle tissue has lower permeability to sodium ions compared to the TTX-sensitive channel NaV1.4 expressed in skeletal muscle tissue. The replacement of a tyrosine with a cysteine at the critical position in domain I of NaV1.4 reduces single channel conductance from 53 pS to the level measured for NaV1.5, 43 pS [, ]. The relationship between channel permeability and the functional requirements of excitable tissues is not clear but it is unlikely that the differences reflect a requirement for TTX resistance in heart muscle.
5. Physiological effects of TTX
The effects of TTX on the human body differ among various excitable tissues based upon the sodium channel isoforms expressed in the cells of that tissue type. The dangers of TTX intoxication include effects on tissues that primarily express TTX-sensitive sodium channel isoforms such as skeletal muscle tissue and peripheral nerves. The major danger is respiratory failure [–]. The diaphragm is composed of skeletal muscle fibers and may become paralyzed during TTX intoxication. Heart muscle tissue that primarily expresses a TTX-resistant channel isoform can in most instances continue to function after TTX is ingested. Other effects include paralysis of the limbs that can progress to generalized flaccid paralysis, dizziness that may be accompanied by a sensation of floating perhaps as proprioceptive input is lost, and numbness of the mouth that can progress to the tongue, face and periphery []. The most severe cases of TTX poisoning can include symptoms of hypotension and bradycardia perhaps as the TTX-sensitive channels found in arterial smooth muscle cells and the sinoatrial node of the heart are blocked [–].
6. Novel mechanisms of TTX resistance
Ingesting newts of the genus Taricha that have high concentrations of TTX in their skin is lethal to almost every one of their potential predators []. One predator, the garter snake Thamnophis sirtalis, has evolved resistance to TTX that allows the snake to eat newts. The effects of attacking a toxic newt can range from reduced locomotor performance to paralysis and death from respiratory failure depending on the level of resistance of the individual snake. Variation in TTX resistance among snake populations is extreme and spans three orders of magnitude []. Thamnophis sirtalis is found across North America, but it is only in snake populations that are sympatric with toxic newts that extreme resistance to TTX has evolved suggesting that snakes have evolved resistance in a coevolutionary arms race with tetrotodoxic newts. Phylogeographic evidence indicates that TTX resistance has evolved independently at least twice within T. sirtalis populations in western North America [, ].
Studying the evolution of TTX resistance in garter snakes has provided an opportunity to determine what changes are possible in a conserved portion of the Nav that still allow for proper channel function. Elevated resistance to TTX is due, at least in part, to mutations in the outer pore of a Nav expressed in the skeletal muscle fibers of resistant snakes [, ]. An analysis of the nucleotide sequence of the channel cloned from snake skeletal muscle demonstrates that the channel has the highest homology to other vertebrate skeletal muscle sodium channel genes (NaV1.4). Tetrodotoxin resistance in garter snakes has evolved through mutations in an important functional region of a TTX-sensitive sodium channel gene, tsNaV1.4 and not through changes in gene expression where a TTX-resistant channel gene is expressed in skeletal muscle tissue.
Mutations observed in TTX-resistant snake sodium channels are at positions in the outer pore [] other than those previously identified, and may therefore have effects on channel function that differ from those imparted by changes in the domain I sequence [, ]. Snakes from three TTX-resistant populations (Warrenton, Benton, and Willow Creek) have an aromatic amino acid in the critical position of the domain I sequence but novel mutations in the domain IV S5–S6 linker of tsNaV1.4 that increase the TTX concentrations required to block the channel. The domain IV outer pore sequence of tsNaV1.4 from a non-resistant snake population (Bear Lake) matches that of rat and human NaV1.4 and is blocked by low concentrations of TTX. Changes in the amino acid sequence of channels from TTX-resistant snakes are in a highly conserved portion of the S5–S6 linkers that forms the outer vestibule and selectivity filter of the channel. Amino acid substitutions that alter the structure or charge in this portion of the pore can affect both TTX-binding and channel function [, ]. For example, TTX binding, single-channel conductance and ion selectivity are all affected by amino acid substitutions that replace the conserved domain IV aspartic acid in the outer ring of charged amino acids in the outer pore with an uncharged residue [, , ]. One of the amino acid substitutions in the Willow Creek channel sequence replaces the aspartic acid in the equivalent position of domain IV with an uncharged asparagine. It is reasonable to predict that this dramatic change at a critical residue may affect other aspects of channel function. Additional substitutions in the channel sequence that affect TTX binding are in regions of the channel that affect the structure of the selectivity filter and outer vestibule. Changes in channel structure may have consequences for channel permeability and selectivity.
