Marine-Inspired Ovothiol Analogs Inhibit Membrane-Bound Gamma-Glutamyl-Transpeptidase and Modulate Reactive Oxygen Species and Glutathione Levels in Human Leukemic Cells
Abstract
1. Introduction
2. Results
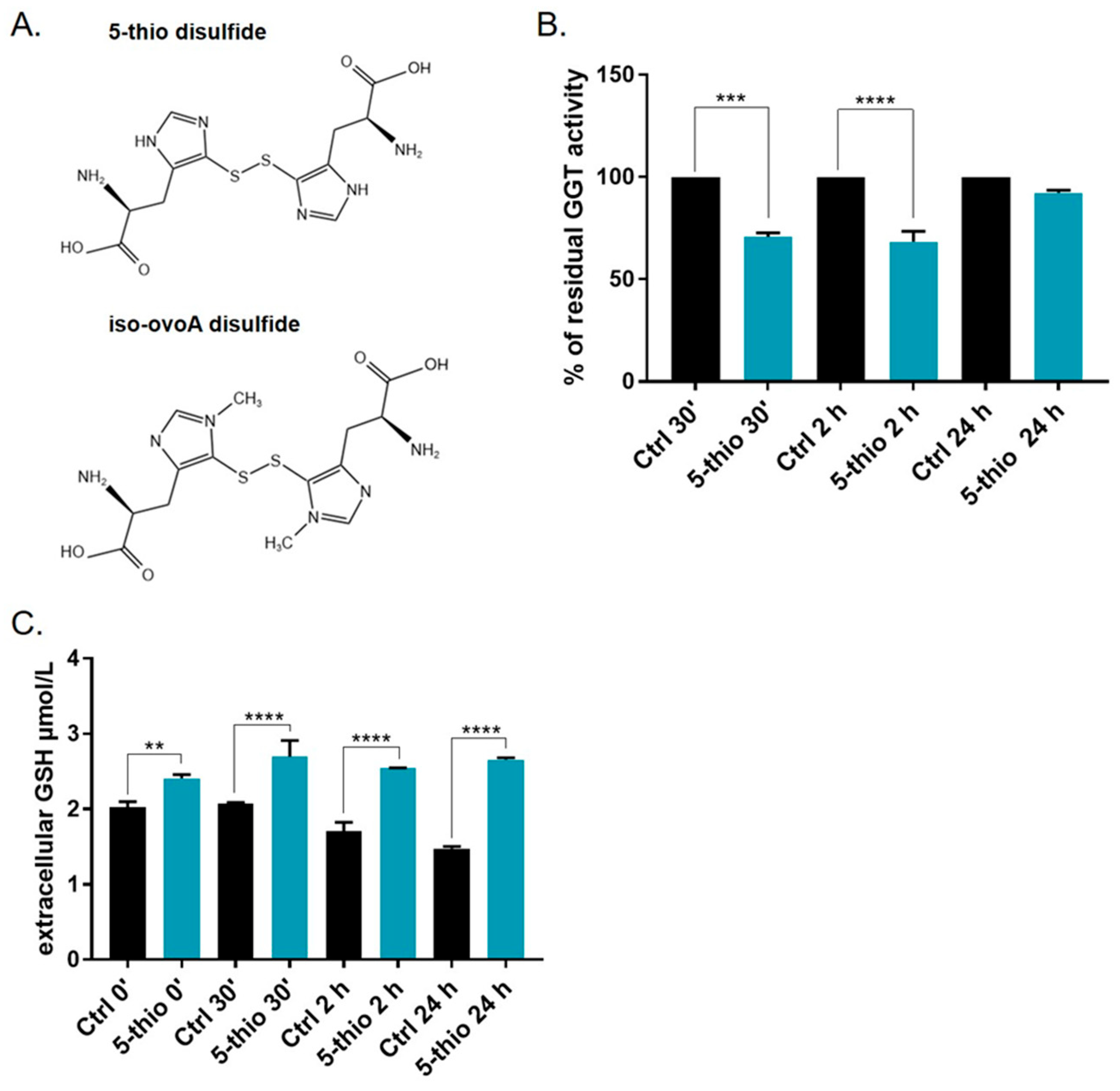
2.1. Marine-Inspired Ovothiol Analogs Inhibit Membrane-Bound GGT in GGT-Positive Cell Lines
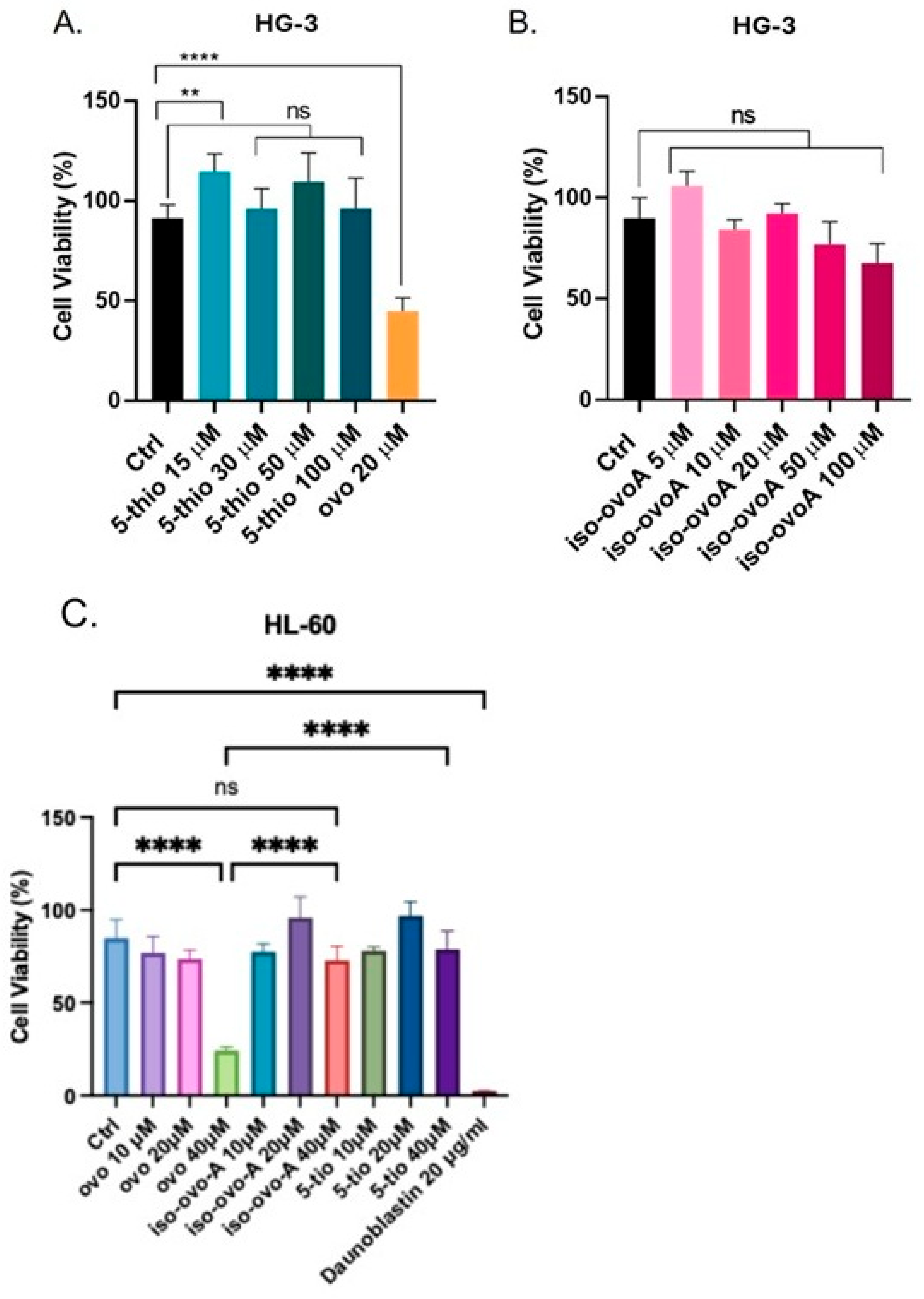
2.2. Ovothiol Analogs Are Not Cytotoxic in Human Leukemia Cells
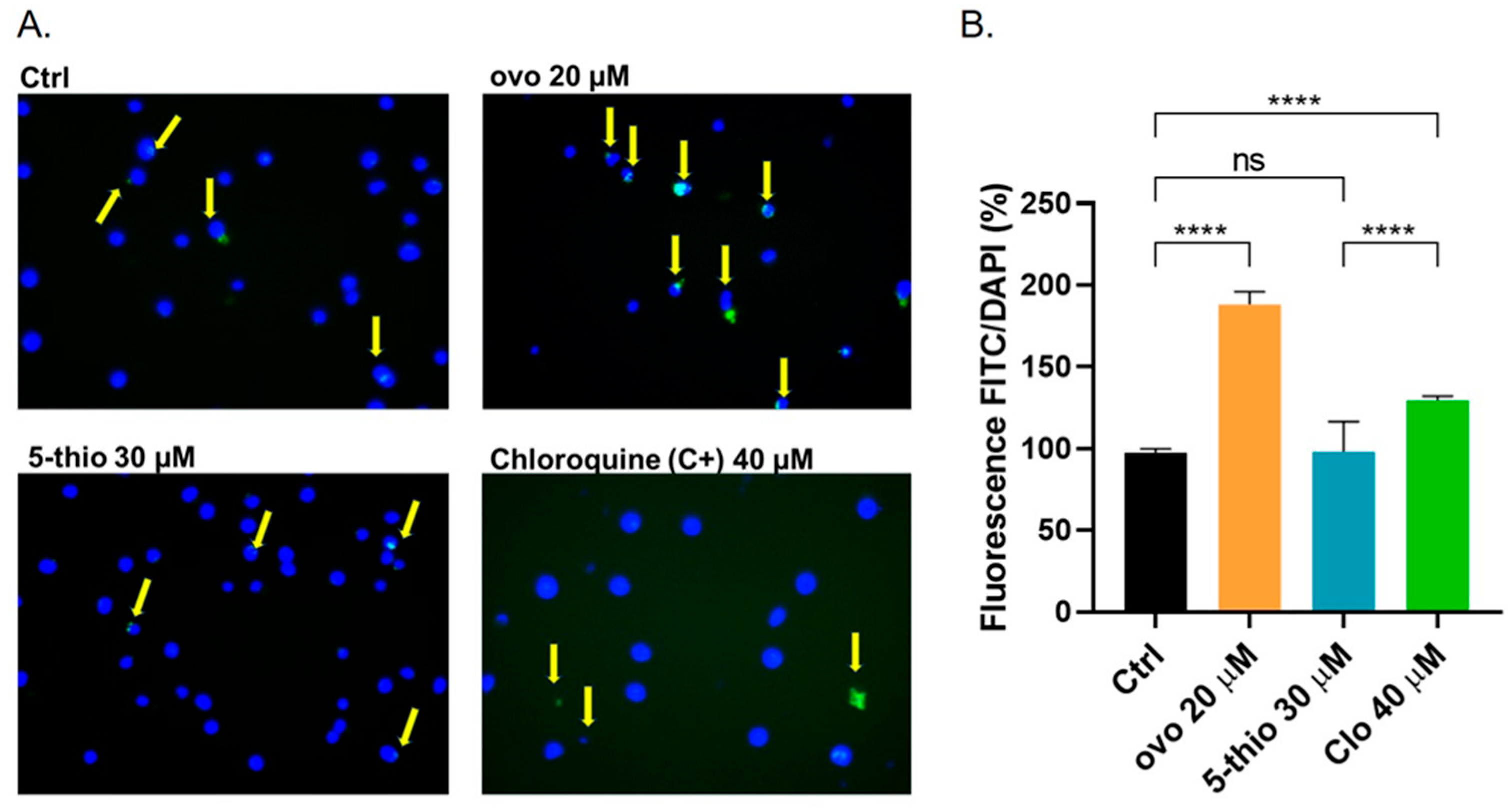
2.3. 5-Thio Does Not Modify Autophagy Flux in GGT-Positive HG-3 Cell Line
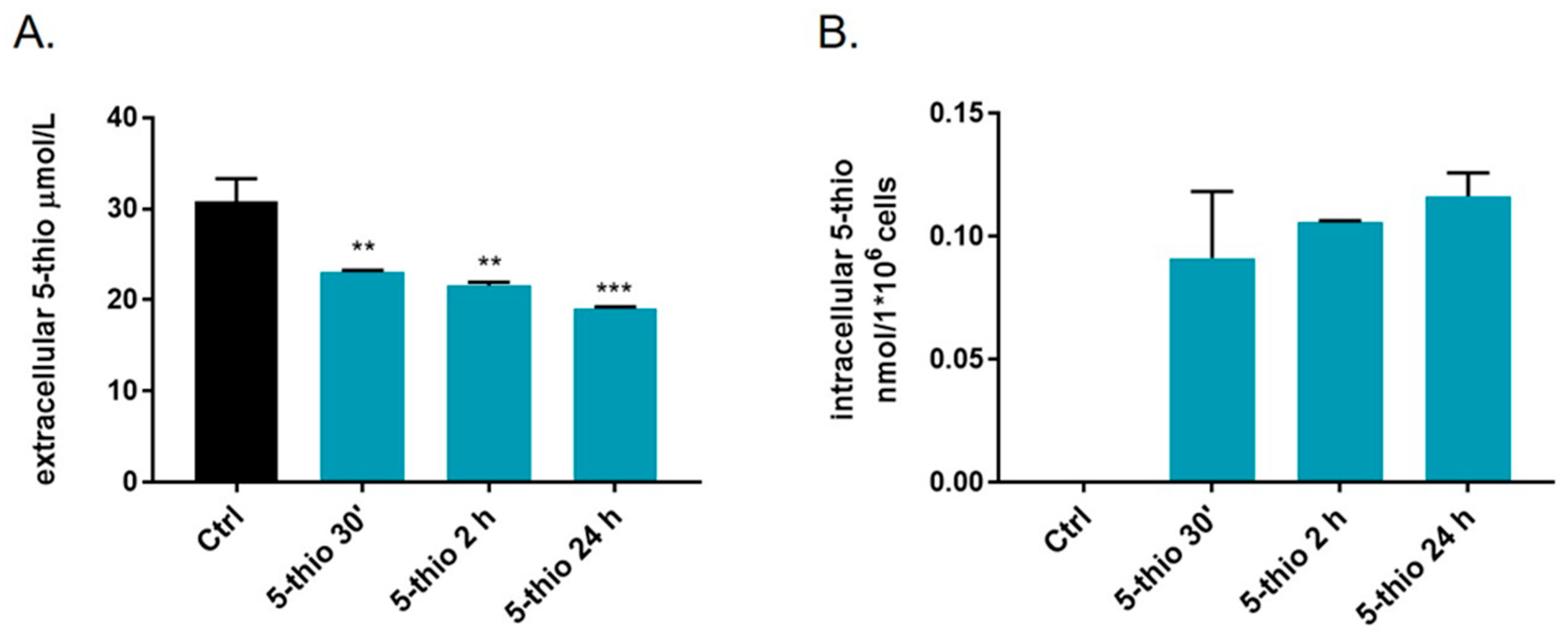
2.4. Bioavailability of 5-Thio in GGT-Positive HG-3 Cell Line
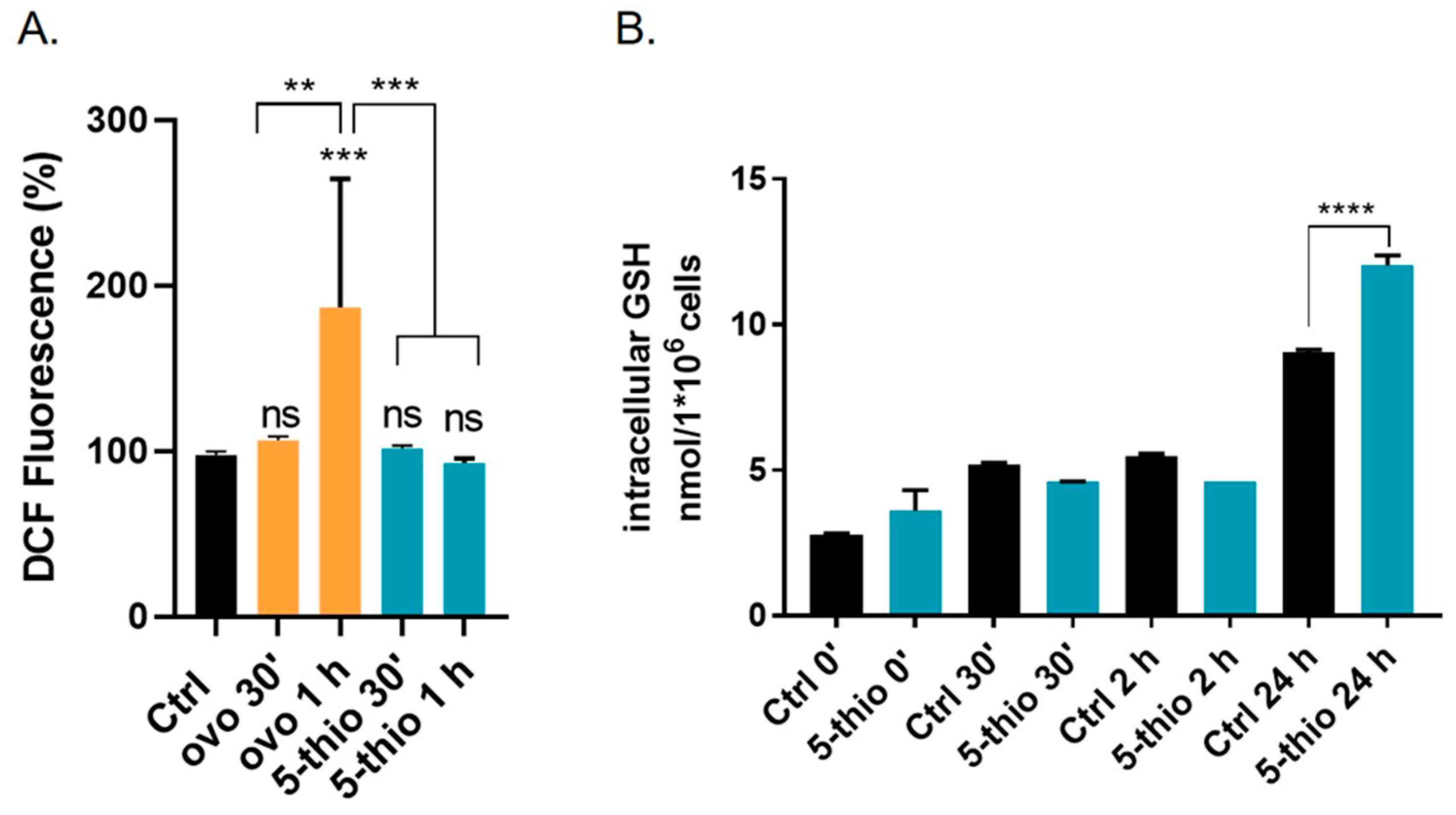
2.5. 5-Thio Modulates Intracellular ROS and GSH Levels in GGT-Positive HG-3 Cell Line
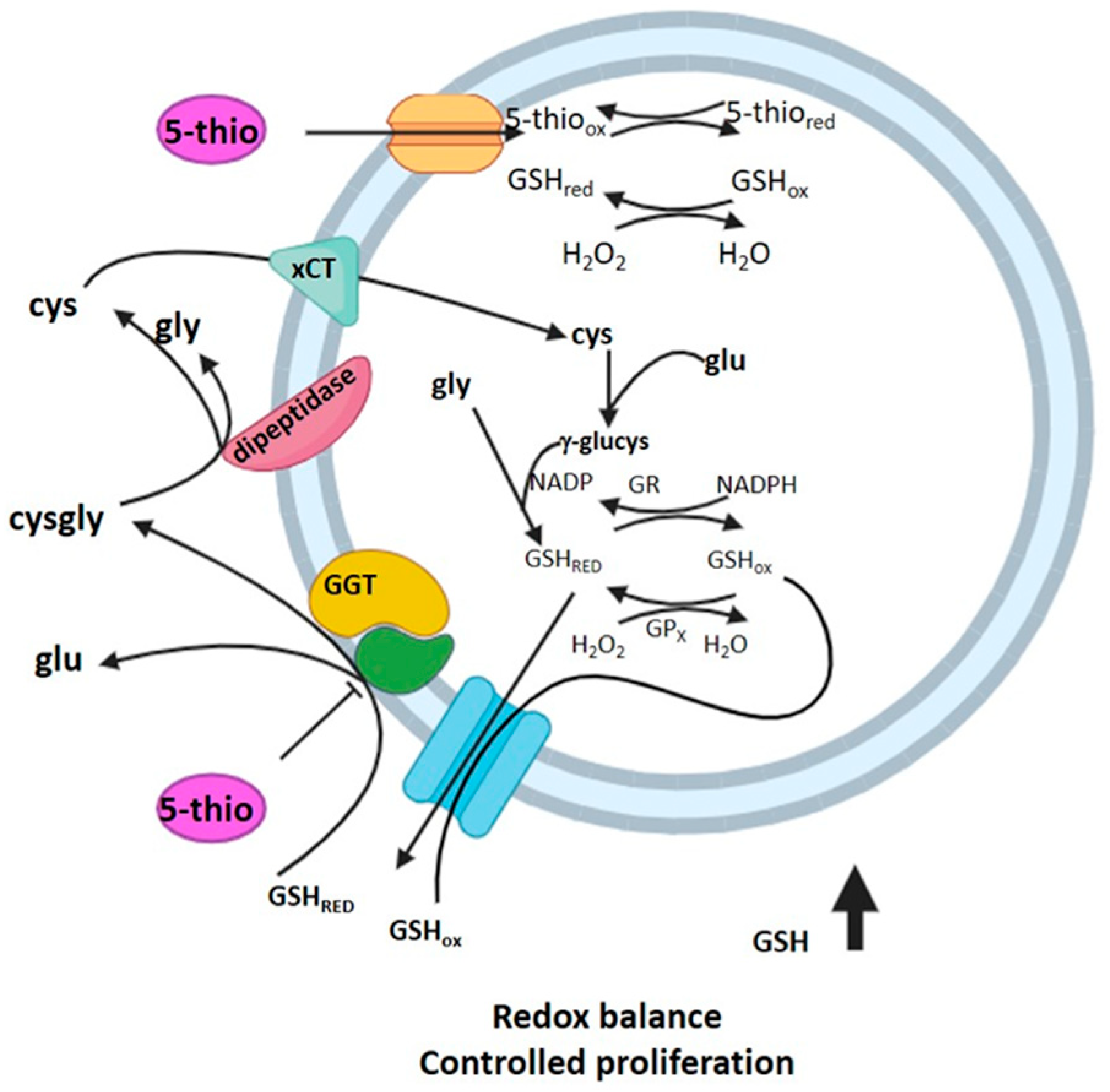
3. Discussion
4. Materials and Methods
4.1. Enzyme Isolation and GGT Activity Assay
4.2. Derivatization and HPLC Analyses
4.3. Cell Culture and Viability Assays
4.4. Intracellular ROS Measurement
4.5. Autophagy Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| BMC | 4-Bromomethyl-7-Methoxycoumarin |
| CO2 | Carbon Dioxide |
| Ctrl | Control |
| DAPI | 4′,6-Diamidino-2-Phenylindole |
| DMSO | Dimethyl Sulfoxide |
| DTT | Dithiothreitol |
| FBS | Fetal Bovine Serum |
| FITC | Fluorescein Isothiocyanate |
| GGT | Gamma-Glutamyl Transpeptidase |
| GSH | Glutathione |
| GSSG | Glutathione Disulfide |
| HPLC | High-Performance Liquid Chromatography |
| iso-ovoA | Iso-Ovothiol A |
| LC-MS | Liquid Chromatography–Mass Spectrometry |
| NMR | Nuclear Magnetic Resonance |
| ns | Not Significant |
| ovo | Ovothiol A |
| OU749 | GGT Inhibitor OU749 |
| PBS | Phosphate-Buffered Saline |
| RPMI | Roswell Park Memorial Institute (Medium) |
| ROS | Reactive Oxygen Species |
| TFA | Trifluoroacetic Acid |
| 5-thio | L-5-Sulfanylhistidine |
References
- Tate, S.S.; Meister, A. γ-Glutamyl Transpeptidase: Catalytic, Structural and Functional Aspects. Mol. Cell. Biochem. 1981, 39, 357–368. [Google Scholar] [CrossRef]
- West, M.B.; Wickham, S.; Quinalty, L.M.; Pavlovicz, R.E.; Li, C.; Hanigan, M.H. Autocatalytic Cleavage of Human Gamma-Glutamyl Transpeptidase Is Highly Dependent on N-Glycosylation at Asparagine 95. J. Biol. Chem. 2011, 286, 28876–28888. [Google Scholar] [CrossRef]
- West, M.B.; Hanigan, M.H. γ-Glutamyl Transpeptidase Is a Heavily N-Glycosylated Heterodimer in HepG2 Cells. Arch. Biochem. Biophys. 2010, 504, 177–181. [Google Scholar] [CrossRef] [PubMed]
- West, M.B.; Segu, Z.M.; Feasley, C.L.; Kang, P.; Klouckova, I.; Li, C.; Novotny, M.V.; West, C.M.; Mechref, Y.; Hanigan, M.H. Analysis of Site-Specific Glycosylation of Renal and Hepatic γ-Glutamyl Transpeptidase from Normal Human Tissue. J. Biol. Chem. 2010, 285, 29511–29524. [Google Scholar] [CrossRef] [PubMed]
- Bachhawat, A.K.; Yadav, S. The Glutathione Cycle: Glutathione Metabolism beyond the γ-Glutamyl Cycle. IUBMB Life 2018, 70, 585–592. [Google Scholar] [CrossRef]
- Ballatori, N.; Krance, S.M.; Marchan, R.; Hammond, C.L. Plasma Membrane Glutathione Transporters and Their Roles in Cell Physiology and Pathophysiology. Mol. Asp. Med. 2009, 30, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Nasr, R.; Lorendeau, D.; Khonkarn, R.; Dury, L.; Pérès, B.; Boumendjel, A.; Cortay, J.-C.; Falson, P.; Chaptal, V.; Baubichon-Cortay, H. Molecular Analysis of the Massive GSH Transport Mechanism Mediated by the Human Multidrug Resistant Protein 1/ABCC1. Sci. Rep. 2020, 10, 7616. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. On the Enzymology of Amino Acid Transport. Science 1973, 180, 33–39. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J.; Choi, J. γ-Glutamyl Transpeptidase in Glutathione Biosynthesis. In Methods in Enzymology; Sies, H., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2005; Volume 401, pp. 468–483. [Google Scholar] [CrossRef]
- Freidman, N.; Chen, I.; Wu, Q.; Briot, C.; Holst, J.; Font, J.; Vandenberg, R.; Ryan, R. Amino Acid Transporters and Exchangers from the SLC1A Family: Structure, Mechanism and Roles in Physiology and Cancer. Neurochem. Res. 2020, 45, 1268–1286. [Google Scholar] [CrossRef]
