Haemostatic and Biocompatibility Evaluation of Alginate-Functionalized Polylactide Composite Containing Zinc Sulphide and Hardystonite
Abstract
1. Introduction
2. Results and Discussion
2.1. Zinc and Calcium Concentration
2.2. Surface Morphology
2.3. Specific Surface Area, Total Pore Volume, and Average Pore Size
2.4. Zeta Potential
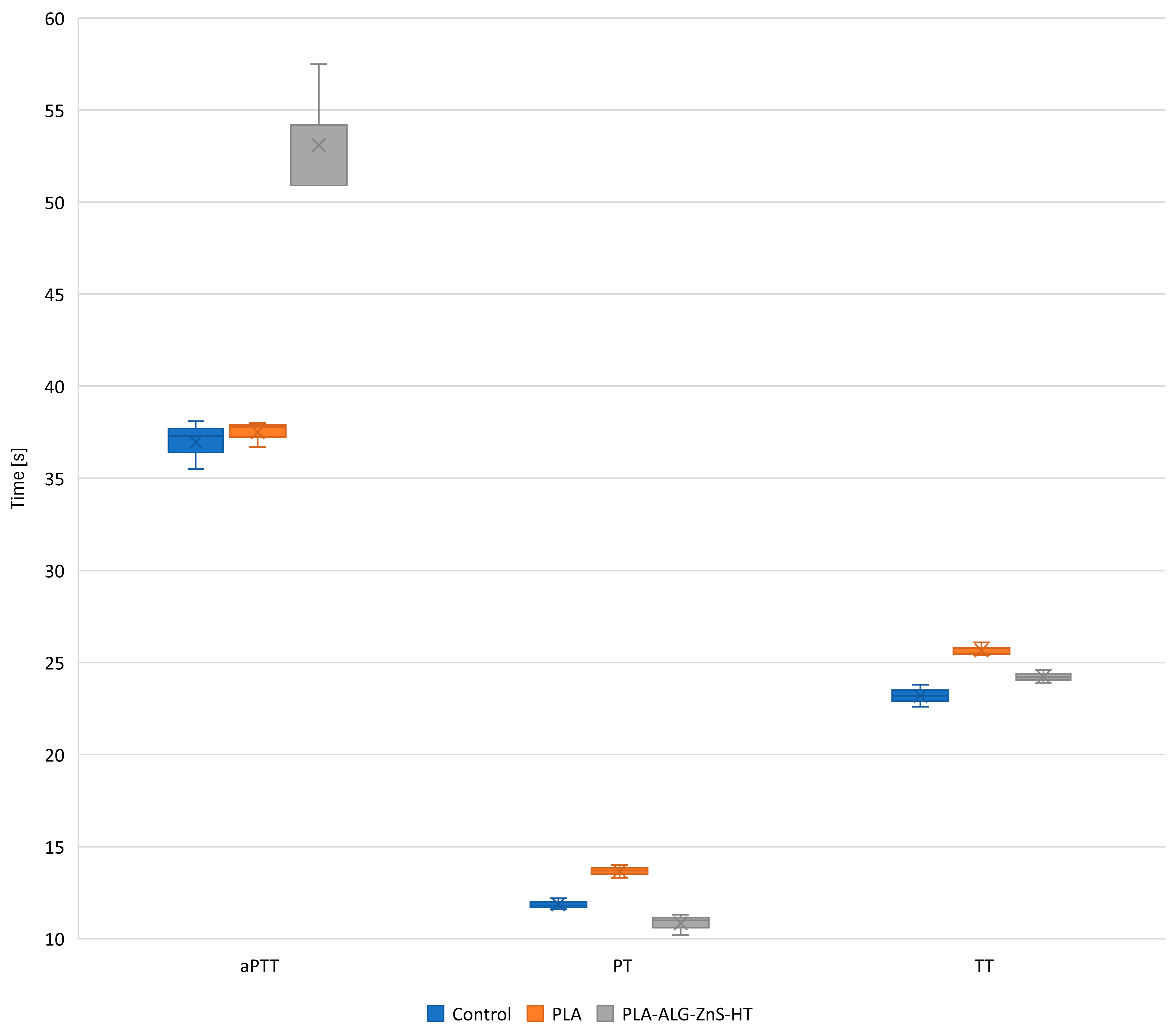
2.5. Blood Plasma Coagulation: Activated Partial Thromboplastin Time, Prothrombin Time, and Thrombin Time
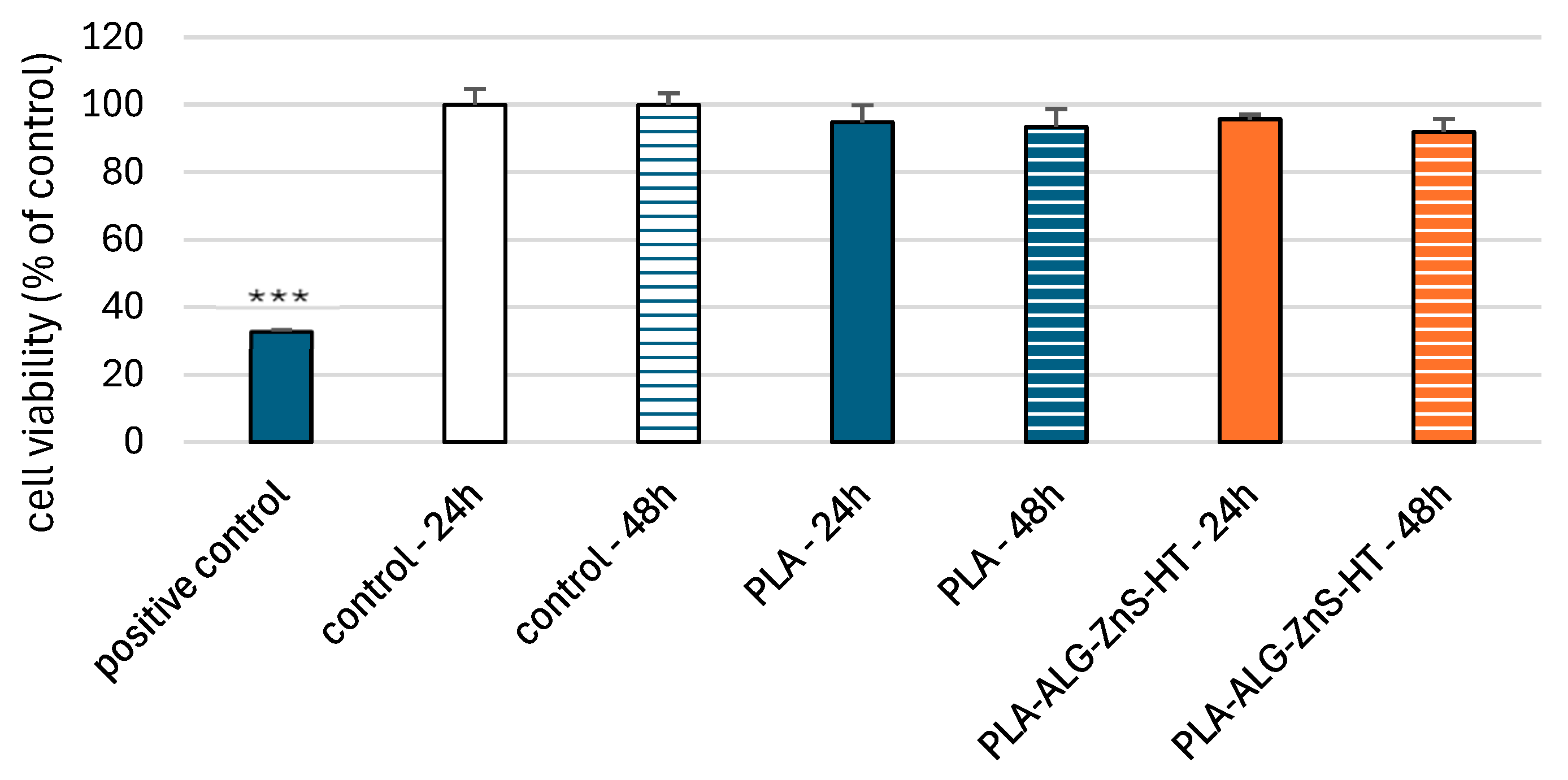
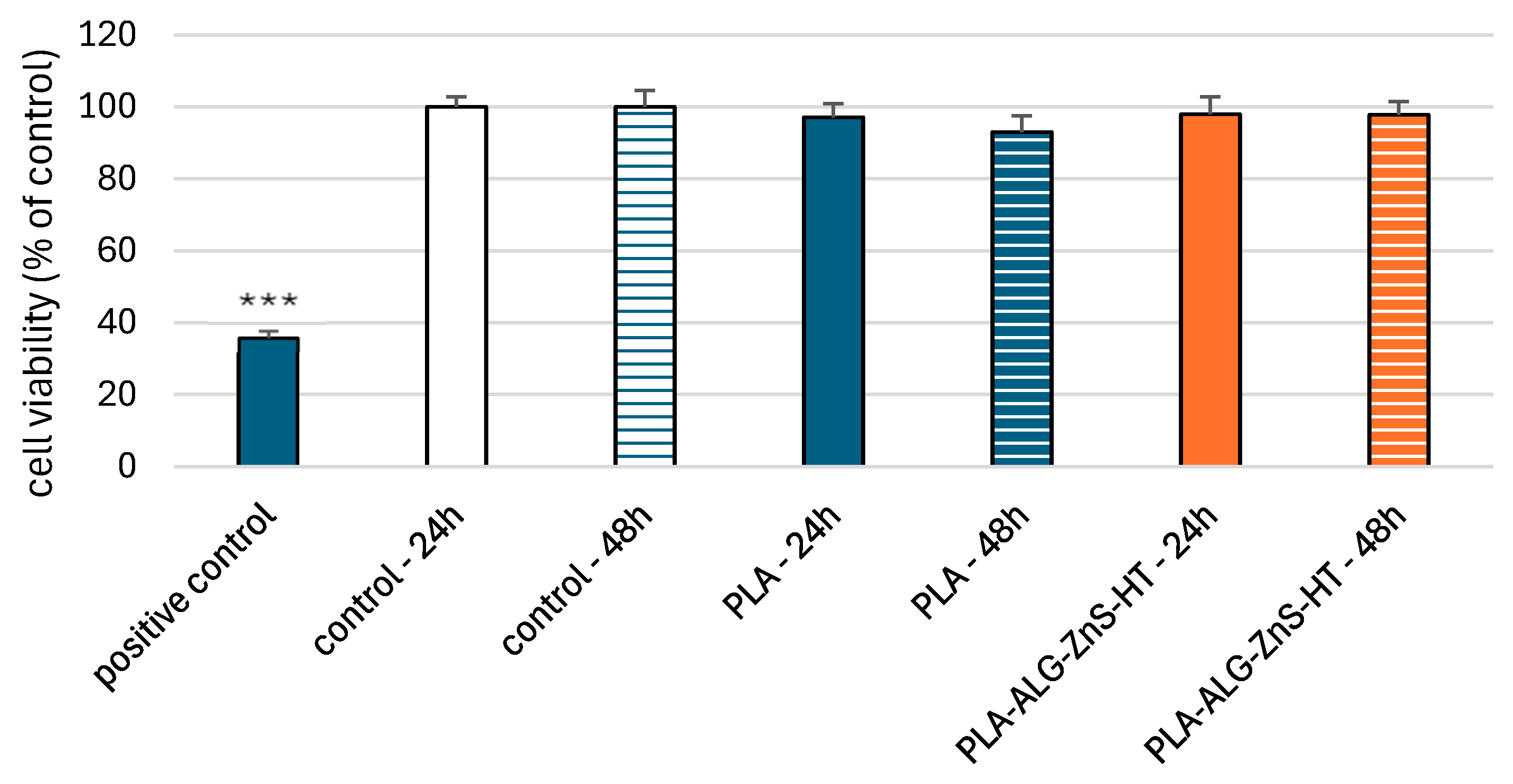
2.6. Viability of PBM and Hs68 Cells
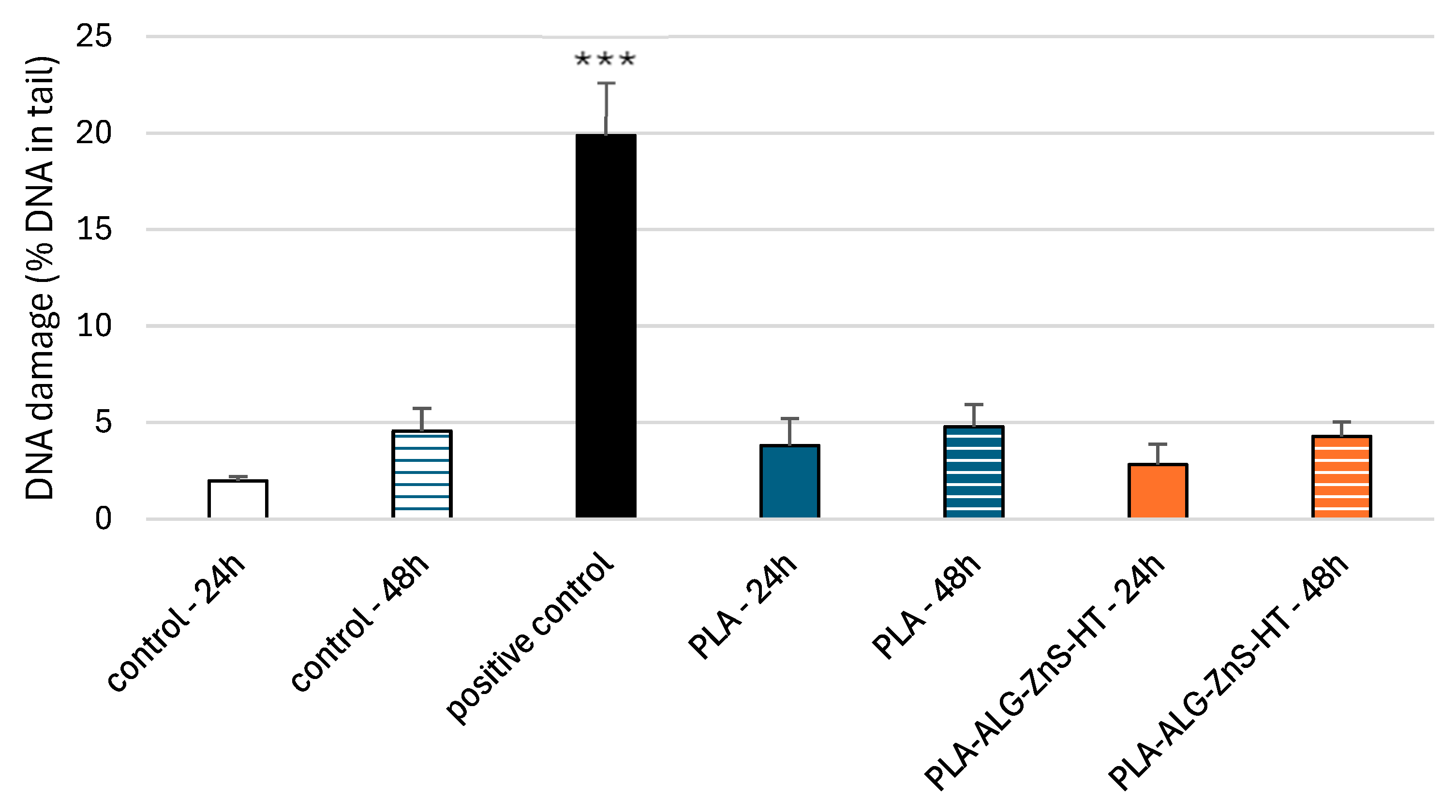
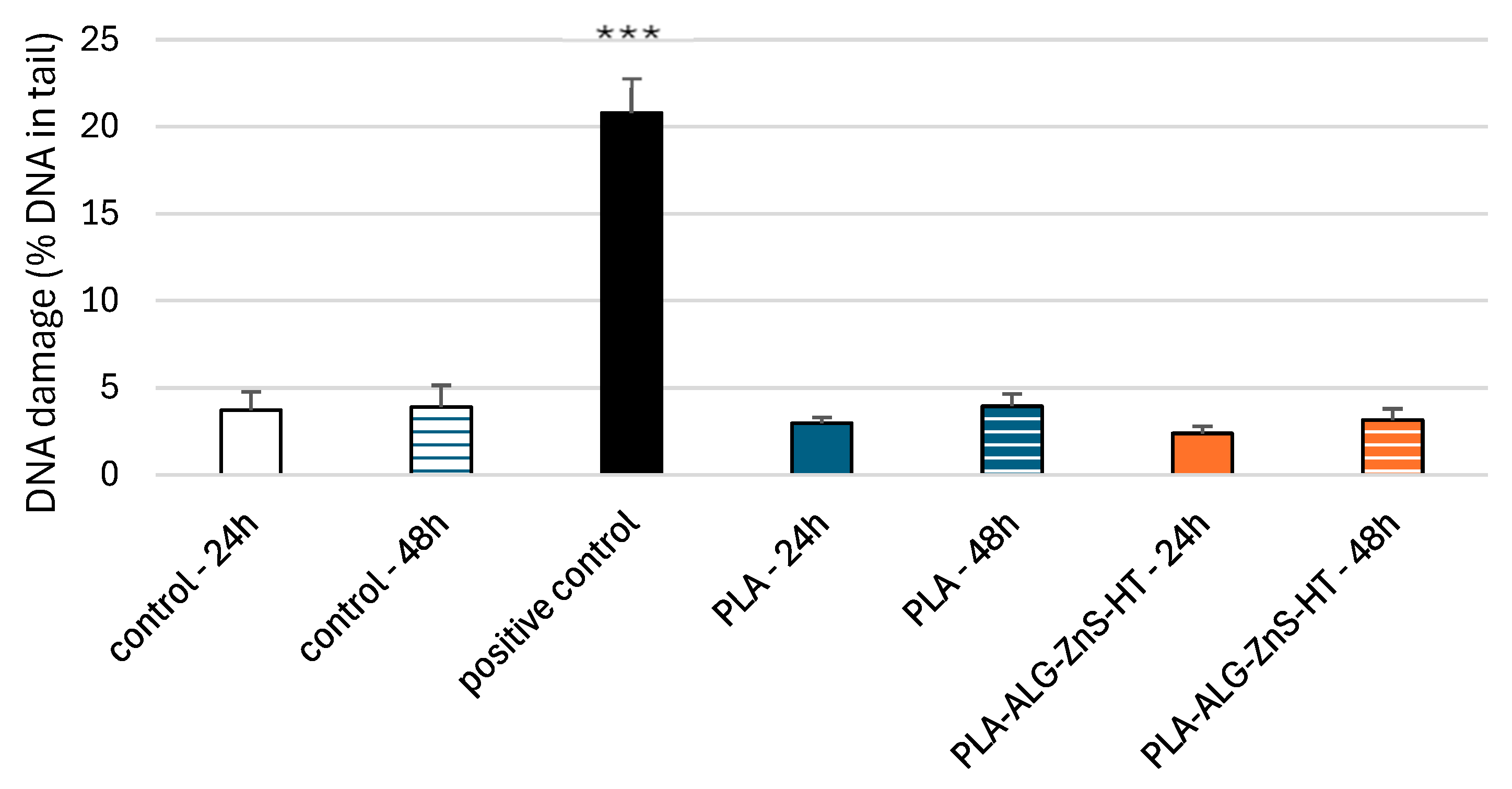
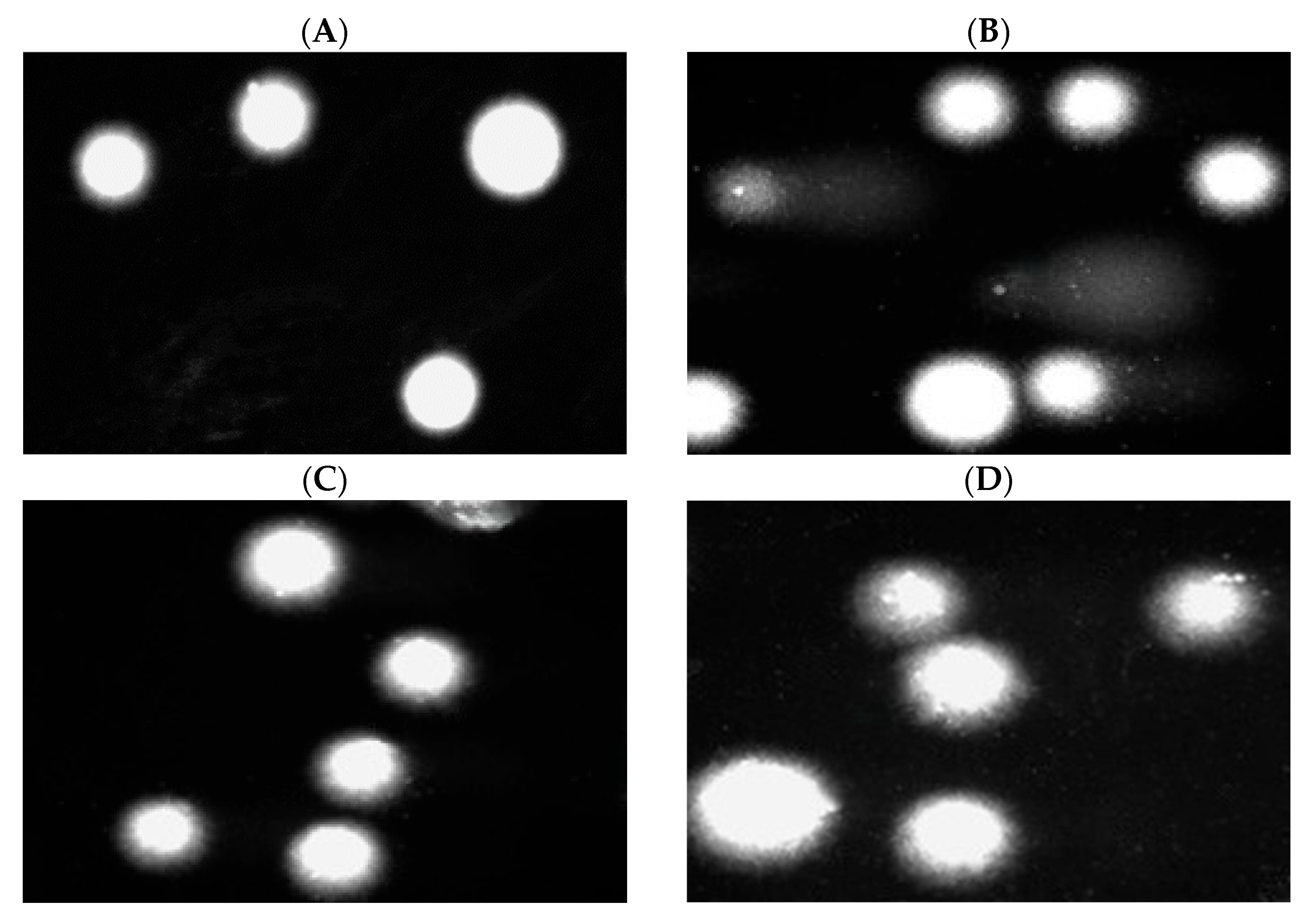
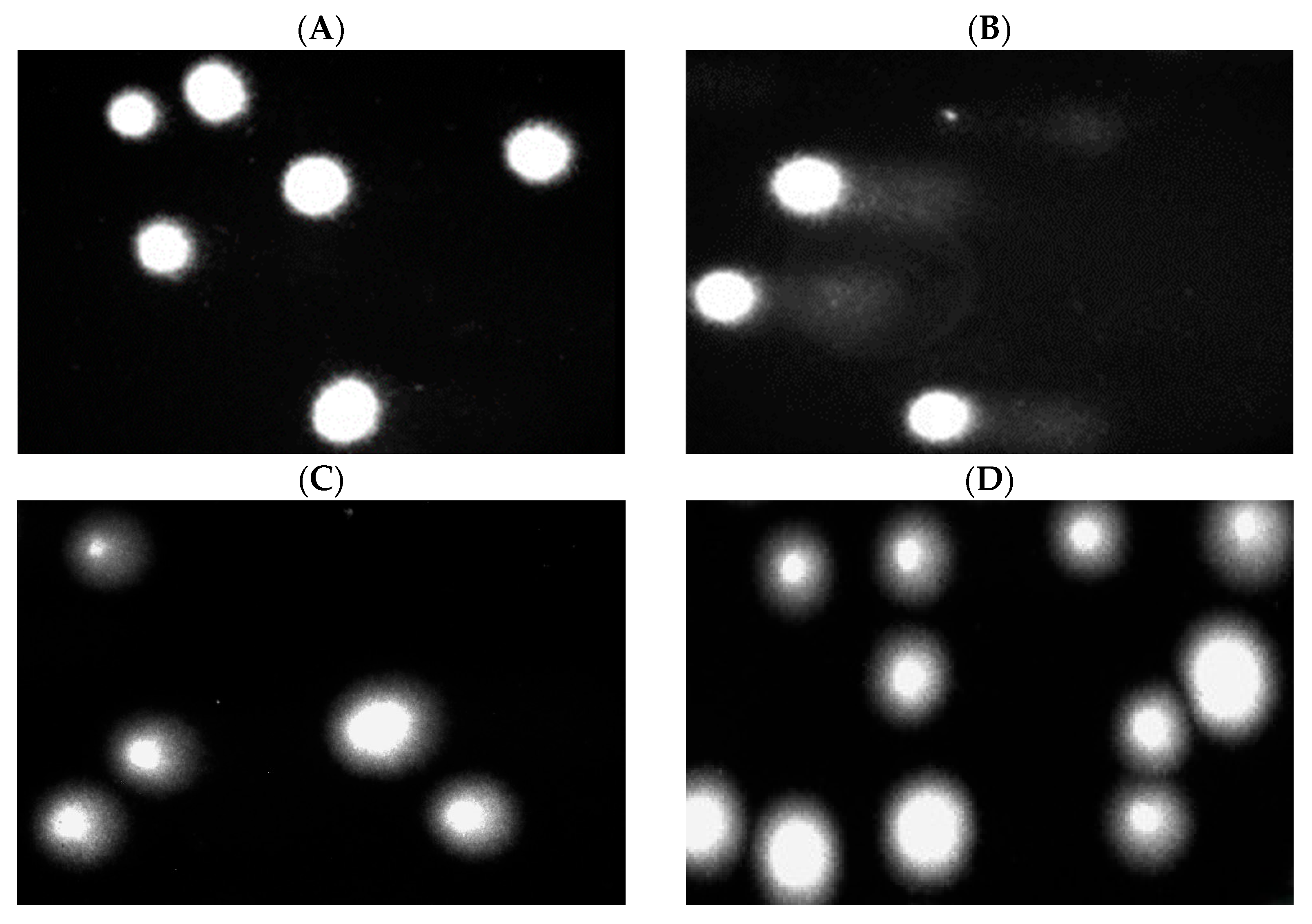
2.7. DNA Damage in PBM Cells and Hs68 Cells
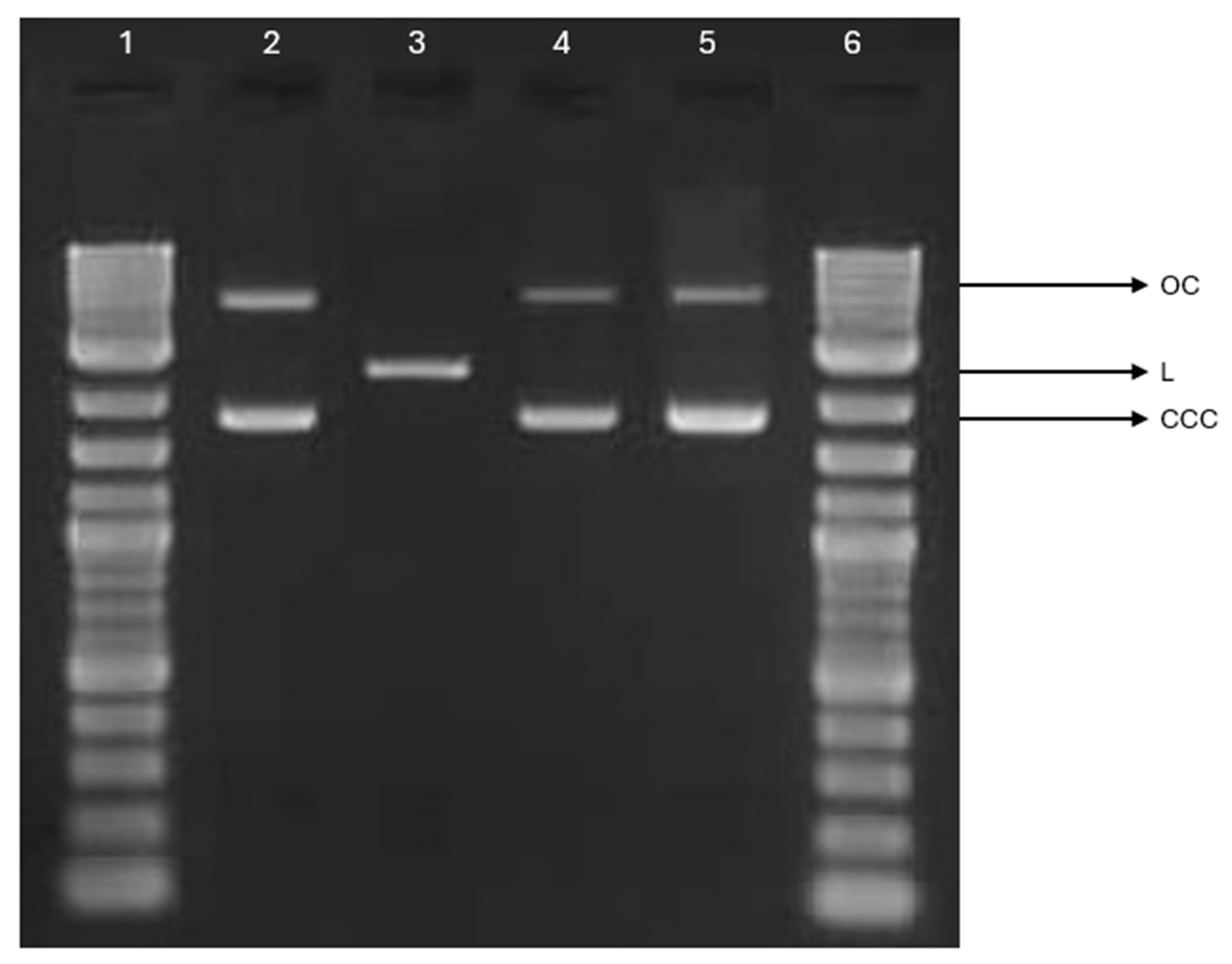
2.8. Plasmid Relaxation Assay
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Sample Preparation via Melt-Blowing Technique and Dip-Coating Method
3.2.2. Zinc and Calcium Concentration
3.2.3. Surface Morphology
3.2.4. Specific Surface Area, Total Pore Volume, and Average Pore Size
3.2.5. Zeta Potential
3.2.6. Blood Plasma Coagulation: Activated Partial Thromboplastin Time, Prothrombin Time, and Thrombin Time
3.2.7. Preparation of Samples for the Assessment of Biological Properties
3.2.8. Cell Culture
3.2.9. Cell Viability Resazurin Assay
3.2.10. DNA Damage
3.2.11. Plasmid Relaxation Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wasyłeczko, M.; Wojciechowski, C.; Chwojnowski, A. Polyethersulfone Polymer for Biomedical Applications and Biotechnology. Int. J. Mol. Sci. 2024, 25, 4233. [Google Scholar] [CrossRef]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current Development of Biodegradable Polymeric Materials for Biomedical Applications. Drug Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and Biocompatible Polymers for Tissue Engineering Application: A Review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Gobi, R.; Ravichandiran, P.; Babu, R.S.; Yoo, D.J. Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review. Polymers 2021, 13, 1962. [Google Scholar] [CrossRef] [PubMed]
- Hammonds, R.L.; Gazzola, W.H.; Benson, R.S. Physical and Thermal Characterization of Polylactic Acid Meltblown Nonwovens. J. Appl. Polym. Sci. 2014, 131, 40593. [Google Scholar] [CrossRef]