The population with the most extreme resistance values (Willow Creek) has the most changes in the outer pore sequence of tsNaV1.4 and the four amino acid substitutions increase the TTX resistance of the channel by three orders of magnitude. This population also has significantly slower action potential (AP) rise rates than the three other populations suggesting that the changes in the structure of the outer pore may have altered channel function. Two populations from another lineage with intermediate TTX resistance (Benton and Warrenton) have more conservative amino acid substitutions and the change in TTX resistance they impart is more modest. The AP rise rates from Benton snakes are the same as those recorded from nonresistant snakes (Bear Lake population), however AP recordings from Warrenton snakes have the surprising result that the AP rise rates are faster than those recorded from other snake populations. This result is not predicted by the single change in the pore sequence of the Warrenton channel and suggests that another mechanism may also play a role in TTX resistance of this snake population. Specifically, snake from this population may express an increased number of sodium channels in skeletal muscle fibers. An increase in the sodium current of skeletal muscle fibers would increase the AP rise rate and could increase the concentration of TTX required to block activity in the skeletal muscle fibers of this population.
The proper functioning of electrically excitable cells depends on maintaining a balance between ion currents that alter the membrane potential. Adaptations that allow common garter snakes to eat tetrodotoxic newts may have altered sodium channel function and this change could alter the magnitude of sodium currents in skeletal muscle cells. Changing sodium current magnitude could alter the balance of currents that influence cell excitability and action potential propagation. There is a physiological trade-off between TTX resistance and locomotor performance that manifests as more resistant snakes having slower maximum crawl speeds []. Differences among populations in AP rise rates suggest that changes that alter the TTX resistance of the skeletal muscle may also affect cell physiology. The slowing of the AP rise rate in one population and its increase in another suggest that changes in channel function and possibly channel expression affect cell physiology. The evolution of TTX resistance in Thamnophis sirtalis has occurred through a series of unique substitutions in a protein that is vital for nerve and muscle cell activity and in a region of the protein that is critical for its function. Further work in this system has the potential to increase our understanding of how ion channel function influences cell physiology through an opportunity to measure the benefits and costs of changes in sodium channel structure on the proper functioning of nerve and skeletal muscle tissue.
Acknowledgements
The authors wish to thank Drs. Gregory Lipkind and Robert French for their generous donation of Figure 2.
Abbreviations
| TTX | tetrodotoxin |
| NaV | voltage-gated sodium channel |
| P-region | pore region |
| S4 | fourth membrane-spanning segment |
- Samples Availability: Available from the authors.
References
- Hille, B. Ion Channels of Excitable Membranes, 3rd ed; Sunderland, M. A., Ed.; Sinauer Associates, 2001; pp. 24–82. [Google Scholar]
- Catterall, W.A. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 2000, 26, 13–25. [Google Scholar]
- Isom, L.L.; De Jongh, K.S.; Patton, D.E.; Reber, B.F.; Offord, J.; Charbonneau, H.; Walsh, K.; Goldin, A.L.; Catterall, W.A. Primary structure and functional expression of the beta 1 subunit of the rat brain sodium channel. Science 1992, 256, 839–842. [Google Scholar]
