Antitumor Activity of Ruditapes philippinarum Polysaccharides Through Mitochondrial Apoptosis in Cellular and Zebrafish Models
Abstract
1. Introduction
2. Results
2.1. Antitumor Activity of ERPP In Vitro
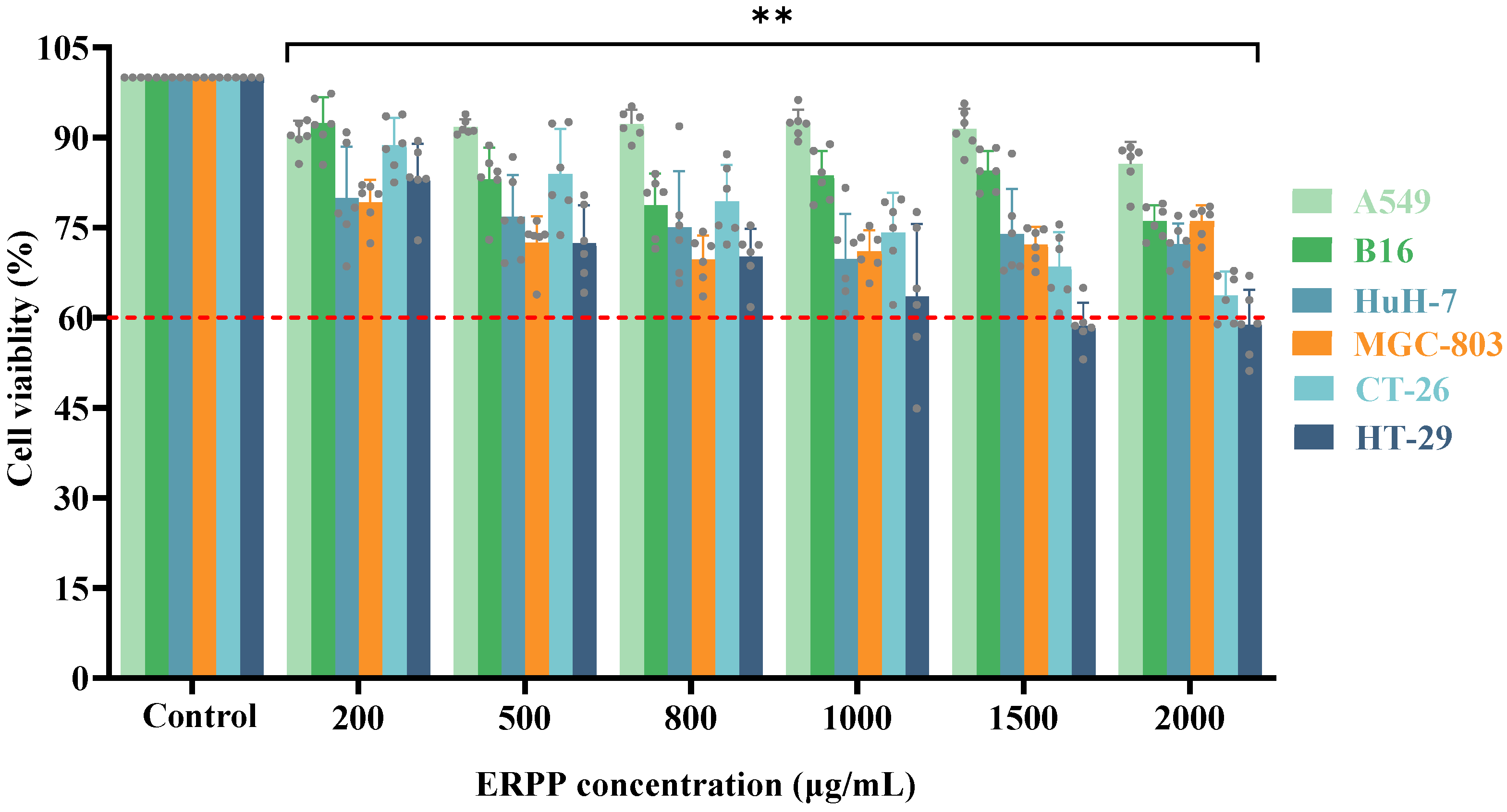
2.1.1. Effects of ERPP on Multiple Cancer Cell Lines
2.1.2. Attenuation of Colony Formation and Migration Capacity in HT-29 Cells by ERPP
