New Strategy for the Degradation of High-Concentration Sodium Alginate with Recombinant Enzyme 102C300C-Vgb and the Beneficial Effects of Its Degradation Products on the Gut Health of Stichopus japonicus
Abstract
1. Introduction
2. Results and Discussion
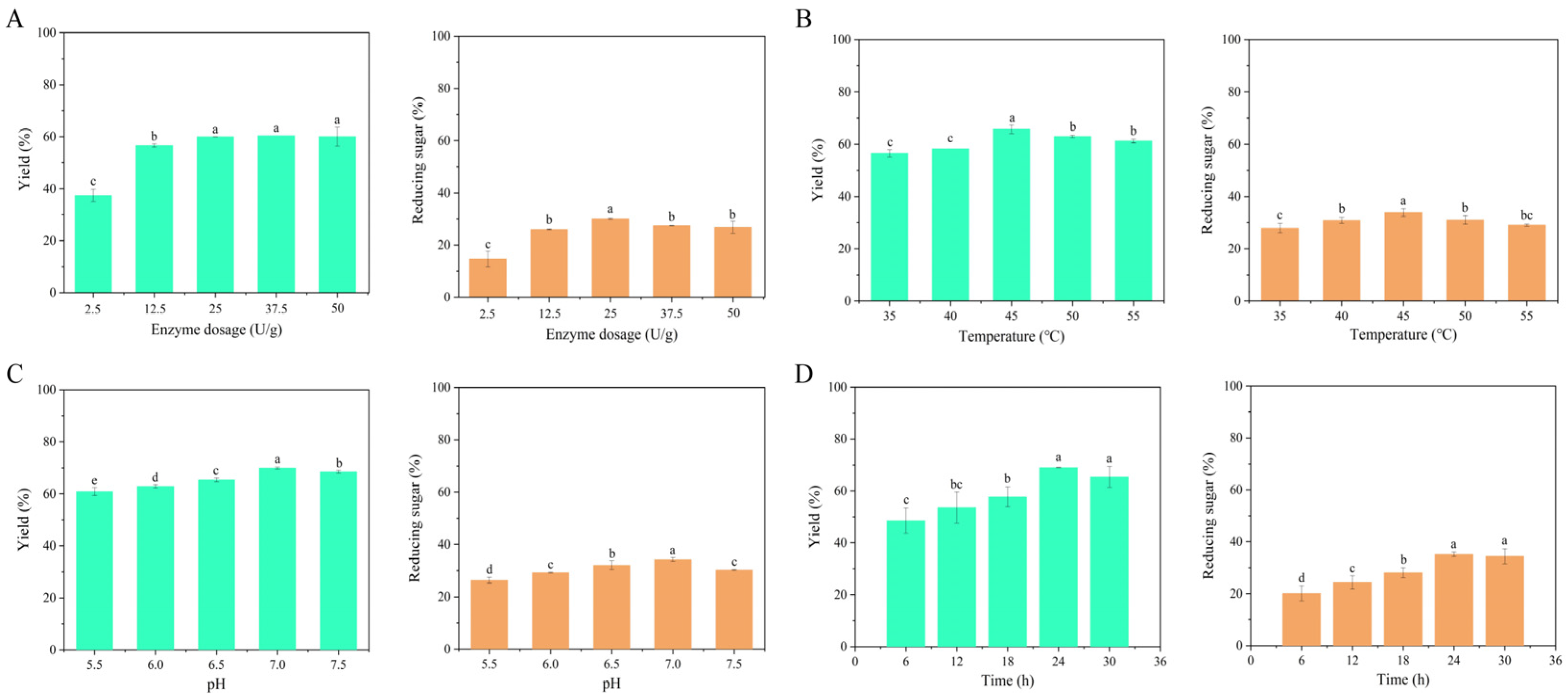
2.1. Optimal Enzymatic Hydrolysis Conditions
2.2. Structural Analysis of Degradation Product
2.2.1. FTIR Analysis
2.2.2. HPLC Analysis of Degradation Product
2.2.3. ESI-MS Analysis of Degradation Product
2.3. Preparation and Compositional Analysis of PSDP
2.4. Structural Analysis of PSDP
2.4.1. HPLC Analysis of PSDP
2.4.2. ESI-MS Analysis of PSDP
2.5. Evaluation of the Feeding Effect of PSDP
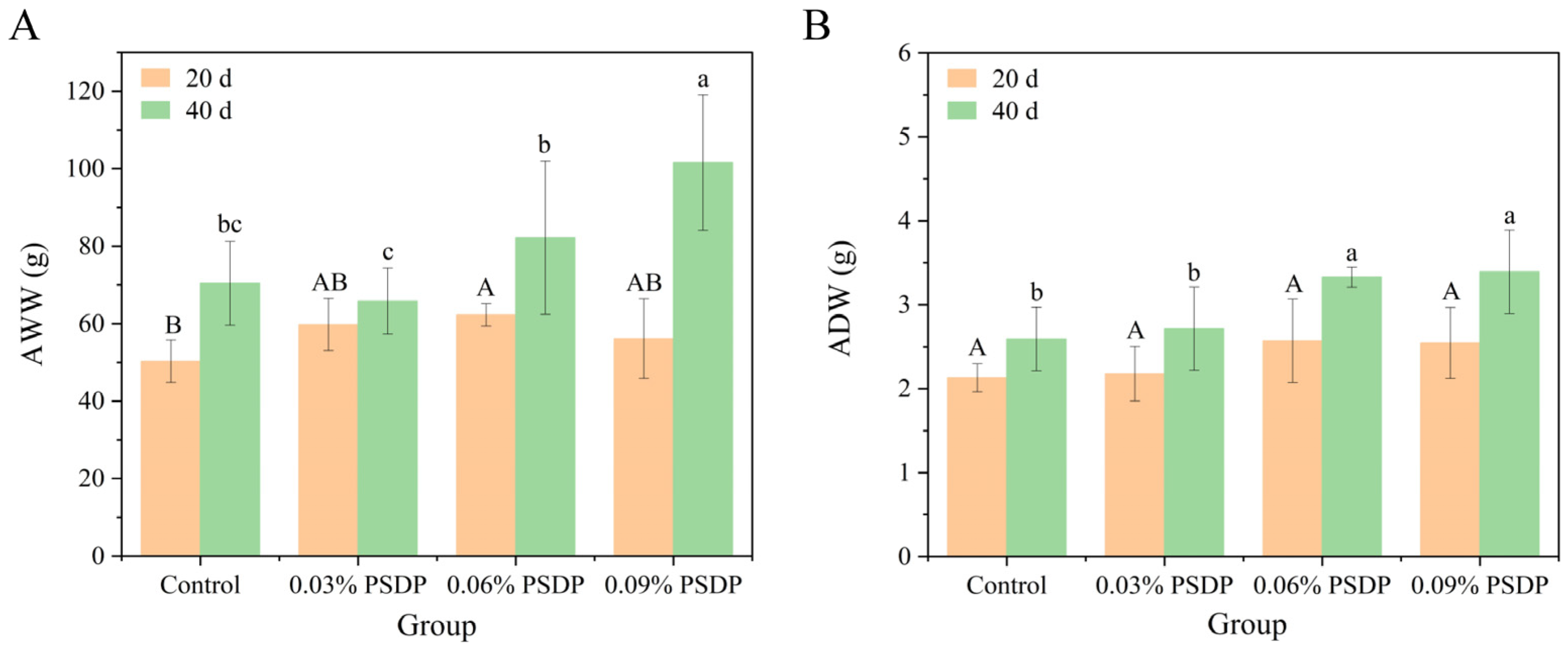
2.5.1. Growth Performance
2.5.2. Body Wall Composition
Protein Aspect
Fat Aspect
Amino-Acid Composition
2.5.3. Gut Health
Intestinal Morphology Analysis
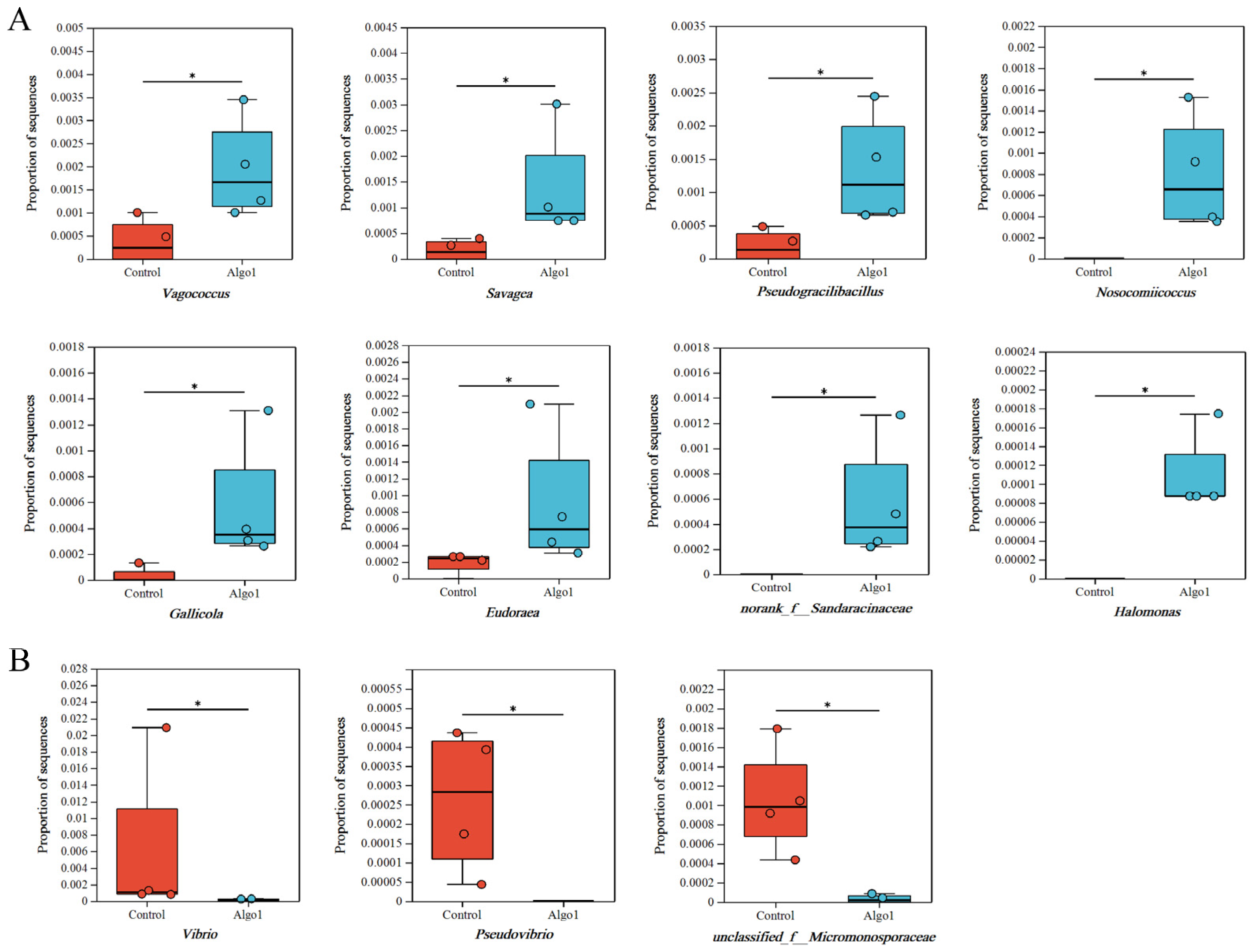
Gut Microbial Diversity Analysis
3. Materials and Methods
3.1. Experimental Materials
3.2. Optimization of Enzymatic Conditions
3.2.1. Enzyme Dosage
3.2.2. Enzymatic Temperature
3.2.3. Enzymatic pH
3.2.4. Enzymatic Time
3.3. 250-L Enlargement Experiment
3.4. S. japonicus Feeding Experiment
3.5. Data Measurement
3.5.1. Yield Analysis of Enzymatic Hydrolysis
3.5.2. Reducing Sugar Content Analysis
3.5.3. Fourier Transform Infrared Analysis
3.5.4. High-Performance Liquid Chromatography Analysis
3.5.5. Electrospray Ionization Mass Spectrometry Analysis
3.5.6. Compositional Analysis of Pilot-Scale Products
3.5.7. Growth Performance
3.5.8. Analyses of Body Wall Compositions
3.5.9. Intestinal Morphometry
3.5.10. Intestinal DNA Extraction and Illumina Sequencing of 16S rRNA Genes
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| S. japonicus | Stichopus japonicus |
| A. japonicus | Apostichopus japonicus |
| SA | Sodium alginate |
| AOS | Alginate oligosaccharides |
| PSDP | Pilot-scale degradation product |
| RSC | Reducing sugar content |
| FTIR | Fourier transform infrared |
| Mw | Molecular weight |
| AWW | Average wet weight: |
| ADW | Average dry weight |
References
- Chen, L.; Wang, X.Y.; Liu, R.Z.; Wang, G.Y. Culturable microorganisms associated with sea cucumbers and microbial natural products. Mar. Drugs 2021, 19, 8. [Google Scholar] [CrossRef]
- Purcell, S.W.; Hair, C.A.; Mills, D.J. Sea cucumber culture, farming and sea ranching in the tropics: Progress, problems and opportunities. Aquaculture 2012, 368–369, 68–81. [Google Scholar] [CrossRef]
- Yin, H.W.; Yue, H.; Wang, M.; Zhang, T.Q.; Zhao, Y.T.; Liu, H.Y.; Wang, J.F.; Zheng, H.W.; Xue, C.H. Preparation of novel sea cucumber intestinal peptides to promote tibial fracture healing in mice by inducing differentiation of hypertrophic chondrocytes to the osteoblast lineage. Mol. Nutr. Food Res. 2024, 68, 2300344. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Dave, D.; Shahidi, F. Northern sea cucumber (Cucumaria frondosa): A potential candidate for functional food, nutraceutical, and pharmaceutical sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, T.R.L.; Dave, D.; Shahidi, F. Sea cucumber derived type I collagen: A comprehensive review. Mar. Drugs 2020, 18, 471. [Google Scholar] [CrossRef]