- Isom, L.L.; Ragsdale, D.S.; De Jongh, K.S.; Westenbroek, R.E.; Reber, B.F.; Scheuer, T.; Catterall, W.A. Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell 1995, 83, 433–442. [Google Scholar]
- Goldin, A.L.; Snutch, T.; Lubbert, H.; Dowsett, A.; Marshall, J.; Auld, V.; Downey, W.; Fritz, L.C.; Lester, H.A.; Dunn, R. Messenger RNA coding for only the alpha subunit of the rat brain Na channel is sufficient for expression of functional channels in Xenopus oocytes. Proc. Natl. Acad. Sci. USA 1986, 83, 7503–7507. [Google Scholar]
- Noda, M.; Ikeda, T.; Kayano, T.; Suzuki, H.; Takeshima, H.; Kurasaki, M.; Takahashi, H.; Numa, S. Existence of distinct sodium channel messenger RNAs in rat brain. Nature 1986, 320, 188–192. [Google Scholar]
- Noda, M.; Ikeda, T.; Suzuki, H.; Takeshima, H.; Takahashi, T.; Kuno, M.; Numa, S. Expression of functional sodium channels from cloned cDNA. Nature 1986, 322, 826–828. [Google Scholar]
- Guy, H.R.; Seetharamulu, P. Molecular model of the action potential sodium channel. Proc. Natl. Acad. Sci. USA 1986, 83, 508–512. [Google Scholar]
- Stuhmer, W.; Conti, F.; Suzuki, H.; Wang, X.D.; Noda, M.; Yahagi, N.; Kubo, H.; Numa, S. Structural parts involved in activation and inactivation of the sodium channel. Nature 1989, 339, 597–603. [Google Scholar]
- Kontis, K.J.; Rounaghi, A.; Goldin, A.L. Sodium channel activation gating is affected by substitutions of voltage sensor positive charges in all four domains. J. Gen. Physiol 1997, 110, 391–401. [Google Scholar]
- Yang, N.; Horn, R. Evidence for voltage-dependent S4 movement in sodium channels. Neuron 1995, 15, 213–218. [Google Scholar]
- Yang, N.; George, A.L., Jr; Horn, R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron 1996, 16, 113–122. [Google Scholar]
- Armstrong, C.M. Sodium channels and gating currents. Physiol. Rev 1981, 61, 644–483. [Google Scholar]
- Vassilev, P.M.; Scheuer, T.; Catterall, W.A. Identification of an intracellular peptide segment involved in sodium channel inactivation. Science 1988, 241, 1658–1661. [Google Scholar]
- Vassilev, P.; Scheuer, T.; Catterall, W.A. Inhibition of inactivation of single sodium channels by a site-directed antibody. Proc. Natl. Acad. Sci. USA 1989, 86, 8147–8151. [Google Scholar]
- West, J.W.; Patton, D.E.; Scheuer, T.; Wang, Y.; Goldin, A.L.; Catterall, W.A. A cluster of hydrophobic amino acid residues required for fast Na+-channel inactivation. Proc. Natl. Acad. Sci. USA 1992, 89, 10910–10914. [Google Scholar]
- Goldin, A.L. Evolution of voltage-gated Na+ channels. J. Exp. Biol. 2002, 205 Pt 5, 575–584. [Google Scholar]
- Yu, F.H.; Catterall, W.A. Overview of the voltage-gated sodium channel family. Genome Biol 2003, 4, 207:1–207:7. [Google Scholar]
- Goldin, A.L. Resurgence of sodium channel research. Annu. Rev. Physiol 2001, 63, 871–894. [Google Scholar]
- Lopreato, G.F.; Lu, Y.; Southwell, A.; Atkinson, N.S.; Hillis, D.M.; Wilcox, T.P.; Zakon, H.H. Evolution and divergence of sodium channel genes in vertebrates. Proc. Natl. Acad. Sci. USA 2001, 98, 7588–7592. [Google Scholar]
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International Union of Pharmacology. XXXIX. Compendium of voltage-gated ion channels: sodium channels. Pharmacol. Rev 2003, 55, 575–578. [Google Scholar]
- George, A.L., Jr; Knittle, T.J.; Tamkun, M.M. Molecular cloning of an atypical voltage-gated sodium channel expressed in human heart and uterus: evidence for a distinct gene family. Proc. Natl. Acad. Sci. USA 1992, 89, 4893–4897. [Google Scholar]
- Hiyama, T.Y.; Watanabe, E.; Ono, K.; Inenaga, K.; Tamkun, M.M.; Yoshida, S.; Noda, M. Nax channel involved in CNS sodium-level sensing. Nat. Neurosci 2002, 5, 511–512. [Google Scholar]
- Watanabe, E.; Hiyama, T.Y.; Kodama, R.; Noda, M. NaX sodium channel is expressed in non-myelinating Schwann cells and alveolar type II cells in mice. Neurosci. Lett 2002, 330, 109–113. [Google Scholar]
- Plummer, N.W.; Meisler, M.H. Evolution and diversity of mammalian sodium channel genes. Genomics 1999, 57, 323–331. [Google Scholar]
- Trimmer, J.S.; Rhodes, K.J. Localization of voltage-gated ion channels in mammalian brain. Annu. Rev. Physiol 2004, 66, 477–519. [Google Scholar]
- Caldwell, J.H.; Schaller, K.L.; Lasher, R.S.; Peles, E.; Levinson, S.R. Sodium channel Nav1.6 is localized at nodes of Ranvier, dendrites, and synapses. Proc. Natl. Acad. Sci. USA 2000, 97, 5616–5620. [Google Scholar]
- Boiko, T.; Rasband, M.N.; Levinson, S.R.; Caldwell, J.H.; Mandel, G.; Trimmer, J.S.; Matthews, G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron 2001, 30, 91–104. [Google Scholar]
- Kaplan, M.R.; Cho, M.H.; Ullian, E.M.; Isom, L.L.; Levinson, S.R.; Barres, B.A. Differential control of clustering of the sodium channels Nav1.2 and Nav1.6 at developing CNS nodes of Ranvier. Neuron 2001, 30, 105–119. [Google Scholar]
- Pugsley, M.K.; Goldin, A.L. Effects of bisaramil, a novel class I antiarrhythmic agent, on heart, skeletal muscle and brain Na+ channels. Eur. J. Pharmacol 1998, 342, 93–104. [Google Scholar]
- Zhou, W.; Goldin, A.L. Use-dependent potentiation of the Nav1.6 sodium channel. Biophys. J 2004, 87, 3862–3872. [Google Scholar]
- Vilin, Y.Y.; Ruben, P.C. Slow inactivation in voltage-gated sodium channels: molecular substrates and contributions to channelopathies. Cell Biochem. Biophys 2001, 35, 171–190. [Google Scholar]
- Goldin, A.L. Mechanisms of sodium channel inactivation. Curr. Opin. Neurobiol 2003, 13, 284–290. [Google Scholar]
- Kallen, R.G.; Sheng, Z.H.; Yang, J.; Chen, L.Q.; Rogart, R.B.; Barchi, R.L. Primary structure and expression of a sodium channel characteristic of denervated and immature rat skeletal muscle. Neuron 1990, 4, 233–242. [Google Scholar]
- Trimmer, J.S.; Cooperman, S.S.; Agnew, W.S.; Mandel, G. Regulation of muscle sodium channel transcripts during development and in response to denervation. Dev. Biol 1990, 142, 360–367. [Google Scholar]
- Ganong, W.F. Review of medical physiology, 22nd ed; New York; McGraw-Hill Medical, 2005; p. p 928. [Google Scholar]
- Jurkat-Rott, K.; Mitrovic, N.; Hang, C.; Kouzmekine, A.; Iaizzo, P.; Herzog, J.; Lerche, H.; Nicole, S.; Vale-Santos, J.; Chauveau, D.; Fontaine, B.; Lehmann-Horn, F. Voltage-sensor sodium channel mutations cause hypokalemic periodic paralysis type 2 by enhanced inactivation and reduced current. Proc. Natl. Acad. Sci. USA 2000, 97, 9549–9554. [Google Scholar]
- Hennig, R.; Lomo, T. Firing patterns of motor units in normal rats. Nature 1985, 314, 164–166. [Google Scholar]
- Richmond, J.E.; Featherstone, D.E.; Hartmann, H.A.; Ruben, P.C. Slow inactivation in human cardiac sodium channels. Biophys. J 1998, 74, 2945–2952. [Google Scholar]
- Purves, D.; Sakmann, B. The effect of contractile activity on fibrillation and extrajunctional acetylcholine-sensitivity in rat muscle maintained in organ culture. J. Physiol 1974, 237, 157–182. [Google Scholar]
- Hayward, L.J.; Brown, R.H., Jr; Cannon, S.C. Slow inactivation differs among mutant Na channels associated with myotonia and periodic paralysis. Biophys. J. 1997, 72, 1204–1219. [Google Scholar]
- Hayward, L.J.; Sandoval, G.M.; Cannon, S.C. Defective slow inactivation of sodium channels contributes to familial periodic paralysis. Neurology 1999, 52, 1447–1453. [Google Scholar]
- Lipkind, G.M.; Fozzard, H.A. KcsA crystal structure as framework for a molecular model of the Na+ channel pore. Biochemistry 2000, 39, 8161–8170. [Google Scholar]
- Tikhonov, D.B.; Zhorov, B.S. Modeling P-loops domain of sodium channel: homology with potassium channels and interaction with ligands. Biophys. J 2005, 88, 184–197. [Google Scholar]
- Terlau, H.; Heinemann, S.H.; Stuhmer, W.; Pusch, M.; Conti, F.; Imoto, K.; Numa, S. Mapping the site of block by tetrodotoxin and saxitoxin of sodium channel II. FEBS Lett 1991, 293, 93–96. [Google Scholar]
- Heinemann, S.H.; Terlau, H.; Stuhmer, W.; Imoto, K.; Numa, S. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature 1992, 356, 441–443. [Google Scholar]
- Penzotti, J.L.; Fozzard, H.A.; Lipkind, G.M.; Dudley, S.C., Jr. Differences in saxitoxin and tetrodotoxin binding revealed by mutagenesis of the Na+ channel outer vestibule. Biophys. J. 1998, 75, 2647–2657. [Google Scholar]
- Choudhary, G.; Yotsu-Yamashita, M.; Shang, L.; Yasumoto, T.; Dudley, S.C., Jr. Interactions of the C-11 hydroxyl of tetrodotoxin with the sodium channel outer vestibule. Biophys. J. 2003, 84, 287–294. [Google Scholar]
- Yamagishi, T.; Li, R.A.; Hsu, K.; Marban, E.; Tomaselli, G.F. Molecular architecture of the voltage-dependent Na channel: functional evidence for alpha helices in the pore. J. Gen. Physiol 2001, 118, 171–181. [Google Scholar]
- Patton, D.E.; Goldin, A.L. A voltage-dependent gating transition induces use-dependent block by tetrodotoxin of rat IIA sodium channels expressed in Xenopus oocytes. Neuron 1991, 7, 637–647. [Google Scholar]
- Dumaine, R.; Hartmann, H.A. Two conformational states involved in the use-dependent TTX blockade of human cardiac Na+channel. Am. J. Physiol. 1996, 270 6 Pt 2, H2029–H2037. [Google Scholar]
- Boccaccio, A.; Moran, O.; Imoto, K.; Conti, F. Tonic and phasic tetrodotoxin block of sodium channels with point mutations in the outer pore region. Biophys. J 1999, 77, 229–240. [Google Scholar]
- Moran, O.; Picollo, A.; Conti, F. Tonic and phasic guanidinium toxin-block of skeletal muscle Na channels expressed in mammalian cells. Biophys. J 2003, 84, 2999–3006. [Google Scholar]
- Armstrong, C.M.; Bezanilla, F. Inactivation of the sodium channel. II. Gating current experiments. J. Gen. Physiol 1977, 70, 567–590. [Google Scholar]
- Heggeness, S.T.; Starkus, J.G. Saxitoxin and tetrodotoxin. Electrostatic effects on sodium channel gating current in crayfish axons. Biophys. J 1986, 49, 629–643. [Google Scholar]
- Rogart, R.B.; Cribbs, L.L.; Muglia, L.K.; Kephart, D.D.; Kaiser, M.W. Molecular cloning of a putative tetrodotoxin-resistant rat heart Na+ channel isoform. Proc. Natl. Acad. Sci. USA 1989, 86, 8170–8174. [Google Scholar]
- Satin, J.; Kyle, J.W.; Chen, M.; Bell, P.; Cribbs, L.L.; Fozzard, H.A.; Rogart, R.B. A mutant of TTX-resistant cardiac sodium channels with TTX-sensitive properties. Science 1992, 256, 1202–1205. [Google Scholar]
- Backx, P.H.; Yue, D.T.; Lawrence, J.H.; Marban, E.; Tomaselli, G.F. Molecular localization of an ion-binding site within the pore of mammalian sodium channels. Science 1992, 257, 248–251. [Google Scholar]
- Sangameswaran, L.; Delgado, S.G.; Fish, L.M.; Koch, B.D.; Jakeman, L.B.; Stewart, G.R.; Sze, P.; Hunter, J.C.; Eglen, R.M.; Herman, R.C. Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. J. Biol. Chem 1996, 271, 5953–5956. [Google Scholar]
- Dib-Hajj, S.D.; Tyrrell, L.; Black, J.A.; Waxman, S.G. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc. Natl. Acad. Sci. USA 1998, 95, 8963–8968. [Google Scholar]
- Kaneko, Y.; Matsumoto, G.; Hanyu, Y. TTX resistivity of Na+ channel in newt retinal neuron. Biochem. Biophys. Res. Commun 1997, 240, 651–656. [Google Scholar]
- Yotsu-Yamashita, M.; Nishimori, K.; Nitanai, Y.; Isemura, M.; Sugimoto, A.; Yasumoto, T. Binding properties of 3H-PbTx-3 and 3H-saxitoxin to brain membranes and to skeletal muscle membranes of puffer fish Fugu pardalis and the primary structure of a voltage-gated Na+ channel α-subunit (fMNa1) from skeletal muscle of F. pardalis. Biochem. Biophys. Res. Commun 2000, 267, 403–412. [Google Scholar]
- Weiss, R.E.; Horn, R. Functional differences between two classes of sodium channels in developing rat skeletal muscle. Science 1986, 233, 361–364. [Google Scholar]
- How, C.K.; Chern, C.H.; Huang, Y.C.; Wang, L.M.; Lee, C.H. Tetrodotoxin poisoning. Am. J. Emerg. Med 2003, 21, 51–54. [Google Scholar]
- Ahasan, H.A.; Mamun, A.A.; Karim, S.R.; Bakar, M.A.; Gazi, E.A.; Bala, C.S. Paralytic complications of puffer fish (tetrodotoxin) poisoning. Singapore Med. J 2004, 45, 73–74. [Google Scholar]
- Isbister, G.K.; Kiernan, M.C. Neurotoxic marine poisoning. Lancet Neurol 2005, 4, 219–228. [Google Scholar]
- Berra-Romani, R.; Blaustein, M.P.; Matteson, D.R. TTX-sensitive voltage-gated Na+ channels are expressed in mesenteric artery smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol 2005, 289, H137–H145. [Google Scholar]
- Maier, S.K.; Westenbroek, R.E.; Schenkman, K.A.; Feigl, E.O.; Scheuer, T.; Catterall, W.A. An unexpected role for brain-type sodium channels in coupling of cell surface depolarization to contraction in the heart. Proc. Natl. Acad. Sci. USA 2002, 99, 4073–4078. [Google Scholar]
- Lei, M.; Jones, S.A.; Liu, J.; Lancaster, M.K.; Fung, S.S.; Dobrzynski, H.; Camelliti, P.; Maier, S.K.; Noble, D.; Boyett, M.R. Requirement of neuronal- and cardiac-type sodium channels for murine sinoatrial node pacemaking. J. Physiol. 2004, 559 Pt 3, 835–848. [Google Scholar]
- Brodie, E.D., Jr. Investigations on the skin toxin of the adult rough-skinned newt Taricha granulosa. Copeia 1968, 2, 307–313. [Google Scholar]
- Brodie, E.D., Jr; Ridenhour, B.J.; Brodie, E.D., III. The evolutionary response of predators to dangerous prey: hotspots and coldspots in the geographic mosaic of coevolution between garter snakes and newts. Evolution Int. J. Org. Evolution 2002, 56, 2067–2082. [Google Scholar]
- Janzen, F.J.; Krenz, J.G.; Haselkorn, T.S.; Brodie, E.D.; Brodie, E.D. Molecular phylogeography of common garter snakes (Thamnophis sirtalis) in western North America: implications for regional historical forces. Mol. Ecol 2002, 11, 1739–1751. [Google Scholar]
- Geffeney, S.; Brodie, E.D., Jr; Ruben, P.C.; Brodie, E.D., III. Mechanisms of adaptation in a predator-prey arms race: TTX-resistant sodium channels. Science 2002, 297, 1336–1339. [Google Scholar]
- Geffeney, S.L.; Fujimoto, E.; Brodie, E.D., 3rd; Brodie, E.D., Jr; Ruben, P.C. Evolutionary diversification of TTX-resistant sodium channels in a predator-prey interaction. Nature 2005, 434, 759–763. [Google Scholar]
- Chiamvimonvat, N.; Perez-Garcia, M.T.; Tomaselli, G.F.; Marban, E. Control of ion flux and selectivity by negatively charged residues in the outer mouth of rat sodium channels. J. Physiol. 1996, 491 Pt 1, 51–59. [Google Scholar]
- Tsushima, R.G.; Li, R.A.; Backx, P.H. Altered ionic selectivity of the sodium channel revealed by cysteine mutations within the pore. J. Gen. Physiol 1997, 109, 463–475. [Google Scholar]
- Brodie, E.D., III; Brodie, E.D., Jr. Costs of exploiting poisonous prey: evolutionary trade-offs in a predator-prey arms race. Evolution 1999, 53, 626–631. [Google Scholar]
- Al-Sabi, A.; McArthur, J.; Ostroumov, V.; French, R.J. Marine toxins that target voltage-gated sodium channels. Mar. Drugs 2006, 4, 157–192. [Google Scholar]
© 2006 by MDPI Reproduction is permitted for noncommercial purposes.