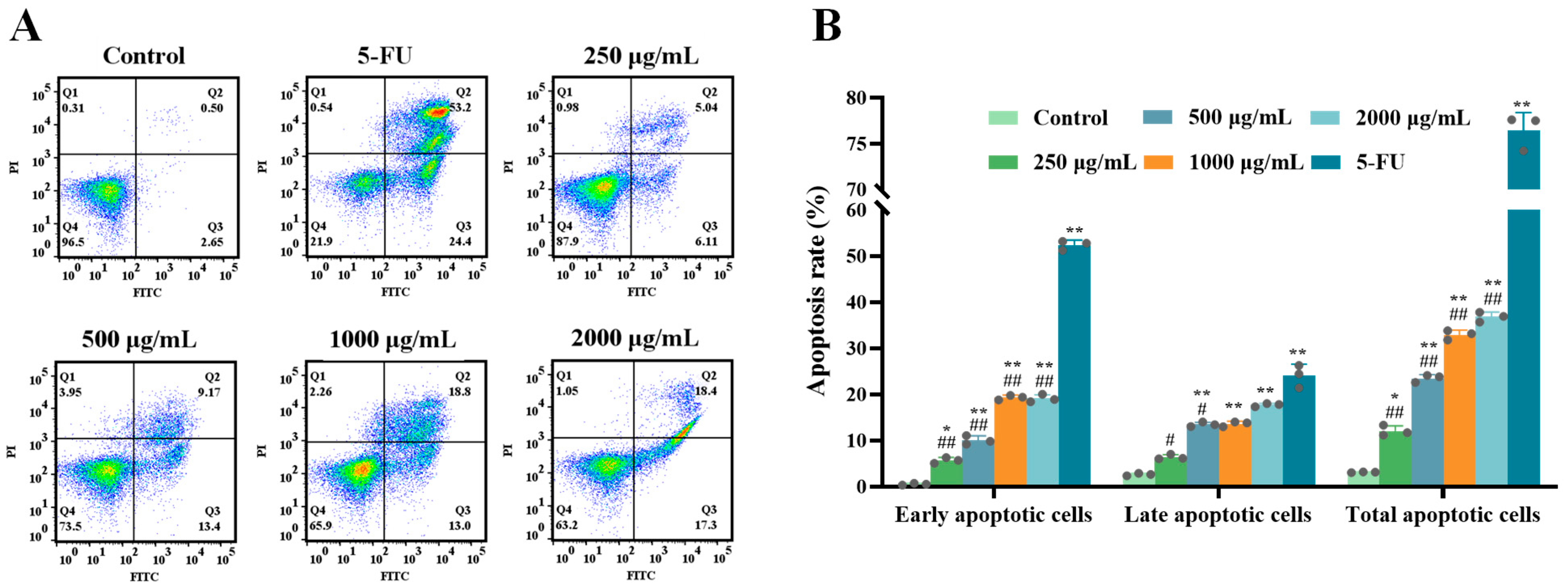
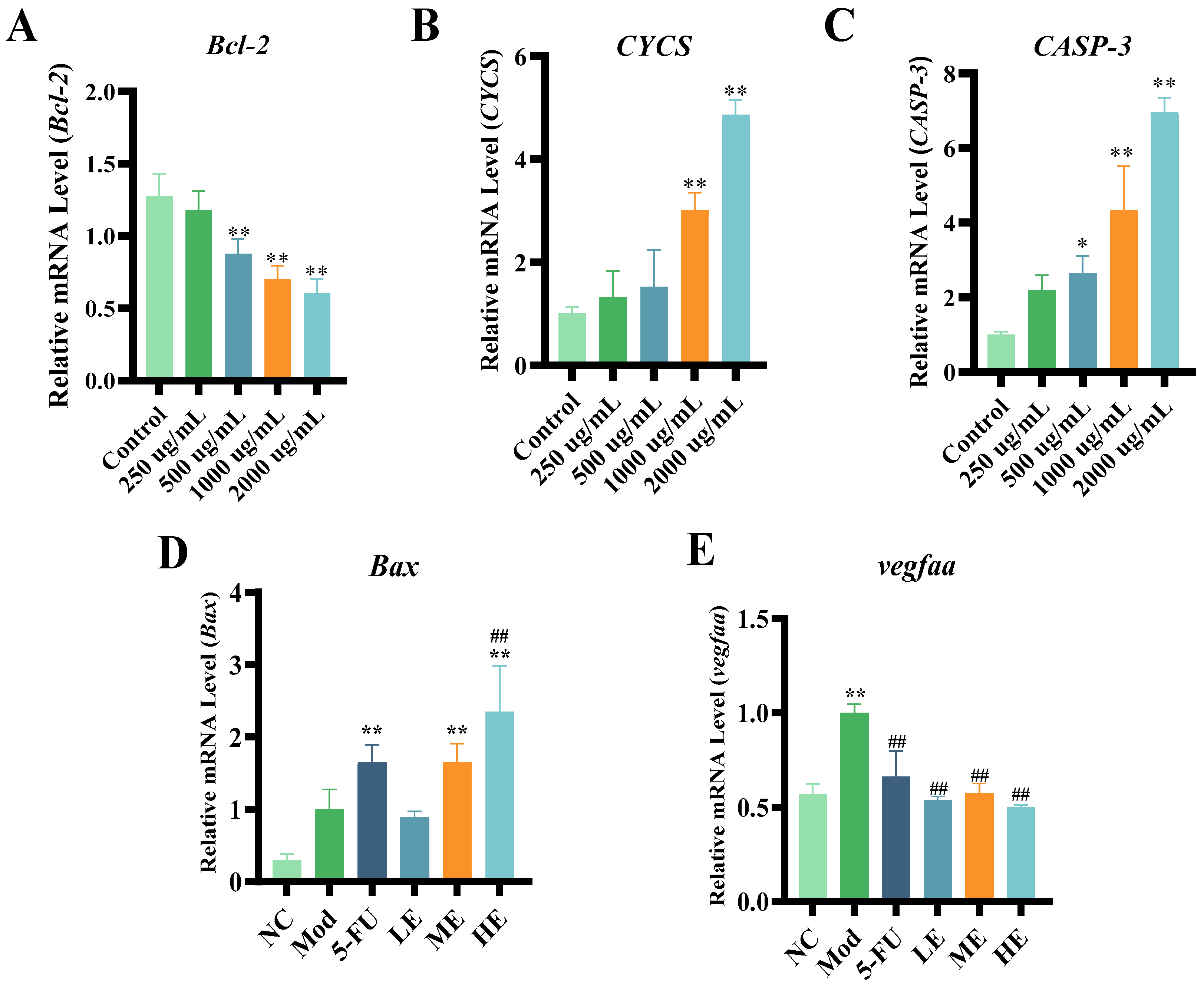
2.1.3. ERPP Induced Apoptosis in HT-29 Cells
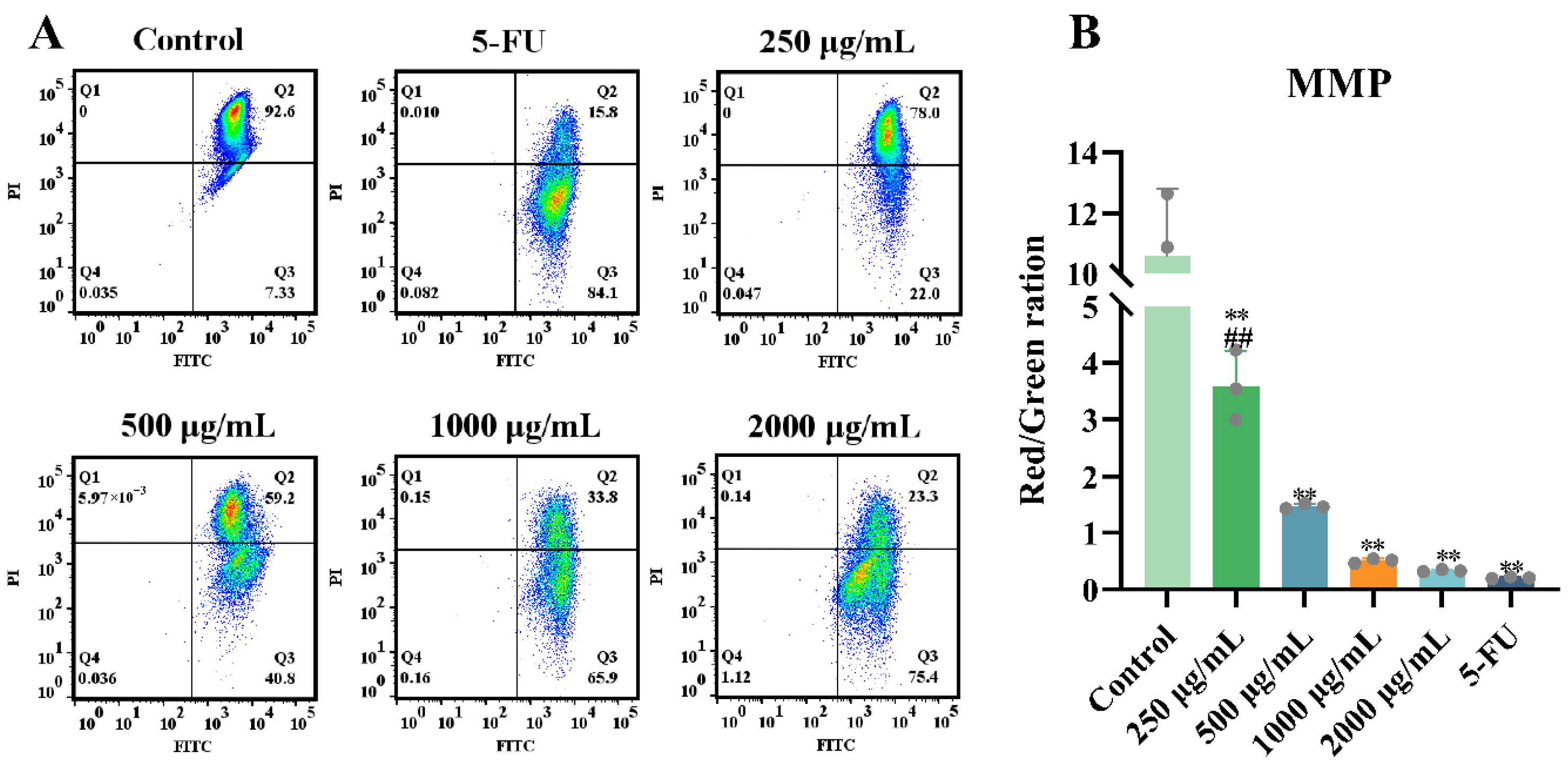
2.1.4. Effects of ERPP on Mitochondrial Membrane Potential (MMP)
2.2. Antitumor Activity of ERPP In Vivo
2.2.1. Effects of ERPP on Tumor Proliferation in HT-29 Zebrafish Xenografts
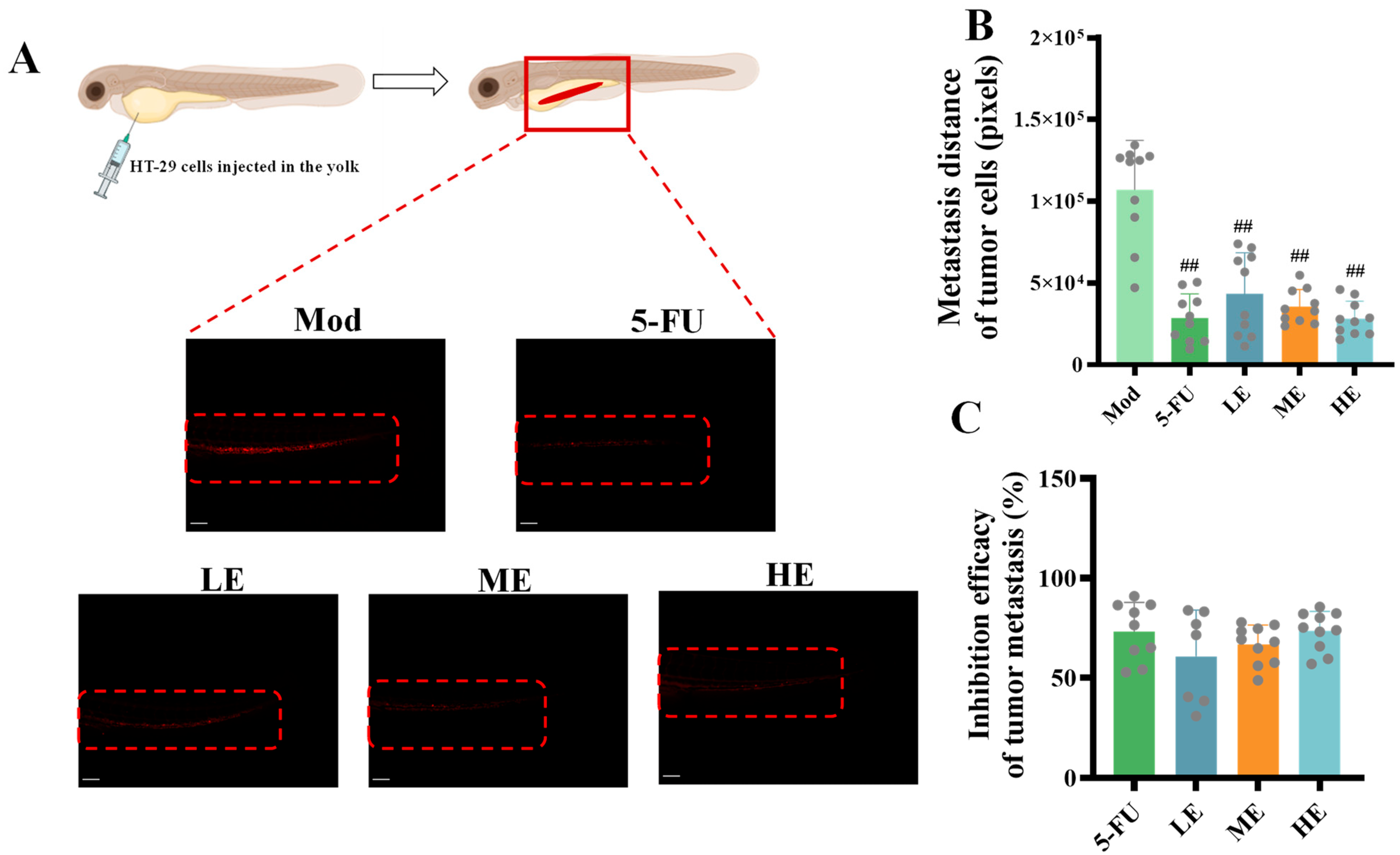