- Hanigan, M.H.; Ricketts, W.A. Extracellular glutathione is a source of cysteine for cells that express gamma-glutamyl transpeptidase. Biochemistry 1993, 32, 6302–6306. [Google Scholar] [CrossRef]
- Franco, R.; Cidlowski, J.A. Apoptosis and glutathione: Beyond an antioxidant. Cell Death Differ. 2009, 16, 1303–1314. [Google Scholar] [CrossRef]
- Zhang, H.; Forman, H.J. Glutathione synthesis and its role in redox signaling. Semin. Cell Dev. Biol. 2012, 23, 722–728. [Google Scholar] [CrossRef]
- Castellano, I.; Merlino, A. γ-Glutamyltranspeptidases: Sequence, structure, biochemical properties, and biotechnological applications. Cell. Mol. Life Sci. 2012, 69, 3381–3394. [Google Scholar] [CrossRef]
- Barrios, R.; Shi, Z.-Z.; Kala, S.V.; Wiseman, A.L.; Welty, S.E.; Kala, G.; Bahler, A.A.; Ou, C.-N.; Lieberman, M.W. Oxygen-induced pulmonary injury in γ-glutamyl transpeptidase-deficient mice. Lung 2001, 179, 319–330. [Google Scholar] [CrossRef]
- Lieberman, M.W.; Wiseman, A.L.; Shi, Z.Z.; Carter, B.Z.; Barrios, R.; Ou, C.N.; Chévez-Barrios, P.; Wang, Y.; Habib, G.M.; Goodman, J.C.; et al. Growth retardation and cysteine deficiency in gamma-glutamyl transpeptidase-deficient mice. Proc. Natl. Acad. Sci. USA 1996, 93, 7923–7926. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, M.H.; Pitot, H.C. Gamma-glutamyl transpeptidase—Its role in hepatocarcinogenesis. Carcinogenesis 1985, 6, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Hanigan, M.H.; Frierson, H.F.; Swanson, P.E.; De Young, B.R. Altered expression of gamma-glutamyl transpeptidase in human tumors. Hum. Pathol. 1999, 30, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Corti, A.; Franzini, M.; Paolicchi, A.; Pompella, A. Gamma-glutamyltransferase of cancer cells at the crossroads of tumor progression, drug resistance and drug targeting. Anticancer Res. 2010, 30, 1169–1181. [Google Scholar]
- Akaydın, S.Y.; Salihoğlu, E.M.; Güngör, D.G.; Karanlık, H.; Demokan, S. Correlation between gamma-glutamyl transferase activity and glutathione levels in molecular subgroups of breast cancer. Eur. J. Breast Health 2020, 16, 72–76. [Google Scholar] [CrossRef]
- Ruoso, P.; Hedley, D.W. Inhibition of γ-glutamyl transpeptidase activity decreases intracellular cysteine levels in cervical carcinoma. Cancer Chemother. Pharmacol. 2004, 54, 49–56. [Google Scholar] [CrossRef]
- Benlloch, M.; Ortega, A.; Ferrer, P.; Segarra, R.; Obrador, E.; Asensi, M.; Carretero, J.; Estrela, J.M. Acceleration of glutathione efflux and inhibition of gamma-glutamyltranspeptidase sensitize metastatic B16 melanoma cells to endothelium-induced cytotoxicity. J. Biol. Chem. 2005, 280, 6950–6959. [Google Scholar] [CrossRef]
- Hanigan, M.H. Gamma-glutamyl transpeptidase: Redox regulation and drug resistance. Adv. Cancer Res. 2014, 122, 103–141. [Google Scholar] [CrossRef]
- Mitrić, A.; Castellano, I. Targeting gamma-glutamyl transpeptidase: A pleiotropic enzyme involved in glutathione metabolism and in the control of redox homeostasis. Free Radic. Biol. Med. 2023, 208, 672–683. [Google Scholar] [CrossRef]
- Castello, G.; Mencoboni, M.; Lerza, R.; Cerruti, A.; Bogliolo, G.; Pannacciulli, I. Suppressive activity of acivicin on murine bone marrow hemopoietic progenitors. Anticancer Res. 1992, 12, 2181–2184. [Google Scholar] [PubMed]
- Terzyan, S.S.; Cook, P.F.; Heroux, A.; Hanigan, M.H. Structure of 6-diazo-5-oxo-norleucine-bound human gamma-glutamyl transpeptidase 1, a novel mechanism of inactivation. Protein Sci. 2017, 26, 1196–1205. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Watanabe, B.; Hiratake, J.; Tanaka, R.; Ohkita, M.; Matsumura, Y. Preventive effect of GGsTop, a novel and selective γ-glutamyl transpeptidase inhibitor, on ischemia/reperfusion-induced renal injury in rats. J. Pharmacol. Exp. Ther. 2011, 339, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Tuzova, M.; Jean, J.-C.; Hughey, R.P.; Brown, L.A.S.; Cruikshank, W.W.; Hiratake, J.; Joyce-Brady, M. Inhibiting lung lining fluid glutathione metabolism with GGsTop as a novel treatment for asthma. Front. Pharmacol. 2014, 5, 179. [Google Scholar] [CrossRef]
- Takeuchi, I.; Kawamata, R.; Makino, K. Effects of GGsTop® on collagen and glutathione in the oral mucosa using a rat model of 5-fluorouracil-induced oral mucositis. In Vivo 2021, 35, 175–180. [Google Scholar] [CrossRef]
- King, J.B.; West, M.B.; Cook, P.F.; Hanigan, M.H. A novel, species-specific class of uncompetitive inhibitors of γ-glutamyl transpeptidase. J. Biol. Chem. 2009, 284, 9059–9065. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; He, S.; He, S.; Wang, Y. Fighting against drug-resistant tumors by the inhibition of γ-glutamyl transferase with supramolecular platinum prodrug nano-assemblies. J. Mater. Chem. B 2021, 9, 4587–4595. [Google Scholar] [CrossRef]
- Castellano, I.; Seebeck, F.P. On ovothiol biosynthesis and biological roles: From life in the ocean to therapeutic potential. Nat. Prod. Rep. 2018, 35, 1241–1250. [Google Scholar] [CrossRef]
- Osik, N.A.; Zelentsova, E.A.; Tsentalovich, Y.P. Kinetic Studies of Antioxidant Properties of Ovothiol A. Antioxidants 2021, 10, 1470. [Google Scholar] [CrossRef]
- Murano, C.; Zuccarotto, A.; Leone, S.; Sollitto, M.; Gerdol, M.; Castellano, I.; Palumbo, A. A Survey on the Distribution of Ovothiol and ovoA Gene Expression in Different Tissues and Cells: A Comparative Analysis in Sea Urchins and Mussels. Mar. Drugs 2022, 20, 268. [Google Scholar] [CrossRef]
- Russo, G.L.; Russo, M.; Castellano, I.; Napolitano, A.; Palumbo, A. Ovothiol Isolated from Sea Urchin Oocytes Induces Autophagy in the Hep-G2 Cell Line. Mar. Drugs 2014, 12, 4069–4085. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; Russo, M.; Masullo, M.; Palumbo, A.; Russo, G.L.; Castellano, I. Sulfur-Containing Histidine Compounds Inhibit γ-Glutamyl Transpeptidase Activity in Human Cancer Cells. J. Biol. Chem. 2019, 294, 14603–14614. [Google Scholar] [CrossRef] [PubMed]
- Milito, A.; Brancaccio, M.; Lisurek, M.; Masullo, M.; Palumbo, A.; Castellano, I. Probing the Interactions of Sulfur-Containing Histidine Compounds with Human Gamma-Glutamyl Transpeptidase. Mar. Drugs 2019, 17, 650. [Google Scholar] [CrossRef]
- Brancaccio, M.; D’Argenio, G.; Lembo, V.; Palumbo, A.; Castellano, I. Antifibrotic Effect of Marine Ovothiol in an In Vivo Model of Liver Fibrosis. Oxid. Med. Cell. Longev. 2018, 2018, 5045734. [Google Scholar] [CrossRef] [PubMed]
- Daunay, S.; Lebel, R.; Farescour, L.; Yadan, J.-C.; Erdelmeier, I. Short Protecting-Group-Free Synthesis of 5-Acetylsulfanyl-Histidines in Water: Novel Precursors of 5-Sulfanyl-Histidine and Its Analogues. Org. Biomol. Chem. 2016, 14, 10473–10480. [Google Scholar] [CrossRef]
- Diederich, M.; el Yaagoubi, M.; Gérardin, P.; Wellman, M.; Siest, G. Characterization and regulatory effect of gamma-glutamyltransferase messenger RNA untranslated regions in human leukemia. Leukemia 1995, 9, 1332–1337. [Google Scholar]
- Pompella, A.; De Tata, V.; Paolicchi, A.; Zunino, F. Expression of Gamma-Glutamyltransferase in Cancer Cells and Its Significance in Drug Resistance. Biochem. Pharmacol. 2006, 71, 231–238. [Google Scholar] [CrossRef]
- Mena, S.; Benlloch, M.; Ortega, A.; Carretero, J.; Obrador, E.; Asensi, M.; Petschen, I.; Brown, B.D.; Estrela, J.M. Bcl-2 and Glutathione Depletion Sensitize B16 Melanoma to Combination Therapy and Eliminate Metastatic Disease. Clin. Cancer Res. 2007, 13, 2658–2666. [Google Scholar] [CrossRef]
- Hanigan, M.H.; Gallagher, B.C.; Townsend, D.M.; Gabarra, V. γ-Glutamyl Transpeptidase Accelerates Tumor Growth and Increases the Resistance of Tumors to Cisplatin In Vivo. Carcinogenesis 1999, 20, 553–559. [Google Scholar] [CrossRef]
- Hanigan, M.H.; Lykissa, E.D.; Townsend, D.M.; Ou, C.-N.; Barrios, R.; Lieberman, M.W. γ-Glutamyl Transpeptidase-Deficient Mice Are Resistant to the Nephrotoxic Effects of Cisplatin. Am. J. Pathol. 2001, 159, 1889–1894. [Google Scholar] [CrossRef]
- Castellano, I.; Di Tomo, P.; Di Pietro, N.; Mandatori, D.; Pipino, C.; Formoso, G.; Napolitano, A.; Palumbo, A.; Pandolfi, A. Anti-Inflammatory Activity of Marine Ovothiol A in an In Vitro Model of Endothelial Dysfunction Induced by Hyperglycemia. Oxid. Med. Cell. Longev. 2018, 2018, 2087373. [Google Scholar] [CrossRef]
- Luccarini, A.; Zuccarotto, A.; Galeazzi, R.; Morresi, C.; Masullo, M.; Castellano, I.; Damiani, E. Insights on the UV-Screening Potential of Marine-Inspired Thiol Compounds. Mar. Drugs 2024, 22, 2. [Google Scholar] [CrossRef]
- Russo, M.T.; Santin, A.; Zuccarotto, A.; Leone, S.; Palumbo, A.; Ferrante, M.I.; Castellano, I. The First Genetic Engineered System for Ovothiol Biosynthesis in Diatoms Reveals a Mitochondrial Localization for the Sulfoxide Synthase OvoA. Open Biol. 2023, 13, 220309. [Google Scholar] [CrossRef] [PubMed]
- Zuccarotto, A.; Sollitto, M.; Leclère, L.; Panzella, L.; Gerdol, M.; Leone, S.; Castellano, I. Molecular Evolution of Ovothiol Biosynthesis in Animal Life Reveals Diversity of the Natural Antioxidant Ovothiols in Cnidaria. Free Radic. Biol. Med. 2024, 227, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, R.; Collins, S.; Trujillo, J.; McCredie, K.; Ahearn, M.; Tsai, S.; Metzgar, R.; Aulakh, G.; Ting, R.; Ruscetti, F.; et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood 1979, 54, 713–733. [Google Scholar] [CrossRef] [PubMed]
- Rosén, A.; Bergh, A.-C.; Gogok, P.; Evaldsson, C.; Myhrinder, A.L.; Hellqvist, E.; Rasul, A.; Björkholm, M.; Jansson, M.; Mansouri, L.; et al. Lymphoblastoid Cell Line with B1 Cell Characteristics Established from a Chronic Lymphocytic Leukemia Clone by In Vitro EBV Infection. OncoImmunology 2012, 1, 18–27. [Google Scholar] [CrossRef]
- Russo, M.; Milito, A.; Spagnuolo, C.; Carbone, V.; Rosén, A.; Minasi, P.; Lauria, F.; Russo, G.L. CK2 and PI3K Are Direct Molecular Targets of Quercetin in Chronic Lymphocytic Leukaemia. Oncotarget 2017, 8, 42571–42587. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Moccia, S.; Tedesco, I.; Lauria, F.; Russo, G.L. Biochemical and Cellular Characterization of New Radio-Resistant Cell Lines Reveals a Role of Natural Flavonoids to Bypass Senescence. Int. J. Mol. Sci. 2021, 23, 301. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Moccia, S.; Bilotto, S.; Spagnuolo, C.; Durante, M.; Lenucci, M.S.; Mita, G.; Volpe, M.G.; Aquino, R.P.; Russo, G.L. A Carotenoid Extract from a Southern Italian Cultivar of Pumpkin Triggers Nonprotective Autophagy in Malignant Cells. Oxid. Med. Cell. Longev. 2017, 2017, 7468538. [Google Scholar] [CrossRef] [PubMed]
- Brancaccio, M.; Milito, A.; Viegas, C.A.; Palumbo, A.; Simes, D.C.; Castellano, I. First Evidence of Dermo-Protective Activity of Marine Sulfur-Containing Histidine Compounds. Free Radic. Biol. Med. 2022, 192, 224–234. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuccarotto, A.; Russo, M.; Di Giacomo, A.; Casale, A.; Mitrić, A.; Leone, S.; Russo, G.L.; Castellano, I. Marine-Inspired Ovothiol Analogs Inhibit Membrane-Bound Gamma-Glutamyl-Transpeptidase and Modulate Reactive Oxygen Species and Glutathione Levels in Human Leukemic Cells. Mar. Drugs 2025, 23, 308. https://doi.org/10.3390/md23080308
Zuccarotto A, Russo M, Di Giacomo A, Casale A, Mitrić A, Leone S, Russo GL, Castellano I. Marine-Inspired Ovothiol Analogs Inhibit Membrane-Bound Gamma-Glutamyl-Transpeptidase and Modulate Reactive Oxygen Species and Glutathione Levels in Human Leukemic Cells. Marine Drugs. 2025; 23(8):308. https://doi.org/10.3390/md23080308
Chicago/Turabian StyleZuccarotto, Annalisa, Maria Russo, Annamaria Di Giacomo, Alessandra Casale, Aleksandra Mitrić, Serena Leone, Gian Luigi Russo, and Immacolata Castellano. 2025. "Marine-Inspired Ovothiol Analogs Inhibit Membrane-Bound Gamma-Glutamyl-Transpeptidase and Modulate Reactive Oxygen Species and Glutathione Levels in Human Leukemic Cells" Marine Drugs 23, no. 8: 308. https://doi.org/10.3390/md23080308
APA StyleZuccarotto, A., Russo, M., Di Giacomo, A., Casale, A., Mitrić, A., Leone, S., Russo, G. L., & Castellano, I. (2025). Marine-Inspired Ovothiol Analogs Inhibit Membrane-Bound Gamma-Glutamyl-Transpeptidase and Modulate Reactive Oxygen Species and Glutathione Levels in Human Leukemic Cells. Marine Drugs, 23(8), 308. https://doi.org/10.3390/md23080308