- Park, M.; Shin, S.K.; Do, Y.H.; Yarish, C.; Kim, J.K. Application of open water integrated multitrophic aquaculture to intensive monoculture: A review of the current status and challenges in Korea. Aquaculture 2018, 497, 174–183. [Google Scholar] [CrossRef]
- Zhu, M.H.; Zhao, H.X.; Chen, J.W.; Xie, H.J.; Du, J. Investigation of antibiotics in sea cucumbers: Occurrence, pollution characteristics, and human risk assessment. Int. J. Environ. Sci. Technol. 2018, 25, 32081–32087. [Google Scholar] [CrossRef]
- Zhu, M.H.; Wang, Z.Y.; Chen, J.W.; Xie, H.J.; Zhao, H.X.; Yuan, X.T. Bioaccumulation, biotransformation, and multicompartmental toxicokinetic model of antibiotics in sea cucumber (Apostichopus japonicus). Environ. Sci. Technol. 2020, 54, 13175–13185. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Li, W.; Liu, B.D.; Liu, A.P.; Han, M.L.; Yin, Y.L.; Xu, G.H.; Cheng, W.X.; Xie, L.W. Dietary supplementation of N-carbamylglutamate promotes growth performance by modulating the homeostasis of gut microbiota in tilapia (Oreochromis niloticus). Aquacult. Rep. 2021, 20, 100750. [Google Scholar] [CrossRef]
- Huang, X.L.; Xie, R.B.; Zou, A.G.; Zhang, S.Q.; Xu, X.H.; Sun, G.H.; Yang, J.M. Effects of polypeptidin feeding on growth and intestinal flora of Apostichopus japonicas. Aquacult. Rep. 2024, 38, 102284. [Google Scholar]
- Amillano-Cisneros, J.M.; Fuentes-Valencia, M.A.; Leyva-Morales, J.B.; Davizón, Y.A.; Marquéz-Pacheco, H.; Valencia-Castañeda, G.; Maldonado-Coyac, J.A.; Ontiveros-García, L.A.; Badilla-Medina, C.N. Prebiotics in Global and Mexican Fish Aquaculture: A Review. Animals 2023, 13, 3607. [Google Scholar] [CrossRef]
- Huang, H.; Li, S.; Bao, S.; Mo, K.; Sun, D.; Hu, Y. Expression and characterization of a cold-adapted alginate Lyase with Exo/Endo-type activity from a novel marine bacterium Alteromonas portus HB161718T. Mar. Drugs 2021, 19, 155. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Li, Q.; Xu, Y.X.; Tang, T.C.; Ning, L.M.; Zhu, B.W. Evolving strategies for marine enzyme engineering: Recent advances on the molecular modifcation of alginate lyase. Mar. Life Sci. Technol. 2022, 4, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.L.; Guo, Y.M.; Yuan, J.M.; Liu, D.; Zhang, B.K. Sodium alginate oligosaccharides from brown algae inhibit Salmonella Enteritidis colonization in broiler chickens. Poultry Sci. 2011, 90, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.Y.; Xiao, M.S.; Ren, X.M.; Secundo, F.; Yu, Y.; Nan, S.H.; Chen, W.M.; Zhu, C.L.; Kong, Q.; Huang, Y.T.; et al. Response of Salmonella enterica serovar Typhimurium to alginate oligosaccharides fermented with fecal inoculum: Integrated transcriptomic and metabolomic analyses. Mar. Life Sci. Technol. 2023, 5, 242–256. [Google Scholar]
- Li, F.L.; Tang, Y.; Wei, L.X.; Yang, M.X.; Lu, Z.J.; Shi, F.; Zhan, F.B.; Li, Y.A.; Liao, W.C.; Lin, L.; et al. Alginate oligosaccharide modulates immune response, fat metabolism, and the gut bacterial community in grass carp (Ctenopharyngodon idellus). Fish Shellfish Immun. 2022, 130, 103–113. [Google Scholar] [CrossRef]
- He, N.; Yang, Y.; Wang, H.; Liu, N.; Yang, Z.; Li, S. Unsaturated alginate oligosaccharides (UAOS) protects against dextran sulfate sodium-induced colitis associated with regulation of gut microbiota. J. Funct. Foods 2021, 83, 104536. [Google Scholar] [CrossRef]
- Yuan, X.; Zheng, J.; Jiao, S. A review on the preparation of chitosan oligosaccharides and application to human health, animal husbandry and agricultural production. Carbohyd. Polym. 2019, 220, 60–70. [Google Scholar] [CrossRef]
- Feng, L.P.; Cao, Y.P.; Xu, D.X.; Wang, S.J.; Zhang, J. Molecular weight distribution, rheological property and structural changes of sodium alginate induced by ultrasound. Ultrason. Sonochem. 2017, 34, 609–615. [Google Scholar] [CrossRef]
- Vasudevan, U.M.; Lee, O.K.; Lee, E.Y. Alginate derived functional oligosaccharides: Recent developments, barriers, and future outlooks. Carbohyd. Polym. 2021, 267, 118158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.W.; Ni, F.; Xiong, Q.; Yao, Z. Marine oligosaccharides originated from seaweeds: Source, preparation, structure, physiological activity and applications. Crit. Rev. Food Sci. 2021, 61, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, S.; Li, X.; Yan, Q.; Reaney, M.J.; Jiang, Z. Alginate oligosaccharides: Production, biological activities, and potential applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1859–1881. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhang, X.; Wu, L.; Li, H.; Chen, Y.; Li, L.; Ni, H.; Li, Q.; Zhu, Y. Exolytic products of alginate by the immobilized alginate lyase confer antioxidant and antiapoptotic bioactivities in human umbilical vein endothelial cells. Carbohyd. Polym. 2021, 251, 116976. [Google Scholar] [CrossRef]
- Liu, Z.M.; Ning, C.; Yuan, M.X.; Yang, S.X.; Wei, X.Y.; Xiao, M.S.; Fu, X.D.; Zhu, C.L.; Mou, H.J. High-level expression of a thermophilic and acidophilic β-mannanase from Aspergillus kawachii IFO 4308 with significant potential in mannooligosaccharide preparation. Bioresour. Technol. 2020, 295, 122257. [Google Scholar] [CrossRef]
- Wang, M.; Chen, L.; Zhang, Z. Potential applications of alginate oligosaccharides for biomedicine-a mini review. Carbohyd. Polym. 2021, 271, 118408. [Google Scholar] [CrossRef]
- Yang, S.X.; Liu, Z.M.; Fu, X.D.; Zhu, C.L.; Kong, Q.; Yang, M.; Mou, H.J. Expression and characterization of an alginate lyase and its thermostable mutant in Pichia pastoris. Mar. Drugs 2020, 18, 305. [Google Scholar] [CrossRef]
- Han, W.J.; Gu, J.Y.; Chen, Y.Y.; Liu, H.H.; Li, Y.Z. Novel alginate lyase (Aly5) from a polysaccharide-degrading marine bacterium, Flammeovirga sp. strain MY04: Effects of module truncation on biochemical characteristics, alginate degradation patterns, and oligosaccharide-yielding properties. Appl. Environ. Microb. 2016, 82, 364–374. [Google Scholar] [CrossRef]
- Mackie, W. Semi-quantitative estimation of the composition of alginates by infra-red spectroscopy. Carbohyd. Res. 1971, 20, 413–415. [Google Scholar] [CrossRef]
- Hifney, A.F.; Fawzy, M.A.; Abdel-Gawad, K.M. Upgrading the antioxidant properties of fucoidan and alginate from Cystoseira trinodis by fungal fermentation or enzymatic pretreatment of the seaweed biomass. Food Chem. 2018, 269, 387–395. [Google Scholar] [CrossRef]