2.2.2. Effects of ERPP on Tumor Metastasis in HT-29 Zebrafish Xenografts
2.2.3. Effects of ERPP Treatments on Tumor Angiogenesis
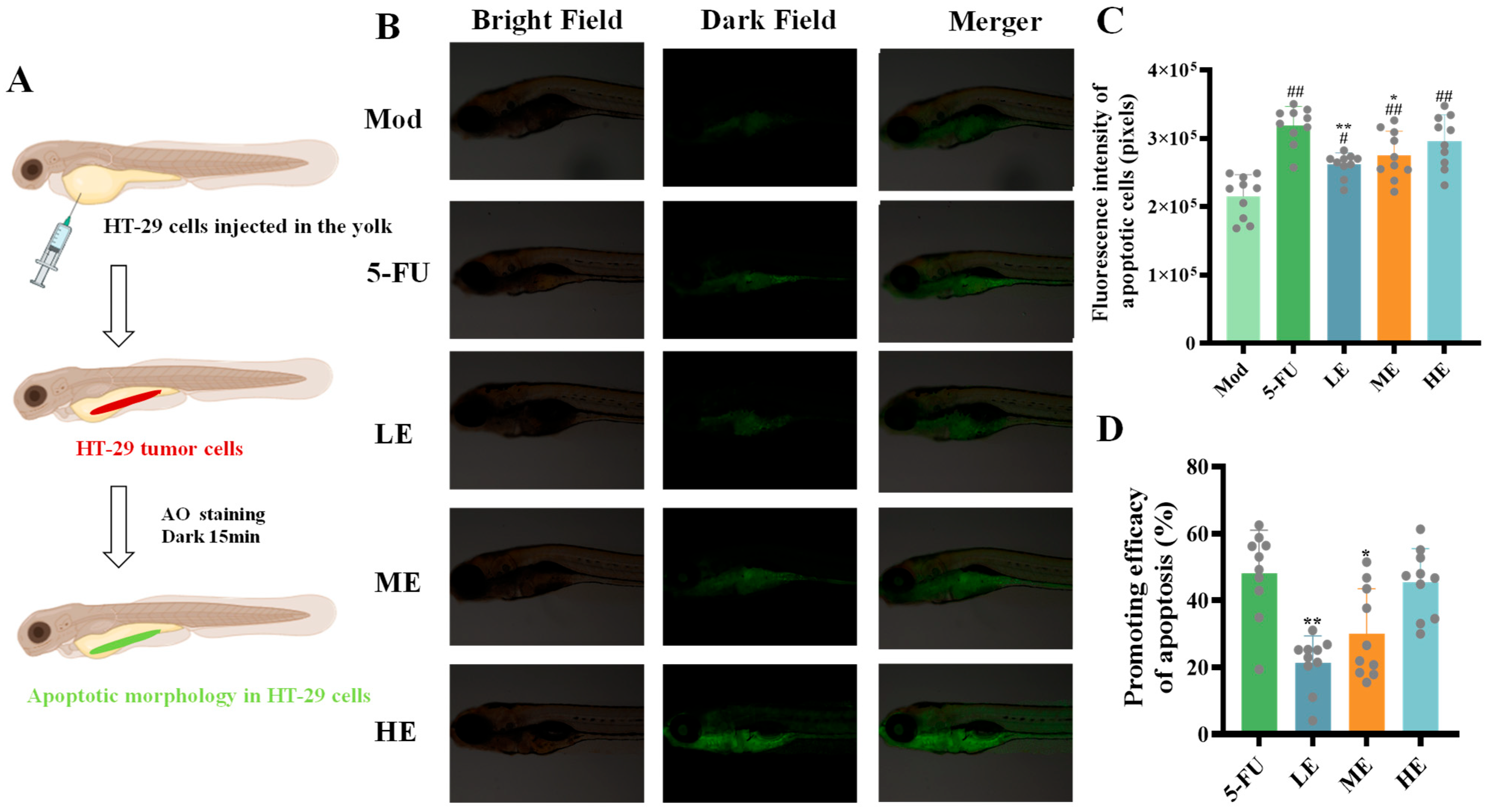
2.2.4. Effects of ERPP Treatments on Cell Apoptosis in HT-29 Zebrafish Xenografts
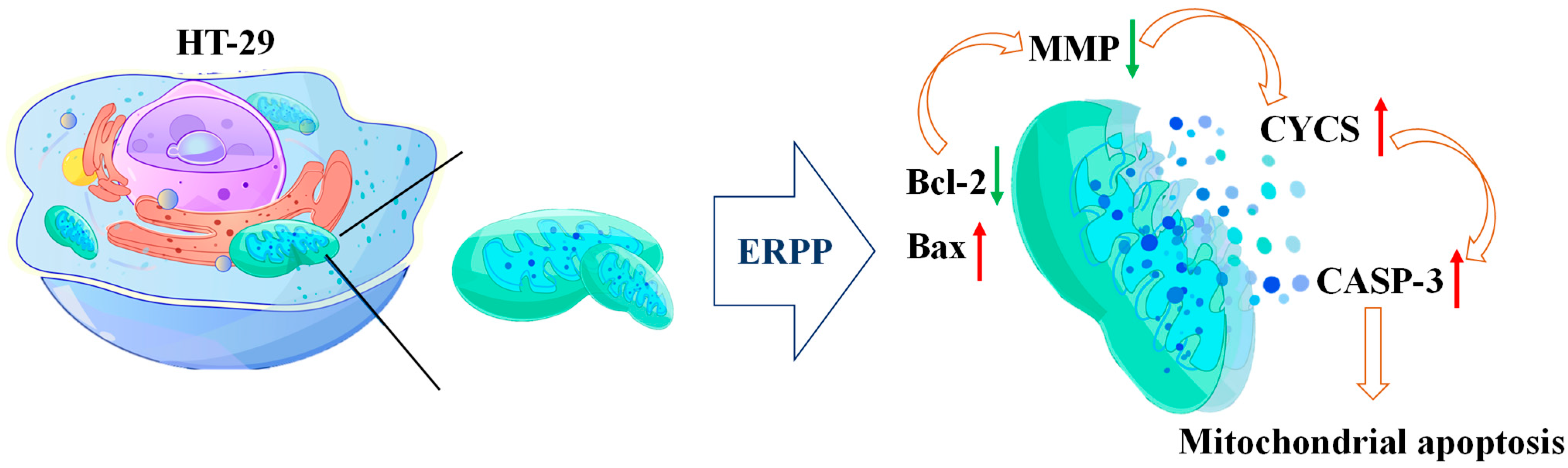
2.3. Research on Antitumor Mechanism of ERPP
2.4. Antioxidant Activities of ERPP
2.4.1. ABTS•+, DPPH•, and Hydroxyl Free Radical Scavenging Rate Analysis