- Morent, R.; De Geyter, N.; Desmet, T.; Dubruel, P.; Leys, C. Plasma Surface Modification of Biodegradable Polymers: A Review. Plasma Process. Polym. 2011, 8, 171–190. [Google Scholar] [CrossRef]
- Badaraev, A.D.; Nemoykina, A.L.; Bolbasov, E.N.; Tverdokhlebov, S.I. PLLA Scaffold Modification Using Magnetron Sputtering of the Copper Target to Provide Antibacterial Properties. Resour.-Effic. Technol. 2017, 3, 204–211. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in Modern Medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Zhou, J.; Han, S.; Dou, Y.; Lu, J.; Wang, C.; He, H.; Li, X.; Zhang, J. Nanostructured Poly(l-Lactide) Matrix as Novel Platform for Drug Delivery. Int. J. Pharm. 2013, 448, 175–188. [Google Scholar] [CrossRef]
- Awadhesh Kumar Pandey; Arun Kumar Dwivedi Recent Advancement in Wound Healing Dressing Material. Int. J. Res. Pharm. Sci. 2019, 10, 2572–2577. [CrossRef]
- Sharif, A.; Mondal, S.; Hoque, M.E. Polylactic Acid (PLA)-Based Nanocomposites: Processing and Properties. In Bio-Based Polymers and Nanocomposites: Preparation, Processing, Properties & Performance; Sanyang, M.L., Jawaid, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 233–254. ISBN 978-3-030-05825-8. [Google Scholar]
- Alam, F.; Shukla, V.R.; Varadarajan, K.M.; Kumar, S. Microarchitected 3D Printed Polylactic Acid (PLA) Nanocomposite Scaffolds for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2020, 103, 103576. [Google Scholar] [CrossRef] [PubMed]
- Essa, D.; Kondiah, P.P.D.; Choonara, Y.E.; Pillay, V. The Design of Poly(Lactide-Co-Glycolide) Nanocarriers for Medical Applications. Front. Bioeng. Biotechnol. 2020, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Spanou, A.; Diez-Escudero, A.; Persson, C. 3D-Printed PLA/HA Composite Structures as Synthetic Trabecular Bone: A Feasibility Study Using Fused Deposition Modeling. J. Mech. Behav. Biomed. Mater. 2020, 103, 103608. [Google Scholar] [CrossRef] [PubMed]
- Davachi, S.M.; Kaffashi, B. Polylactic Acid in Medicine. Polym.-Plast. Technol. Eng. 2015, 54, 944–967. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and Medical Applications of Polylactic Acid: A Review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(Lactic Acid) Nanofibrous Scaffolds for Tissue Engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef]
- Hassani, F.; Mousavi, S.M.; Saghatoleslami, N.; Ghaffarian, V. Polyethersulfone/Poly(D,L-Lactide) Blend Membranes: Preparation, Characterization, and Performance. Chem. Eng. Technol. 2014, 37, 1065–1071. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(Lactic Acid) Modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Ramot, Y.; Haim-Zada, M.; Domb, A.J.; Nyska, A. Biocompatibility and Safety of PLA and Its Copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153–162. [Google Scholar] [CrossRef]
- Bergström, J.S.; Hayman, D. An Overview of Mechanical Properties and Material Modeling of Polylactide (PLA) for Medical Applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, C.; Singh, P.; Mukhopadhyay, S.; Gupta, A.; Gupta, B. Alginate Based Biomaterials for Hemostatic Applications: Innovations and Developments. Int. J. Biol. Macromol. 2024, 264, 130771. [Google Scholar] [CrossRef]
- Hegde, V.; Uthappa, U.T.; Altalhi, T.; Jung, H.-Y.; Han, S.S.; Kurkuri, M.D. Alginate Based Polymeric Systems for Drug Delivery, Antibacterial/Microbial, and Wound Dressing Applications. Mater. Today Commun. 2022, 33, 104813. [Google Scholar] [CrossRef]
- He, Q.; Tong, T.; Yu, C.; Wang, Q. Advances in Algin and Alginate-Hybrid Materials for Drug Delivery and Tissue Engineering. Mar. Drugs 2022, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, D.R.; Biswal, T. Alginate and Its Application to Tissue Engineering. SN Appl. Sci. 2021, 3, 30. [Google Scholar] [CrossRef]
- Li, J.; He, J.; Huang, Y. Role of Alginate in Antibacterial Finishing of Textiles. Int. J. Biol. Macromol. 2017, 94, 466–473. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- Soleimanpour, M.; Mirhaji, S.S.; Jafari, S.; Derakhshankhah, H.; Mamashli, F.; Nedaei, H.; Karimi, M.R.; Motasadizadeh, H.; Fatahi, Y.; Ghasemi, A.; et al. Designing a New Alginate-Fibrinogen Biomaterial Composite Hydrogel for Wound Healing. Sci. Rep. 2022, 12, 7213. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, P.; He, F.; Zhang, C. Application of Alginate-Based Hydrogels in Hemostasis. Gels 2022, 8, 109. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Aljabali, A.A.A.; Mishra, V.; Tambuwala, M.M.; Serrano-Aroca, Á. Alginate: Enhancement Strategies for Advanced Applications. Int. J. Mol. Sci. 2022, 23, 4486. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-Based Composite Materials for Wound Dressing Application: A Mini Review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and Alginate Composites for Biomedical Applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Zdiri, K.; Cayla, A.; Elamri, A.; Erard, A.; Salaun, F. Alginate-Based Bio-Composites and Their Potential Applications. J. Funct. Biomater. 2022, 13, 117. [Google Scholar] [CrossRef]
- Rinaudo, M. Biomaterials Based on a Natural Polysaccharide: Alginate. TIP 2014, 17, 92–96. [Google Scholar] [CrossRef]
- Lv, C.; Zhou, X.; Wang, P.; Li, J.; Wu, Z.; Jiao, Z.; Guo, M.; Wang, Z.; Wang, Y.; Wang, L.; et al. Biodegradable Alginate-Based Sponge with Antibacterial and Shape Memory Properties for Penetrating Wound Hemostasis. Compos. Part B Eng. 2022, 247, 110263. [Google Scholar] [CrossRef]
- Orlowska, J.; Kurczewska, U.; Derwinska, K.; Orlowski, W.; Orszulak-Michalak, D. In Vitro Evaluation of Immunogenic Properties of Active Dressings. Curr. Issues Pharm. Med. Sci. 2014, 27, 55–60. [Google Scholar] [CrossRef]
- Shahriari-Khalaji, M.; Alassod, A.; Nozhat, Z. Cotton-Based Health Care Textile: A Mini Review. Polym. Bull. 2022, 79, 10409–10432. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, W.; Liu, Y.; Yang, Z.; Ma, L.; Zhuang, H.; Wang, E.; Wu, C.; Huan, Z.; Guo, F.; et al. Design of a Biofluid-Absorbing Bioactive Sandwich-Structured Zn–Si Bioceramic Composite Wound Dressing for Hair Follicle Regeneration and Skin Burn Wound Healing. Bioact. Mater. 2021, 6, 1910–1920. [Google Scholar] [CrossRef]
- Yao, Q.; Wang, C.; Yu, B.; Wang, P.; Tang, X.; Shi, D.; Xia, Y.; Hu, Y.; Li, S.; Zhou, W. Well-Ordered and Visual Poly(ε-Caprolactone) Composite Fibrous Membranes for the Treatment of Skin Wounds. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131940. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Wang, X.; Peng, J.; Xu, Y.; Chang, J. Multifunctional Hydrogels Prepared by Dual Ion Cross-Linking for Chronic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 16054–16062. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-Based Biomaterials for Regeneration and Therapy. Trends Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Sun, Y.; Ge, Z.; Tian, X.; Shen, J.; Yuan, J. Antioxidant and Antibacterial PU/ZnS@Keratin Mats with H2S and Zn2+ Release for Infected Diabetic Wound Healing. Int. J. Biol. Macromol. 2025, 304, 140787. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, G.; Tang, Y.; Xu, Y.; Ma, T.; Ma, W.; Zhu, H.; Chen, J.; Lei, C.; Wang, Y. pH-Responsive Medical Dressing Based on Zinc Sulfide Nanoparticle/Silk Fibroin Composite Fibers. ACS Appl. Nano Mater. 2024, 7, 15615–15625. [Google Scholar] [CrossRef]