- Liang, Q.P.; Huang, Y.T.; Liu, Z.M.; Xiao, M.S.; Ren, X.M.; Liu, T.H.; Li, H.Y.; Yu, D.X.; Wang, Y.; Zhu, C.L. A recombinant alginate lyase Algt1 with potential in preparing alginate oligosaccharides at high concentration substrate. Foods 2023, 12, 4039. [Google Scholar] [CrossRef]
- Kaur, S.; Abraham, R.E.; Franco, C.M.M.; Puri, M. Production of alginate oligosaccharides (AOSs) using enhanced physicochemical properties of immobilized alginate lyase for industrial application. Mar. Drugs 2024, 22, 120. [Google Scholar] [CrossRef]
- Qiao, X.W.; Li, X.X.; Liu, S.Q.; Li, Y.; Zheng, M.L.; Fu, W.Y.; Long, H.M.; Luo, F.X. Study on storage stability of spray dried hemoglobin powder. Feed Res. 2023, 46, 107–111. (In Chinese) [Google Scholar]
- Falkeborg, M.; Cheong, L.Z.; Gianfico, C.; Sztukiel, K.M.; Kristensen, K.; Glasius, M.; Xu, X.B.; Guo, Z. Alginate oligosaccharides: Enzymatic preparation and antioxidant property evaluation. Food Chem. 2014, 164, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Wang, H.Y.; Yang, Z.Z.; Zhao, K.Y.; Li, S.Y.; He, N.N. The role of functional oligosaccharides as prebiotics in ulcerative colitis. Food Funct. 2022, 13, 6875–6893. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Vazvani, M.G.; Ebrahimi-Zarandi, M.; Skorik, Y.A. Alginate-induced disease resistance in plants. Polymers 2022, 14, 661. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, J.; Xu, Q.; Yin, H.; Chen, D.; Yu, B.; He, J. Alginate oligosaccharide protects against enterotoxigenic Escherichia coli-induced porcine intestinal barrier injury. Carbohyd. Polym. 2021, 270, 118316. [Google Scholar] [CrossRef]
- Bai, N.; Deng, W.; Qi, Z.; Pan, S.; Li, Q.; Gu, M. The effect of alginate oligosaccharides on intestine barrier function and Vibrio parahaemolyticus infections in the white shrimp Litopenaeus vannamei. Fish Shellfish Immun. 2023, 141, 109011. [Google Scholar] [CrossRef]
- Shi, L.D.; Zhai, H.J.; Wei, L.B.; Zeng, F.S.; Ren, T.J.; Han, Y.Z. Effects of dietary folic acid on growth performance, body wall folate content, immune and antioxidant enzyme activity of juvenile sea cucumber, Apostichopus japonicas. Aquac. Res. 2022, 53, 6219–6226. [Google Scholar] [CrossRef]
- Gao, X.B.; Zhai, H.; Wei, L.; Shi, L.; Yan, L.; Peng, Z.; Wang, W.; Ren, T.; Han, Y. Effects of dietary mannan oligosaccharides on growth, non-specific immunity, and intestinal health in juveniles of the Japanese sea cucumber (Apostichopus japonicus). Aquacult. Int. 2023, 31, 1705–1727. [Google Scholar] [CrossRef]
- Feng, J.H.; Zhang, L.; Xia, X.B.; Hu, W.; Zhou, P. Effect of geographic variation on the proteome of sea cucumber (Stichopus japonicus). Food Res. Int. 2020, 136, 109498. [Google Scholar] [CrossRef]
- Li, M.; Qi, Y.; Mu, L.; Li, Z.; Zhao, Q.; Sun, J.; Jiang, Q. Effects of processing method on chemical compositions and nutritional quality of ready-to-eat sea cucumber (Apostichopus japonicus). Food Sci. Nutr. 2019, 7, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Hu, C.; Fan, S. Chemical composition and nutritional quality of sea cucumbers. J. Sci. Food Agr. 2010, 90, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods-a review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Z.; Qi, Y.; Guo, Z.; Lin, Y.; Li, W.; Hu, Y.; Zhao, Q. Proximate composition and nutritional quality of deep sea growth sea cucumbers (Stichopus japonicus) from different origins. J. Sci. Food Agr. 2016, 96, 2378–2383. [Google Scholar] [CrossRef]
- Nishanthan, G.; Kumara, P.A.D.A.; de Croos, M.D.S.T.; Prasada, D.V.P.; Dissanayake, D.C.T. Effects of processing on proximate and fatty acid compositions of six commercial sea cucumber species of Sri Lanka. J. Food Sci. Technol. 2018, 55, 1933–1941. [Google Scholar] [CrossRef]
- Li, M.; Gao, Y.; Qi, Y.X.; Song, Z.Y.; Li, Z.B.; Lin, Y.T.; Zhao, Q.C. Assessment of the nutritional value of cultured sea cucumber Apostichopus japonicas. J. Aquat. Food Prod. T. 2021, 30, 868–879. [Google Scholar] [CrossRef]
- Lee, M.H.; Kim, Y.K.; Moon, H.S.; Kim, K.D.; Kim, G.G.; Cho, H.A.; Yoon, N.Y.; Sim, K.B.; Park, H.Y.; Lee, D.S. Comparison on proximate composition and nutritional profile of red and black sea cucumbers (Apostichopus Japonicus) from Ulleungdo (Island) and Dokdo (Island), Korea. Food Sci. Biotechnol. 2012, 21, 1285–1291. [Google Scholar] [CrossRef]
- Sugano, M.; Ishiwaki, N.; Nakashima, K. Dietary Protein-dependent modification of serum cholesterol level in rats. Significance of the arginine/lysine ratio. Ann. Nutr. Metab. 1984, 28, 192–199. [Google Scholar] [CrossRef]
- Heidarieh, M.; Mirvaghef, A.R.; Akbari, M.; Sheikhzadeh, N.; Kamyabi-Moghaddam, Z.; Askari, H.; Shahbazfar, A.A. Evaluations of Hilyses™, fermented Saccharomyces cerevisiae, on rainbow trout (Oncorhynchus mykiss) growth performance, enzymatic activities and gastrointestinal structure. Aquacult. Nutr. 2012, 19, 343–348. [Google Scholar] [CrossRef]
- Torrecillas, S.; Makol, A.; Caballero, M.J.; Montero, D.; Robaina, L.; Real, F.; Sweetman, J.; Tort, L.; Izquierdo, M.S. Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immun. 2007, 23, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; He, J. Effects of alginate oligosaccharide on the growth performance, antioxidant capacity and intestinal digestion-absorption function in weaned pigs. Anim. Feed Sci. Tech. 2017, 234, 118–127. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, J.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; He, J. Alginate oligosaccharide enhances intestinal integrity of weaned pigs through altering intestinal inflammatory responses and antioxidant status. RSC Adv. 2018, 8, 13482–13492. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Zhao, Z.H.; Yang, Y.J.; Song, Q.Z.; Ding, J.; Han, B.; Zhao, C. Dietary allicin improves behavior, physiology, growth, and disease resistance in the sea cucumber Apostichopus japonicas. Aquaculture 2024, 593, 741321. [Google Scholar] [CrossRef]
- Chang, Y.Q.; Ding, J.; Song, J. Biological Research and Culture of Sea Cucumbers and Sea Urchins; China Ocean Press: Beijing, China, 2004. [Google Scholar]
- Zhou, M.; Hernandez-Sanabria, E.; Guan, L.L. Assessment of the microbial ecology of ruminal methanogens in cattle with different feed efficiencies. Appl. Environ. Microb. 2009, 75, 6524–6533. [Google Scholar] [CrossRef]
- Zhang, Z.; Xing, R.L.; Lv, Z.M.; Shao, Y.N.; Zhang, W.W.; Zhao, X.L.; Li, C.H. Analysis of gut microbiota revealed Lactococcus garvieae could be an indicative of skin ulceration syndrome in farmed sea cucumber Apostichopus japonicus. Fish Shellfish Immun. 2018, 80, 148–154. [Google Scholar] [CrossRef]
- Tsagaraki, T.M.; Pree, B.; Leiknes, Ø.; Larsen, A.; Bratbak, G.; Øvreås, L.; Egge, J.K.; Spanek, R.; Paulsen, M.L.; Olsen, Y.; et al. Bacterial community composition responds to changes in copepod abundance and alters ecosystem function in an arctic mesocosm study. ISME J. 2018, 12, 2694–2705. [Google Scholar] [CrossRef]
- Bi, D.C.; Yang, X.; Lu, J. Preparation and potential applications of alginate oligosaccharides. Crit. Rev. Food Sci. 2023, 63, 10130–10147. [Google Scholar] [CrossRef]
- Tao, L.; Chai, J.; Liu, H.Y.; Huang, W.H.; Zou, Y.; Wu, M.L.; Peng, B.Q.; Wang, Q.; Tang, K.Y. Characterization and dynamics of the gut microbiota in rice fishes at different developmental stages in rice-fish coculture systems. Microorganisms 2022, 10, 2373. [Google Scholar] [CrossRef]
- Foysal, M.J.; Alam, M.; Robiul Kawser, A.Q.M.; Hasan, F.; Rahman, M.M.; Tay, C.Y.; Prodhan, M.S.H.; Gupta, S.K. Meta-omics technologies reveals beneficiary effects of Lactobacillus plantarum as dietary supplements on gut microbiota, immune response and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 520, 734974. [Google Scholar] [CrossRef]
- Adak, A.; Khan, M.R. An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 2019, 76, 473–493. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.G.; Li, J.N. Advances in the understanding of the intestinal microenvironment and inflammatory bowel disease. Chin. Med. J. 2020, 133, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, X.W.; Zhu, J.Y.; Liu, L.L.; Liu, Y.C.; Zhu, H. Dietary sanguinarine affected immune response, digestive enzyme activity and intestinal microbiota of Koi carp (Cyprinus carpiod). Aquaculture 2018, 502, 72–79. [Google Scholar] [CrossRef]
- Thompson, F.L.; Iida, T.; Swings, J. Biodiversity of vibrios. Microbiol. Mol. Biol. R. 2004, 68, 403–431. [Google Scholar] [CrossRef]
- Li, Z.; Ren, H.Y.; Li, Q.; Murtaza, B.; Li, X.Y.; Zhang, J.C.; Xu, Y.P. Exploring the effects of phage cocktails in preventing Vibrio infections in juvenile sea cucumber (Apostichopus japonicus) farming. Aquaculture 2020, 515, 734599. [Google Scholar] [CrossRef]
- Bondarev, V.; Richter, M.; Romano, S.; Piel, J.; Schwedt, A.; Schulz-Vogt, H.N. The genus Pseudovibrio contains metabolically versatile bacteria adapted for symbiosis. Environ. Microbiol. 2013, 15, 2095–2113. [Google Scholar] [CrossRef]
- Domínguez-Borbor, C.; Ardiles, V.; Bermeo, M.; Bolívar-Alvarado, C.; Tomalá, C.; Sonnenholzner, S.; Rodríguez, J.A. The marine symbiont Pseudovibrio denitrificans, is effective to control pathogenic Vibrio spp. in shrimp aquaculture. Aquaculture 2019, 508, 127–136. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ye, C.; Yuan, J.; Qin, S. Alginate oligosaccharide improves lipid metabolism and inflammation by modulating gut microbiota in high-fat diet fed mice. Appl. Microbiol. Biot. 2020, 104, 3541–3554. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, J.; Xiong, B.; Zhang, C.; Kang, B.; Gao, Y.; Li, Z.; Ge, W.; Cheng, S.; Hao, Y. Microbiota from alginate oligosaccharide-dosed mice successfully mitigated small intestinal mucositis. Microbiome 2020, 8, 112. [Google Scholar] [CrossRef]
- Zhang, C.; Xiong, B.; Chen, L.; Ge, W.; Yin, S.; Feng, Y.; Sun, Z.; Sun, Q.; Zhao, Y.; Shen, W. Rescue of male fertility following faecal microbiota transplantation from alginate oligosaccharide-dosed mice. Gut 2021, 70, 2213–2215. [Google Scholar] [CrossRef]
- Gu, Z.Q.; Niu, F.Y.; Yu, Z.H.; Bao, Z.; Mukhtar, H.; Yang, P.; Wang, S.T.; Mou, H.J.; Yang, M. High-efficiency expression of alginate lyase in Pichia pastoris facilitated by Vitreoscilla hemoglobin. Int. J. Biol. Macromol. 2024, 282, 137027. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Zhao, M.J.; Kuang, F.Y.; Zhang, Y.Y.; Lv, G.P. Effects of hydrolysis condition and detection method on the monosaccharide composition analysis of polysaccharides from natural sources. Separations 2024, 11, 2. [Google Scholar] [CrossRef]
- Acheampong, R.; Tutu, C.O.; Amissah, J.G.N.; Danquah, A.O.; Saalia, F.K. Physicochemical and sensory characteristics of a breakfast cereal made from sprouted finger millet-maize composite flour. Cogent Food Agricult. 2024, 10, 2363003. [Google Scholar] [CrossRef]
- Liu, W.; Pan, Q.S.; Zhang, P.; Huang, D.S.; Fan, A.P.; Yi, P. Determination of total arsenic in Chinese traditional herbs by high pressure digestion-hydride generation atomic fluorescence spectrometry. Adv. Mat. Res. 2012, 554–556, 1967–1970. [Google Scholar] [CrossRef]
- Hodges, D.J.; Skelding, D. Determination of lead in blood by atomic-absorption spectroscopy with electrothermal atomisation. Analyst 1983, 108, 813–820. [Google Scholar] [CrossRef]
- Marzo, L.D.; Pranata, J.; Barbano, D.M. Measurement of casein in milk by Kjeldahl and sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J. Dairy Sci. 2021, 104, 7448–7456. [Google Scholar] [CrossRef]
- Marco, A.; Rubio, R.; Compañó, R.; Casals, I. Comparison of the Kjeldahl method and a combustion method for total nitrogen determination in animal feed. Talanta 2002, 57, 1019–1026. [Google Scholar] [CrossRef]
- Shin, J.M.; Hwang, Y.O.; Tu, O.J.; Jo, H.B.; Kim, J.H.; Chae, Y.Z.; Rhu, K.H.; Park, S.K. Comparison of different methods to quantify fat classes in bakery products. Food Chem. 2013, 136, 703–709. [Google Scholar] [CrossRef]






| Index | Detection Result |
|---|---|
| Sensory requirements | |
| Sensation | Powder, light yellowish-brown in color, free from mold, spoilage, and off odors or smells |
| Physicochemical parameters | |
| AOS content (%) | 66.7 ± 0.1 |
| Water content (%) | 5.10 ± 0.85 |
| Crude ash content (%) | 20.95 ± 3.32 |
| Particle size (80 mesh pass rate, %) | 99.50 ± 0.42 |
| Sanitary indicators | |
| Total arsenic (mg/kg) | 0.29 ± 0.01 |
| Lead (mg/kg) | ND |
| Salmonella (/25g) | ND |
| 20 Day | Control (%) | 0.03% PSDP (%) | 0.06% PSDP (%) | 0.09% PSDP (%) | 40 Day | Control (%) | 0.03% PSDP (%) | 0.06% PSDP (%) | 0.09% PSDP (%) |
|---|---|---|---|---|---|---|---|---|---|
| Asp | 4.60 | 4 | 4.80 | 4.70 | Asp | 4.80 | 4.70 | 4.30 | 4.50 |
| Thr | 2.40 | 2.10 | 2.50 | 2.60 | Thr | 2.50 | 2.50 | 2.20 | 2.30 |
| Ser | 2.30 | 2 | 2.40 | 2.50 | Ser | 2.30 | 2.30 | 2.1 | 2.30 |
| Glu | 7.20 | 6.20 | 7.40 | 7.06 | Glu | 7.40 | 7.30 | 6.60 | 7 |
| Gly | 6.50 | 5.60 | 6.60 | 7.10 | Gly | 6.10 | 6.50 | 5.90 | 6.10 |
| Ala | 3 | 2.60 | 3 | 3.70 | Ala | 2.90 | 3 | 2.70 | 2.70 |
| Cys | 0.60 | 0.40 | 0.50 | 0.80 | Cys | 0.40 | 0.30 | 0.30 | 0.30 |
| Val | 1.90 | 1.60 | 1.90 | 1.90 | Val | 2 | 1.80 | 1.60 | 1 |
| Met | 0.80 | 0.70 | 0.70 | 1.20 | Met | 0.90 | 0.80 | 0.70 | 0.40 |
| Ile | 1.40 | 1.50 | 1.50 | 1.90 | Ile | 1.50 | 1.30 | 1.20 | 0.70 |
| Leu | 2 | 2 | 2.20 | 2.70 | Leu | 2.40 | 2.20 | 2 | 2.10 |
| Tyr | 1.50 | 1.20 | 1.40 | 1.90 | Tyr | 1.40 | 1.30 | 1.20 | 1.90 |
| Phe | 1.50 | 1.20 | 1.50 | 1.50 | Phe | 1.50 | 1.30 | 1.30 | 1.80 |
| Lys | 1.90 | 1.50 | 1.90 | 1.80 | Lys | 2 | 1.80 | 1.60 | 1.50 |
| His | 0.50 | 0.50 | 0.60 | 0.70 | His | 0.60 | 0.70 | 0.50 | 0.30 |
| Arg | 3.40 | 2.90 | 3.50 | 3.50 | Arg | 3.40 | 2.40 | 3.10 | 2.90 |
| Hypro | 2.40 | 2 | 2.30 | 2.90 | Hypro | 2 | 2.40 | 2.10 | 2.20 |
| Pro | 3.20 | 2.80 | 3.30 | 3.20 | Pro | 3.10 | 3.40 | 3 | 2.80 |
| Group | Serosa Layer Thickness (μm) | Muscularis Propria Thickness (μm) | Submucosa Thickness (μm) | Folds Width (μm) |
|---|---|---|---|---|
| Control | 22.25 ± 10.37 b | 9.64 ± 1.7 b | 23.61 ± 3.42 b | 211.85 ± 25.8 c |
| 0.03% PSDP | 25.45 ± 2.58 b | 9.41 ± 1.55 b | 37.78 ± 9.06 a | 184.23 ± 13.5 c |
| 0.06% PSDP | 29.49 ± 9.99 b | 12.58 ± 2.13 a | 43.83 ± 7.82 a | 246.62 ± 23.4 b |
| 0.09% PSDP | 39.72 ± 3.55 a | 11.92 ± 2.56 ab | 41.26 ± 3.89 a | 311.01 ± 3.99 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Z.; Niu, F.; Yang, P.; Gong, W.; Mukhtar, H.; Li, S.; Zheng, Y.; Zhong, Y.; Cui, H.; Li, J.; et al. New Strategy for the Degradation of High-Concentration Sodium Alginate with Recombinant Enzyme 102C300C-Vgb and the Beneficial Effects of Its Degradation Products on the Gut Health of Stichopus japonicus. Mar. Drugs 2025, 23, 339. https://doi.org/10.3390/md23090339
Gu Z, Niu F, Yang P, Gong W, Mukhtar H, Li S, Zheng Y, Zhong Y, Cui H, Li J, et al. New Strategy for the Degradation of High-Concentration Sodium Alginate with Recombinant Enzyme 102C300C-Vgb and the Beneficial Effects of Its Degradation Products on the Gut Health of Stichopus japonicus. Marine Drugs. 2025; 23(9):339. https://doi.org/10.3390/md23090339
Chicago/Turabian StyleGu, Ziqiang, Feiyu Niu, Peng Yang, Wenling Gong, Hina Mukhtar, Siyu Li, Yanwen Zheng, Yiling Zhong, Hanyi Cui, Jichao Li, and et al. 2025. "New Strategy for the Degradation of High-Concentration Sodium Alginate with Recombinant Enzyme 102C300C-Vgb and the Beneficial Effects of Its Degradation Products on the Gut Health of Stichopus japonicus" Marine Drugs 23, no. 9: 339. https://doi.org/10.3390/md23090339
APA StyleGu, Z., Niu, F., Yang, P., Gong, W., Mukhtar, H., Li, S., Zheng, Y., Zhong, Y., Cui, H., Li, J., Mou, H., & Li, D. (2025). New Strategy for the Degradation of High-Concentration Sodium Alginate with Recombinant Enzyme 102C300C-Vgb and the Beneficial Effects of Its Degradation Products on the Gut Health of Stichopus japonicus. Marine Drugs, 23(9), 339. https://doi.org/10.3390/md23090339