2.4.2. Effects of ERPP on Intracellular ROS
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Sample Preparation of ERPP
4.3. Cell Culture
4.3.1. Cell Counting Kit-8 Assay
4.3.2. Colony Formation
4.3.3. Wound Healing Assay
4.3.4. Cell Apoptosis
4.3.5. Detection of Mitochondrial Membrane Potential (MMP)
4.4. Animal Experiment
4.4.1. Fluorescent Labeling of HT-29 Cells
4.4.2. The Establishment of a Zebrafish Tumor Transplantation Model
4.4.3. Tumor Growth, Angiogenesis, and Metastatic Potential Quantification
4.4.4. Tumor Cells Apoptosis
4.5. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (RT-qPCR)
4.6. Antioxidant Activity Analysis
4.6.1. ABTS•+, DPPH•, and Hydroxyl Free Radical Scavenging Rate Analysis
4.6.2. Intracellular Reactive Oxygen Species (ROS) Detection
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Byrne, R.M.; Ruhl, R.; Lanciault, C.; Anand, S.; Nellore, A.; Tsikitis, V.L. Age-related differences in gene expression in colorectal cancer (CRC). J. Clin. Oncol. 2018, 36, 654. [Google Scholar] [CrossRef]
- Killock, D. Early MRD predicts disease recurrence and benefit from adjuvant chemotherapy in CRC. Nat. Rev. Clin. Oncol. 2023, 20, 137. [Google Scholar] [CrossRef]
- Ramos García, L.; Webster, R.M. The colorectal cancer drug market. Nat. Rev. Drug Discov. 2024, 23, 414–415. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, N.; Hou, J.; Cao, R.; Jia, L.; Guo, Y.; Xu, J. Structure and antitumor activity of a polysaccharide from Rosa roxburghii. Int. J. Biol. Macromol. 2024, 273, 132807. [Google Scholar] [CrossRef] [PubMed]
- Reilley, M.; