Abstract
The popularity of bioactive compounds extracted from sea cucumbers is growing due to their wide application in the pharmaceutical industry, particularly in the development of drugs for neurological disorders. Different types of compounds, such as saponins, phenolic compounds, cerebrosides, and glucocerebrosides, are being studied intensively for their efficacy in assessing the treatment of neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, and brain tumors, among others. Positive results have been observed in the upregulation in the content of p-CREB, p-PL3K, BDNF, SOD, and MDA. Furthermore, the neuroprotective mechanism of the compounds against Alzheimer’s disease revealed that suppressing the phosphorylation of tau protein by the PI3K/Akt/GSK3β pathway leads to improved synaptic plasticity and reduced nerve fiber tangles. This comprehensive review explores recent findings on the therapeutic potential of sea cucumber bioactives in the treatment of brain-related disorders.
1. Introduction
Neurodegeneration is a progressive dysfunction and loss of neuronal structure and function that results in neuronal cell death. It is linked to deficiencies in particular brain processes (memory, mobility, and cognition), which can occur in several disorders affecting the central nervous system (CNS) [1,2,3,4]. The global prevalence of neurodegenerative diseases (NDs) such as Alzheimer’s disease (AD), Parkinson’s disease (PD), stroke, and depression has witnessed a significant rise, making them a serious health threat due to their chronic nature and severity [5]. As a result, these diseases have emerged as a research hotspot in biomedical science, particularly in the pursuit of effective therapeutic approaches. New research shows that a person’s genetic composition and environmental factors can significantly raise their risk of developing NDs [6], whereas age is the single biggest risk factor for the development of all NDs [7].
Although NDs differ clinically, there is a basic pathological mechanism for the occurrence of such disorders, including aberrant protein deposition, intracellular calcium (Ca2+) overload, mitochondrial dysfunction, imbalanced redox homeostasis, and neuroinflammation [8,9]. The main contributing factor is the accumulation and deposition of toxic proteins in the brain, and mitochondrial dysfunction is an important variable in the progression of the disease [6,10]. Previous studies have indicated that the survival rate of NDs is limited [11,12,13]. A current investigation in 2019 stated that there were 349.2 million people affected by severe neurological illnesses and 10 million fatalities globally [14,15]. Among all these medical conditions, AD was the leading cause of mortality, next to cognitive impairment and neonatal dementia [16]. The number of fatalities has risen by 39% in absolute terms during the last 30 years, and the number of disability-adjusted life-years has increased by 15% [17].
Depending entirely on the disease’s nature and stage, these disorders may have a variety of neuropathological traits that can be extremely dangerous or even fatal in some cases. Currently, several management strategies are recognized which either focus on the pathophysiology of the disease or try to alleviate its symptoms [6]. However, the rate at which these diseases are becoming more common is outpacing the rate at which they can be managed and treated. While several neuroprotective and disease-modifying drugs have shown significant promise in preclinical research, finding novel chemical compounds to serve as design templates for drug development initiatives is becoming a growing challenge [18]. This initiates the need for the exploration of natural bioactive compounds to treat disease progression and also to achieve a complete remission.
Since ancient times, natural products have been used and recognized for their healing properties. The protective effects of natural products and the isolated bioactive components against various diseases, including cancer, diabetes, heart disease, reproductive disorders, and neurological diseases, have been the subject of numerous studies in recent decades [19,20]. In the case of neurodegenerative diseases, the mode of action may involve multiple mechanisms, including antioxidant, anti-inflammatory, and antiapoptotic activities. Due to the diverse spectrum of pharmacological and biological actions, natural products are seen as potential alternatives for the treatment of neurodegeneration, aiding in the creation and discovery of new medications [21,22]. Currently, in addition to terrestrial sources of natural products, marine organisms such as microorganisms, vertebrates, invertebrates, and algae are gaining prominence in the extraction of pharmacologically active substances as a research interest [23]. Given the growing burden of infections, metabolic abnormalities, and age- and lifestyle-associated diseases, there is a pressing need for ongoing exploration of marine bioactives through both conventional and contemporary approaches [24]. Numerous chemical substances like bioactive peptides, fatty acids, pigments, alkaloids, and polysaccharides have previously been identified from marine sources and could be beneficial in preventing and treating a range of neuroinflammatory diseases [25]. These findings have brought the sea cucumber, a marine organism, into the spotlight of scientific circles due to its rich nutritional content and potential to support neurological health [26].
Among different valuable marine resources, sea cucumbers are one of the most promising ones, belonging to the class Holothuroidea. These organisms are soft-bodied marine invertebrates with leathery skin and an elongated shape with a single, branched gonad. There are 1716 species of sea cucumbers, with the Asia–Pacific region having the highest biodiversity [27]. Due to the exposure to the challenging and dynamic environment, sea cucumbers have evolved to produce distinct secondary metabolites with biological activity [28]. Sea cucumbers have been used for their medicinal properties since ancient times. According to the Ming dynasty account (1368–1644), sea cucumbers were known as “haishen,” or “ocean ginseng,” since they had the same medical qualities as ginseng [28]. Within the framework of Traditional Chinese Medicine, sea cucumbers have been utilized as a revitalizing agent to treat skeletal and joint weakness, especially that associated with age-related inflexibility, renal system abnormalities, sexual disorders, dry-stool constipation, poor lipid digestion, and circulatory complications [29].
However, the creation and discovery of novel medications greatly benefit from their taxonomic variety, diverse biological activity, high production, and chemical distinctiveness [30,31]. The active compounds isolated from these organisms exhibit broad chemical diversity, including polysaccharides like glycosaminoglycans (mucopolysaccharides), neutral glycans, fucosylated chondroitin sulfates (FCSs), and sulfated fucans, as well as peptides, phospholipids, and glycolipids, including glycosphingolipids (cerebrosides), polyunsaturated fatty acids, phenols, and triterpene glycosides (saponins) [32,33,34,35,36,37,38]. For example, FCS is a distinct type of sulfated glycosaminoglycan that is only present in echinoderm sea cucumbers, which differs from other known mammalian glycosaminoglycans in both structure and function [39]. One of the most unique features of sea cucumbers is their ability to regenerate internal organs and body parts which is far higher than that of sea stars and sea urchins, making them excellent models for regeneration [40]. For instance, the presence of a unique genomic profile related to intestinal regeneration in Apostichopus japonicus holds promising biomedical implications [41]. These tissue-healing capabilities may play a role in hindering tumorigenesis by preventing the transformation of epithelial cells into cancerous cells [42].
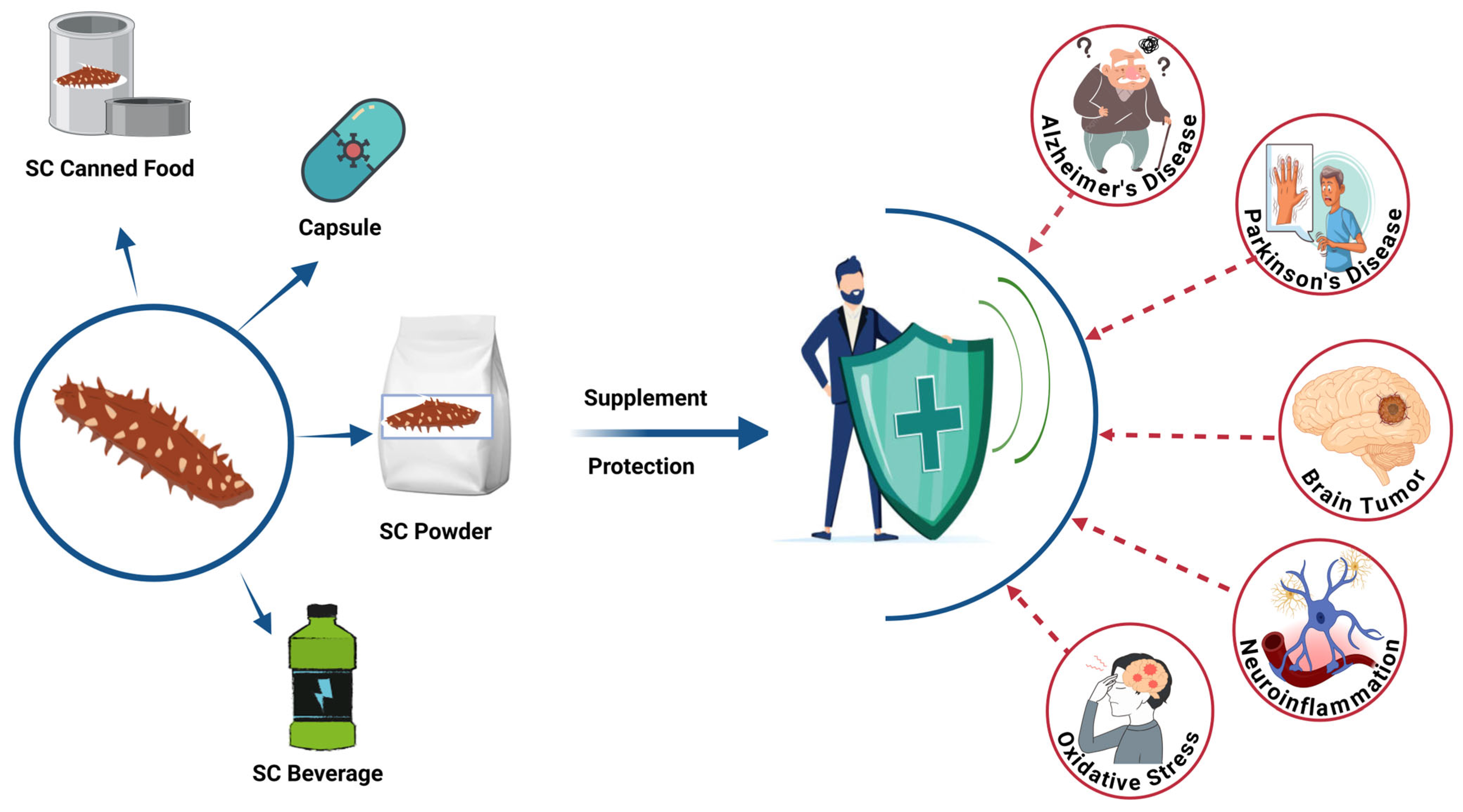
The neuroprotection potency of sea cucumbers’ bioactives (SCBs) was shown in several animal models [43,44]. Thus, this review aims to examine evidence of potential therapeutic bioactives derived from sea cucumber for the prevention and management of brain-related disorders such as Alzheimer’s disease, Parkinson’s disease, brain tumors, neuroinflammation, and oxidative-stress modulated brain disorders (Figure 1).
Figure 1.
Potential neuroprotective applications of sea cucumber-based products. SC, sea cucumber. The figure was generated from the concept of Man et al. (2022) [45] using Biorender.com (Agreement number: FF28KHIJ6E).
Sea cucumber (SC)-derived products, including canned food, capsules, powders, and beverages, can be formulated as dietary supplements for brain health [35,45,46,47,48]. These bioactive-rich formulations may provide protection against various brain-related disorders through their multifunctional therapeutic properties [45,47]. While research on the development of SCBs is attracting more attention, the ability to translate these new findings into industrial practice and new products remains immature. Challenges, including validating health claims, unclarified mechanisms, unsatisfactory sensory properties, and high production costs, must be solved. This review provides a comprehensive and up-to-date overview of the biological functions of SCBs and discusses potential solutions to the identified issues. This information will provide a general perspective on developing SCB nutraceuticals and functional foods.
2. Research Methodology
In order to search the relevant published articles, multiple search engines such as Scopus, PubMed, Web of Science, and Google Scholar were used. The search strategy was focused only on English-language scientific articles published between 2000 and 2025. More than 100 keywords containing one- to seven-word phrases like “toxic protein deposition in the brain”, “alpha-synuclein (α-Syn) aggregates”, etc., were used (Table 1).

Table 1.
Keyword and search phrase selection list.
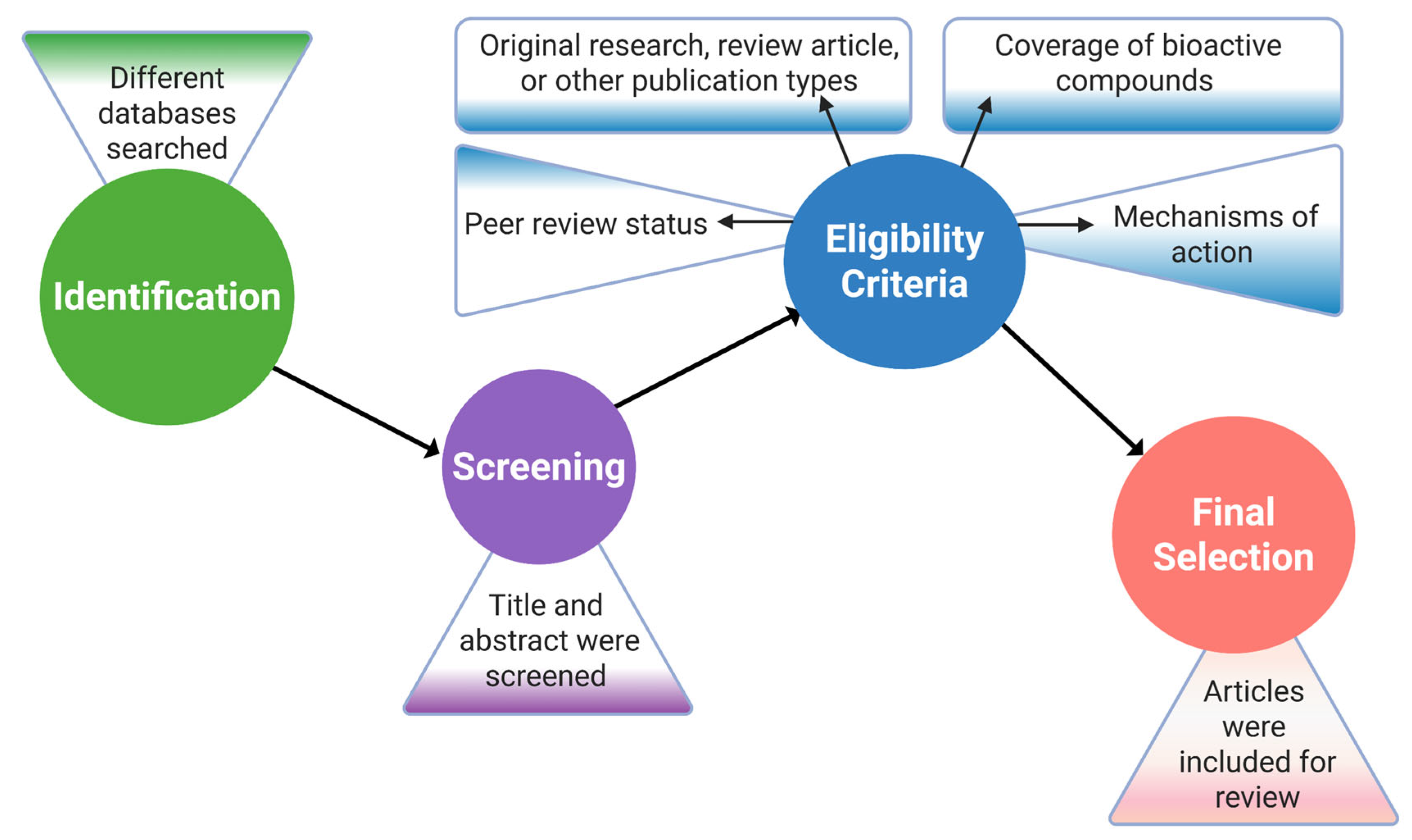
The article selection process was based on important criteria, for example, the peer review status, the type of publication, including original research, review article, or other publication types, the coverage of bioactive compounds extracted from the target organism, and the effects as well as the mechanisms of action of the compounds in the body. Following that, the selected articles were further assessed based on the study objectives, appropriateness of the methods, accuracy of the data analysis, and effectiveness of the findings [49]. In order to increase the credibility and coherence of the results by finding parallels and differences among studies, a meticulous cross-referencing and validation process was employed, and studies with inconsistent results or a poor methodology were omitted (Figure 2).
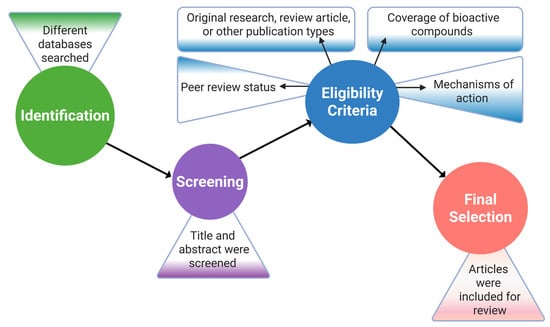
Figure 2.
Research article selection process flowchart. This figure was generated using Biorender.com (Agreement No: MX28J5UCP2).
3. Bioactive Compounds in Sea Cucumbers
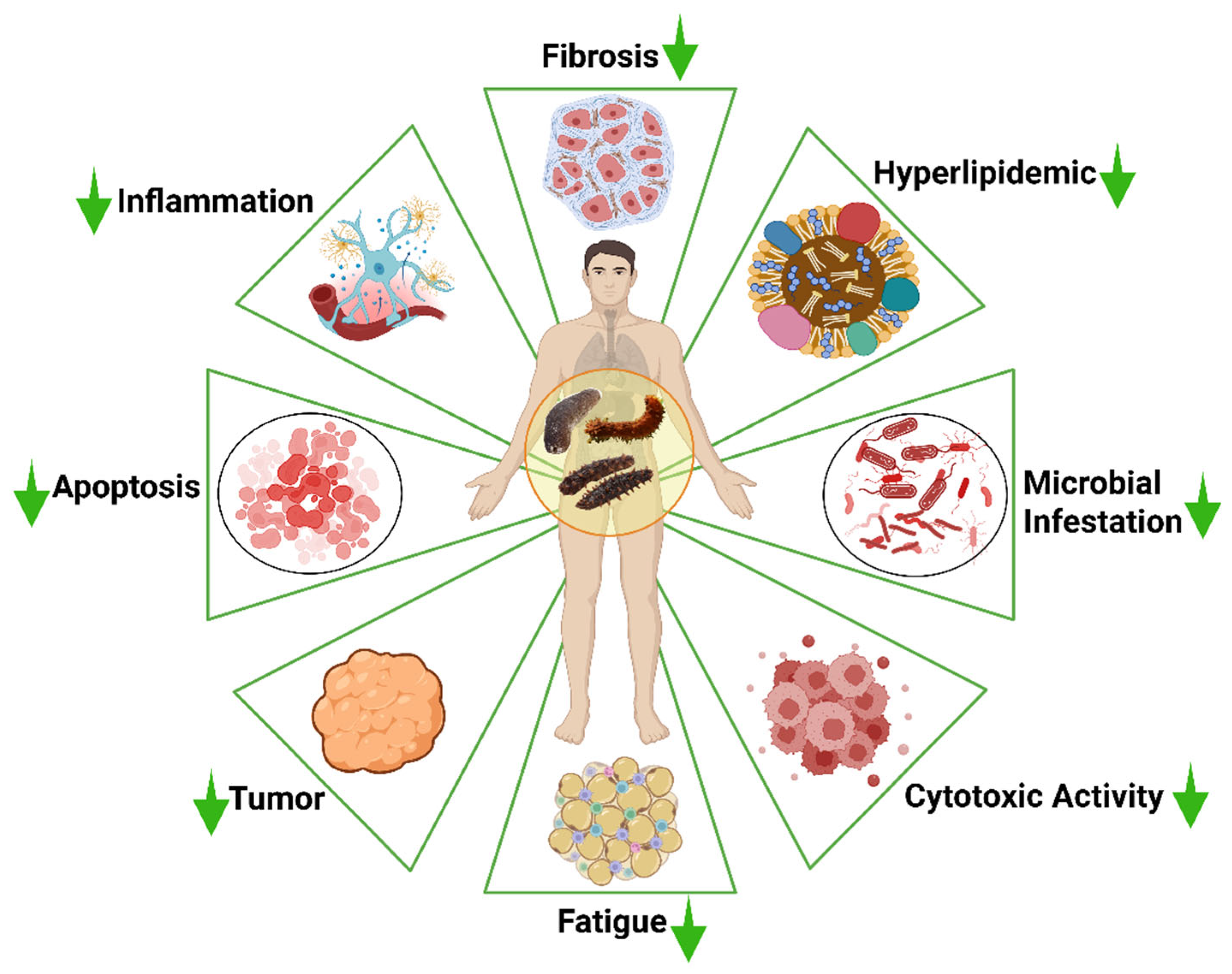
Most of the body parts of the sea cucumber, including the processing discards, which account for over 50% of the body weight of sea cucumbers [37], may contain a wide range of bioactive compounds. These bioactive compounds may include peptides, phenols, triterpene glycosides, fucoidan, fucosylated chondroitin sulfate (FCS), cerebrosides, and sphingoids. These bioactive compounds extracted from sea cucumbers have multifunctional therapeutic potential in biomedical and nutraceutical applications, such as anti-cancer, antihypertensive, antioxidant, antidiabetic, anti-tumor, anti-inflammatory, anti-microbial, and wound healing properties (Figure 3).
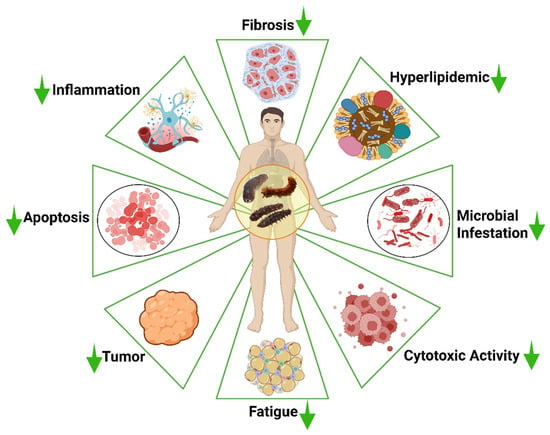
Figure 3.
Bioactive compounds derived from sea cucumbers and their associated overall pharmacological properties. The concept of the figure was adopted based on the general approach described by Salindeho et al. (2022) [50] using Biorender.com (Agreement number: MQ28J0DC2Z).
3.1. Saponin
Sea cucumbers are among the few animal lineages that produce saponins, secondary metabolites, which are widely found in plants [51]. In contrast to the typical creation of lanosterol in animal cholesterol synthesis, Li et al. (2018) noted that the sea cucumber has “plant-like” patterns that are characteristic of evolutionary convergence, which enable it to produce parkeol for saponin synthesis through oxidosqualene cyclase [52]. The molecular structure of sea cucumber saponins is typically a triterpenoid oligoglycoside, joined by sugar chains via the β-glycosidic link and a non-polar (fat-soluble) aglycone. Based on the carbon structure of the non-polar aglycone region, these glycosylated molecules, also known as glycosides, are classified into three primary groups: triterpenoidal glycosides, steroidal glycosides, and steroidal alkaloid glycosides [42,53,54]. The majority of sea cucumber saponins exist in the holostane form; however, they are frequently separated into holostane and nonholostane forms based on the varying positions of the aglycone lactones [55]. Currently, several newer saponins can be isolated and characterized from sea cucumber through techniques like liquid–liquid extraction with various solvents, Soxhlet extraction with 70% solvents, solid phase chromatography with silica gel or resins, etc. [56]. Apart from these, different advanced methods are used in the extraction, optimization, and characterization of these bioactive compounds like high-performance liquid chromatography (HPLC), time-of-flight mass spectrometry (TOF/MS), matrix-assisted laser desorption ionization–time-of-flight mass spectrometry (MALDI-TOF MS), and high-performance centrifugal partition chromatography (HPCPC), high-resolution mass spectrometry (HRMS), and nuclear magnetic resonance (NMR) [56,57,58]. Numerous sea cucumbers and their various body parts have been identified to contain a variety of saponins, including Frondoside A, Echinoside A, Cucumarioside A2-2, Holotoxin A1, Stichoposide C, Acetylated Lessoniosides A-E, and Non-acetylated Lessoniosides F and G [59,60], and there is a correlation between the molecular structure and bioavailability of these compounds. For instance, in the rat model, Echinoside A, with a lower molecular mass and less complex glycan structure, exhibited higher bioavailability than Holotoxin A1, likely due to reduced sterically branched chains [61].
Due to the potential of saponin for neuroprotective management and effects on the attenuation of diseases of the central nervous system, saponins accounted for 72% of sea cucumber research in the field of tumor and cancer management [60,62].
Saponin extracted from Holothuria leucospilota showed antioxidant activity in 2,2-Diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid (ABTS), and the reducing power assay, indicating that it can lower reactive oxygen species (ROS), a major contributor to brain stress. Additionally, another study found that the extract activates the daf-16/Forkhead box O (FOXO) pathway in Caenorhabditis worms, hence mediating lifespan extension and stress tolerance [63,64]. Together with other bioactive substances from the body wall and Cuvierian tubule of H. leucospilota, saponin-rich extract improved dopaminergic (DA) neuronal function in food-sensing behavior and reduced α-synuclein aggregation in in vivo PD models [65]. In a study on a transgenic C. elegans AD model, the effects of frondoside A from sea cucumber (C. frondosa), a saponin, on amyloid-beta (Aβ) aggregation and proteotoxicity were assessed, in which frondoside A considerably postponed the worm paralysis brought on by Aβ aggregation and restored chemotaxis failure in worms whose neurones produce Aβ, and shielded the worms from oxidative stress [66].
3.2. Peptides and Proteins
Biologically active peptides are parts of naturally occurring proteins that are inactive in their precursor form but exert a physiological effect upon enzymatic release or transport to the active site [67]. They are generally a group of peptides, in most cases consisting of fewer than 50 residues, that have a function in a living organism or cell. Although some of these peptides are found in a bare format, many of them are hidden in the intact structure of protein molecules [68]. Since they have nutraceutical potential, these protein hydrolysates and peptide fractions are widely used as functional food additives in the pharmaceutical industry [69]. As such, their widespread potential has led to their increasing application in disease prevention and quality health promotion, while growing scientific and commercial interest [70].
There are several techniques for synthesizing bioactive peptides from natural sources, which fall into three categories: in vitro, in vivo, and in silico. To create bioactive peptides, particularly commercial enzymes, in vitro techniques, including microbial fermentation, chemical hydrolysis, and enzymatic hydrolysis, are frequently employed. Enzymatic hydrolysis is considered the crucial step for sea cucumber peptides (SCP) production, where a variety of parameters influence the ultimate yield and bioactivity of SCP. Hence, a majority of research on SCP has used enzymatic hydrolysis techniques [45]. In the majority of studies, commercial food-grade enzymes such as Trypsin, Flavorzyme, and Alcalase were used to ensure a better yield of peptides, due to their better efficiency and operational suitability, as the resulting peptides demonstrated potent antioxidative activity [71,72,73,74].
Bioactive peptides isolated from the sea cucumber show antioxidative and neuroprotective properties by targeting various mechanisms such as reduced ROS production, enhanced acetylcholinesterase (AChE) activity in mouse brains, regulating acetylcholine (Ach) and AChE activity to protect the cholinergic system, and choline acetyltransferase (ChAT) upregulation, which is associated with improved memory performance [73,75,76]. Furthermore, neurodegenerative illnesses can be improved by sea cucumber-derived biopeptides or enzymatic hydrolysates in many ways, such as preserving the redox balance, reducing mitophagy, boosting cell survival, encouraging neuron organization and form, and controlling the cholinergic system. In silico bioinformatics analysis revealed that SCPs were found to be ACE inhibitory, antioxidative, and most importantly, demonstrated the structural attributes including hydrophobicity, low molecular size, and amino acid (AA) composition, which addresses their strong correlation with absorption, distribution, metabolism and excretion (ADME) performance, and oral bioavailability, aligning closely with the pharmacokinetic properties of captopril [77]. It has been discovered that short peptides have a strong neuroprotective effect [78]. In a study, the effect of SCP extracted from S. japonicus on memory impairment was evaluated, where over 92% of short peptides enhanced synaptic plasticity and controlled dopamine/serotonin metabolization through the TH/VMAT2 pathway, and the possibility of blood–brain barrier (BBB) crossing was suggested [79].
Again, SCP shows antioxidant properties by compensating for glutathione depletion, lowering mitochondrial superoxide levels, reducing mitophagy, and protecting human neuroblastoma cells against hydrogen peroxide (H2O2) [80]. In H2O2-exposed Vero cells, Lee et al. (2021) discovered that αchymotrypsin-assisted biopeptides from sea cucumber (S. japonicus) significantly reduced intracellular ROS levels and deoxyribonucleic acid (DNA) damage, preserved cell integrity, and boosted cell viability [72]. Moreover, in vivo research showed that the peptide fraction of S. variegatus and C. frondosa effectively increased longevity in both normal and D-galactose-induced aging fruit flies and reduced oxidative damage in mice by upregulating Klotho expression, activating superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), and preventing protein oxidation and lipid peroxidation [81,82]. Apart from this, SCP from A. leucoprocta improved cognitive dysfunction in D-gal-induced aging mice. SCP activated a GABABR/cAMP/PKA/CREB pathway (γ-aminobutyric acid type B receptors (GABABR)/cyclic adenosine monophosphate (cAMP)/cAMP-dependent protein kinase A (PKA)/cAMP response element-binding protein (CREB)) that boosts the release of GABA (gamma-aminobutyric acid), a brain chemical that calms the nervous system [83].
3.3. Polysaccharides
Polysaccharides such as sulfated polysaccharides (fucosylated chondroitin sulfate, or FCS), sulfated fucan or fucoidan, non-sulfated polysaccharides, or neutral glucan are abundant in sea cucumbers, mostly in the body wall [33,84,85,86]. The sulfation pattern of the monosaccharide composition determines the bioactivity of FCS, an exclusive glycosaminoglycan [87]. Despite interspecific variations, all FCSs share a core backbone of {3)-D-GalNAc-(β1,4)-D-GlcA-(β1,}, with the branching regions differing in the sulfation pattern and glycosylation of fucosyl groups [33]. As opposed to branching FCSs, sulfated fucans from sea cucumbers are frequently linear polymers made up of repeating structural components [88,89,90]. A previous study found that compared to native FCS polysaccharides (~9%), sea cucumber-derived FCS oligomers demonstrated a greater absorption rate (~32%), leading to improved bioavailability [91].
In addition to their well-documented bioactivities, including anticoagulant, anti-cancer, antithrombotic, and antibacterial effects, sea cucumber-derived polysaccharides also exert neuroprotective properties, though the neuroprotective effects are influenced by the species-specific structural variations within the polysaccharide molecules. According to Li et al. (2020), S. chloronotus fucoidan mostly consists of L-fucose and sulfate esters, exhibiting immunoregulatory and lipid peroxidation inhibition [92]. Furthermore, the gonadal polysaccharide of sea cucumbers (A. japonicus) demonstrated reducing power, DPPH, and hydroxyl radical-scavenging properties, maintaining a lower molecular weight while having a larger sulfate group concentration increased the activity [93]. Li et al. (2021) reported that polysaccharides derived from the sea cucumber C. frondosa lessen the cytotoxicity and aggregation of Aβ40, one of the factors that causes AD [94]. Neural stem cell (NSC) is a strong contender for cell replacement treatment [95,96] for several untreatable CNS conditions. In a study, Cui et al. (2016) found that a polysaccharide from S. japonicus helped NSCs move to damaged areas, turning into nerve and support cells and helping to repair long-term nerve injuries [97]. FCS from various sea cucumbers also helps to reduce inflammation and tissue damage by modifying the expression of important genes, including NF-ĸb (nuclear factor kappa-light-chain-enhancer of activated B cells), TNFα (tumor necrosis factor alpha), iNOS (inducible nitric oxide synthase), and COX-2 (cyclooxygenase-2) [32].
3.4. Phenolic Compound
The body parts, including the body wall, tentacles, and viscera, of sea cucumbers all contain substantial amounts of phenolics with potent antioxidant properties [85]. The amount of phenolic compounds and their antioxidant properties vary depending on factors such as species, habitat, food habits, and harvesting period [98]. Several classes of phenolics may be distinguished from sea cucumbers, including phenolic acids, flavonoids, tannins, stilbenes, lignans, and coumarins [99]. The most common phenolic compounds found in sea cucumbers are chlorogenic acid (up to 93% by weight), gallic acid, p-coumaric acid, protocatechuic acid, ferulic acid, ellagic acid, cinnamic acid, catechin, rutin, quercetin, and pyrogallol [85], while the ascorbic acid content is minimal [100,101,102]. The antioxidant properties of sea cucumber phenolics have been studied by multiple researchers, which may have greater potential in neuroprotection by reducing ROS. Hossain et al. (2022) reported that around 23 phenolic compounds were isolated from the C. frondosa, containing mostly phenolic acids and flavonoids. These compounds showed antioxidant properties along with anti-tyrosinase and antiglycation properties, and inhibitory activities against low-density lipoprotein (LDL) cholesterol oxidation and DNA damage [37]. Again, 12 phenolic compounds were extracted from the aqueous extract of H. tubulosa, comprising mainly flavonoids and phenolic acids, showing high antioxidant activities. The total phenolic and flavonoid contents of AEs were reported to correlate with their antioxidant activity values [103].
The synergistic effect of these phenolic compounds with other bioactives in the in vivo C. elegans model, to treat diseases like AD and PD, suggested potential for natural preventive and therapeutic agents for neurorestoration [44,104,105]. The high radical scavenging capability (13.14 ± 2.17%) of the ethanolic extracts and fractions of H. atra demonstrated strong primary antioxidants, making them suitable for usage in the food and pharmaceutical sectors, as well as being a natural antioxidant that can be refined [106].
3.5. Fatty Acid and Phospholipid
Fatty acids in sea cucumbers can effectively improve impaired learning and memory functions related to aging and NDs [107,108,109]. Wang et al. (2020) found different phospholipid (PL) classes, along with an ether-PL sub-class from six different sea cucumbers by using a Normal Phase Liquid Chromatography–Triple-Quadrupole-Time-of-Flight Mass Spectrometry/Mass Spectrometry (NPLC-Triple-TOF-MS/MS) method [110]. The highest PL levels were rich in ether-phospholipids, which were obtained from the species C. frondosa (8.05 μmol/g) and rare phosphonoethanolamines were found for the first time in sea cucumbers [110]. Ermolenko et al. (2022) revealed the phospholipid profile of A. japonicus, analyzing the major structural PL glycerophosphoethanolamines (PEs), glycerophosphocholines (PCs), glycerophosphoserines (PSs), and glycerophosphoinositols (PIs) in tissues of wild and cultured sea cucumbers, mentioning that a diet with ω-3 polyunsaturated fatty acids (PUFAs) influences the PL profile, enhancing nutritional properties [111]. For example, eicosapentaenoic acid-enriched phospholipids (EPA-PLs) from sea cucumber, C. frondosa, help to improve Aβ-induced cognitive deficiency in a similar mechanism to docosahexaenoic acid phospholipids (DHA-PLs) in rats [107]. Moreover, in the PD mice model, EPA-PL improved behavioral deficiency by suppressing oxidative stress and apoptosis, thereby alleviating the loss of DA neurons via the mitochondria-mediated pathway and mitogen-activated protein kinase pathway [36]. Che et al. (2018) found that the neuroprotective effects of DHA/EPA-PLs depend on the molecular form, as eicosapentaenoic acid phosphatidylserine (EPA-PS) and docosahexaenoic acid phosphatidylserine (DHA-PS) could effectively protect PC12 from apoptosis [109]. Another study reported by Zhou et al. (2016) stated that phosphatidylcholine (PC) from sea cucumber A. molpadioides showed positive results in treating scopolamine-induced hippocampus impairment [108]. Due to higher level of EPA and DHA in PC, it showed better improvement, suppressing the malondialdehyde (MDA) level (28.80%) and monoamine oxidase (MAO) (33.64%) activity, and simultaneously increasing SOD (95.53 U/mg·prot.) activity [108]. Another saturated medium-chain fatty acid, decanoic acid, was isolated from H. leucospilota by Sanguanphun et al. (2022), which exhibited effectiveness against C. elegans PD models by inhibiting neurodegeneration [112].
3.6. Cerebrosides
Cerebrosides (Cers) are neutral substances that are vital to brain function. They are made up of ceramide (sphingosine and FA) and a monosaccharide that is connected to the C1 of esfingol by a β-glycosidic link. The white matter of the brain and the myelin sheaths surrounding nerves are rich in cerebrosides, while the cell membranes of other tissues contain trace amounts of these substances [113]. The level of sphingolipid (ceramides, cerebrosides, and gangliosides) in the brain rises with increased dietary intake of these compounds [114,115]. After consumption, it gets partially metabolized to glucosylated glucosylceramide (GlcCer) and sphingomyelin (SM), making it suitable to cross the blood–brain barrier (BBB) [116]. Several studies have been conducted on the protective role of cerebrosides in regulating brain functions. According to Li et al. (2019), Cers could significantly ameliorate Aβ1-42-induced cognitive deficiency from neuronal damage, suppressing the induced apoptosis [35]. Wu et al. (2013) showed that Cer from A. molpadioides protected brain cells from H2O2 and t-BHP-induced damage and increased the activity of a protective enzyme called SOD [117].
3.7. Other
Aside from the above groups of bioactives, other active compounds could be found in sea cucumber, which may exert a neuroprotective effect in pre-clinical and clinical models. For example, ethanol (ET), ethyl acetate (EA), butanol (BU), and aqueous (AQ) extract of H. leucospilota prevented the degeneration of DA neurons in the PD model, where terpenoids, steroids, saponins, and glycosides were identified from the EA extract [65]. Together with other phenolic compounds, the terpene friedelin, which was isolated from H. scabra, demonstrated possible antioxidant qualities [118].
A small cyclic ether, 2-butoxytetrahydrofuran (2-BTHF), was extracted from H. scabra and demonstrated its therapeutic potential against AD through the attenuation of Aβ aggregation in a transgenic C. elegans model [119]. Again, Jattujan et al. (2022) isolated five compounds: diterpene glycosides (holothuria A and B), palmitic acid, bis (2-ethylhexyl) phthalate (DEHP), and 2-butoxytetrahydrofuran (2-BTHF) from H. scabra. Among these five compounds, two of them, 2-BTHF and palmitic acid, demonstrated anti-aging activities [120]. Again, 14 carotenoids were identified from C. frondosa japonica using supercritical CO2 extraction, where cucumariaxanthin and canthaxanthin were abundant [121]. In addition, the fatty acid composition and carotenoids of 12 sea cucumbers revealed the cytotoxic activity of the carotenoids, and two particular fatty acid compounds (DHA and EPA) [122]. Previous studies demonstrated various biological activities and functions in different model systems, presented in Table 2.

Table 2.
Overall biological activities and functions of bioactive compounds derived from different sea cucumber species.
4. Potential Therapeutic Applications
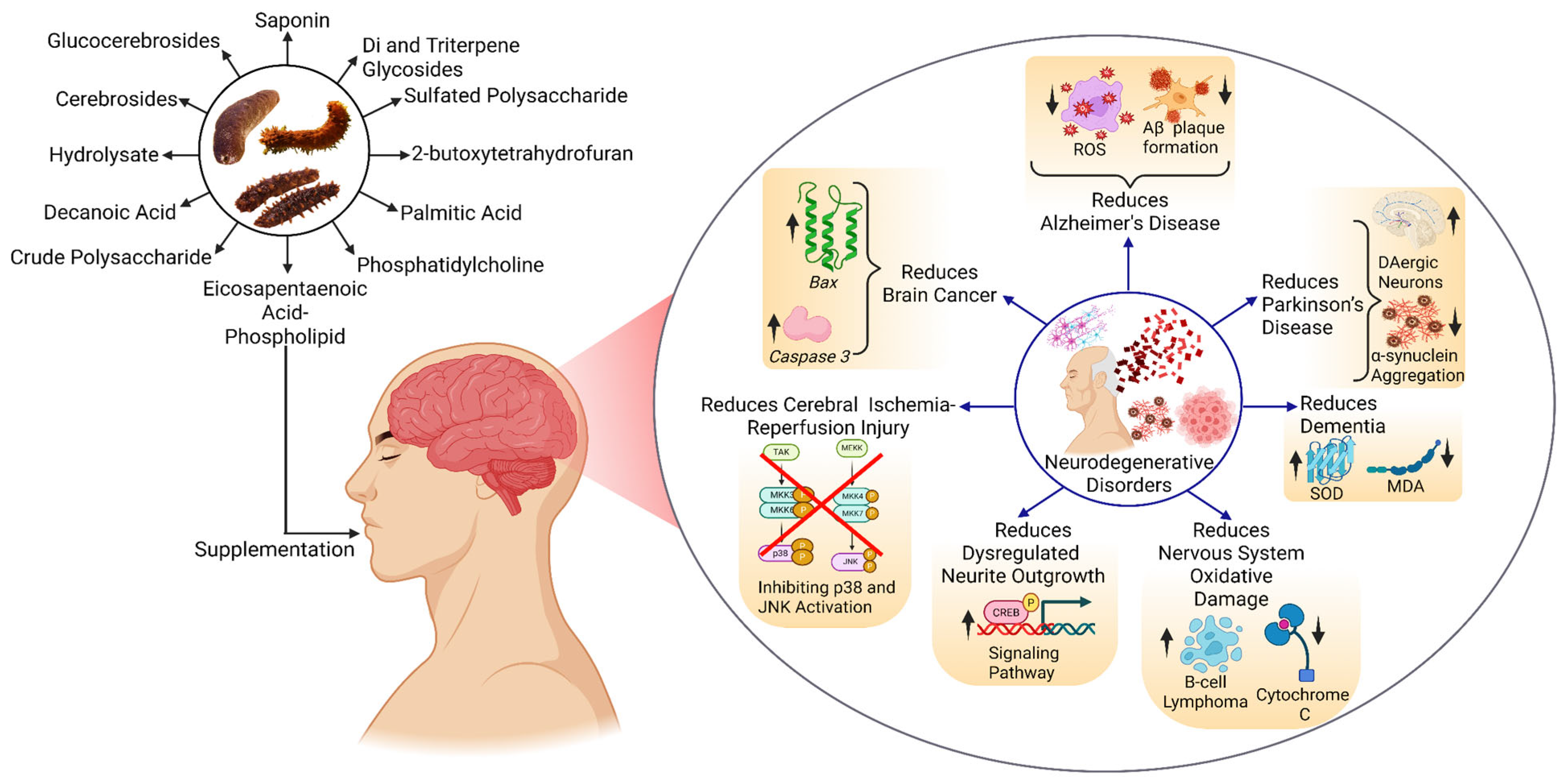
The bioactive compounds from different sea cucumbers have shown positive results in combating NDs such as AD, PD, brain cancer, brain tumors, and other disorders. In treating AD, these compounds reduce ROS, which causes inflammation in the brain, and inhibit the formation of Aβ plaque, which is the main reason behind AD. These compounds also reduce the aggregation of alpha-synuclein (α-Syn), resulting in the improvement in the PD condition. Apart from these two important disorders, these compounds also play a role in treating brain cancer, reducing cerebral ischemia–reperfusion injury, dysregulating neurite outgrowth, and oxidative damage by modulating different signaling pathways or gene expression (Figure 4).
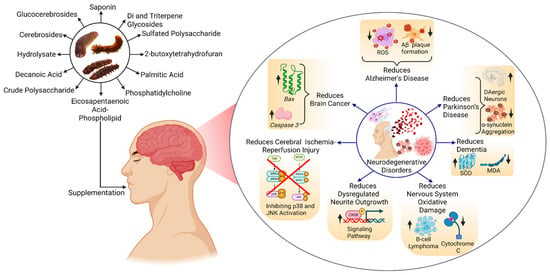
Figure 4.
Effect of bioactive compounds on NDs. Abbreviations: CREB—cAMP Response Element-Binding Protein, MDA—Malondialdehyde, JNK—c-Jun N-terminal Kinase, SOD—Superoxide Dismutase, ROS—Reactive Oxygen Species. The figure was generated from the concept of Bonetto et al. (2025) [171] using Biorender.com (Agreement number: ZR28J5NO9Q).
Despite having high potential for treating disease disorders, the neuroprotective effect is still in the preclinical research stage, with significant findings in AD and PD. Human clinical studies are very limited, and none of these focus on ND, limiting the implications or benefits of SCBs on human health [60,172]. Even though encouraging outcomes in preclinical studies using animal models and cell cultures are present, no bioactives as neurodrugs have yet advanced to the stage of clinical approval. In vivo research of SCBs is mostly focused on anti-tumor/anti-cancer activities, followed by lipid metabolism modulation and glucose metabolism management [173]. As a result, little is being explored regarding the mechanisms of action of these compounds.
4.1. Alzheimer’s Disease
Alzheimer’s disease, a progressive and incurable neurological condition, is diagnosed by a gradual breakdown of mental functions, leading to impaired decision making, communication, motor planning, and visual comprehension, which ultimately advances into dementia [174]. Numerous mechanisms, including mitochondrial breakdown, oxidative cellular stress, harmful accumulation of amyloid/tau (τ) proteins, and cholinergic deficits, are attributed to the etiology of AD [175]. A range of proposed theories have been suggested to explain the pathophysiology of AD, including the cholinergic hypothesis, Aβ deposition hypothesis, tau protein hypothesis, oxidative stress hypothesis, metal ion hypothesis, and neuroinflammation hypothesis [176], but the two primary ones are the hyperphosphorylation of the tau protein and the amyloid-β (Aβ) cascade. As AD progresses into advanced phases, it is linked to extensive Aβ plaques and tau aggregates as neurofibrillary tangles (NFTs), characterized by dementia [177,178].
Aβ is often a soluble short peptide that is created through a proteolytic process of α-secretase, β-secretase, and γ-secretase, cleaving the transmembrane protein, amyloid precursor protein (APP) [179]. However, the tau protein is mostly found in axons and is a member of the microtubule-associated protein family [180]. Along with the abnormal accumulation of Aβ, the hyperphosphorylation of the tau protein promotes the degeneration of neuronal stability. Depending on how much oligomerisation occurs, the imbalance between the synthesis and clearance of Aβ results in several kinds of harmful oligomers, including protofibrils, fibrils, and plaques [181]. Simultaneously, hyperphosphorylation of the tau protein creates an imbalance in the functions and stability of microtubules, resulting in neuronal death through the formation of an impaired double-helix fiber [182,183]. Research has revealed the functional interplay between Aβ and tau, linking them to the progressive deterioration of neuronal circuits and the impairment of cognitive functions observed in AD [184,185].
Other processes have been demonstrated to occur before the development of senile plaques and the deposition of NFTs, including oxidative stress, which is elevated in the aging brain [186,187,188]. The neuronal structural molecules are composed of a high proportion of PUFAs, which are highly susceptible to ROS and eventually lead to lipid peroxidation and subsequent apoptosis at the molecular level [189,190]. ROS generation in AD is both a cause and an effect of nuclear factor erythroid 2-related factor 2 (Nrf2) activation through the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/glycogen synthase kinase 3 beta (GSK3β), p62, p38, Mitogen-Activated Protein Kinase (MAPK)/Nuclear Factor kappa-light-chain-enhancer of activated B cell (NF-Κb) pathways, which are deeply associated with AD pathogenesis [191]. Additionally, the imbalance in metal ions (Fe, Cu, Zn, and Ca) triggers oxidative stress, evident by the elevated ROS and decreased levels of GSH, SOD, and antioxidant protein (ATOX). In addition to promoting Aβ overproduction by activating β- and γ-secretases and/or inhibiting α-secretase, oxidative stress can cause tau hyperphosphorylation by activating protein kinases (e.g., GSK-3β, cyclin-dependent kinase 5 (CDK5), MAPK, etc.) and/or inhibiting Protein Phosphatase 2A (PP2A) [192], suggesting one of the root causes of AD, supporting the metal ions hypothesis.