- Stoica, A.E.; Grumezescu, A.M.; Hermenean, A.O.; Andronescu, E.; Vasile, B.S. Scar-Free Healing: Current Concepts and Future Perspectives. Nanomaterials 2020, 10, 2179. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Khan, M.A.; Jathoi, N.R.; Rasool, U.; Almaary, K.S.; Bahkali, N.A.; Ihsan, J.; Bokhari, S.A.I. A Comparative Study of Green and Chemically Prepared PEGylated Zinc Sulfide Nanoparticles (ZnS-NPs) for Biological Applications. ChemistrySelect 2024, 9, e202403736. [Google Scholar] [CrossRef]
- Panthi, G.; Ranjit, R.; Khadka, S.; Gyawali, K.R.; Kim, H.-Y.; Park, M. Characterization and Antibacterial Activity of Rice Grain-Shaped ZnS Nanoparticles Immobilized inside the Polymer Electrospun Nanofibers. Adv. Compos. Hybrid Mater. 2020, 3, 8–15. [Google Scholar] [CrossRef]
- Niveditha, S.; Veetil, V.T.; Rajeeve, A.D.; Cheriyan, S.; Yamuna, R.; Karthega, M. Wound Healing Applications of β-Cyclodextrin Capped Zinc Sulphide Nanoparticles Impregnated Electrospun Polymeric Nanofibrous Scaffold. J. Drug Deliv. Sci. Technol. 2024, 95, 105597. [Google Scholar] [CrossRef]
- Han, B.; Fang, W.H.; Zhao, S.; Yang, Z.; Hoang, B.X. Zinc Sulfide Nanoparticles Improve Skin Regeneration. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102263. [Google Scholar] [CrossRef]
- Ghobashy, M.M.; Elbarbary, A.M.; Hegazy, D.E.; Maziad, N.A. Radiation Synthesis of pH-Sensitive 2-(Dimethylamino)Ethyl Methacrylate/Polyethylene Oxide/ZnS Nanocomposite Hydrogel Membrane for Wound Dressing Application. J. Drug Deliv. Sci. Technol. 2022, 73, 103399. [Google Scholar] [CrossRef]
- Hu, Q.; Lukesh, J.C. H2S Donors with Cytoprotective Effects in Models of MI/R Injury and Chemotherapy-Induced Cardiotoxicity. Antioxidants 2023, 12, 650. [Google Scholar] [CrossRef]
- Huang, T.; Holden, J.A.; Reynolds, E.C.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J. Multifunctional Antimicrobial Polypeptide-Selenium Nanoparticles Combat Drug-Resistant Bacteria. ACS Appl. Mater. Interfaces 2020, 12, 55696–55709. [Google Scholar] [CrossRef]
- Alijani, H.Q.; Pourseyedi, S.; Torkzadeh Mahani, M.; Khatami, M. Green Synthesis of Zinc Sulfide (ZnS) Nanoparticles Using Stevia Rebaudiana Bertoni and Evaluation of Its Cytotoxic Properties. J. Mol. Struct. 2019, 1175, 214–218. [Google Scholar] [CrossRef]
- Kudzin, M.H.; Giełdowska, M.; Mrozińska, Z.; Boguń, M. Poly(Lactic Acid)/Zinc/Alginate Complex Material: Preparation and Antimicrobial Properties. Antibiotics 2021, 10, 1327. [Google Scholar] [CrossRef]
- Airaksinen, S. Role of Excipients in Moisture Sorption and Physical Stability of Solid Pharmaceutical Formulations. Academic Dissertation, University of Helsinky, Helsinki, Finland, 2005. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore Characterization of Different Types of Coal from Coal and Gas Outburst Disaster Sites Using Low Temperature Nitrogen Adsorption Approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Rachmawati, H.; Yanda, Y.L.; Rahma, A.; Mase, N. Curcumin-Loaded PLA Nanoparticles: Formulation and Physical Evaluation. Sci. Pharm. 2016, 84, 191–202. [Google Scholar] [CrossRef]
- Chaudhry, R.; Killeen, R.B.; Babiker, H.M. Physiology, Coagulation Pathways. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Raber, M.N. Coagulation Tests. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; ISBN 978-0-409-90077-4. [Google Scholar]
- Barmore, W.; Bajwa, T.; Burns, B. Biochemistry, Clotting Factors. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Ambrus, A.; Bányai, I.; Weiss, M.S.; Hilgenfeld, R.; Keresztessy, Z.; Muszbek, L.; Fésüs, L. Calcium Binding of Transglutaminases: A 43Ca NMR Study Combined with Surface Polarity Analysis. J. Biomol. Struct. Dyn. 2001, 19, 59–74. [Google Scholar] [CrossRef]
- Varga-szabo, D.; Braun, A.; Nieswandt, B. Calcium Signaling in Platelets. J. Thromb. Haemost. 2009, 7, 1057–1066. [Google Scholar] [CrossRef]
- Singh, S.; Dodt, J.; Volkers, P.; Hethershaw, E.; Philippou, H.; Ivaskevicius, V.; Imhof, D.; Oldenburg, J.; Biswas, A. Structure Functional Insights into Calcium Binding during the Activation of Coagulation Factor XIII A. Sci. Rep. 2019, 9, 11324. [Google Scholar] [CrossRef]
- Mutch, N.J.; Waters, E.K.; Morrissey, J.H. Immobilized Transition Metals Stimulate Contact Activation and Drive Factor XII-Mediated Coagulation. J. Thromb. Haemost. 2012, 10, 2108–2115. [Google Scholar] [CrossRef]
- Mrozińska, Z.; Ponczek, M.B.; Kaczmarek, A.; Świerczyńska, M.; Kudzin, M.H. Activity in the Field of Blood Coagulation Processes of Poly(Lactide)-Zinc Fiber Composite Material Obtained by Magnetron Sputtering. Coatings 2024, 14, 666. [Google Scholar] [CrossRef]
- Hamvar, M.; Bakhsheshi-Rad, H.R.; Omidi, M.; Ismail, A.F.; Aziz, M.; Berto, F.; Chen, X. Biocompatibility and Bioactivity of Hardystonite-Based Nanocomposite Scaffold for Tissue Engineering Applications. Biomed. Phys. Eng. Express 2020, 6, 035011. [Google Scholar] [CrossRef]
- Bagherpour, I.; Yaghtin, A.; Naghib, S.M.; Molaabasi, F. Synthesis and Investigation on Microstructural, Mechanical Features of Mesoporous Hardystonite/Reduced Graphene Oxide Nanocomposite for Medical Applications. Front. Bioeng. Biotechnol. 2023, 11, 1073435. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A Simple Technique for Quantitation of Low Levels of DNA Damage in Individual Cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Dash, S.K.; Ghosh, T.; Roy, S.; Chattopadhyay, S.; Das, D. Zinc Sulfide Nanoparticles Selectively Induce Cytotoxic and Genotoxic Effects on Leukemic Cells: Involvement of Reactive Oxygen Species and Tumor Necrosis Factor Alpha. J. Appl. Toxicol. 2014, 34, 1130–1144. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Chhabra, H.; Kadam, S.S.; Londhe, K.; Soni, V.P.; Bellare, J.R. Hardystonite Improves Biocompatibility and Strength of Electrospun Polycaprolactone Nanofibers over Hydroxyapatite: A Comparative Study. Mater. Sci. Eng. C 2013, 33, 2926–2936. [Google Scholar] [CrossRef]
- Mrozińska, Z.; Ponczek, M.; Kaczmarek, A.; Boguń, M.; Sulak, E.; Kudzin, M.H. Blood Coagulation Activities of Cotton–Alginate–Copper Composites. Mar. Drugs 2023, 21, 625. [Google Scholar] [CrossRef]
- Mrozińska, Z.; Kudzin, M.H.; Ponczek, M.B.; Kaczmarek, A.; Król, P.; Lisiak-Kucińska, A.; Żyłła, R.; Walawska, A. Biochemical Approach to Poly(Lactide)–Copper Composite—Impact on Blood Coagulation Processes. Materials 2024, 17, 608. [Google Scholar] [CrossRef]
- Mrozińska, Z.; Kaczmarek, A.; Świerczyńska, M.; Juszczak, M.; Kudzin, M.H. Biochemical Behavior, Influence on Cell DNA Condition, and Microbiological Properties of Wool and Wool–Copper Materials. Materials 2024, 17, 2878. [Google Scholar] [CrossRef]
- Kluska, M.; Juszczak, M.; Wysokiński, D.; Żuchowski, J.; Stochmal, A.; Woźniak, K. Kaempferol Derivatives Isolated from Lens Culinaris Medik. Reduce DNA Damage Induced by Etoposide in Peripheral Blood Mononuclear Cells. Toxicol. Res. 2019, 8, 896–907. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (Resazurin) Fluorescent Dye for the Assessment of Mammalian Cell Cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Tokarz, P.; Piastowska-Ciesielska, A.W.; Kaarniranta, K.; Blasiak, J. All-Trans Retinoic Acid Modulates DNA Damage Response and the Expression of the VEGF-A and MKI67 Genes in ARPE-19 Cells Subjected to Oxidative Stress. Int. J. Mol. Sci. 2016, 17, 898. [Google Scholar] [CrossRef]
- Collins, A.R. The Comet Assay for DNA Damage and Repair. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
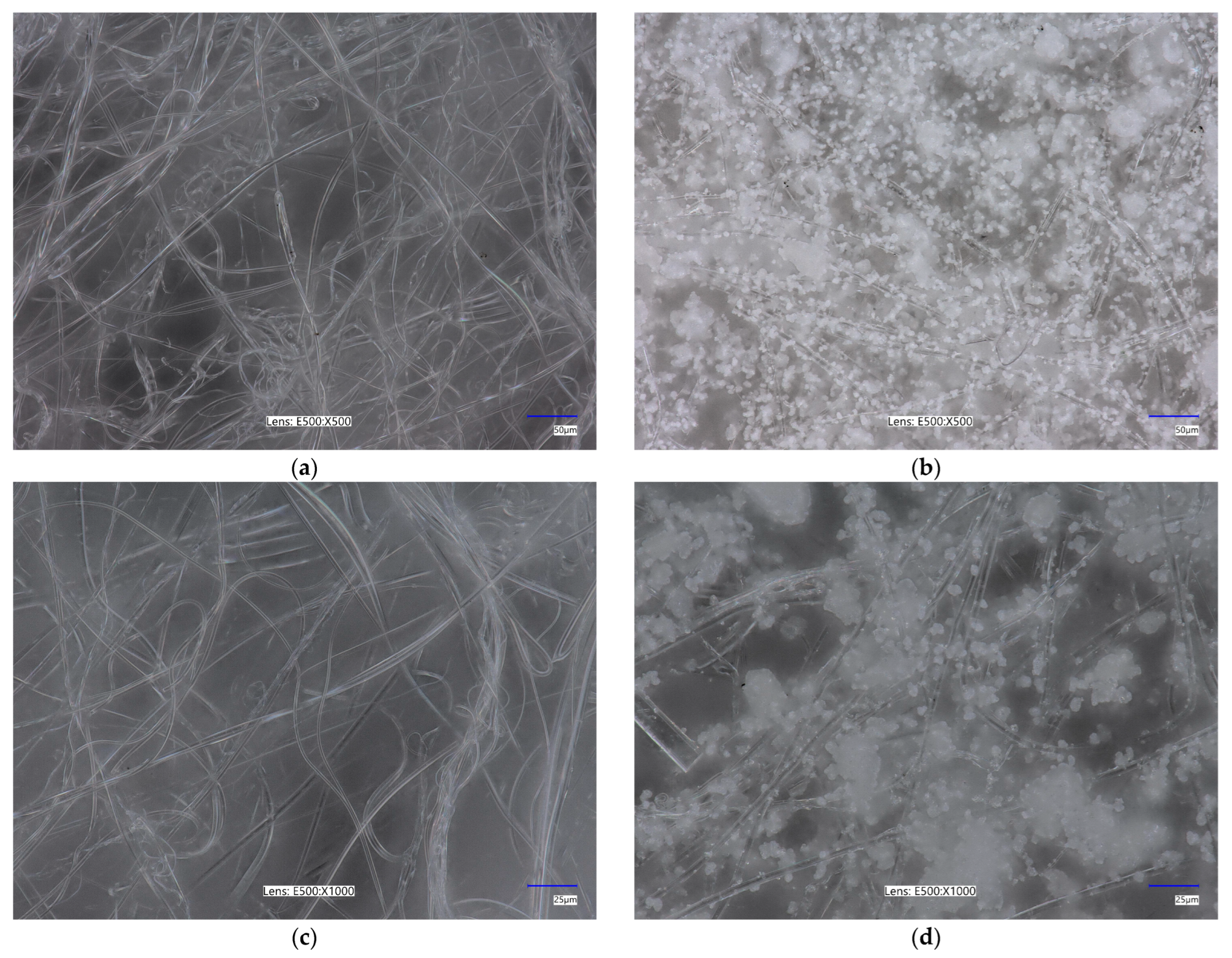
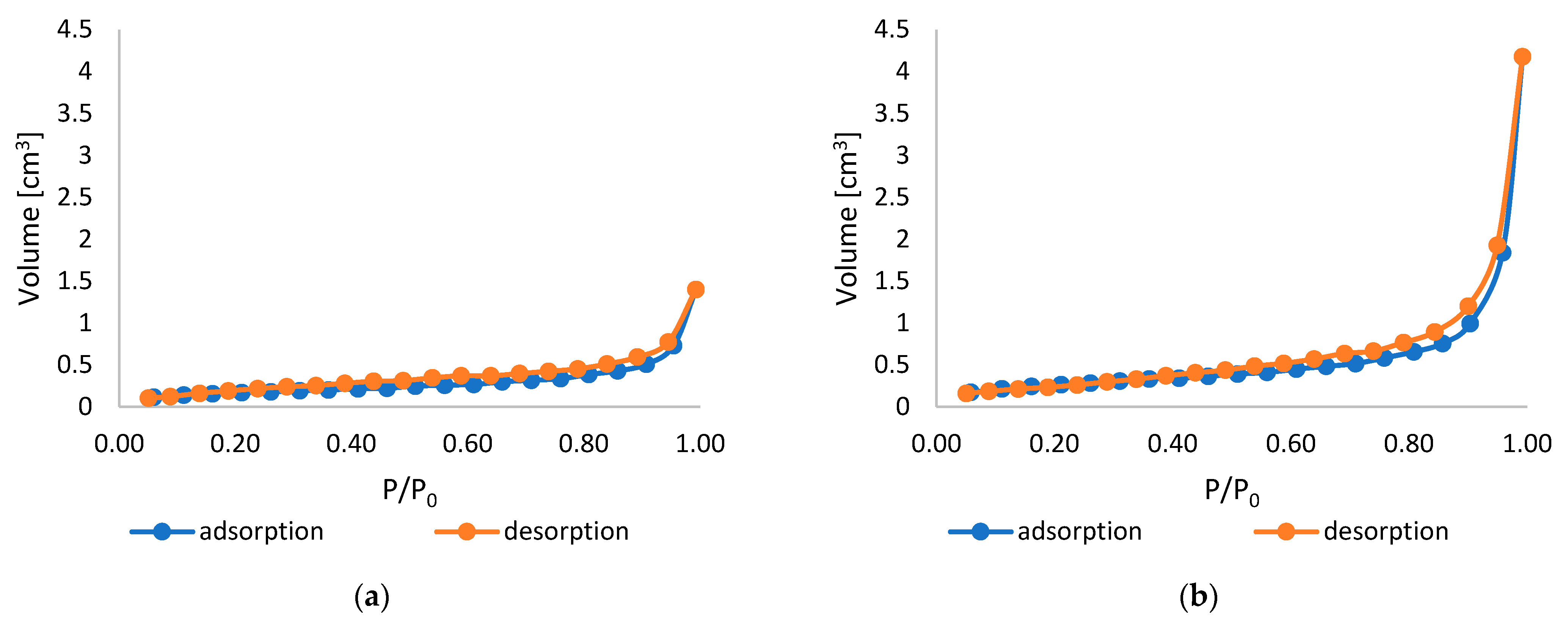
| Sample Name | Specific Surface Area [m2/g] | Total Pore Volume [cm3/g] | Average Pore Size [nm] |
|---|---|---|---|
| PLA | 1.082 | 3.985 × 10−3 | 14.73 |
| PLA-ALG-ZnS-HT | 1.217 | 8.210 × 10−3 | 26.99 |
| Values | Processing Parameters |
|---|---|
| 195 °C | Extruder temperature—zone 1 |
| 245 °C | Extruder temperature—zone 2 |
| 260 °C | Extruder temperature—zone 3 |
| 260 °C | Die head temperature |
| 260 °C | Air heater temperature |
| 7–8 m3/h | Airflow rate |
| 95 g/m2 | Basis weight of nonwovens |
| 6 g/min | Polymer throughput |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, A.; Mrozińska, Z.; Chruściel, J.J.; Juszczak, M.; Woźniak, K.; Kudzin, M.H. Haemostatic and Biocompatibility Evaluation of Alginate-Functionalized Polylactide Composite Containing Zinc Sulphide and Hardystonite. Mar. Drugs 2025, 23, 349. https://doi.org/10.3390/md23090349
Kaczmarek A, Mrozińska Z, Chruściel JJ, Juszczak M, Woźniak K, Kudzin MH. Haemostatic and Biocompatibility Evaluation of Alginate-Functionalized Polylactide Composite Containing Zinc Sulphide and Hardystonite. Marine Drugs. 2025; 23(9):349. https://doi.org/10.3390/md23090349
Chicago/Turabian StyleKaczmarek, Anna, Zdzisława Mrozińska, Jerzy J. Chruściel, Michał Juszczak, Katarzyna Woźniak, and Marcin H. Kudzin. 2025. "Haemostatic and Biocompatibility Evaluation of Alginate-Functionalized Polylactide Composite Containing Zinc Sulphide and Hardystonite" Marine Drugs 23, no. 9: 349. https://doi.org/10.3390/md23090349
APA StyleKaczmarek, A., Mrozińska, Z., Chruściel, J. J., Juszczak, M., Woźniak, K., & Kudzin, M. H. (2025). Haemostatic and Biocompatibility Evaluation of Alginate-Functionalized Polylactide Composite Containing Zinc Sulphide and Hardystonite. Marine Drugs, 23(9), 349. https://doi.org/10.3390/md23090349