Given the importance of fundamental forebrain cholinergic neurones (BFCNs) in memory, learning, and cognitive function, the cholinergic hypothesis of AD states that acetylcholine (ACh) is necessary for cholinergic signal transduction associated with memory and learning [193]. It serves as a potent regulator and prerequisite for the complete expression of sensation-induced neurovascular coupling response [194]. Ach is synthesized by the enzyme choline acetyltransferase, whose catalytic activity depends on substrates like choline, acetyl-CoA, and ATP [176]. Along with the gradual and substantial decline in cognitive and behavioral abilities, Ach deficiency in AD patients is linked to abnormal cholinergic system activity that controls and encourages alterations in tau phosphorylation and APP metabolism, which results in neurotoxicity, neuroinflammation, and neuronal death [195,196].
Furthermore, elevated levels of inflammatory cytokines and related genes have also been linked to the onset of AD [197]. Under the neuroinflammatory theory, neuroinflammation, an innate host mechanism that helps shield and restore the brain’s normal structure and function from infections and injuries, is what drives the start of neurodegeneration [198]. It is distinguished by the activation of innate immune cells, permeable endothelium cells, microglia and astrocytes, and invading blood cells that result from mechanical or pharmacological damage to the BBB or brain structures [199,200]. Consequently, neuroinflammation induces the various immune system cells to produce and release inflammatory mediators, such as cytokines (IL-1β, IL-6, IL-18), chemokines (CCL1, CCL5, CXCL1), small-molecule messengers (prostaglandins and nitric oxide), and reactive oxygen [199]. These mediators can aggravate Aβ and τ pathologies [201].
Moreover, there are several components of the AlzPathway, which include the Aβ cleavage and degradation, apolipoprotein E (ApoE)-cholesterol pathway and the NFT accumulation, acetylcholine production, Wnt signaling pathway, the ubiquitin-mediated proteolysis, apoptosis, and calcium signaling pathway, the notch signaling pathway, the MAPK signaling pathway, the abnormal ceramide accumulation, reactive oxidation process, neurotrophin signaling pathway, the cell cycle, mammalian/mechanistic target of rapamycin (mTOR) signaling pathway, the lipid pathway, the insulin pathway, and the inflammation pathway [202]. Multiple comprehensive reviews reported that a clearly defined underlying cause of AD has not been identified yet [203,204], and no definitive treatment exists to date [205,206]. Nevertheless, recent studies are shifting towards the exploration of natural compounds with potential therapeutic efficacy [207,208].
4.2. Parkinson’s Disease
As an incurable neurodegenerative disease, PD comes after AD, with the second-highest incidence rate [209]. It is characterized by symptomatology involving both motor and non-motor aspects. The clinical manifestations of motor abnormalities are bradykinesia, hypokinesia, akinesia, hypomimia, hypophonia, drooling, swallowing issues, micrographia, stiffness (stiffness of limbs), unstable posture, and resting tremors [210]. However, nonmotor symptoms might express sooner and exert a substantial impact on quality of life. These symptoms include sadness, constipation, sleep difficulties, odd sensations, weariness, and dementia [211]. Multiple pathophysiological cascades underlie the progression of PD, which may include oxidative stress [212], mitochondrial damage [213], toxic exposure [214], aberrant protein folding and aggregation [215], dopamine neuron disruptions [216,217], the impairment of protein clearance pathways [218], autonomous cellular dysfunction [219], and the intracellular transmission of the prion-like protein [220]. Notably, one of the most prominent theories for PD is the buildup of misfolded proteins in intracellular regions [220,221].
The hallmark of PD is the loss of DA neurones in the midbrain’s substantia nigra pars compacta (SNpc), which is linked to Lewy bodies (LBs), a cytoplasmic inclusions that include insoluble misfolded alpha-synuclein (α-Syn) aggregates [222]. But PD also affects non-DA neurones and is typified by a more extensive pathology in other parts of the brain [223]. Based on the most pronounced mechanism of PD, LBs are spherical, eosinophilic, intraneuronal inclusions with a hyaline center and a pale peripheral halo made up of over 90 proteins [224]. The chronological formation of LBs and the subsequent deposition of α-Syn begins from the anterior olfactory nucleus and the dorsal motor nucleus of the glossopharyngeal and vagal nerves, and progresses to the brain stem, mesocortex, allocortex, and neocortex in later stages [225]. Based on the comprehensive review of the existing literature, Srinivasan et al. (2021) reported that the hereditary predisposition to early-onset PD is brought on by mutations in the genes for alpha-synuclein (SNCA), ATPase cation transporting 13A2 (ATP13A2), glucocerebrosidase (GBA), F-box protein 7 (FBX07), vacuolar protein sorting-associated protein 35 (VPS35), phospholipase A2, group VI (PLA2G6), DnaJ (Hsp40) homolog, subfamily C, member 6 (DNAJC6), Synaptojanin 1 (SYNJ1), Ubiquitin C-terminal hydrolase L1 (UCHL1), parkin (PRKN), leucine-rich repeat kinase 2 (LRRK2), PTEN-induced kinase 1 (PINK1), and DJ-1 which results in the abnormal protein conformations and interfere with the inherent cellular mechanistic ability to remove the misfolded proteins [226].
Despite the significant role of degenerative protein formation, dopamine persists as one of the crucial factors in the multifactorial etiology of PD progression. The functional role of dopamine in PD remains obscure due to its complex mechanism of action. The depletion of the dopamine level in the substantia nigra results in the disrupted coordination between the direct and indirect pathways, which is considered the leading cause of PD and reduced thalamocortical input [217,227]. Till now, clinically used anti-PD medications include monoamine oxidase B (MAO-B) inhibitors (selegiline, rasagiline, and safinamide), catechol-O-methyl transferase (COMT) inhibitors, dopamine precursors (levodopa and carbidopa), and dopamine agonists (pramipexole, ropinirole, rotigotine, and apomorphine) [228]. Due to the restricted penetration of exogenous DA and other catecholamines through the BBB, DA replacement therapy provides the foundation for the pharmacologic treatment of PD, which is mostly symptomatic [229].
4.3. Stroke and Ischemic Injuries
A stroke is a clinically observed condition presenting with acute and localized neurological impairment attributed to central nervous system vascular injury (infarction, hemorrhage) [230]. After a stroke, neurological functional abnormalities include hemiplegia, the loss of sensory and vibratory feeling, balance issues, verbal issues, ataxia, impaired reflexes, ptosis (of the eyelid), field of vision deficits, aphasia, apraxia, facial numbness or paraesthesia, which exert a negative influence on quality of life [231]. The underlying pathology of the stroke determines whether it is ischaemic or haemorrhagic [232]. Approximately 87% of strokes are attributed to the predominant subtype, ischemic stroke, with intracerebral haemorrhagic (ICH) stroke contributing 10%, while only 3% are subarachnoid hemorrhages (SAHs) [233].
Cerebral ischemia is brought about by cerebral artery blockage, which prevents blood flow to a particular region of the brain. This deprives neurons of oxygen and energy, which negatively impacts energy-dependent functions in neuronal cells [234,235]. Consequently, after ischemia–reperfusion, survivors are still at a high risk for neurological impairments, disability, and other repercussions [236]. Restoring blood flow to the ischaemic penumbra as soon as possible is a key component of the clinical care of ischaemic stroke in order to save damaged neurons [237]. A number of factors can contribute to ischaemic stroke, such as large-artery atherosclerosis (which accounts for 45% of all events), cardioembolism (15–30%), systemic hypoperfusion, penetrating artery disease, carotid dissection, hypercoagulability of genetic syndromes, or other unpredictable causes [238]. In terms of the source, time interval, location, and intensity of ischemia, as well as age and comorbidities, the neurological impairment and clinical presentation following an ischaemic stroke show significant variety [239].
Bioactive properties of sea cucumber, such as anti-atherosclerosis, anti-coagulation, and anti-inflammation, could be a major source of post-stroke management [32,123,153,170]. These active biomolecules may be used as a dietary supplement to delay the onset of illness.
4.4. Brain Cancer and Brain Tumors
Brain cancer represents a severe malignancy to the CNS, comprising primary tumors that originate within the neural tissue and metastatic secondary tumors that spread from extracranial cancers [240]. Over decades of scientific research, brain tumors have remained the most fatal form of cancer [241]. Additionally, the prevalence of brain tumors is rising in some populations, maybe as a result of improvements in systemic cancer therapy and survival or in the detection of primary brain tumors [242,243].
Tumors are not just collections of cancer cells; they are also a diverse collection of an extracellular matrix, secreted proteins, and resident and invading host cells [244]. A brain tumor in the patient may cause specific neurological symptoms such as headaches, seizures, aphasia, weakness, sensory loss, visual problems, and ataxia, or no symptoms if it happens accidentally [245].
Mounting evidence suggests that the microenvironment of the tumor accelerates the progression of cancer as a potent facilitator [246]. The three most common types of brain tumors are brain metastases, meningiomas, and gliomas or glioblastomas (GBMs) [245]. As outlined in the world health organization (WHO) classification system, brain tumors could be classified as either grade I or grade IV on a morphological, immunological, molecular, and genetic profiling basis [247], where GBMs, the most common and malignant tumor of the central nervous system, affect both children and adults with a slight predominance in males. Various malignant gliomas, which make up approximately 50.1% of all malignant brain tumors [248], are classified as a grade IV tumor based on histopathological features [249].
When a brain tumor (BT) develops in the cranium, the micro-vessels in the peritumoral areas are compressed, which reduces the cerebral blood flow (CBF) locally [250,251,252]. The term “blood–tumor barrier” (BTB) refers to another malfunction of the BBB that occurs during tumor growth [253] and is distinguished by the loss of astrocytic endfeet and neural connections, as well as an abnormal pericyte distribution [254]. Glioma cells that invade the body can physically push out astrocytic endfeet and damage the integrity of the BBB [255]. Although certain large and small molecules can pass through the BTB, it is not sufficiently permeable to permit the accumulation of substantial drug concentrations from the periphery inside the BT [256].
As an effective treatment approach, efforts have been concentrated on developing the next-generation targeted therapies or medications that could have superior BBB penetration capabilities. The several methods for enhancing medication transport across the BBB/BTB can be divided into two categories: those that are minimally invasive and those that are non-invasive. Despite not having reached their full potential, intrusive methods, which include direct access to the site of affliction, are presently undergoing significant optimization and improvement, with encouraging preclinical results [256]. These bioactive compounds have shown significant potential to fight against brain-oriented diseases with varying efficacy depending on the type of model and administered dosage (Table 3).

Table 3.
List of bioactive compounds in sea cucumbers in the prevention of NDs using different models and their dose-responsive effects.
5. Neuroprotective Mechanism Involved
5.1. Alzheimer’s Disease
5.1.1. In Lowering Oxidative Stress
Many studies have been performed to evaluate Aβ-induced cognitive dysfunction by observing learning and memory ability through different behavioral experiments [267,268,269]. The bioactive compounds from sea cucumber improve this cytotoxic condition by regulating the mitochondria-dependent apoptotic pathway [35,60,270]. The generation of Aβ in the brain is affected by oxidative stress, which leads to lipid peroxidation, protein oxidation, and DNA oxidation in the nervous system [271]. The mitochondrial respiratory chain is the main source of ROS-generated oxidative stress [272], and according to Che et al. (2017), cerebrosides from sea cucumber inhibited Aβ-induced cell apoptosis by the mitochondria-dependent apoptotic pathway. These compounds downregulated Caspase-9, cleaved Caspase-3, total Caspase-3, and Bax, and upregulated Bcl-2 protein in the target cell, leading to the breakdown of the membrane integrity of mitochondria, thus resulting in Aβ-induced cell apoptosis [270]. Caspase-9 initiates apoptosis in the cell, while Caspase-3 degrades neuronal components that are important for the brain, and Bax promotes mitochondrial membrane permeabilisation [273,274,275]. However, Bcl-2 is an anti-apoptotic protein that plays a role in stabilizing mitochondrial integrity and inhibiting Bax activity, thereby preventing cell death [276,277]. In the case of the progression of AD, higher levels of Caspase-9, cleaved Caspase-3, and Bax, and lower levels of Bcl-2 cause neurodegenerative problems. In a study on evaluating the impact of cerebroside on mice, the level of Caspase-9 and Caspase-3 was found to be decreasing from approximately 280% to 100% of Normal and approximately 240% to 120% of Normal, respectively, indicating the inhibition of Aβ-induced cell apoptosis [270].
Moreover, oxidative stress indicators like SOD, nitric oxide (NO), nitric oxide synthase (NOS), MDA, 8-hydroxy-2′-deoxyguanosine (8-OHdG), and 8-oxo-guanine (8-oxo-G) play vital roles in assessing the success of preventing Aβ-induced oxidative stress [270]. According to Che et al. (2017), cerebrosides were found to lower the level of SOD activity (from 40 U/mgprot to around 35 U/mgprot), MDA (from approximately 1.3 nmol/mgprot to around 1 nmol/mgprot), and the amount of NO (from 20 μmol/gprot to around 8 μmol/gprot), and elevated the content of NOS (from 10 μmol/gprot to around 12 μmol/gprot), 8-OhdG (from approximately 8 ng/mgprot to around 9 ng/mgprot), and 8-oxo-G (from approximately 43 pg/mgprot to around 52 pg/mgprot) in brain tissue, suggesting that these compounds can reverse cognitive impairment as well as reduce or stop the production of the Aβ protein in the brain [270].
5.1.2. In Improving Synaptic Plasticity and Ameliorating Nerve Fiber Tangles
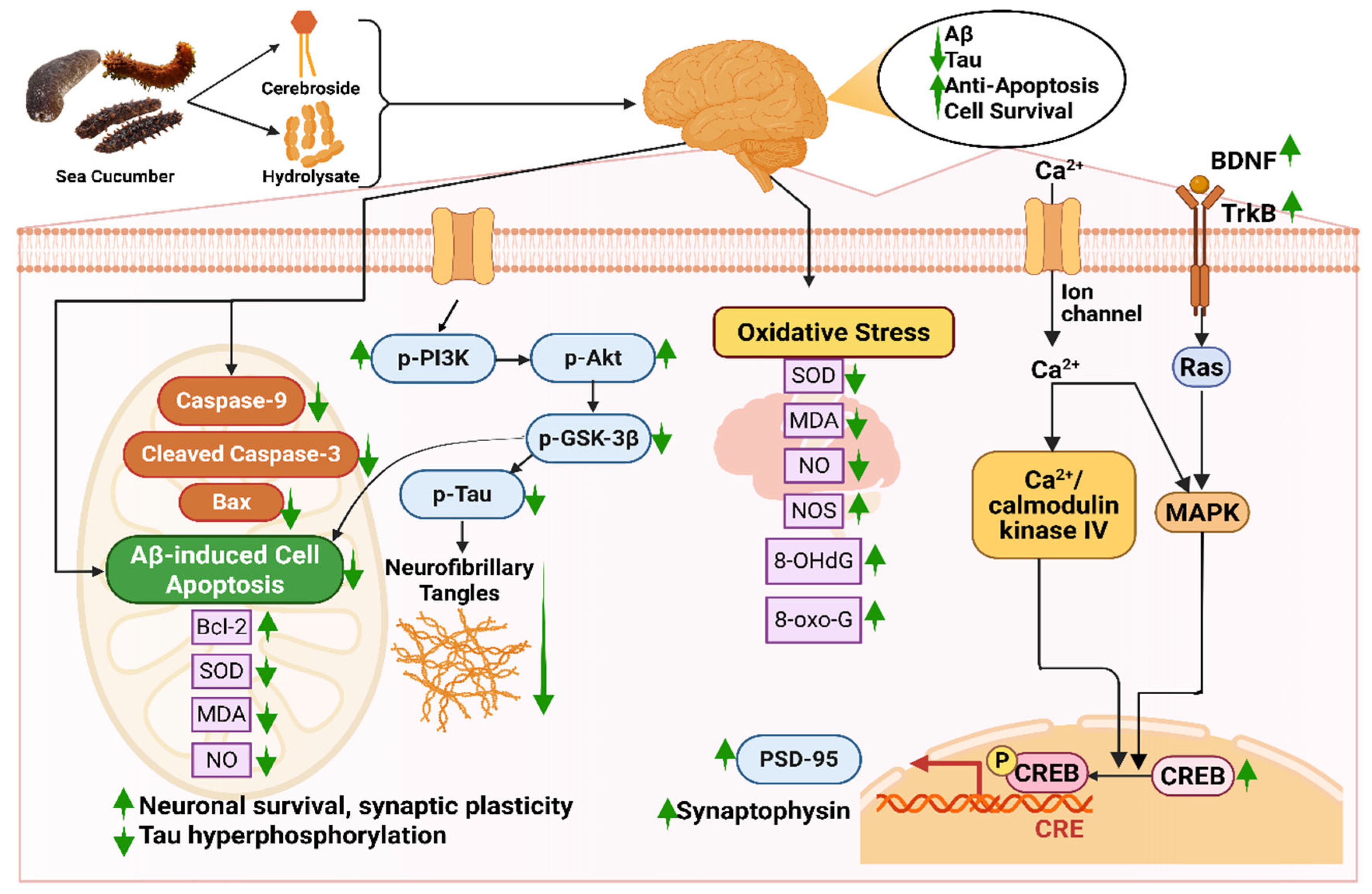
Bioactive compounds like hydrolysate, cerebrosides, or glucocerebrosides from sea cucumber reduce Aβ-induced cognitive deficiency by triggering the BDNF/TrkB/CREB pathway and escalating the expression of PSD-95 and synaptophysin [35]. Firstly, these compounds increase the amount of brain-derived neurotrophic factor (BDNF) protein in bioactive compound-injected mice [35,278]. BDNF plays a role in neuronal survival and growth, which later binds with specific tropomyosin kinase B (TrkB) receptors and elevates the TrkB level [279]. The binding of these two proteins elevates the level of cAMP response element-binding protein (CREB), which is essential for maintaining the activity of synapses [280]. A higher level of CREB improves the level of Postsynaptic density protein-95 (PSD-95) and synaptophysin, indicating improved synaptic plasticity [281]. Moreover, the bioactive compounds ameliorate nerve fiber tangles via the PI3K/Akt/GSK-3β pathway, in which levels of p-P13K and p-Akt are increased; on the contrary, p-Tau and p-GSK3β are decreased [35]. The protein kinases, phosphatidylinositol 3-kinase (PI3K) and phosphorylated Akt (p-Akt), help in cell survival and protection [282]. Interaction between these two kinases is the main reason behind neuronal survival, synaptic plasticity, and resistance to oxidative stress. Any form of dysregulation or breakdown in the PI3K/AKT signaling pathway results in Aβ accumulation, tau hyperphosphorylation, and mitochondrial dysfunction [282]. Again, phosphorylated tau protein (p-Tau) is a key biomarker in diagnosing AD that is responsible for the degeneration of neurons and synaptic impairment [283], whereas due to glycogen synthase kinase-3β (GSK-3β), hyperphosphorylation of the tau protein occurs, resulting in the formation of abnormal clumps of tau protein called neurofibrillary tangles (NFTs) (Figure 5) [284].
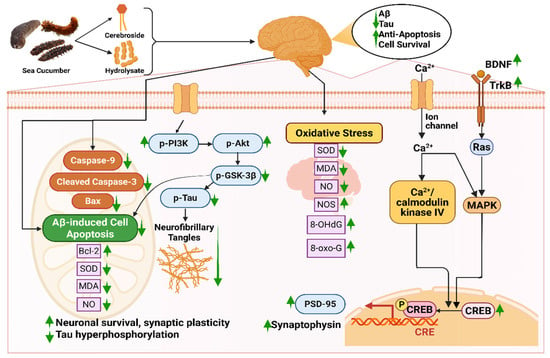
Figure 5.
Neuroprotective mechanisms of sea cucumber-derived bioactive compounds in Alzheimer’s disease models. Abbreviations: 8-OHdG, 8-Hydroxy-2′-deoxyguanosine; 8-oxo-G, 8-oxo-guanine; Aβ, amyloid-beta; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma 2; BDNF, brain-derived neurotrophic factor; CRE, cAMP response element; CREB, cAMP response element-binding protein; MDA, malondialdehyde; MAPK, mitogen-activated protein kinase; NO, nitric oxide; NOS, nitric oxide synthase; p-Akt, phosphorylated protein kinase; p-PI3K, phosphorylated phosphoinositide 3-kinase; p-Tau, Phosphorylated tau protein; PSD-95, postsynaptic density protein 95; Ras, renin-angiotensin system; SOD, superoxide dismutase; TrkB, tropomyosin receptor kinase B. The figure was generated from the concept of Li et al. (2019) [35] using Biorender.com (Agreement number: ZG28FPN3IU).
In a study by Li et al. (2019), the positive neuroprotective effect of sea cucumber-derived cerebrosides was studied in which levels of BDNF, p-CERB, and PSD95 were increased from approximately 1 to 1.2 folds, 1 to a 1.5 folds, and 1 to 1.1 folds, respectively, indicating enhanced neuronal survival, transcriptional activity, and synaptic integrity [35]. In the same study, the content of p-PI3K and p-Akt was found to upregulate from 1 to 1.1 folds to 1 to 1.25 folds, respectively. It was suggested that cerebrosides suppressed the phosphorylation of the tau protein by the PI3K/Akt/GSK3β pathway.
In another study, Gong et al. (2025) provided insights on how the gut–brain axis affects the gut microbiota in aging rats with cognitive impairment through the oral administration of sea cucumber hydrolysate (SCH). SCH restored gut microbiota homeostasis, balancing the Bacillota/Bacteroidota ratio and increasing beneficial taxa such as Lachnospiraceae and Verrucomicrobiota, which was also accompanied by enhancing cholinergic function, activating BDNF/TrkB signaling, and attenuating neuroinflammation mediated via inhibition of NF-κB and microglial activation. The study potentially addresses the neuroprotective effect of low-molecular-weight peptides containing key amino acids (Gly, Glu, Pro, Arg) to support neurotransmission, immune modulation, and BBB integrity [285].
5.2. Neuroprotective Mechanism Involved in Parkinson’s Disease
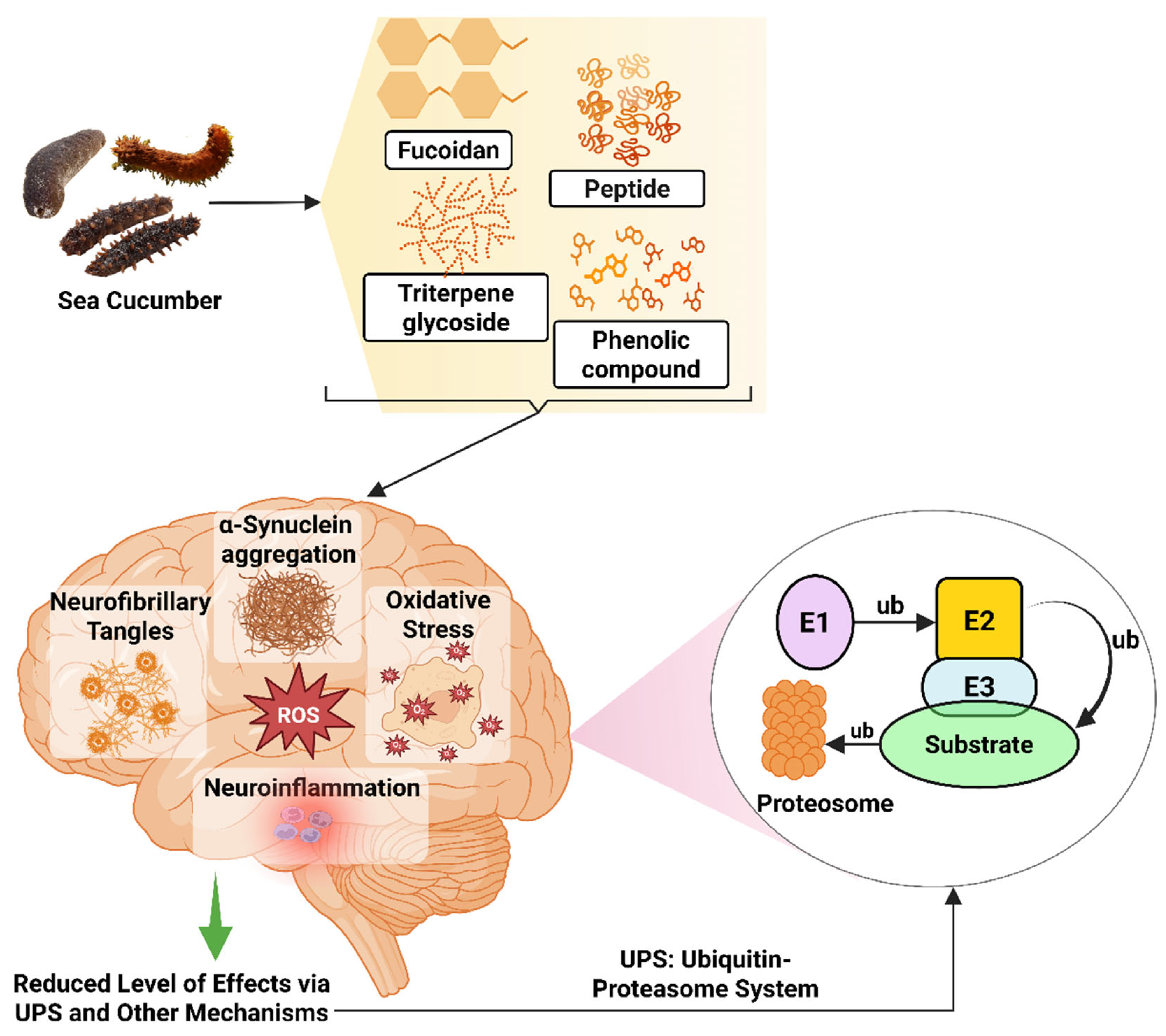
Different bioactive compounds from sea cucumber, such as extracts of H. leucospilota, H. scabra, C. elegans model, and S. japonicus, are being studied for treating PD [65,262,286]; however, the neuroprotective mechanism is yet to be discovered. According to Malaiwong et al. (2019), activation of the ubiquitin-proteasome system (UPS) is one of the mechanisms behind the success of H. leucospilota extract in having anti-Parkinson effects [65]. Ubiquitin in the UPS is a 76-amino-acid conserved protein that plays a role in controlling and assisting the protein denaturation process [287]. The ubiquitin binds with the targeted protein and, via catalytic reactions, breaks down the protein. For this reaction, three types of enzymes, such as E1 (ubiquitin-activating enzyme), E2 (ubiquitin-conjugating enzyme), and E3 (ubiquitin–protein ligase), are used [288,289]. Toxic intracellular proteins can accumulate due to abnormal protein stability, resulting in hampering neural homeostasis. Accumulation of the aggregated and misfolded brain protein, α-synuclein, is one of the main reasons behind PD-linked mutations of the α-synuclein gene, which can interfere with different cellular and molecular functions, thus inducing neurotoxicity [290].
Moreover, the generation of ROS inflicts damage on the substantia nigra of the brain via the peroxidative degradation of lipid, protein, and DNA oxidation [291]. Monoamine oxidase (MAO) activation, mitochondrial malfunction, alterations in the brain’s iron level, or even modifications to the antioxidant defense system appear to be the primary causes of this occurrence [292,293,294,295]. It has also been noted that NFTs with hyperphosphorylated tau protein are prevalent in PD brains [296,297]. Additionally, inflammatory cytokines, chemokines, GFAP, and nNOS are reported to be abundant in PD brains [298,299]. The corresponding evidence of the elevated expression of α-synuclein in enteric neurites parallels the intensity of intestinal wall inflammation, besides neuroinflammation [300,301].
Studies have been carried out to understand how specific compounds from sea cucumbers provide neuroprotective effects in PD models. Diterpene glycosides extracted from H. scabra decrease α-synuclein accumulation and protect α-synuclein-mediated DA neuronal loss and its toxicities via lgg-1 and atg-7 in C. elegans PD model [261]. The ethanolic extract showed neurorestoration effects on maintaining the numbers of DA neurons and fibers in both substantia nigra pars compacta (SNpc) and striatum in both mice and cellular models induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), as determined by a grid walk test, in a cellular model of PD [104]. Another study reported by Sanguanphun et al. (2022) revealed that decanoic acid isolated from H. leucospilota exerts an anti-Parkinson effect in C. elegans PD models by partly modulating the IIS/DAF-16 pathway, attenuating DA neurodegeneration, improving DA-dependent behaviors, and reducing oxidative stress in 6-OHDA-induced C. elegans (Figure 6) [112].
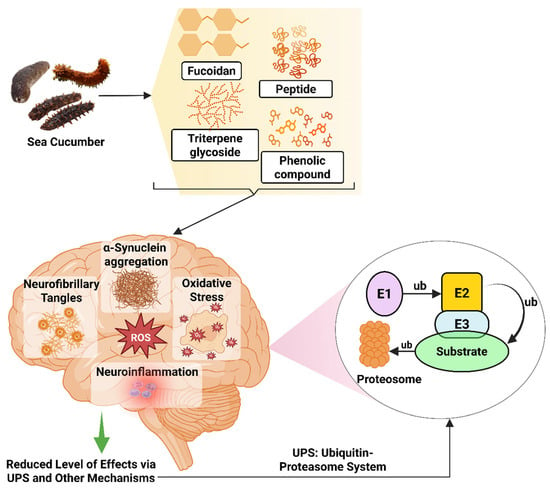
Figure 6.
Neuroprotective effects of sea cucumber-derived bioactive compounds in PD models. E1, Ubiquitin-activating enzyme; E2, Ubiquitin-conjugating enzyme; E3, Ubiquitin ligase; ub, ubiquitin. The figure was generated from the concept of Lim and Tan (2007) [302] using Biorender.com (Agreement number: FH28FPNE87).
The regulation of cognitive health via gut microbiome alterations has been brought on by multiple studies [303,304,305]. Imbalance or dysbiosis of the intestinal microbiome is associated with functional changes in the CNS, facilitated by microbial metabolites and bidirectional interactions with the nervous, immune, and endocrine system along the gut–brain axis [306,307]. Some of the bioactive compounds from sea cucumber have been found to play a role in reducing this dysbiosis. For instance, FCS from sea cucumbers reduces inflammation linked to dysbiosis by lowering Staphylococcus levels through gut–brain axis regulation, which could mitigate PD symptoms. Dysbiosis can be measured by assessing the ratio of the phylum Firmicutes to the Bacteroidota. A study on the effect of FCS in treating PD showed a greater percentage of Bacteroidota and fewer Firmicutes, reducing the dysbiosis and resulting in improved gut and brain health [308].
6. Preclinical and Clinical Evidence
6.1. Alzheimer’s Disease
Bioactive compounds from sea cucumber have shown a promising effect in several AD models targeting specific disease-related pathways. In a transgenic C. elegans model of AD, Frondoside A considerably postponed the worm paralysis brought on by Aβ aggregation, reducing the level of small oligomeric forms, the most toxic species of Aβ, and subsequently turning down ROS production at a low dose of 1 µM [66]. Hydrolysates from sea cucumber (S. japonicus) exhibited higher β-secretase inhibitory activity, which serves as a key regulator in the amyloidogenic pathway and catalyzes the formation of neurotoxic Aβ peptide, and the reduction in BACE, sAPPβ, β-amyloid, p-JNK, and p-p38 in SH-SY5Y cells [258]. Sea cucumber peptides (SCP) derived from A. leucoprocta improved cognitive dysfunction in D-gal-induced aging mice. SCP plays role in releasing GABA by activating the GABABR/cAMP/PKA/CREB pathway and alleviate neuronal and oxidative stress damage, subsequently ameliorating cognitive dysfunction [83]. Sulfated polysaccharides from C. frondosa disrupt preformed Aβ40 fibrils by disassembling mature fibrils [94].
A study described by Li et al. (2019) found that Cer from the body wall of the sea cucumber (A. molpadioides) ameliorated Aβ1-42-induced neuronal damage and suppressed induced apoptosis by decreasing the Bax/Bcl-2 ratio [35]. Additionally, Cer enhanced the expressions of PSD-95 and synaptophysin by activating the BDNF/TrkB/CREB signaling pathway, thereby ameliorating Aβ1-42-induced synaptic dysfunction. Furthermore, Cer attenuated Aβ1-42-induced tau hyperphosphorylation by activating the PI3K/Akt/GSK3β signaling pathway in male SD rats as an Alzheimer’s disease model. Additionally, 2-BTHF, a cyclic ether from H. scabra, has been suggested as a possible treatment for AD at 1 µg/mL and may shield C. elegans from Aβ toxicity by inhibiting its aggregation through an HSF-1-regulated autophagic mechanism [119].
Furthermore, in a study on the in vivo model of AD, dietary glucocerebrosides (SCGs) from sea cucumber (C. frondosa) influenced fatty acid hydroxylation or exosome-mediated Aβ clearance, resulting in a 30.7% reduction in hippocampus Aβ42 when compared to untreated AD animals. Fatty acid hydroxylation and exosome-mediated Aβ clearance are thought to be modulated to produce this effect, although there is no concrete evidence that SCGs are connected to any particular enzymatic pathways, such as β-secretase inhibition. SCGs may also help preserve neurones by maintaining myelin integrity, controlling ceramide/sulfatide ratios and lipid remodeling in the brain’s sphingolipid profile [259]. The study described by Li et al. (2019) found that cerebrosides from the body wall of the sea cucumber (A. molpadioides) ameliorated Aβ1-42-induced neuronal damage and suppressed apoptosis by decreasing the Bax/Bcl-2 ratio. Additionally, Cer enhanced the levels of PSD-95 and synaptophysin by activating the BDNF/TrkB/CREB signaling pathway, thereby ameliorating Aβ1-42-induced synaptic dysfunction. In another study, it was found that Cer attenuated Aβ1-42-induced tau hyperphosphorylation by activating the PI3K/Akt/GSK3β signaling pathway in male AD-infected rats [35].
Another bioactive, Frondoside A (FA), has been proven to postpone the worm paralysis brought on by Aβ aggregation in a transgenic C. elegans model of AD and subsequently turns down ROS production at a low dose of 1 µM. The study also implies that FA may target the early stages of Aβ peptide formation, preventing the development of these neurotoxic species [66].
In addition, inhibition of β-secretase (BACE1) in the amyloidogenic pathway has been studied to reduce pathogenic Aβ formation. According to Ma et al. (2021), the BACE1 inhibitory activity of S. japonicus crude polysaccharide (IC50: 16.13 µg/mL) was higher than trypsin hydrolysate (IC50: 93.59 µg/mL) due to the interference of sulfate-rich structural characteristics with the interactions between the enzyme and the substrate [258].
6.2. Parkinson’s Disease
As the bioactives from the body part of the sea cucumber possess anti-PD therapeutic potentials, multiple studies have been conducted to explore the pharmacokinetics and pharmacodynamic effects of these compounds. Using the toxin 6-OHDA to damage the nigrostriatal pathway and cause motor impairment, the striatal injection is one of the most commonly used animal models of PD [309].
Together with other bioactive substances from the body wall and Cuvierian tubule of H. leucospilota, saponin-rich extract improved DA neuronal function in food-sensing behavior and reduced α-synuclein aggregation, which in turn promoted the neuroprotection and regeneration of DA neurons in 6-OHDA-treated C. elegans PD models by downregulating the apoptosis gene (egl-1) and upregulating the genes that govern DA-synthesis (cat-2) and free-radical scavenging (sod-3) [65]. Chalorak et al. (2018) elucidated that triterpene glycosides and phenolic substances found in H. scabra extracts considerably reduced the degeneration of DA neurones in the BZ555 strain caused by the selective catecholamine neurotoxin 6-hydroxydopamine (6-OHDA) while improving food-sensing behavior, extending longevity, decreasing α-synuclein aggregation, and restoring lipid content in NL5901 [44]. Saponin from C. frondosa in the C. elegans PD model was tested for toxicity and optimal concentration by food clearance assay, and used to treat 6-OHDA-induced BZ555 strain and transgenic α-synuclein NL5901 strains in C. elegans. Treatment with the extract significantly attenuated DA neurodegeneration induced by 6-OHDA in the BZ555 strain, improved the basal slowing rate, and prolonged lifespan in the 6-OHDA-induced wild-type strain with the downregulation of the apoptosis mediators, egl-1 and ced-3, and the upregulation of sod-3 and cat-2. Interestingly, only FA reduced α-synuclein aggregation, rescued lifespan in NL5901, and upregulated the protein degradation regulators, including ubh-4, hsf-1, hsp-16.1, and hsp-16.2 [260].
EPA-PL was extracted from the sea cucumber (C. frondosa) and applied to PD mice induced by MPTP, which improved behavioral deficiency by suppressing oxidative stress and apoptosis, thereby alleviating the loss of DA neurons via the mitochondria–mediated pathway and mitogen-activated protein kinase pathway [36]. At 100 μM, 2-BTHF significantly decreased αsynuclein accumulation and DA neurodegeneration, as molecular docking revealed that 2-BTHF may bind to HSF-1 and DAF-16 transcription factors. It also increased the mRNA transcripts of genes that encode proteins involved in proteostasis, such as the ubiquitination/SUMOylation-related ubc-9 gene, the autophagy-related genes atg-7 and lgg-1, and the molecular chaperones hsp-16.2 and hsp-16.49. However, PPAR signaling pathways that mediated fatty acid metabolism were upregulated, according to transcriptome profiling. The 2-BTHF improved gcs-1-mediated glutathione production, increased the fat-7 gene, and markedly reversed lipid accumulation in the C. elegans PD model [266]. These results suggested that sea cucumber extracts and their active ingredient compounds may have anti-PD potential.
Chalorak et al., in 2021, demonstrated that HSEA-P1 and HSEA-P2, diterpene glycosides from H. scabra, attenuated α-synuclein accumulation with the protection of DA neurons, and restored dopamine-dependent behaviors in C. elegans models, specifically through autophagy-related genes (lgg-1 and atg-7). This occurred due to the mechanism involved in enhancing protein clearance as a critical pathway for α-synuclein degradation, while upregulating genes like bec-1, lgg-1, and atg-7 [261]. This inconsistency suggests that HSEA compounds target a specific subset of the autophagy pathway, potentially omitting unc-51-mediated vesicle nucleation [310] or atg-18-mediated lysosomal fusion [311].
According to Promtang et al. (2024), 2-BTHF from H. scabra substantially lowered oxidative stress markers and α-synuclein accumulation in the muscle cells and DA neurones of the transgenic C. elegans model of the PD brain. The antioxidative capabilities of this compound are expressed by promoting lipid restoration and increasing glutathione production. However, 2-BTHF demonstrated relatively minor, non-significant effects on DA neurones (UA44), indicating cell-type-specific action or variable sensitivity, while significantly decreasing monomeric α-synuclein in muscle cells (NL5901) [266]. Additionally, this study suggests that the small molecule 2-BTHF may be able to cross the BBB by diffusion without restriction due to its low molecular weight and the formation of fewer than eight hydrogen bonds. These results imply that 2-BTHF may interact with the transcription factors HSF-1 and DAF-16 [312] and also correspond with the evidence of anti-aging properties of 2-BTHF [120]. However, there is still a lack of evaluation of the effectiveness of the drug due to a lack of a physiologically intact BBB [313].
Another bioactive compound, Frondoside A (FA), demonstrated neurorescue effects on DA neurodegeneration. In worms with α-synuclein overexpression, it significantly increased the mRNA levels of protein degradation regulators (hsp-1, ubh-4, hsp-16.1, hsp-16.2), indicating protein degradation pathways, an enhanced ubiquitin-proteasome system (UPS), and heat shock proteins (HSPs). Instead of using direct experimental exposure in the C. elegans model, this study claims that FA’s sulfate and acetyl groups are responsible for its better efficacy on broader structure–function relationships [260]. Again, two saturated fatty acids, decanoic acid (C10:0) and palmitic acid (C16:0), employ different but converging pathways for reducing oxidative stress, α-synuclein aggregation, and DA neurodegeneration in the C. elegans PD model. In that study, palmitic acid regulated autophagy and lipid metabolism, whereas decanoic acid was associated with conferring neuroprotection by activating the insulin/IGF-1 signaling (IIS) pathway, specifically through the nuclear translocation of the transcription factor DAF-16. Although both FAs exhibited non-linear dosage responses, palmitic acid’s dual autophagy-lipid regulation could render it more effective in synucleinopathies [112,265].
6.3. Stroke and Ischemic Injuries
As a leading cause of stroke globally, atherosclerosis in the major intracranial arteries induces the physiological regulation of blood flow that results in considerable luminal stenosis as well as modest wall thickening [314]. Recent studies suggest therapeutic applications against the arterial plaque formations that eventually lead to stroke. In a comprehensive review, Wang et al. (2022) reported that naturally occurring active compounds could inhibit atherosclerotic plaque formation within the arteries that supply blood to the brain by regulating autophagy [315]. Additionally, Hahn and Hill (2015) stated that patients with smaller infarcts seem to benefit from anticoagulation within two weeks following an acute stroke, which also helps to avoid the early recurrence of infarction [316]. Multiple active biomolecules of different ranges have been characterized and explored from different species of sea cucumber to find out the subsequent anticoagulant properties, such as FCSs [86,146,168], sulfated fucan [150], and fucosylated glycosaminoglycan [165]. A potential anticoagulant action was demonstrated by the glycosaminoglycan that was isolated from A. japonicus, where anticoagulant activity was nearly identical to that of heparin at the same quantity, below 170 µg/mL [123].
By inhibiting the activation of MAPKs, another bioactive sulfated polysaccharide from S. japonicus may be able to significantly reduce cell apoptosis brought on by Na2S2O4-induced hypoxia/reoxygenation (H/R) injury. This could lead to a significant decrease in the Bax/Bcl-2 ratio, cleaved caspase-3/caspase-3, p53 phosphorylation, and cytochrome c release, making it a potential medication to prevent or treat cerebral ischemia–reperfusion injury [263].
6.4. Brain Cancer and Brain Tumors
There is a lack of research on the therapeutic potential of sea cucumber as an anti-tumor or anti-cancer agent for brain tumors but their antioxidative nature and chemopreventive potentials for other organs or tissues have been studied extensively [88,126,139]. However, a few studies have shown the positive effect of sea cucumber’s bioactives in the PC12 cell line to reveal their potential as anti-tumor and anti-cancer agents. In a study by Che et al. (2018), it was found that the neuroprotective effects of DHA/EPA-PLs depend on the molecular form, as EPA-PS and DHA-PS (phosphatidylserine) could downregulate the messenger RNA level of caspase-3, caspase-9, and Bax, and upregulate Bcl-2 at the protein level [317]. This modulation protects PC12 cells from oxidative stress and prevents mitochondrial-mediated apoptosis and perhaps helps in crossing the BBB [109].
Additionally, the anti-cancer efficacy of ethyl acetate extract from H. scabra body wall has been shown on human GBM cell lines (A172 and U87MG) through the mitochondria-mediated pathway [143]. But in this study, the BBB permeability of the compounds remains unaddressed, which is a critical gap for GBM therapeutics [318].
7. Challenges and Future Outlook
Preclinical research has demonstrated the positive impact of using bioactive compounds extracted from sea cucumbers to address and control brain-related disorders. However, there are still some research gaps that are inhibiting the bringing of these advances to patients and the use of these marine natural products in medicine; such challenges must first be tackled. Several research studies have been conducted on evaluating the role of some common bioactive compounds extracted from sea cucumbers in fighting brain-oriented diseases. Future studies should include the isolation, identification, and chemical and biological characterization of new bioactive compounds such as alkaloids, antioxidant phenolics, functional peptides, and other health-promoting components using advanced methods [319].
Inadequate knowledge is available about the role of bioactive peptides from sea cucumber in molecular mechanisms as potential nutraceutical agents for memory impairment using proteomics technology. Integrated omics tool, along with molecular docking, could be used in the future, as proteomics technology offers a promising technique for studying molecular mechanisms through large-scale protein analysis [79,93]. In addition, more studies should be performed to understand the exact mechanism of action, metabolism, distribution, and transport of the existing and newly found bioactive compounds from sea cucumbers in the central nervous system. Comprehensive studies should be performed to evaluate the safety of the compounds through proper and long-term clinical trials to observe the interaction of the compounds with the food matrix and movement in the digestive system. The molecular and cellular mechanisms will reveal information about the impact on neuroinflammation and synaptic plasticity, the microbiota–gut–brain axis, the interactive pathway, and the mechanism of action [45].
Different kinds of bioactive compounds are extracted from sea cucumbers; however, knowledge of the exact mechanisms of action and optimal dosages for these compounds in supporting brain health is limited. Again, some of the compounds, like holothurians, a type of triterpene glycoside, can be toxic for human health when applied at a high concentration, causing skin irritation or other adverse effects [27]. Moreover, only a few neurotransmitter-based drugs are approved by the FDA, among which some of the drugs possess a serious negative impact and show deteriorated conditions after 12 months of administration [320]. Even though bioactive compounds from sea cucumber have high potential in treating NDs, the ability to cross the blood–brain barrier (BBB) remains unclear, which is essential to be acknowledged as neurotransmitter-based drugs. To attain a successful transition to first-in-human (FIH) trials, sponsors must strategically coordinate an approach across a variety of disciplines, such as toxicological assessment, biomarker assessment, pharmacokinetic studies, and global compliance etc., all of which must be seamlessly incorporated.
Moreover, many studies on the positive impact of sea cucumbers in inhibiting the aggregation of Aβ peptides have been conducted in in vivo and in vitro models. However, human clinical trials have not been performed to validate these findings and investigate their potential therapeutic applications [60]. Due to their efficacy and potential contribution to the nutraceutical sector and logical dosage recommendations, marine pharmaceuticals have sparked growing attention in recent decades. Many of the naturally occurring compounds, like bioactive compounds from sea cucumbers, are of tremendous interest for prospective medication development as well as ingredients for novel leads and commercially successful products for industrial purposes, particularly medicines, agrochemicals, functional foods, and nutraceuticals [321]. However, in order to use the compounds for treating brain health, more long-term clinical studies need to be performed. Comprehensive human studies must be conducted, taking into account dosage, safety, and long-term efficacy. Except for such trials, the medicinal potential of sea cucumber-derived chemicals remains uncertain, despite promising bioactivities in laboratory conditions.
8. Conclusions
Neurodegenerative brain disorders are complex, for which new and multidirectional treatments are needed, and sea cucumber bioactives have shown good potential in this regard. The promising effect of bioactive compounds extracted from sea cucumbers in health management is yet to be fully explored. Potential in vivo studies, along with in vitro studies that describe the molecular- and cellular-level mechanisms, may increase the utilization of these highly nutritional marine species for the development of highly efficacious therapeutics. Interdisciplinary collaboration among marine biology for optimized cultivation, neuropharmacology for multi-target delivery, and clinical medicine for early intervention should be performed to accelerate the translation of sea cucumber bioactives into therapies. Apart from these, the collaboration of the experts from bioengineering, toxicology studies, and the pharmaceutical industry is also crucial to increase the bioavailability and targeted delivery of these compounds. Incorporation of advanced omics technologies along with computational modeling can be carried out to gain deeper insights into the mode of action of these compounds, facilitating the rationality of clinical trials. Knowledge of marine biologists in the identification and sustainable extraction of these compounds and neuropharmacologists in formulating targeted delivery strategies for treating ND provides a critical foundation for exploring the therapeutic potential of sea cucumber-derived bioactives in the prevention and management of brain-related disorders. This review serves as a bridge between marine natural products and neuroscience. It highlights evidence-based research on sea cucumber-derived bioactives in the management of brain-related disorders, emphasizing their multimodal therapeutic potential. The study also underscores the urgent need to elucidate the exact mechanisms of action to prevent neurodegeneration, improve prognostic outcomes, and reduce systemic side effects. The bioactive ingredients from sea cucumbers could greatly benefit from better use in protecting the nervous system and boosting brain function. Harnessing the therapeutic potential of sea cucumber bioactives could pave the way for novel, nature-based therapies in the prevention and management of brain-related illnesses.
Author Contributions
P.R.D.—writing, methodology, data curation, formal analysis, and original draft preparation; H.B.—investigation, validation, and writing; M.K.A.—supervision, conceptualization, visualization, software, data interpretation, review and editing; S.B.—writing, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- Przedborski, S.; Vila, M.; Jackson-Lewis, V. Series Introduction: Neurodegeneration: What Is It and Where Are We? J. Clin. Investig. 2003, 111, 3–10. [Google Scholar] [CrossRef]
- Gao, H.-M.; Hong, J.-S. Why Neurodegenerative Diseases Are Progressive: Uncontrolled Inflammation Drives Disease Progression. Trends Immunol. 2008, 29, 357–365. [Google Scholar] [CrossRef]
- Yildiz-Unal, A.; Korulu, S.; Karabay, A. Neuroprotective Strategies against Calpain-Mediated Neurodegeneration. Neuropsychiatr. Dis. Treat. 2015, 11, 297–310. [Google Scholar] [CrossRef]
- Gadhave, D.G.; Sugandhi, V.V.; Kokare, C.R. Potential Biomaterials and Experimental Animal Models for Inventing New Drug Delivery Approaches in the Neurodegenerative Disorder: Multiple Sclerosis. Brain Res. 2024, 1822, 148674. [Google Scholar] [CrossRef]
- Chou, S.-C.; Aggarwal, A.; Dawson, V.L.; Dawson, T.M.; Kam, T.-I. Recent Advances in Preventing Neurodegenerative Diseases. Fac. Rev. 2021, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Mayne, K.; White, J.A.; McMurran, C.E.; Rivera, F.J.; De La Fuente, A.G. Aging and Neurodegenerative Disease: Is the Adaptive Immune System a Friend or Foe? Front. Aging Neurosci. 2020, 12, 572090. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, M.; Papi, L.; Gori, F.; Turillazzi, E. Natural Products in Neurodegenerative Diseases: A Great Promise but an Ethical Challenge. Int. J. Mol. Sci. 2019, 20, 5170. [Google Scholar] [CrossRef]
- Goldsteins, G.; Hakosalo, V.; Jaronen, M.; Keuters, M.H.; Lehtonen, Š.; Koistinaho, J. CNS Redox Homeostasis and Dysfunction in Neurodegenerative Diseases. Antioxidants 2022, 11, 405. [Google Scholar] [CrossRef]
- Mahmood, N.; Nasir, S.B.; Hefferon, K. Conjugated Recombinant Proteins as Emerging New Drugs. In Bioeconomy for Sustainable Development; Keswani, C., Ed.; Springer: Singapore, 2020; pp. 347–357. ISBN 978-981-13-9430-0. [Google Scholar]
- Bäckström, D.; Granåsen, G.; Domellöf, M.E.; Linder, J.; Jakobson Mo, S.; Riklund, K.; Zetterberg, H.; Blennow, K.; Forsgren, L. Early Predictors of Mortality in Parkinsonism and Parkinson Disease: A Population-Based Study. Neurology 2018, 91, e2045–e2056. [Google Scholar] [CrossRef]
- Huh, T.H.; Yoon, J.L.; Cho, J.J.; Kim, M.Y.; Ju, Y.S. Survival Analysis of Patients with Alzheimer’s Disease: A Study Based on Data from the Korean National Health Insurance Services’ Senior Cohort Database. Korean J. Fam. Med. 2020, 41, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.G.; Sugandhi, V.V.; Jha, S.K.; Nangare, S.N.; Gupta, G.; Singh, S.K.; Dua, K.; Cho, H.; Hansbro, P.M.; Paudel, K.R. Neurodegenerative Disorders: Mechanisms of Degeneration and Therapeutic Approaches with Their Clinical Relevance. Ageing Res. Rev. 2024, 99, 102357. [Google Scholar] [CrossRef]
- Ding, C.; Wu, Y.; Chen, X.; Chen, Y.; Wu, Z.; Lin, Z.; Kang, D.; Fang, W.; Chen, F. Global, Regional, and National Burden and Attributable Risk Factors of Neurological Disorders: The Global Burden of Disease Study 1990–2019. Front. Public Health 2022, 10, 952161. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, Y.; Pan, H.; Han, L. Global, Regional, and National Burden of Neurological Disorders in 204 Countries and Territories Worldwide. J. Glob. Health 2023, 13, 04160. [Google Scholar] [CrossRef]
- Castelpietra, G.; Knudsen, A.K.S.; Agardh, E.E.; Armocida, B.; Beghi, M.; Iburg, K.M.; Logroscino, G.; Ma, R.; Starace, F.; Steel, N.; et al. The Burden of Mental Disorders, Substance Use Disorders and Self-Harm among Young People in Europe, 1990-2019: Findings from the Global Burden of Disease Study 2019. Lancet Reg. Health Eur. 2022, 16, 100341. [Google Scholar] [CrossRef]
- Feigin, V.L.; Vos, T.; Nichols, E.; Owolabi, M.O.; Carroll, W.M.; Dichgans, M.; Deuschl, G.; Parmar, P.; Brainin, M.; Murray, C. The Global Burden of Neurological Disorders: Translating Evidence into Policy. Lancet Neurol. 2020, 19, 255–265. [Google Scholar] [CrossRef]
- Amtul, Z. Atta-ur-Rahman Nutraceuticals Neuroprotect Naturally. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2016; Volume 50, pp. 373–397. ISBN 978-0-444-63749-9. [Google Scholar]
- Singh, K.; Gupta, J.K.; Chanchal, D.K.; Shinde, M.G.; Kumar, S.; Jain, D.; Almarhoon, Z.M.; Alshahrani, A.M.; Calina, D.; Sharifi-Rad, J.; et al. Natural Products as Drug Leads: Exploring Their Potential in Drug Discovery and Development. Naunyn. Schmiedebergs Arch. Pharmacol. 2025, 398, 4673–4687. [Google Scholar] [CrossRef]
- Tufail, T.; Bader Ul Ain, H.; Ashraf, J.; Mahmood, S.; Noreen, S.; Ijaz, A.; Ikram, A.; Arshad, M.T.; Abdullahi, M.A. Bioactive Compounds in Seafood: Implications for Health and Nutrition. Food Sci. Nutr. 2025, 13, e70181. [Google Scholar] [CrossRef]
- Angeloni, C.; Vauzour, D. Natural Products and Neuroprotection. Int. J. Mol. Sci. 2019, 20, 5570. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sairazi, N.S.; Sirajudeen, K.N.S. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid. Based Complement. Alternat. Med. 2020, 2020, 6565396. [Google Scholar] [CrossRef]
- Gong, H.; Luo, Z.; Chen, W.; Feng, Z.-P.; Wang, G.-L.; Sun, H.-S. Marine Compound Xyloketal B as a Potential Drug Development Target for Neuroprotection. Mar. Drugs 2018, 16, 516. [Google Scholar] [CrossRef]
- Nii-Trebi, N.I. Emerging and Neglected Infectious Diseases: Insights, Advances, and Challenges. BioMed Res. Int. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chen, Y.; Chan, W.Y. Marine Natural Products with Anti-Inflammatory Activity. Appl. Microbiol. Biotechnol. 2016, 100, 1645–1666. [Google Scholar] [CrossRef]
- Khotimchenko, Y.S. The Nutritional Value of Holothurians. Russ. J. Mar. Biol. 2015, 41, 409–423. [Google Scholar] [CrossRef]
- Pangestuti, R.; Arifin, Z. Medicinal and Health Benefit Effects of Functional Sea Cucumbers. J. Tradit. Complement. Med. 2018, 8, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, Y.; Zhang, W.; Franco, C. Discovery of Novel Saponins from the Viscera of the Sea Cucumber Holothuria Lessoni. Mar. Drugs 2014, 12, 2633–2667. [Google Scholar] [CrossRef]
- Gopakumar, K.; Gopakumar, B. Health Foods from Ocean Animals, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020; ISBN 978-1-003-08424-2. [Google Scholar]
- Resoles, J.A.A.; Yu, E.T. The Neuropeptidomes of the Sea Cucumbers Stichopus Cf. horrens and Holothuria scabra. Sci. Rep. 2025, 15, 7032. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Zelepuga, E.A.; Chingizova, E.A.; Menchinskaya, E.S.; Tabakmakher, K.M.; Kalinovsky, A.I.; Avilov, S.A.; Popov, R.S.; Dmitrenok, P.S.; Kalinin, V.I. Cladolosides of Groups S and T: Triterpene Glycosides from the Sea Cucumber Cladolabes Schmeltzii with Unique Sulfation; Human Breast Cancer Cytotoxicity and QSAR. Mar. Drugs 2025, 23, 265. [Google Scholar] [CrossRef] [PubMed]
- Olivera-Castillo, L.; Grant, G.; Kantún-Moreno, N.; Barrera-Pérez, H.A.; Montero, J.; Olvera-Novoa, M.A.; Carrillo-Cocom, L.M.; Acevedo, J.J.; Puerto-Castillo, C.; May Solís, V.; et al. A Glycosaminoglycan-Rich Fraction from Sea Cucumber Isostichopus Badionotus Has Potent Anti-Inflammatory Properties In Vitro and In Vivo. Nutrients 2020, 12, 1698. [Google Scholar] [CrossRef]
- Cui, L.; Sun, H.; Shang, X.; Wen, J.; Li, P.; Yang, S.; Chen, L.; Huang, X.; Li, H.; Yin, R.; et al. Purification and Structural Analyses of Sulfated Polysaccharides from Low-Value Sea Cucumber Stichopus Naso and Anticoagulant Activities of Its Oligosaccharides. Mar. Drugs 2024, 22, 265. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.-X.; Xu, H.-P.; Li, Y.; Zhang, Q.-W.; Xie, H. Preparation and Evaluation of Peptides with Potential Antioxidant Activity by Microwave Assisted Enzymatic Hydrolysis of Collagen from Sea Cucumber Acaudina Molpadioides Obtained from Zhejiang Province in China. Mar. Drugs 2019, 17, 169. [Google Scholar] [CrossRef]
- Li, Q.; Che, H.-X.; Wang, C.-C.; Zhang, L.-Y.; Ding, L.; Xue, C.-H.; Zhang, T.-T.; Wang, Y.-M. Cerebrosides from Sea Cucumber Improved Aβ1-42 -Induced Cognitive Deficiency in a Rat Model of Alzheimer’s Disease. Mol. Nutr. Food Res. 2019, 63, e1800707. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, D.; Zhang, T.-T.; Yanagita, T.; Xue, C.-H.; Chang, Y.-G.; Wang, Y.-M. A Comparative Study about EPA-PL and EPA-EE on Ameliorating Behavioral Deficits in MPTP-Induced Mice with Parkinson’s Disease by Suppressing Oxidative Stress and Apoptosis. J. Funct. Foods 2018, 50, 8–17. [Google Scholar] [CrossRef]
- Hossain, A.; Yeo, J.; Dave, D.; Shahidi, F. Phenolic Compounds and Antioxidant Capacity of Sea Cucumber (Cucumaria frondosa) Processing Discards as Affected by High-Pressure Processing (HPP). Antioxidants 2022, 11, 337. [Google Scholar] [CrossRef] [PubMed]
- Mert Ozupek, N.; Cavas, L. Triterpene Glycosides Associated Antifouling Activity from Holothuria tubulosa and H. Polii. Reg. Stud. Mar. Sci. 2017, 13, 32–41. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Zhi, Z.; Yan, L.; Ye, X.; Ding, T.; Yan, L.; Linhardt, R.J.; Chen, S. Depolymerization of Fucosylated Chondroitin Sulfate with a Modified Fenton-System and Anticoagulant Activity of the Resulting Fragments. Mar. Drugs 2016, 14, 170. [Google Scholar] [CrossRef]
- Medina-Feliciano, J.G.; García-Arrarás, J.E. Regeneration in Echinoderms: Molecular Advancements. Front. Cell Dev. Biol. 2021, 9, 768641. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, L.; Yuan, J.; Sun, Y.; Gao, Y.; Zhang, L.; Li, S.; Dai, H.; Hamel, J.-F.; Liu, C.; et al. The Sea Cucumber Genome Provides Insights into Morphological Evolution and Visceral Regeneration. PLoS Biol. 2017, 15, e2003790. [Google Scholar] [CrossRef]
- Janakiram, N.B.; Mohammed, A.; Rao, C.V. Sea Cucumbers Metabolites as Potent Anti-Cancer Agents. Mar. Drugs 2015, 13, 2909–2923. [Google Scholar] [CrossRef]
- Jattujan, P.; Chalorak, P.; Siangcham, T.; Sangpairoj, K.; Nobsathian, S.; Poomtong, T.; Sobhon, P.; Meemon, K. Holothuria scabra Extracts Possess Anti-Oxidant Activity and Promote Stress Resistance and Lifespan Extension in Caenorhabditis elegans. Exp. Gerontol. 2018, 110, 158–171. [Google Scholar] [CrossRef]
- Chalorak, P.; Jattujan, P.; Nobsathian, S.; Poomtong, T.; Sobhon, P.; Meemon, K. Holothuria scabra Extracts Exhibit Anti-Parkinson Potential in C. elegans: A Model for Anti-Parkinson Testing. Nutr. Neurosci. 2018, 21, 427–438. [Google Scholar] [CrossRef]
- Man, J.; El-Aty, A.M.A.; Wang, Z.; Tan, M. Recent Advances in Sea Cucumber Peptide: Production, Bioactive Properties, and Prospects. Food Front. 2023, 4, 131–163. [Google Scholar] [CrossRef]
- Slater, M. Use and Exploitation of Sea Cucumbers. In Echinoderm Aquaculture; Brown, N.P., Eddy, S.D., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 57–73. ISBN 978-0-470-96038-7. [Google Scholar]
- Yu, E.Y.; Liao, Z.L.; Tan, Y.F.; Qiu, Y.J.; Zhu, J.P.; Chen, Y.; Lin, S.S.; Wu, M.H. Efficacy and tolerance of Memantine monotherapy and combination therapy with Reinhartdt And Sea Cucumber Capsule on agitation in moderate to severe Alzheimer disease. Zhonghua Yi Xue Za Zhi 2017, 97, 2091–2094. [Google Scholar] [CrossRef]
- Chen, F.; Lin, L.; Zhao, M.; Zhu, Q. Modification of Cucumaria frondosa Hydrolysate through Maillard Reaction for Sea Cucumber Peptide Based-Beverage. LWT 2021, 136, 110329. [Google Scholar] [CrossRef]
- Barua, H.; Acharjee, M.R.; Giteru, S.G.; Chowdhury, M.; Wu, H.; Kumar, L.; Ahmmed, M.K. Dietary Phospholipids and Their Impact on Crustacean Physiology: Growth, Metabolism, Immunity, and Beyond. Aquac. Nutr. 2025, 2025, 8180797. [Google Scholar] [CrossRef]
- Salindeho, N.; Nurkolis, F.; Gunawan, W.B.; Handoko, M.N.; Samtiya, M.; Muliadi, R.D. Anticancer and Anticholesterol Attributes of Sea Cucumbers: An Opinion in Terms of Functional Food Applications. Front. Nutr. 2022, 9, 986986. [Google Scholar] [CrossRef]
- Osbourn, A.; Goss, R.J.M.; Field, R.A. The Saponins—Polar Isoprenoids with Important and Diverse Biological Activities. Nat. Prod. Rep. 2011, 28, 1261. [Google Scholar] [CrossRef]
- Li, Y.; Wang, R.; Xun, X.; Wang, J.; Bao, L.; Thimmappa, R.; Ding, J.; Jiang, J.; Zhang, L.; Li, T.; et al. Sea Cucumber Genome Provides Insights into Saponin Biosynthesis and Aestivation Regulation. Cell Discov. 2018, 4, 29. [Google Scholar] [CrossRef]
- Bahrami, Y.; Zhang, W.; Chataway, T.; Franco, C. Structural Elucidation of Novel Saponins in the Sea Cucumber Holothuria Lessoni. Mar. Drugs 2014, 12, 4439–4473. [Google Scholar] [CrossRef]
- Caulier, G.; Mezali, K.; Soualili, D.L.; Decroo, C.; Demeyer, M.; Eeckhaut, I.; Gerbaux, P.; Flammang, P. Chemical Characterization of Saponins Contained in the Body Wall and the Cuvierian Tubules of the Sea Cucumber Holothuria (Platyperona) Sanctori (Delle Chiaje, 1823). Biochem. Syst. Ecol. 2016, 68, 119–127. [Google Scholar] [CrossRef]
- Mondol, M.A.M.; Shin, H.J.; Rahman, M.A.; Islam, M.T. Sea Cucumber Glycosides: Chemical Structures, Producing Species and Important Biological Properties. Mar. Drugs 2017, 15, 317. [Google Scholar] [CrossRef]
- Fagbohun, O.F.; Joseph, J.S.; Oriyomi, O.V.; Rupasinghe, H.P.V. Saponins of North Atlantic Sea Cucumber: Chemistry, Health Benefits, and Future Prospectives. Mar. Drugs 2023, 21, 262. [Google Scholar] [CrossRef]
- Bahrami, Y.; Zhang, W.; Franco, C.M.M. Distribution of Saponins in the Sea Cucumber Holothuria Lessoni; the Body Wall Versus the Viscera, and Their Biological Activities. Mar. Drugs 2018, 16, 423. [Google Scholar] [CrossRef]
- Khattab, R.A.; Elbandy, M.; Lawrence, A.; Paget, T.; Rae-Rho, J.; Binnaser, Y.S.; Ali, I. Extraction, Identification and Biological Activities of Saponins in Sea Cucumber Pearsonothuria Graeffei. Comb. Chem. High Throughput Screen. 2018, 21, 222–231. [Google Scholar] [CrossRef]
- Bahrami, Y.; Franco, C.M.M. Structure Elucidation of New Acetylated Saponins, Lessoniosides A, B, C, D, and E, and Non-Acetylated Saponins, Lessoniosides F and G, from the Viscera of the Sea Cucumber Holothuria Lessoni. Mar. Drugs 2015, 13, 597–617. [Google Scholar] [CrossRef]
- Liang, Q.; Ahmed, F.; Zhang, M.; Sperou, N.; Franco, C.M.M.; Feng, Q.; Zhang, W. In Vivo and Clinical Studies of Sea Cucumber-Derived Bioactives for Human Health and Nutrition From 2012–2021. Front. Mar. Sci. 2022, 9, 917857. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Jiang, T.; Wang, H.; Yang, S.; Lv, Z. Absorption and Transport of Sea Cucumber Saponins from Apostichopus japonicus. Mar. Drugs 2016, 14, 114. [Google Scholar] [CrossRef]
- Sun, A.; Xu, X.; Lin, J.; Cui, X.; Xu, R. Neuroprotection by Saponins: NEUROPROTECTION BY SAPONINS. Phytother. Res. 2015, 29, 187–200. [Google Scholar] [CrossRef]
- Soltani, M.; Parivar, K.; Baharara, J.; Kerachian, M.A.; Asili, J. Putative Mechanism for Apoptosis-Inducing Properties of Crude Saponin Isolated from Sea Cucumber (Holothuria leucospilota) as an Antioxidant Compound. Iran. J. Basic Med. Sci. 2015, 18, 180–187. [Google Scholar]
- Kitisin, T.; Suphamungmee, W.; Meemon, K. Saponin-Rich Extracts from Holothuria leucospilota Mediate Lifespan Extension and Stress Resistance in Caenorhabditis elegans via Daf-16. J. Food Biochem. 2019, 43, e13075. [Google Scholar] [CrossRef]
- Malaiwong, N.; Chalorak, P.; Jattujan, P.; Manohong, P.; Niamnont, N.; Suphamungmee, W.; Sobhon, P.; Meemon, K. Anti-Parkinson Activity of Bioactive Substances Extracted from Holothuria leucospilota. Biomed. Pharmacother. 2019, 109, 1967–1977. [Google Scholar] [CrossRef]
- Tangrodchanapong, T.; Sobhon, P.; Meemon, K. Frondoside A Attenuates Amyloid-β Proteotoxicity in Transgenic Caenorhabditis elegans by Suppressing Its Formation. Front. Pharmacol. 2020, 11, 553579. [Google Scholar] [CrossRef]
- Karaś, M. Influence of Physiological and Chemical Factors on the Absorption of Bioactive Peptides. Int. J. Food Sci. Technol. 2019, 54, 1486–1496. [Google Scholar] [CrossRef]
- Daliri, E.B.-M.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Karaś, M.; Rybczyńska-Tkaczyk, K.; Zielińska, E.; Zieliński, D. Current Trends of Bioactive Peptides—New Sources and Therapeutic Effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Small Bioactive Peptides for Biomaterials Design and Therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef]
- Hernández-Sámano, A.C.; Hernández-Ledesma, B. Release of Antioxidant Peptides from the Body Wall Proteins of the Sea Cucumber Isostichopus fuscus. Nat. Prod. Commun. 2015, 10, 1934578X1501000829. [Google Scholar] [CrossRef]
- Lee, H.-G.; Kim, H.-S.; Oh, J.-Y.; Lee, D.-S.; Yang, H.-W.; Kang, M.-C.; Kim, E.-A.; Kang, N.; Kim, J.; Heo, S.-J.; et al. Potential Antioxidant Properties of Enzymatic Hydrolysates from Stichopus japonicus against Hydrogen Peroxide-Induced Oxidative Stress. Antioxidants 2021, 10, 110. [Google Scholar] [CrossRef]
- Safari, R.; Yaghoubzadeh, Z. Antioxidant Activity of Bioactive Peptides Extracted from Sea Cucumber (Holothuria leucospilata). Int. J. Pept. Res. Ther. 2020, 26, 2393–2398. [Google Scholar] [CrossRef]
- Zhang, Y.; He, S.; Bonneil, É.; Simpson, B.K. Generation of Antioxidative Peptides from Atlantic Sea Cucumber Using Alcalase versus Trypsin: In Vitro Activity, de Novo Sequencing, and in Silico Docking for in Vivo Function Prediction. Food Chem. 2020, 306, 125581. [Google Scholar] [CrossRef]
- Wang, Q.; Shi, J.; Zhong, H.; Abdullah; Zhuang, J.; Zhang, J.; Wang, J.; Zhang, X.; Feng, F. High-Degree Hydrolysis Sea Cucumber Peptides Improve Exercise Performance and Exert Antifatigue Effect via Activating the NRF2 and AMPK Signaling Pathways in Mice. J. Funct. Foods 2021, 86, 104677. [Google Scholar] [CrossRef]
- Ghanbari, R.; Zarei, M.; Ebrahimpour, A.; Abdul-Hamid, A.; Ismail, A.; Saari, N. Angiotensin-I Converting Enzyme (ACE) Inhibitory and Anti-Oxidant Activities of Sea Cucumber (Actinopyga lecanora) Hydrolysates. Int. J. Mol. Sci. 2015, 16, 28870–28885. [Google Scholar] [CrossRef]
- Senadheera, T.R.L.; Hossain, A.; Dave, D.; Shahidi, F. In Silico Analysis of Bioactive Peptides Produced from Underutilized Sea Cucumber By-Products—A Bioinformatics Approach. Mar. Drugs 2022, 20, 610. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun-Waterhouse, D.; Neil Waterhouse, G.I.; Zheng, L.; Su, G.; Zhao, M. Effects of Food-Derived Bioactive Peptides on Cognitive Deficits and Memory Decline in Neurodegenerative Diseases: A Review. Trends Food Sci. Technol. 2021, 116, 712–732. [Google Scholar] [CrossRef]
- Lu, Z.; Lv, R.; Dong, L.; Chen, D.; Lin, S. Sea Cucumber Peptides Protect against Memory Impairment by Regulating Dopamine/Serotonin Metabolization and Synapse Plasticity of Mice Hippocampus. J. Funct. Foods 2023, 108, 105732. [Google Scholar] [CrossRef]
- Lu, M.; Mishra, A.; Boschetti, C.; Lin, J.; Liu, Y.; Huang, H.; Kaminski, C.F.; Huang, Z.; Tunnacliffe, A.; Kaminski Schierle, G.S. Sea Cucumber-Derived Peptides Alleviate Oxidative Stress in Neuroblastoma Cells and Improve Survival in C. elegans Exposed to Neurotoxic Paraquat. Oxid. Med. Cell. Longev. 2021, 2021, 8842926. [Google Scholar] [CrossRef]
- Lin, L.; Yang, K.; Zheng, L.; Zhao, M.; Sun, W.; Zhu, Q.; Liu, S. Anti-Aging Effect of Sea Cucumber (Cucumaria frondosa) Hydrolysate on Fruit Flies and d-Galactose-Induced Aging Mice. J. Funct. Foods 2018, 47, 11–18. [Google Scholar] [CrossRef]
- Lin, L.; Zhu, Q.; Zheng, L.; Zhao, M.; Fan, J.; Liu, S. Preparation of Sea Cucumber (Stichopus variegates) Peptide Fraction with Desired Organoleptic Property and Its Anti-Aging Activity in Fruit Flies and D-Galactose-Induced Aging Mice. J. Funct. Foods 2020, 69, 103954. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Wu, Y.; Zhao, M.; Wang, J.; Du, J.; Bao, Z.; Min, W.; Shen, F.; Feng, F. Upregulation of cAMP/PKA/CREB Signaling Mediated by GABABR Might Contribute to the Amelioration Effects of Sea Cucumber Peptides (Acaudina leucoprocta) on Cognitive Dysfunction in Mice. Food Biosci. 2025, 63, 105632. [Google Scholar] [CrossRef]
- Song, S.; Wu, S.; Ai, C.; Xu, X.; Zhu, Z.; Cao, C.; Yang, J.; Wen, C. Compositional Analysis of Sulfated Polysaccharides from Sea Cucumber (Stichopus japonicus) Released by Autolysis Reaction. Int. J. Biol. Macromol. 2018, 114, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Dave, D.; Shahidi, F. Antioxidant Potential of Sea Cucumbers and Their Beneficial Effects on Human Health. Mar. Drugs 2022, 20, 521. [Google Scholar] [CrossRef]
- Luo, L.; Wu, M.; Xu, L.; Lian, W.; Xiang, J.; Lu, F.; Gao, N.; Xiao, C.; Wang, S.; Zhao, J. Comparison of Physicochemical Characteristics and Anticoagulant Activities of Polysaccharides from Three Sea Cucumbers. Mar. Drugs 2013, 11, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Dave, D.; Shahidi, F. Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef]
- Thinh, P.D.; Ly, B.M.; Usoltseva, R.V.; Shevchenko, N.M.; Rasin, A.B.; Anastyuk, S.D.; Malyarenko, O.S.; Zvyagintseva, T.N.; San, P.T.; Ermakova, S.P. A Novel Sulfated Fucan from Vietnamese Sea Cucumber Stichopus variegatus: Isolation, Structure and Anticancer Activity in Vitro. Int. J. Biol. Macromol. 2018, 117, 1101–1109. [Google Scholar] [CrossRef]
- Mansour, M.B.; Balti, R.; Yacoubi, L.; Ollivier, V.; Chaubet, F.; Maaroufi, R.M. Primary Structure and Anticoagulant Activity of Fucoidan from the Sea Cucumber Holothuria Polii. Int. J. Biol. Macromol. 2019, 121, 1145–1153. [Google Scholar] [CrossRef]
- Ning, Z.; Wang, P.; Zuo, Z.; Tao, X.; Gao, L.; Xu, C.; Wang, Z.; Wu, B.; Gao, N.; Zhao, J. A Fucan Sulfate with Pentasaccharide Repeating Units from the Sea Cucumber Holothuriafloridana and Its Anticoagulant Activity. Mar. Drugs 2022, 20, 377. [Google Scholar] [CrossRef]
- Yan, L.; Zhu, M.; Wang, D.; Tao, W.; Liu, D.; Zhang, F.; Linhardt, R.J.; Ye, X.; Chen, S. Oral Administration of Fucosylated Chondroitin Sulfate Oligomers in Gastro-Resistant Microcapsules Exhibits a Safe Antithrombotic Activity. Thromb. Haemost. 2021, 121, 015–026. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, S.; Shi, W.; Qi, X.; Song, W.; Mou, J.; Yang, J. Structure Characterization, Antioxidant and Immunoregulatory Properties of a Novel Fucoidan from the Sea Cucumber Stichopus Chloronotus. Carbohydr. Polym. 2020, 231, 115767. [Google Scholar] [CrossRef]
- Wang, J.; Shi, S.; Li, F.; Du, X.; Kong, B.; Wang, H.; Xia, X. Physicochemical Properties and Antioxidant Activity of Polysaccharides Obtained from Sea Cucumber Gonads via Ultrasound-Assisted Enzymatic Techniques. LWT 2022, 160, 113307. [Google Scholar] [CrossRef]
- Li, G.; Zhou, Y.; Yang, W.-Y.; Zhang, C.; Hong, L.; Jia, L. Inhibitory Effects of Sulfated Polysaccharides from the Sea Cucumber Cucumaria frondosa against Aβ40 Aggregation and Cytotoxicity. ACS Chem. Neurosci. 2021, 12, 1854–1859. [Google Scholar] [CrossRef] [PubMed]
- Cecerska-Heryć, E.; Pękała, M.; Serwin, N.; Gliźniewicz, M.; Grygorcewicz, B.; Michalczyk, A.; Heryć, R.; Budkowska, M.; Dołęgowska, B. The Use of Stem Cells as a Potential Treatment Method for Selected Neurodegenerative Diseases: Review. Cell. Mol. Neurobiol. 2023, 43, 2643–2673. [Google Scholar] [CrossRef]
- Tang, X.; Deng, P.; Li, L.; He, Y.; Wang, J.; Hao, D.; Yang, H. Advances in Genetically Modified Neural Stem Cell Therapy for Central Nervous System Injury and Neurological Diseases. Stem Cell Res. Ther. 2024, 15, 482. [Google Scholar] [CrossRef]
- Cui, C.; Wang, P.; Cui, N.; Song, S.; Liang, H.; Ji, A. Stichopus japonicus Polysaccharide, Fucoidan, or Heparin Enhanced the SDF-1α/CXCR4 Axis and Promoted NSC Migration via Activation of the PI3K/Akt/FOXO3a Signaling Pathway. Cell. Mol. Neurobiol. 2016, 36, 1311–1329. [Google Scholar] [CrossRef]
- Ceesay, A.; Nor Shamsudin, M.; Aliyu-Paiko, M.; Ismail, I.S.; Nazarudin, M.F.; Mohamed Alipiah, N. Extraction and Characterization of Organ Components of the Malaysian Sea Cucumber Holothuria leucospilota Yielded Bioactives Exhibiting Diverse Properties. BioMed Res. Int. 2019, 2019, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Vamadevan, V.; Oh, W.Y.; Peng, H. Phenolic Compounds in Agri-Food by-Products, Their Bioavailability and Health Effects. J. Food Bioact. 2019, 5, 57–119. [Google Scholar] [CrossRef]
- Esmat, A.Y.; Said, M.M.; Soliman, A.A.; El-Masry, K.S.H.; Badiea, E.A. Bioactive Compounds, Antioxidant Potential, and Hepatoprotective Activity of Sea Cucumber (Holothuria atra) against Thioacetamide Intoxication in Rats. Nutrition 2013, 29, 258–267. [Google Scholar] [CrossRef]
- Fahmy, S.R. Anti-Fibrotic Effect of Holothuria Arenicola Extract against Bile Duct Ligation in Rats. BMC Complement. Altern. Med. 2015, 15, 14. [Google Scholar] [CrossRef]
- Dakrory, A.I.; Fahmy, S.R.; Soliman, A.M.; Mohamed, A.S.; Amer, S.A.M. Protective and Curative Effects of the Sea Cucumber Holothuria atra Extract against DMBA-Induced Hepatorenal Diseases in Rats. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Aatab, F.; Bellali, F.; Aboudamia, F.Z.; Errhif, A.; Kharroubi, M. Phenolic Compounds and in Vitro Antioxidant Activity of Spray-Dried and Freeze-Dried Aqueous Extracts of Sea Cucumber (Holothuria tubulosa). J. Appl. Biol. Biotechnol. 2023, 11, 158–167. [Google Scholar] [CrossRef]
- Noonong, K.; Sobhon, P.; Sroyraya, M.; Chaithirayanon, K. Neuroprotective and Neurorestorative Effects of Holothuria scabra Extract in the MPTP/MPP+-Induced Mouse and Cellular Models of Parkinson’s Disease. Front. Neurosci. 2020, 14, 575459. [Google Scholar] [CrossRef] [PubMed]
- Kleawyothatis, W.; Jattujan, P.; Chumphoochai, K.; Chalorak, P.; Sobhon, P.; Meemon, K. Holothuria scabra Extracts Confer Neuroprotective Effect in C. elegans Model of Alzheimer’s Disease by Attenuating Amyloid-β Aggregation and Toxicity. J. Tradit. Complement. Med. 2023, 13, 93–104. [Google Scholar] [CrossRef]
- Pangestuti, R.; Murniasih, T.; Yunovilsa Putra, M.; Rasyid, A.; Tri Wibowo, J.; Ardiansyah, A.; Untari, F. Free Radical Scavenging Activity of Selected Sea Cucumber Species from Mataram-Lombok, Indonesia. J. Teknol. 2016, 78. [Google Scholar] [CrossRef]
- Wen, M.; Xu, J.; Ding, L.; Zhang, L.; Du, L.; Wang, J.; Wang, Y.; Xue, C. Eicosapentaenoic Acid-Enriched Phospholipids Improve Aβ1–40-Induced Cognitive Deficiency in a Rat Model of Alzheimer’s Disease. J. Funct. Foods 2016, 24, 537–548. [Google Scholar] [CrossRef]
- Zhou, M.; Xue, Y.; Sun, S.; Wen, M.; Li, Z.; Xu, J.; Wang, J.; Yanagita, T.; Wang, Y.; Xue, C. Effects of Different Fatty Acids Composition of Phosphatidylcholine on Brain Function of Dementia Mice Induced by Scopolamine. Lipids Health Dis. 2016, 15, 135. [Google Scholar] [CrossRef]
- Che, H.; Fu, X.; Zhang, L.; Gao, X.; Wen, M.; Du, L.; Xue, C.; Xu, J.; Wang, Y. Neuroprotective Effects of N-3 Polyunsaturated Fatty Acid-Enriched Phosphatidylserine Against Oxidative Damage in PC12 Cells. Cell. Mol. Neurobiol. 2018, 38, 657–668. [Google Scholar] [CrossRef]
- Wang, X.; Cong, P.; Chen, Q.; Li, Z.; Xu, J.; Xue, C. Characterizing the Phospholipid Composition of Six Edible Sea Cucumbers by NPLC-Triple TOF-MS/MS. J. Food Compos. Anal. 2020, 94, 103626. [Google Scholar] [CrossRef]
- Ermolenko, E.V.; Sikorskaya, T.V.; Grigorchuk, V.P. The Phospholipid Molecular Species Profile of Apostichopus japonicus Tissues Modifies through Exposure to N-3 Polyunsaturated Fatty Acid-Deficient Diet. Mar. Drugs 2022, 20, 578. [Google Scholar] [CrossRef]
- Sanguanphun, T.; Sornkaew, N.; Malaiwong, N.; Chalorak, P.; Jattujan, P.; Niamnont, N.; Sobhon, P.; Meemon, K. Neuroprotective Effects of a Medium Chain Fatty Acid, Decanoic Acid, Isolated from H. Leucospilota against Parkinsonism in C. elegans PD Model. Front. Pharmacol. 2022, 13, 1004568. [Google Scholar] [CrossRef] [PubMed]
- Blanco, A.; Blanco, G. Lipids. In Medical Biochemistry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 99–119. ISBN 978-0-12-803550-4. [Google Scholar]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Imran, A.; Qasim, M.; Zafar, S.; Kamran, S.K.S.; Razzaq, A.; Aziz, N.; et al. Role of Cholesterol and Sphingolipids in Brain Development and Neurological Diseases. Lipids Health Dis. 2019, 18, 26. [Google Scholar] [CrossRef]
- Zheng, L.; Fleith, M.; Giuffrida, F.; O’Neill, B.V.; Schneider, N. Dietary Polar Lipids and Cognitive Development: A Narrative Review. Adv. Nutr. Bethesda Md 2019, 10, 1163–1176. [Google Scholar] [CrossRef]
- Eguchi, K.; Mikami, D.; Sun, H.; Tsumita, T.; Takahashi, K.; Mukai, K.; Yuyama, K.; Igarashi, Y. Blood-Brain Barrier Permeability Analysis of Plant Ceramides. PLoS ONE 2020, 15, e0241640. [Google Scholar] [CrossRef]
- Wu, F.-J.; Xue, Y.; Tang, Q.-J.; Xu, J.; Du, L.; Xue, C.-H.; Takahashi, K.; Wang, Y.-M. The Protective Effects of Cerebrosides from Sea Cucumber and Starfish on the Oxidative Damage in PC12 Cells. J. Oleo Sci. 2013, 62, 717–727. [Google Scholar] [CrossRef]
- Nobsathian, S.; Tuchinda, P.; Sobhon, P.; Tinikul, Y.; Poljaroen, J.; Tinikul, R.; Sroyraya, M.; Poomton, T.; Chaichotranunt, S. An Antioxidant Activity of the Whole Body of Holothuria scabra. Chem. Biol. Technol. Agric. 2017, 4, 4. [Google Scholar] [CrossRef]
- Tangrodchanapong, T.; Sornkaew, N.; Yurasakpong, L.; Niamnont, N.; Nantasenamat, C.; Sobhon, P.; Meemon, K. Beneficial Effects of Cyclic Ether 2-Butoxytetrahydrofuran from Sea Cucumber Holothuria scabra against Aβ Aggregate Toxicity in Transgenic Caenorhabditis elegans and Potential Chemical Interaction. Molecules 2021, 26, 2195. [Google Scholar] [CrossRef] [PubMed]
- Jattujan, P.; Srisirirung, S.; Watcharaporn, W.; Chumphoochai, K.; Kraokaew, P.; Sanguanphun, T.; Prasertsuksri, P.; Thongdechsri, S.; Sobhon, P.; Meemon, K. 2-Butoxytetrahydrofuran and Palmitic Acid from Holothuria scabra Enhance C. elegans Lifespan and Healthspan via DAF-16/FOXO and SKN-1/NRF2 Signaling Pathways. Pharmaceuticals 2022, 15, 1374. [Google Scholar] [CrossRef]
- Zakharenko, A.; Romanchenko, D.; Thinh, P.D.; Pikula, K.; Hang, C.T.T.; Yuan, W.; Xia, X.; Chaika, V.; Chernyshev, V.; Zakharenko, S.; et al. Features and Advantages of Supercritical CO2 Extraction of Sea Cucumber Cucumaria frondosa Japonica Semper, 1868. Molecules 2020, 25, 4088. [Google Scholar] [CrossRef]
- Chasanah, E.; Fawzya, Y.N.; Tarman, K.; Januar, H.I.; Nursid, M. Fatty Acid Profile, Carotenoid Content, and In Vitro Anticancer Activity of Karimunjawa and Lampung Sea Cucumber. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2016, 11, 117. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Jiang, T.; Lv, Z. Novel Branch Patterns and Anticoagulant Activity of Glycosaminoglycan from Sea Cucumber Apostichopus japonicus. Int. J. Biol. Macromol. 2015, 72, 911–918. [Google Scholar] [CrossRef]
- Wang, X.-H.; Zou, Z.-R.; Yi, Y.-H.; Han, H.; Li, L.; Pan, M.-X. Variegatusides: New Non-Sulphated Triterpene Glycosides from the Sea Cucumber Stichopus variegates Semper. Mar. Drugs 2014, 12, 2004–2018. [Google Scholar] [CrossRef]
- Siregar, M.; Bachtiar, E.; Nurhayati, A.; Lewaru, M. Antioxidant Activity of Gamat (Stichopus variegatus) and Milk Sea Cucumbers (Holothuria fuscocinerea) from the Thousand Islands National Park Waters. J. Aquac. Fish Health 2023, 12, 390–404. [Google Scholar] [CrossRef]
- Pringgenies, D.; Rudiyanti, S.; Yudiati, E. Exploration of Sea Cucumbers Stichopus hermanii from Karimunjawa Islands as Production of Marine Biological Resources. IOP Conf. Ser. Earth Environ. Sci. 2018, 116, 012039. [Google Scholar] [CrossRef]
- Althunibat, O.; Ridzwan, B.; Taher, M.; Daud, J.; Jauhari Arief Ichwan, S.; Qaralleh, H. Antioxidant and Cytotoxic Properties of Two Sea Cucumbers, Holothuria edulis Lesson and Stichopus horrens Selenka. Acta Biol. Hung. 2013, 64, 10–20. [Google Scholar] [CrossRef]
- Keshavarz, M.; Shamsizadeh, F.; Tavakoli, A.; Baghban, N.; Khoradmehr, A.; Kameli, A.; Rasekh, P.; Daneshi, A.; Nabipour, I.; Vahdat, K.; et al. Chemical Compositions and Experimental and Computational Modeling Activity of Sea Cucumber Holothuria Parva Ethanolic Extract against Herpes Simplex Virus Type 1. Biomed. Pharmacother. 2021, 141, 111936. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, H.; Vazirizadeh, A.; Nabipour, I.; Najafi, A.; Tajbakhsh, S.; Bahabadi, M. In Vitro Study of Antibacterial Activities of Ethanol, Methanol and Acetone Extracts from Sea Cucumber Holothuria Parva. Iran. J. Fish. Sci. 2018, 17, 542–551. [Google Scholar] [CrossRef]
- Salimi, A.; Motallebi, A.; Ayatollahi, M.; Seydi, E.; Mohseni, A.R.; Nazemi, M.; Pourahmad, J. Selective Toxicity of Persian Gulf Sea Cucumber Holothuria Parva on Human Chronic Lymphocytic Leukemia b Lymphocytes by Direct Mitochondrial Targeting. Environ. Toxicol. 2017, 32, 1158–1169. [Google Scholar] [CrossRef] [PubMed]
- Amidi, S.; Hashemi, Z.; Motallebi, A.; Nazemi, M.; Farrokhpayam, H.; Seydi, E.; Pourahmad, J. Identification of (Z)-2,3-Diphenylacrylonitrile as Anti-Cancer Molecule in Persian Gulf Sea Cucumber Holothuria Parva. Mar. Drugs 2017, 15, 314. [Google Scholar] [CrossRef]
- Telahigue, K.; Ghali, R.; Nouiri, E.; Labidi, A.; Hajji, T. Antibacterial Activities and Bioactive Compounds of the Ethyl Acetate Extract of the Sea Cucumber Holothuria forskali from Tunisian Coasts. J. Mar. Biol. Assoc. UK 2020, 100, 229–237. [Google Scholar] [CrossRef]
- García, J.; Méndez, D.; Álvarez, M.; Sanmartin, B.; Vázquez, R.; Regueiro, L.; Atanassova, M. Design of Novel Functional Food Products Enriched with Bioactive Extracts from Holothurians for Meeting the Nutritional Needs of the Elderly. LWT 2019, 109, 55–62. [Google Scholar] [CrossRef]
- Mou, J.; Wang, C.; Li, W.; Yang, J. Purification, Structural Characterization and Anticoagulant Properties of Fucosylated Chondroitin Sulfate Isolated from Holothuria Mexicana. Int. J. Biol. Macromol. 2017, 98, 208–215. [Google Scholar] [CrossRef]
- Ben Mansour, M.; Balti, R.; Ollivier, V.; Ben Jannet, H.; Chaubet, F.; Maaroufi, R.M. Characterization and Anticoagulant Activity of a Fucosylated Chondroitin Sulfate with Unusually Procoagulant Effect from Sea Cucumber. Carbohydr. Polym. 2017, 174, 760–771. [Google Scholar] [CrossRef]
- Omran, N.E.; Khedr, A.M. Structure Elucidation, Protein Profile and the Antitumor Effect of the Biological Active Substance Extracted from Sea Cucumber Holothuria polii. Toxicol. Ind. Health 2015, 31, 1–8. [Google Scholar] [CrossRef]
- Li, C.; Niu, Q.; Li, S.; Zhang, X.; Liu, C.; Cai, C.; Li, G.; Yu, G. Fucoidan from Sea Cucumber Holothuria Polii: Structural Elucidation and Stimulation of Hematopoietic Activity. Int. J. Biol. Macromol. 2020, 154, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- El Barky, A.R.; Hussein, S.A.; Alm-Eldeen, A.A.; Hafez, Y.A.; Mohamed, T.M. Anti-Diabetic Activity of Holothuria Thomasi Saponin. Biomed. Pharmacother. 2016, 84, 1472–1487. [Google Scholar] [CrossRef]
- Alper, M.; Güneş, M. Evaluation of Cytotoxic, Apoptotic Effects and Phenolic Compounds of Sea Cucumber Holothuria tubulosa (Gmelin, 1791) Extracts. Turk. J. Vet. Anim. Sci. 2020, 44, 641–655. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Silchenko, A.S.; Grebnev, B.B.; Stonik, V.A.; Nifantiev, N.E.; Usov, A.I. Fucosylated Chondroitin Sulfates from the Sea Cucumbers Paracaudina Chilensis and Holothuria Hilla: Structures and Anticoagulant Activity. Mar. Drugs 2020, 18, 540. [Google Scholar] [CrossRef]
- Dhinakaran, D.I.; Lipton, A.P. Bioactive Compounds from Holothuria atra of Indian Ocean. SpringerPlus 2014, 3, 673. [Google Scholar] [CrossRef] [PubMed]
- Assawasuparerk, K.; Vanichviriyakit, R.; Chotwiwatthanakun, C.; Nobsathian, S.; Rawangchue, T.; Wittayachumnankul, B. Scabraside D Extracted from Holothuria scabra Induces Apoptosis and Inhibits Growth of Human Cholangiocarcinoma Xenografts in Mice. Asian Pac. J. Cancer Prev. 2016, 17, 511–517. [Google Scholar] [CrossRef]
- Sangpairoj, K.; Chaithirayanon, K.; Vivithanaporn, P.; Siangcham, T.; Jattujan, P.; Poomtong, T.; Nobsathian, S.; Sobhon, P. Extract of the Sea Cucumber, Holothuria scabra, Induces Apoptosis in Human Glioblastoma Cell Lines. Funct. Foods Health Dis. 2016, 6, 452. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.A.; Eltamany, E.E.; Hal, D.M.; Ibrahim, A.K.; AboulMagd, A.M.; Al-Warhi, T.; Youssif, K.A.; Abd El-kader, A.M.; Hassanean, H.A.; Fayez, S.; et al. New Cytotoxic Cerebrosides from the Red Sea Cucumber Holothuria Spinifera Supported by In-Silico Studies. Mar. Drugs 2020, 18, 405. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Abdelmohsen, U.R.; Hal, D.M.; Ibrahim, A.K.; Hassanean, H.A.; Abdelhameed, R.F.A.; Temraz, T.A.; Hajjar, D.; Makki, A.A.; Hendawy, O.M.; et al. Holospiniferoside: A New Antitumor Cerebroside from The Red Sea Cucumber Holothuria Spinifera: In Vitro and In Silico Studies. Molecules 2021, 26, 1555. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Tsvetkova, E.A.; Nikogosova, S.P.; Hang, C.T.T.; Thinh, P.D.; Trung, D.T.; Van, T.T.T.; Shashkov, A.S.; et al. Fucose-Rich Sulfated Polysaccharides from Two Vietnamese Sea Cucumbers Bohadschia Argus and Holothuria (Theelothuria) Spinifera: Structures and Anticoagulant Activity. Mar. Drugs 2022, 20, 380. [Google Scholar] [CrossRef] [PubMed]
- Vien, L.T.; Hanh, T.T.H.; Huong, P.T.T.; Van Thanh, N.; Cuong, N.X.; Nam, N.H.; Thung, D.C. Triterpene Tetraglycosides from the Vietnamese Sea Cucumber Holothuria Impatiens. Vietnam J. Chem. 2018, 56, 612–616. [Google Scholar] [CrossRef]
- Dai, Y.-L.; Kim, E.-A.; Luo, H.-M.; Jiang, Y.-F.; Oh, J.-Y.; Heo, S.-J.; Jeon, Y.-J. Characterization and Anti-Tumor Activity of Saponin-Rich Fractions of South Korean Sea Cucumbers (Apostichopus japonicus). J. Food Sci. Technol. 2020, 57, 2283–2292. [Google Scholar] [CrossRef]
- Wan, H.; Han, J.; Tang, S.; Bao, W.; Lu, C.; Zhou, J.; Ming, T.; Li, Y.; Su, X. Comparisons of Protective Effects between Two Sea Cucumber Hydrolysates against Diet Induced Hyperuricemia and Renal Inflammation in Mice. Food Funct. 2020, 11, 1074–1086. [Google Scholar] [CrossRef]
- He, W.; Sun, H.; Su, L.; Zhou, D.; Zhang, X.; Shanggui, D.; Chen, Y. Structure and Anticoagulant Activity of a Sulfated Fucan from the Sea Cucumber Acaudina leucoprocta. Int. J. Biol. Macromol. 2020, 164, 87–94. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, J.; Xu, C.; Li, Q.; Ma, Y.; Zhao, S.; Zhuang, J.; Shen, F.; Wang, Q.; Feng, F.; et al. Sea Cucumber (Acaudina leucoprocta) Peptides Extended the Lifespan and Enhanced Antioxidant Capacity via DAF-16/DAF-2/SOD-3/OLD-1/PEPT-1 in Caenorhabditis elegans. Front. Nutr. 2022, 9, 1065145. [Google Scholar] [CrossRef]
- Zhang, B.; Xue, C.; Hu, X.; Xu, J.; Li, Z.; Wang, J.; Yanagita, T.; Xue, Y.; Wang, Y. Dietary Sea Cucumber Cerebroside Alleviates Orotic Acid-Induced Excess Hepatic Adipopexis in Rats. Lipids Health Dis. 2012, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, J.; Xu, Y.; Yang, H.; Wang, J.; Xue, C.; Yan, X.; Su, L. Anti-Inflammation Effects of Fucosylated Chondroitin Sulphate from Acaudina molpadioides by Altering Gut Microbiota in Obese Mice. Food Funct. 2019, 10, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, S.; Wang, J.; Li, S.; Jiang, W. Fucoidan from Acaudina Molpadioides Protects Pancreatic Islet against Cell Apoptosis via Inhibition of Inflammation in Type 2 Diabetic Mice. Food Sci. Biotechnol. 2016, 25, 293–300. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andryjaschenko, P.V.; Dmitrenok, P.S.; Menchinskaya, E.S.; Aminin, D.L.; Kalinin, V.I. Structure of Cucumarioside I 2 from the Sea Cucumber Eupentacta fraudatrix (Djakonov et Baranova) and Cytotoxic and Immunostimulatory Activities of This Saponin and Relative Compounds. Nat. Prod. Res. 2013, 27, 1776–1783. [Google Scholar] [CrossRef]
- Elbandy, M.; Rho, J.R.; Afifi, R. Analysis of Saponins as Bioactive Zoochemicals from the Marine Functional Food Sea Cucumber Bohadschia Cousteaui. Eur. Food Res. Technol. 2014, 238, 937–955. [Google Scholar] [CrossRef]
- Yang, W.-S.; Qi, X.-R.; Xu, Q.-Z.; Yuan, C.-H.; Yi, Y.-H.; Tang, H.-F.; Shen, L.; Han, H. A New Sulfated Triterpene Glycoside from the Sea Cucumber Colochirus Quadrangularis, and Evaluation of Its Antifungal, Antitumor and Immunomodulatory Activities. Bioorg. Med. Chem. 2021, 41, 116188. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Andrijaschenko, P.V.; Popov, R.S.; Dmitrenok, P.S.; Chingizova, E.A.; Ermakova, S.P.; Malyarenko, O.S.; Dautov, S.S.; et al. Structures and Bioactivities of Quadrangularisosides A, A1, B, B1, B2, C, C1, D, D1–D4, and E from the Sea Cucumber Colochirus Quadrangularis: The First Discovery of the Glycosides, Sulfated by C-4 of the Terminal 3-O-Methylglucose Residue. Synergetic Effect on Colony Formation of Tumor HT-29 Cells of These Glycosides with Radioactive Irradiation. Mar. Drugs 2020, 18, 394. [Google Scholar] [CrossRef]
- Silchenko, A.S.; Kalinovsky, A.I.; Avilov, S.A.; Kalinin, V.I.; Andrijaschenko, P.V.; Dmitrenok, P.S.; Chingizova, E.A.; Ermakova, S.P.; Malyarenko, O.S.; Dautova, T.N. Nine New Triterpene Glycosides, Magnumosides A1–A4, B1, B2, C1, C2 and C4, from the Vietnamese Sea Cucumber Neothyonidium (=Massinium) Magnum: Structures and Activities against Tumor Cells Independently and in Synergy with Radioactive Irradiation. Mar. Drugs 2017, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Chim-Chi, Y.; Olivera-Castillo, L.; Betancur-Ancona, D.; Chel-Guerrero, L. Protein Hydrolysate Fractions from Sea Cucumber (Isostichopus badionotus) Inhibit Angiotensin-Converting Enzyme. J. Aquat. Food Prod. Technol. 2017, 26, 1199–1209. [Google Scholar] [CrossRef]
- He, M.; Wang, J.; Hu, S.; Wang, Y.; Xue, C.; Li, H. The Effects of Fucosylated Chondroitin Sulfate Isolated from Isostichopus Badionotus on Antimetastatic Activity via Down-Regulation of Hif-1α and Hpa. Food Sci. Biotechnol. 2014, 23, 1643–1651. [Google Scholar] [CrossRef]
- Mildenberger, J.; Remm, M.; Atanassova, M. Self-Assembly Potential of Bioactive Peptides from Norwegian Sea Cucumber Parastichopus Tremulus for Development of Functional Hydrogels. LWT 2021, 148, 111678. [Google Scholar] [CrossRef]
- Sottorff, I.; Aballay, A.; Hernández, V.; Roa, L.; Muñoz, L.X.; Silva, M.; Becerra, J.; Astuya, A. Characterization of Bioactive Molecules Isolated from Sea Cucumber Athyonidium Chilensis. Rev. Biol. Mar. Oceanogr. 2013, 48, 23–35. [Google Scholar] [CrossRef]
- Abedin, M.Z.; Karim, A.A.; Latiff, A.A.; Gan, C.-Y.; Ghazali, F.C.; Barzideh, Z.; Ferdosh, S.; Akanda, M.J.H.; Zzaman, W.; Karim, M.R.; et al. Biochemical and Radical-Scavenging Properties of Sea Cucumber (Stichopus vastus) Collagen Hydrolysates. Nat. Prod. Res. 2014, 28, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Lai, S.; Huang, R.; Wu, M.; Gao, N.; Xu, L.; Qin, H.; Peng, W.; Zhao, J. Structure and Anticoagulant Activity of Fucosylated Glycosaminoglycan Degraded by Deaminative Cleavage. Carbohydr. Polym. 2013, 98, 1514–1523. [Google Scholar] [CrossRef]
- Huang, N.; Wu, M.-Y.; Zheng, C.-B.; Zhu, L.; Zhao, J.-H.; Zheng, Y.-T. The Depolymerized Fucosylated Chondroitin Sulfate from Sea Cucumber Potently Inhibits HIV Replication via Interfering with Virus Entry. Carbohydr. Res. 2013, 380, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.; Wu, M.; Huang, N.; Gao, N.; Xiao, C.; Li, Z.; Zhang, Z.; Zheng, Y.; Peng, W.; Zhao, J. Anti-HIV-1 Activity and Structure–Activity-Relationship Study of a Fucosylated Glycosaminoglycan from an Echinoderm by Targeting the Conserved CD4 Induced Epitope. Biochim. Biophys. Acta BBA—Gen. Subj. 2013, 1830, 4681–4691. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Gao, N.; Xiao, C.; Lin, L.; Purcell, S.W.; Wu, M.; Zhao, J. Modulating the Degree of Fucosylation of Fucosylated Chondroitin Sulfate Enhances Heparin Cofactor II-Dependent Thrombin Inhibition. Eur. J. Med. Chem. 2018, 154, 133–143. [Google Scholar] [CrossRef]
- Han, Q.; Li, K.; Dong, X.; Luo, Y.; Zhu, B. Function of Thelenota Ananas Saponin Desulfated Holothurin A in Modulating Cholesterol Metabolism. Sci. Rep. 2018, 8, 9506. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-A.; Jia, S.; Li, K.; Sui, Y.; Hong, H.; Dong, X.; Luo, Y.; Zhu, B. Thelenota Ananas Saponin Extracts Attenuate the Atherosclerosis in apoE−/− Mice by Modulating Lipid Metabolism. J. Funct. Foods 2019, 58, 238–247. [Google Scholar] [CrossRef]
- Bonetto, V.; Ferraresi, A.; Sampò, S.; Isidoro, C. Fungal Bioactive Compounds as Emerging Therapeutic Options for Age-Related Neurodegenerative Disorders. Int. J. Mol. Sci. 2025, 26, 4800. [Google Scholar] [CrossRef]
- Desai, A.V.; Lu, M.; Marcus, S.; Saran, A.; Malankar, A.; Mazumder, A. A Phase II Trial of TBL12 Sea Cucumber Extract in Patients with Untreated Asymptomatic Myeloma. Blood 2014, 124, 5733. [Google Scholar] [CrossRef]
- Shen, J.; Swift, B.; Mamelok, R.; Pine, S.; Sinclair, J.; Attar, M. Design and Conduct Considerations for First-in-Human Trials. Clin. Transl. Sci. 2019, 12, 6–19. [Google Scholar] [CrossRef]
- Twohig, D.; Nielsen, H.M. α-Synuclein in the Pathophysiology of Alzheimer’s Disease. Mol. Neurodegener. 2019, 14, 23. [Google Scholar] [CrossRef]
- Mohamed, T.; Shakeri, A.; Rao, P.P.N. Amyloid Cascade in Alzheimer’s Disease: Recent Advances in Medicinal Chemistry. Eur. J. Med. Chem. 2016, 113, 258–272. [Google Scholar] [CrossRef]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and Progress of Hypotheses and Clinical Trials for Alzheimer’s Disease. Signal Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef]
- Ittner, L.M.; Götz, J. Amyloid-β and Tau--a Toxic Pas de Deux in Alzheimer’s Disease. Nat. Rev. Neurosci. 2011, 12, 65–72. [Google Scholar] [CrossRef]
- Hyman, B.T.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Carrillo, M.C.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; et al. National Institute on Aging-Alzheimer’s Association Guidelines for the Neuropathologic Assessment of Alzheimer’s Disease. Alzheimers Dement. J. Alzheimers Assoc. 2012, 8, 1–13. [Google Scholar] [CrossRef]
- Hardy, J. Has the Amyloid Cascade Hypothesis for Alzheimers Disease Been Proved? Curr. Alzheimer Res. 2006, 3, 71–73. [Google Scholar] [CrossRef]
- Konzack, S.; Thies, E.; Marx, A.; Mandelkow, E.-M.; Mandelkow, E. Swimming against the Tide: Mobility of the Microtubule-Associated Protein Tau in Neurons. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 9916–9927. [Google Scholar] [CrossRef]
- Haass, C.; Selkoe, D.J. Soluble Protein Oligomers in Neurodegeneration: Lessons from the Alzheimer’s Amyloid Beta-Peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef]
- Hanger, D.P.; Anderton, B.H.; Noble, W. Tau Phosphorylation: The Therapeutic Challenge for Neurodegenerative Disease. Trends Mol. Med. 2009, 15, 112–119. [Google Scholar] [CrossRef]
- Roberson, E.D.; Scearce-Levie, K.; Palop, J.J.; Yan, F.; Cheng, I.H.; Wu, T.; Gerstein, H.; Yu, G.-Q.; Mucke, L. Reducing Endogenous Tau Ameliorates Amyloid Beta-Induced Deficits in an Alzheimer’s Disease Mouse Model. Science 2007, 316, 750–754. [Google Scholar] [CrossRef]
- Busche, M.A.; Hyman, B.T. Synergy between Amyloid-β and Tau in Alzheimer’s Disease. Nat. Neurosci. 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of Oxidative Stress in Alzheimer’s Disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Castellani, R.; Perry, G. Oxidative Stress and Alzheimer’s Disease. In Inflammation, Aging, and Oxidative Stress; Bondy, S.C., Campbell, A., Eds.; Oxidative Stress in Applied Basic Research and Clinical Practice; Springer International Publishing: Cham, Switzerland, 2016; pp. 189–198. ISBN 978-3-319-33484-4. [Google Scholar]
- Uddin, M.S.; Kabir, M.T. Oxidative Stress in Alzheimer’s Disease: Molecular Hallmarks of Underlying Vulnerability. In Biological, Diagnostic and Therapeutic Advances in Alzheimer’s Disease; Ashraf, G.M., Alexiou, A., Eds.; Springer Singapore: Singapore, 2019; pp. 91–115. ISBN 978-981-13-9635-9. [Google Scholar]
- Nunomura, A.; Castellani, R.J.; Zhu, X.; Moreira, P.I.; Perry, G.; Smith, M.A. Involvement of Oxidative Stress in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2006, 65, 631–641. [Google Scholar] [CrossRef]
- Pocernich, C.B.; Butterfield, D.A. Elevation of Glutathione as a Therapeutic Strategy in Alzheimer Disease. Biochim. Biophys. Acta BBA—Mol. Basis Dis. 2012, 1822, 625–630. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhen, Y.; Wang, G.; Liu, B. Deconvoluting the Complexity of Reactive Oxygen Species (ROS) in Neurodegenerative Diseases. Front. Neuroanat. 2022, 16, 910427. [Google Scholar] [CrossRef]
- Wang, L.; Yin, Y.-L.; Liu, X.-Z.; Shen, P.; Zheng, Y.-G.; Lan, X.-R.; Lu, C.-B.; Wang, J.-Z. Current Understanding of Metal Ions in the Pathogenesis of Alzheimer’s Disease. Transl. Neurodegener. 2020, 9, 10. [Google Scholar] [CrossRef]
- Li, Y.; Fan, H.; Sun, J.; Ni, M.; Zhang, L.; Chen, C.; Hong, X.; Fang, F.; Zhang, W.; Ma, P. Circular RNA Expression Profile of Alzheimer’s Disease and Its Clinical Significance as Biomarkers for the Disease Risk and Progression. Int. J. Biochem. Cell Biol. 2020, 123, 105747. [Google Scholar] [CrossRef]
- Lecrux, C.; Sandoe, C.H.; Neupane, S.; Kropf, P.; Toussay, X.; Tong, X.-K.; Lacalle-Aurioles, M.; Shmuel, A.; Hamel, E. Impact of Altered Cholinergic Tones on the Neurovascular Coupling Response to Whisker Stimulation. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 1518–1531. [Google Scholar] [CrossRef]
- Hung, S.-Y.; Fu, W.-M. Drug Candidates in Clinical Trials for Alzheimer’s Disease. J. Biomed. Sci. 2017, 24, 47. [Google Scholar] [CrossRef] [PubMed]
- Majdi, A.; Sadigh-Eteghad, S.; Rahigh Aghsan, S.; Farajdokht, F.; Vatandoust, S.M.; Namvaran, A.; Mahmoudi, J. Amyloid-β, Tau, and the Cholinergic System in Alzheimer’s Disease: Seeking Direction in a Tangle of Clues. Rev. Neurosci. 2020, 31, 391–413. [Google Scholar] [CrossRef] [PubMed]
- López González, I.; Garcia-Esparcia, P.; Llorens, F.; Ferrer, I. Genetic and Transcriptomic Profiles of Inflammation in Neurodegenerative Diseases: Alzheimer, Parkinson, Creutzfeldt-Jakob and Tauopathies. Int. J. Mol. Sci. 2016, 17, 206. [Google Scholar] [CrossRef]
- Thakur, S.; Dhapola, R.; Sarma, P.; Medhi, B.; Reddy, D.H. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2023, 46, 1–17. [Google Scholar] [CrossRef]
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The Devil Is in the Details. J. Neurochem. 2016, 139 (Suppl. S2), 136–153. [Google Scholar] [CrossRef] [PubMed]
- Gilhus, N.E.; Deuschl, G. Neuroinflammation—A Common Thread in Neurological Disorders. Nat. Rev. Neurol. 2019, 15, 429–430. [Google Scholar] [CrossRef]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 Inflammasome Activation Drives Tau Pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, S.; Iijima, R.; Ogishima, S.; Kikuchi, M.; Matsuoka, Y.; Ghosh, S.; Miyamoto, T.; Miyashita, A.; Kuwano, R.; Tanaka, H. AlzPathway: A Comprehensive Map of Signaling Pathways of Alzheimer’s Disease. BMC Syst. Biol. 2012, 6, 52. [Google Scholar] [CrossRef]
- Srivastava, S.; Ahmad, R.; Khare, S.K. Alzheimer’s Disease and Its Treatment by Different Approaches: A Review. Eur. J. Med. Chem. 2021, 216, 113320. [Google Scholar] [CrossRef]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s Disease: Treatment Strategies and Their Limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef]
- Husna Ibrahim, N.; Yahaya, M.F.; Mohamed, W.; Teoh, S.L.; Hui, C.K.; Kumar, J. Pharmacotherapy of Alzheimer’s Disease: Seeking Clarity in a Time of Uncertainty. Front. Pharmacol. 2020, 11, 261. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, J.; Xia, Y.; Zhang, J.; Chen, L. Recent Advances in Alzheimer’s Disease: Mechanisms, Clinical Trials and New Drug Development Strategies. Signal Transduct. Target. Ther. 2024, 9, 211. [Google Scholar] [CrossRef]
- Jia, C.; Chai, J.; Zhang, S.; Sun, Y.; He, L.; Sang, Z.; Chen, D.; Zheng, X. The Advancements of Marine Natural Products in the Treatment of Alzheimer’s Disease: A Study Based on Cell and Animal Experiments. Mar. Drugs 2025, 23, 91. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Ghanem, A.W.; AbuMadi, S.; Thaher, D.; Jaghama, W.; Karaman, D.; Karaman, R. Promising Natural Remedies for Alzheimer’s Disease Therapy. Molecules 2025, 30, 922. [Google Scholar] [CrossRef]
- Wright Willis, A.; Evanoff, B.A.; Lian, M.; Criswell, S.R.; Racette, B.A. Geographic and Ethnic Variation in Parkinson Disease: A Population-Based Study of US Medicare Beneficiaries. Neuroepidemiology 2010, 34, 143–151. [Google Scholar] [CrossRef]
- Liu, S.; Ninan, I.; Antonova, I.; Battaglia, F.; Trinchese, F.; Narasanna, A.; Kolodilov, N.; Dauer, W.; Hawkins, R.D.; Arancio, O. α-Synuclein Produces a Long-Lasting Increase in Neurotransmitter Release. EMBO J. 2004, 23, 4506–4516. [Google Scholar] [CrossRef]
- Pfeiffer, R.F. Non-Motor Symptoms in Parkinson’s Disease. Parkinsonism Relat. Disord. 2016, 22, S119–S122. [Google Scholar] [CrossRef]
- Percário, S.; Da Silva Barbosa, A.; Varela, E.L.P.; Gomes, A.R.Q.; Ferreira, M.E.S.; De Nazaré Araújo Moreira, T.; Dolabela, M.F. Oxidative Stress in Parkinson’s Disease: Potential Benefits of Antioxidant Supplementation. Oxid. Med. Cell. Longev. 2020, 2020, 1–23. [Google Scholar] [CrossRef]
- Chen, C.; Turnbull, D.M.; Reeve, A.K. Mitochondrial Dysfunction in Parkinson’s Disease—Cause or Consequence? Biology 2019, 8, 38. [Google Scholar] [CrossRef]
- Muddapu, V.R.; Mandali, A.; Chakravarthy, V.S.; Ramaswamy, S. A Computational Model of Loss of Dopaminergic Cells in Parkinson’s Disease Due to Glutamate-Induced Excitotoxicity. Front. Neural Circuits 2019, 13, 11. [Google Scholar] [CrossRef]
- Tsoi, P.S.; Quan, M.D.; Ferreon, J.C.; Ferreon, A.C.M. Aggregation of Disordered Proteins Associated with Neurodegeneration. Int. J. Mol. Sci. 2023, 24, 3380. [Google Scholar] [CrossRef]
- Surmeier, D.J. Determinants of Dopaminergic Neuron Loss in Parkinson’s Disease. FEBS J. 2018, 285, 3657–3668. [Google Scholar] [CrossRef]
- Ramesh, S.; Arachchige, A.S.P.M. Depletion of Dopamine in Parkinson’s Disease and Relevant Therapeutic Options: A Review of the Literature. AIMS Neurosci. 2023, 10, 200–231. [Google Scholar] [CrossRef]
- Hardy, J. Failures in Protein Clearance Partly Underlie Late Onset Neurodegenerative Diseases and Link Pathology to Genetic Risk. Front. Neurosci. 2019, 13, 1304. [Google Scholar] [CrossRef]
- Michel, P.P.; Hirsch, E.C.; Hunot, S. Understanding Dopaminergic Cell Death Pathways in Parkinson Disease. Neuron 2016, 90, 675–691. [Google Scholar] [CrossRef]
- Chauhan, A.; Jeans, A.F. Is Parkinson’s Disease Truly a Prion-Like Disorder? An Appraisal of Current Evidence. Neurol. Res. Int. 2015, 2015, 345285. [Google Scholar] [CrossRef]
- Martin, I.; Dawson, V.L.; Dawson, T.M. Recent Advances in the Genetics of Parkinson’s Disease. Annu. Rev. Genomics Hum. Genet. 2011, 12, 301–325. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson Disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef]
- Simon, D.K.; Tanner, C.M.; Brundin, P. Parkinson Disease Epidemiology, Pathology, Genetics, and Pathophysiology. Clin. Geriatr. Med. 2020, 36, 1–12. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Tanji, K.; Odagiri, S.; Miki, Y.; Mori, F.; Takahashi, H. The Lewy Body in Parkinson’s Disease and Related Neurodegenerative Disorders. Mol. Neurobiol. 2013, 47, 495–508. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K.; Rüb, U.; de Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of Brain Pathology Related to Sporadic Parkinson’s Disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Srinivasan, E.; Chandrasekhar, G.; Chandrasekar, P.; Anbarasu, K.; Vickram, A.S.; Karunakaran, R.; Rajasekaran, R.; Srikumar, P.S. Alpha-Synuclein Aggregation in Parkinson’s Disease. Front. Med. 2021, 8, 736978. [Google Scholar] [CrossRef]
- Obeso, J.A.; Marin, C.; Rodriguez-Oroz, C.; Blesa, J.; Benitez-Temiño, B.; Mena-Segovia, J.; Rodríguez, M.; Olanow, C.W. The Basal Ganglia in Parkinson’s Disease: Current Concepts and Unexplained Observations. Ann. Neurol. 2008, 64 (Suppl. S2), S30–S46. [Google Scholar] [CrossRef]
- Homayoun, H. Parkinson Disease. Ann. Intern. Med. 2018, 169, ITC33–ITC48. [Google Scholar] [CrossRef]
- Iversen, L.L. Dopamine Handbook; Oxford University Press: Oxford, UK, 2010; ISBN 978-0-19-537303-5. [Google Scholar]
- Murphy, S.J.X.; Werring, D.J. Stroke: Causes and Clinical Features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef]
- Teasell, R.W.; Foley, N.C.; Bhogal, S.K.; Speechley, M.R. An Evidence-Based Review of Stroke Rehabilitation. Top. Stroke Rehabil. 2003, 10, 29–58. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Caplan, L.R.; Donnan, G.A.; Hennerici, M.G. Classification of Stroke Subtypes. Cerebrovasc. Dis. 2009, 27, 493–501. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Hossmann, K.-A. Cerebral Ischemia: Models, Methods and Outcomes. Neuropharmacology 2008, 55, 257–270. [Google Scholar] [CrossRef]
- Murphy, T.H.; Li, P.; Betts, K.; Liu, R. Two-Photon Imaging of Stroke Onset in Vivo Reveals That NMDA-Receptor Independent Ischemic Depolarization Is the Major Cause of Rapid Reversible Damage to Dendrites and Spines. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 1756–1772. [Google Scholar] [CrossRef]
- Sun, S.; Lv, W.; Li, S.; Zhang, Q.; He, W.; Min, Z.; Teng, C.; Chen, Y.; Liu, L.; Yin, J.; et al. Smart Liposomal Nanocarrier Enhanced the Treatment of Ischemic Stroke through Neutrophil Extracellular Traps and Cyclic Guanosine Monophosphate-Adenosine Monophosphate Synthase-Stimulator of Interferon Genes (cGAS-STING) Pathway Inhibition of Ischemic Penumbra. ACS Nano 2023, 17, 17845–17857. [Google Scholar] [CrossRef]
- Wang, C.; Sun, C.; Ding, Z.; Wu, X.; Liu, K.; Cao, J. Bioactive Materials Facilitate the Restoration of Neurological Function Post Cerebral Ischemic Stroke. Int. J. Nanomedicine 2024, 19, 14171–14191. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Puleo, M.G.; Velardo, M.C.; Corpora, F.; Daidone, M.; Pinto, A. Molecular Biology of Atherosclerotic Ischemic Strokes. Int. J. Mol. Sci. 2020, 21, 9372. [Google Scholar] [CrossRef]
- Sommer, C.J. Ischemic Stroke: Experimental Models and Reality. Acta Neuropathol. 2017, 133, 245–261. [Google Scholar] [CrossRef]
- Ownsworth, T.; Goadby, E.; Chambers, S.K. Support after Brain Tumor Means Different Things: Family Caregivers’ Experiences of Support and Relationship Changes. Front. Oncol. 2015, 5, 33. [Google Scholar] [CrossRef]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C.; et al. Challenges to Curing Primary Brain Tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef]
- Arvold, N.D.; Lee, E.Q.; Mehta, M.P.; Margolin, K.; Alexander, B.M.; Lin, N.U.; Anders, C.K.; Soffietti, R.; Camidge, D.R.; Vogelbaum, M.A.; et al. Updates in the Management of Brain Metastases. Neuro-Oncology 2016, 18, 1043–1065. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-Oncology 2015, 17 (Suppl. S4), iv1–iv62. [Google Scholar] [CrossRef]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- McFaline-Figueroa, J.R.; Lee, E.Q. Brain Tumors. Am. J. Med. 2018, 131, 874–882. [Google Scholar] [CrossRef]
- Truffi, M.; Sorrentino, L.; Corsi, F. Fibroblasts in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1234, 15–29. [Google Scholar] [CrossRef]
- Wesseling, P.; Capper, D. WHO 2016 Classification of Gliomas. Neuropathol. Appl. Neurobiol. 2018, 44, 139–150. [Google Scholar] [CrossRef]
- Nguyen, H.-M.; Guz-Montgomery, K.; Lowe, D.B.; Saha, D. Pathogenetic Features and Current Management of Glioblastoma. Cancers 2021, 13, 856. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A Summary. Acta Neuropathol. (Berl.) 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Noh, T.; Walbert, T. Brain Metastasis: Clinical Manifestations, Symptom Management, and Palliative Care. Handb. Clin. Neurol. 2018, 149, 75–88. [Google Scholar] [CrossRef]
- Seano, G.; Nia, H.T.; Emblem, K.E.; Datta, M.; Ren, J.; Krishnan, S.; Kloepper, J.; Pinho, M.C.; Ho, W.W.; Ghosh, M.; et al. Solid Stress in Brain Tumours Causes Neuronal Loss and Neurological Dysfunction and Can Be Reversed by Lithium. Nat. Biomed. Eng. 2019, 3, 230–245. [Google Scholar] [CrossRef]
- Van Roost, D.; Hartmann, A.; Quade, G. Changes of Cerebral Blood Flow Following Dexamethasone Treatment in Brain Tumour Patients. A Xe/CT Study. Acta Neurochir. 2001, 143, 37–43; discussion 43–44. [Google Scholar] [CrossRef]
- Da Ros, M.; De Gregorio, V.; Iorio, A.L.; Giunti, L.; Guidi, M.; de Martino, M.; Genitori, L.; Sardi, I. Glioblastoma Chemoresistance: The Double Play by Microenvironment and Blood-Brain Barrier. Int. J. Mol. Sci. 2018, 19, 2879. [Google Scholar] [CrossRef]
- Dubois, L.G.; Campanati, L.; Righy, C.; D’Andrea-Meira, I.; Spohr, T.C.L.d.S.E.; Porto-Carreiro, I.; Pereira, C.M.; Balça-Silva, J.; Kahn, S.A.; DosSantos, M.F.; et al. Gliomas and the Vascular Fragility of the Blood Brain Barrier. Front. Cell. Neurosci. 2014, 8, 418. [Google Scholar] [CrossRef]
- Watkins, S.; Robel, S.; Kimbrough, I.F.; Robert, S.M.; Ellis-Davies, G.; Sontheimer, H. Disruption of Astrocyte-Vascular Coupling and the Blood-Brain Barrier by Invading Glioma Cells. Nat. Commun. 2014, 5, 4196. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The Blood–Brain Barrier and Blood–Tumour Barrier in Brain Tumours and Metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Lin, X.; Yao, M.; Lu, J.-H.; Wang, Y.; Yin, X.; Liang, M.; Yuan, E.; Ren, J. Identification of Novel Oligopeptides from the Simulated Digestion of Sea Cucumber (Stichopus japonicus) to Alleviate Aβ Aggregation Progression. J. Funct. Foods 2019, 60, 103412. [Google Scholar] [CrossRef]
- Ma, E.-H.; Rathnayake, A.U.; Lee, J.K.; Lee, S.-M.; Byun, H.-G. Characterization of β-Secretase Inhibitory Extracts from Sea Cucumber (Stichopus japonicus) Hydrolysis with Their Cellular Level Mechanism in SH-SY5Y Cells. Eur. Food Res. Technol. 2021, 247, 2039–2052. [Google Scholar] [CrossRef]
- Song, Y.; Cong, P.; Lu, L.; Wang, Y.; Tang, Q.; Zhang, H.; Xu, J.; Xue, C. Effects of Dietary Glucocerebrosides from Sea Cucumber on the Brain Sphingolipid Profiles of Mouse Models of Alzheimer’s Disease. Food Funct. 2017, 8, 1271–1281. [Google Scholar] [CrossRef]
- Chalorak, P.; Sanguanphun, T.; Limboonreung, T.; Meemon, K. Neurorescue Effects of Frondoside A and Ginsenoside Rg3 in C. elegans Model of Parkinson’s Disease. Molecules 2021, 26, 4843. [Google Scholar] [CrossRef]
- Chalorak, P.; Sornkaew, N.; Manohong, P.; Niamnont, N.; Malaiwong, N.; Limboonreung, T.; Sobhon, P.; Aschner, M.; Meemon, K. Diterpene Glycosides from Holothuria scabra Exert the α-Synuclein Degradation and Neuroprotection against α-Synuclein-Mediated Neurodegeneration in C. elegans Model. J. Ethnopharmacol. 2021, 279, 114347. [Google Scholar] [CrossRef]
- Cui, C.; Cui, N.; Wang, P.; Song, S.; Liang, H.; Ji, A. Neuroprotective Effect of Sulfated Polysaccharide Isolated from Sea Cucumber Stichopus japonicus on 6-OHDA-Induced Death in SH-SY5Y through Inhibition of MAPK and NF-κB and Activation of PI3K/Akt Signaling Pathways. Biochem. Biophys. Res. Commun. 2016, 470, 375–383. [Google Scholar] [CrossRef]
- Cui, C.; Cui, N.; Wang, P.; Song, S.; Liang, H.; Ji, A. Sulfated Polysaccharide Isolated from the Sea Cucumber Stichopus japonicus Against PC12 Hypoxia/Reoxygenation Injury by Inhibition of the MAPK Signaling Pathway. Cell. Mol. Neurobiol. 2015, 35, 1081–1092. [Google Scholar] [CrossRef]
- Wang, X.; Cong, P.; Liu, Y.; Tao, S.; Chen, Q.; Wang, J.; Xu, J.; Xue, C. Neuritogenic Effect of Sea Cucumber Glucocerebrosides on NGF-Induced PC12 Cells via Activation of the TrkA/CREB/BDNF Signalling Pathway. J. Funct. Foods 2018, 46, 175–184. [Google Scholar] [CrossRef]
- Sanguanphun, T.; Promtang, S.; Sornkaew, N.; Niamnont, N.; Sobhon, P.; Meemon, K. Anti-Parkinson Effects of Holothuria leucospilota-Derived Palmitic Acid in Caenorhabditis elegans Model of Parkinson’s Disease. Mar. Drugs 2023, 21, 141. [Google Scholar] [CrossRef]
- Promtang, S.; Sanguanphun, T.; Chalorak, P.; Pe, L.S.; Niamnont, N.; Sobhon, P.; Meemon, K. 2-Butoxytetrahydrofuran, Isolated from Holothuria scabra, Attenuates Aggregative and Oxidative Properties of α-Synuclein and Alleviates Its Toxicity in a Transgenic Caenorhabditis elegans Model of Parkinson’s Disease. ACS Chem. Neurosci. 2024, 15, 2182–2197. [Google Scholar] [CrossRef]
- Wesson, D.W.; Nixon, R.A.; Levy, E.; Wilson, D.A. Mechanisms of Neural and Behavioral Dysfunction in Alzheimer’s Disease. Mol. Neurobiol. 2011, 43, 163–179. [Google Scholar] [CrossRef]
- Puzzo, D.; Lee, L.; Palmeri, A.; Calabrese, G.; Arancio, O. Behavioral Assays with Mouse Models of Alzheimer’s Disease: Practical Considerations and Guidelines. Biochem. Pharmacol. 2014, 88, 450–467. [Google Scholar] [CrossRef]
- Ford, L.; Crossley, M.; Williams, T.; Thorpe, J.R.; Serpell, L.C.; Kemenes, G. Effects of Aβ Exposure on Long-Term Associative Memory and Its Neuronal Mechanisms in a Defined Neuronal Network. Sci. Rep. 2015, 5, 10614. [Google Scholar] [CrossRef]
- Che, H.; Du, L.; Cong, P.; Tao, S.; Ding, N.; Wu, F.; Xue, C.; Xu, J.; Wang, Y. Cerebrosides from Sea Cucumber Protect Against Oxidative Stress in SAMP8 Mice and PC12 Cells. J. Med. Food 2017, 20, 392–402. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of Oxidative Damage in Alzheimer’s Disease Brain: Central Role for Amyloid β-Peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef]
- Khan, A.; Vaibhav, K.; Javed, H.; Moshahid Khan, M.; Tabassum, R.; Ahmed, M.E.; Srivastava, P.; Khuwaja, G.; Islam, F.; Saeed Siddiqui, M.; et al. Attenuation of Aβ-Induced Neurotoxicity by Thymoquinone via Inhibition of Mitochondrial Dysfunction and Oxidative Stress. Mol. Cell. Biochem. 2012, 369, 55–65. [Google Scholar] [CrossRef]
- Cory, S.; Adams, J.M. The Bcl2 Family: Regulators of the Cellular Life-or-Death Switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Friedlander, R.M. Apoptosis and Caspases in Neurodegenerative Diseases. N. Engl. J. Med. 2003, 348, 1365–1375. [Google Scholar] [CrossRef]
- Antonsson, B. Mitochondria and the Bcl-2 Family Proteins in Apoptosis Signaling Pathways. Mol. Cell. Biochem. 2004, 256–257, 141–155. [Google Scholar] [CrossRef]
- Liao, W.; Lai, T.; Chen, L.; Fu, J.; Sreenivasan, S.T.; Yu, Z.; Ren, J. Synthesis and Characterization of a Walnut Peptides-Zinc Complex and Its Antiproliferative Activity against Human Breast Carcinoma Cells through the Induction of Apoptosis. J. Agric. Food Chem. 2016, 64, 1509–1519. [Google Scholar] [CrossRef]
- Lu, J.; Wu, D.; Zheng, Y.; Hu, B.; Zhang, Z. Purple Sweet Potato Color Alleviates D-Galactose-Induced Brain Aging in Old Mice by Promoting Survival of Neurons via PI3K Pathway and Inhibiting Cytochrome C-Mediated Apoptosis. Brain Pathol. Zurich Switz. 2010, 20, 598–612. [Google Scholar] [CrossRef]
- Ho, V.M.; Lee, J.-A.; Martin, K.C. The Cell Biology of Synaptic Plasticity. Science 2011, 334, 623–628. [Google Scholar] [CrossRef]
- Adasme, T.; Haeger, P.; Paula-Lima, A.C.; Espinoza, I.; Casas-Alarcón, M.M.; Carrasco, M.A.; Hidalgo, C. Involvement of Ryanodine Receptors in Neurotrophin-Induced Hippocampal Synaptic Plasticity and Spatial Memory Formation. Proc. Natl. Acad. Sci. USA 2011, 108, 3029–3034. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Choi, B.; Yang, H.; Hwang, Y.; Park, J.H.Y.; LaFerla, F.M.; Han, J.; Lee, K.W.; Kim, J. Sulforaphane Epigenetically Enhances Neuronal BDNF Expression and TrkB Signaling Pathways. Mol. Nutr. Food Res. 2017, 61, 1600194. [Google Scholar] [CrossRef]
- Shao, C.Y.; Mirra, S.S.; Sait, H.B.R.; Sacktor, T.C.; Sigurdsson, E.M. Postsynaptic Degeneration as Revealed by PSD-95 Reduction Occurs after Advanced Aβ and Tau Pathology in Transgenic Mouse Models of Alzheimer’s Disease. Acta Neuropathol. 2011, 122, 285–292. [Google Scholar] [CrossRef]
- Bhatia, V.; Vikram, V.; Chandel, A.; Rattan, A. Interplay between PI3k/AKT Signaling and Caspase Pathway in Alzheimer Disease: Mechanism and Therapeutic Implications. Inflammopharmacology 2025, 33, 1785–1802. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- De Simone, A.; Tumiatti, V.; Andrisano, V.; Milelli, A. Glycogen Synthase Kinase 3β: A New Gold Rush in Anti-Alzheimer’s Disease Multitarget Drug Discovery?: Miniperspective. J. Med. Chem. 2021, 64, 26–41. [Google Scholar] [CrossRef]
- Gong, H.; Zhao, H.; Mao, X. Sea Cucumber Hydrolysates Alleviate Cognitive Deficits in D-Galactose-Induced C57BL/6J Aging Mice Associated with Modulation of Gut Microbiota. Foods 2025, 14, 1938. [Google Scholar] [CrossRef]
- Han, H.; Yi, Y.-H.; Li, L.; Liu, B.-S.; Pan, M.-X.; Yan, B.; Wang, X.-H. Triterpene Glycosides from Sea Cucumber Holothuria leucospilota: Triterpene Glycosides from Sea Cucumber Holothuria leucospilota. Chin. J. Nat. Med. 2009, 7, 346–350. [Google Scholar] [CrossRef]
- Bedford, L.; Lowe, J.; Dick, L.R.; Mayer, R.J.; Brownell, J.E. Ubiquitin-like Protein Conjugation and the Ubiquitin–Proteasome System as Drug Targets. Nat. Rev. Drug Discov. 2011, 10, 29–46. [Google Scholar] [CrossRef]
- Glickman, M.H.; Ciechanover, A. The Ubiquitin-Proteasome Proteolytic Pathway: Destruction for the Sake of Construction. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [CrossRef]
- Hegde, A.N.; Upadhya, S.C. The Ubiquitin–Proteasome Pathway in Health and Disease of the Nervous System. Trends Neurosci. 2007, 30, 587–595. [Google Scholar] [CrossRef]
- Lim, K.L.; Lim, T.M. Molecular Mechanisms of Neurodegeneration in Parkinson’s Disease: Clues from Mendelian Syndromes. IUBMB Life 2003, 55, 315–322. [Google Scholar] [CrossRef]
- Umeno, A.; Biju, V.; Yoshida, Y. In Vivo ROS Production and Use of Oxidative Stress-Derived Biomarkers to Detect the Onset of Diseases Such as Alzheimer’s Disease, Parkinson’s Disease, and Diabetes. Free Radic. Res. 2017, 51, 413–427. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative Stress, Mitochondrial Dysfunction and Neurodegenerative Diseases; a Mechanistic Insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Domanskyi, A.; Parlato, R. Oxidative Stress in Neurodegenerative Diseases. Antioxidants 2022, 11, 504. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; You, L.; Zhang, J.; Chang, Y.-Z.; Yu, P. Brain Iron Metabolism, Redox Balance and Neurological Diseases. Antioxidants 2023, 12, 1289. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.J.; Andersen, J.K. Perspectives on MAO-B in Aging and Neurological Disease: Where Do We Go From Here? Mol. Neurobiol. 2004, 30, 077–090. [Google Scholar] [CrossRef]
- Cisbani, G.; Maxan, A.; Kordower, J.H.; Planel, E.; Freeman, T.B.; Cicchetti, F. Presence of Tau Pathology within Foetal Neural Allografts in Patients with Huntington’s and Parkinson’s Disease. Brain J. Neurol. 2017, 140, 2982–2992. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Meng, L.; He, M.; Zhang, Z. Tau in the Pathophysiology of Parkinson’s Disease. J. Mol. Neurosci. 2021, 71, 2179–2191. [Google Scholar] [CrossRef]
- Choi, D.-Y.; Lee, M.K.; Hong, J.T. Lack of CCR5 Modifies Glial Phenotypes and Population of the Nigral Dopaminergic Neurons, but Not MPTP-Induced Dopaminergic Neurodegeneration. Neurobiol. Dis. 2013, 49, 159–168. [Google Scholar] [CrossRef]
- Kouli, A.; Camacho, M.; Allinson, K.; Williams-Gray, C.H. Neuroinflammation and Protein Pathology in Parkinson’s Disease Dementia. Acta Neuropathol. Commun. 2020, 8, 211. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [PubMed]
- Stolzenberg, E.; Berry, D.; Yang, D.; Lee, E.Y.; Kroemer, A.; Kaufman, S.; Wong, G.C.L.; Oppenheim, J.J.; Sen, S.; Fishbein, T.; et al. A Role for Neuronal Alpha-Synuclein in Gastrointestinal Immunity. J. Innate Immun. 2017, 9, 456–463. [Google Scholar] [CrossRef]
- Lim, K.-L.; Tan, J.M. Role of the Ubiquitin Proteasome System in Parkinson’s Disease. BMC Biochem. 2007, 8, S13. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Yuan, Y.; Liu, L.; Zhao, Y.; Wang, X. Gut Microbiota Alteration Is Associated With Cognitive Deficits in Genetically Diabetic (Db/Db) Mice During Aging. Front. Aging Neurosci. 2022, 13, 815562. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.-C.; Lin, C.-C.; Chiu, Y.-L.; Koh, S.-H.; Liu, Y.-C.; Chuang, Y.-F. Compositional and Functional Gut Microbiota Alterations in Mild Cognitive Impairment: Links to Alzheimer’s Disease Pathology. Alzheimers Res. Ther. 2025, 17, 122. [Google Scholar] [CrossRef]
- Zhang, R.; Ding, N.; Feng, X.; Liao, W. The Gut Microbiome, Immune Modulation, and Cognitive Decline: Insights on the Gut-Brain Axis. Front. Immunol. 2025, 16, 1529958. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The Gut Microbiome in Neurological Disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Picón, M.; Laguna, A. New Avenues for Parkinson’s Disease Therapeutics: Disease-Modifying Strategies Based on the Gut Microbiota. Biomolecules 2021, 11, 433. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Ye, Q.; Wang, Y.; Zhang, J.; Lin, S.; Wang, G.; Yang, X.; Zhang, J.; Chen, S.; et al. Fucosylated Chondroitin Sulfate against Parkinson’s Disease through Inhibiting Inflammation Induced by Gut Dysbiosis. J. Agric. Food Chem. 2022, 70, 13676–13691. [Google Scholar] [CrossRef]
- Blandini, F.; Armentero, M.-T. Animal Models of Parkinson’s Disease. FEBS J. 2012, 279, 1156–1166. [Google Scholar] [CrossRef]
- Zou, L.; Liao, M.; Zhen, Y.; Zhu, S.; Chen, X.; Zhang, J.; Hao, Y.; Liu, B. Autophagy and beyond: Unraveling the Complexity of UNC-51-like Kinase 1 (ULK1) from Biological Functions to Therapeutic Implications. Acta Pharm. Sin. B 2022, 12, 3743–3782. [Google Scholar] [CrossRef]
- Kriegenburg, F.; Ungermann, C.; Reggiori, F. Coordination of Autophagosome–Lysosome Fusion by Atg8 Family Members. Curr. Biol. 2018, 28, R512–R518. [Google Scholar] [CrossRef]
- Pardridge, W.M. Drug Transport across the Blood-Brain Barrier. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2012, 32, 1959–1972. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Li, P.; Wu, S.; Lei, L.; He, D. Tris(2-Chloroethyl) Phosphate (TCEP) and Tris(2-Chloropropyl) Phosphate (TCPP) Induce Locomotor Deficits and Dopaminergic Degeneration in Caenorhabditis elegans. Toxicol. Res. 2017, 6, 63–72. [Google Scholar] [CrossRef]
- Gorelick, P.B.; Wong, K.S.; Bae, H.-J.; Pandey, D.K. Large Artery Intracranial Occlusive Disease: A Large Worldwide Burden but a Relatively Neglected Frontier. Stroke 2008, 39, 2396–2399. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yuan, R.; Liu, M.; Zhang, Y.; Jia, B.; Ruan, J.; Shen, J.; Zhang, Y.; Liu, M.; Wang, T. Targeting Autophagy in Atherosclerosis: Advances and Therapeutic Potential of Natural Bioactive Compounds from Herbal Medicines and Natural Products. Biomed. Pharmacother. 2022, 155, 113712. [Google Scholar] [CrossRef] [PubMed]
- Hahn, C.; Hill, M.D. Early Anti-Coagulation after Ischemic Stroke Due to Atrial Fibrillation Is Safe and Prevents Recurrent Stroke. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 2015, 42, 92–95. [Google Scholar] [CrossRef]
- Giordano, G.; Costa, L.G. Primary Neurons in Culture and Neuronal Cell Lines for in Vitro Neurotoxicological Studies. Methods Mol. Biol. Clifton NJ 2011, 758, 13–27. [Google Scholar] [CrossRef]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma Multiforme (GBM): An Overview of Current Therapies and Mechanisms of Resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef]
- Bordbar, S.; Anwar, F.; Saari, N. High-Value Components and Bioactives from Sea Cucumbers for Functional Foods—A Review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, F.; Han, Z.; Yin, Z.; Ge, X.; Lei, P. Relationship Between Amyloid-β Deposition and Blood-Brain Barrier Dysfunction in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 695479. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, V. Marine Products for Healthcare: Functional and Bioactive Nutraceutical Compounds from the Ocean, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-0-429-14048-8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).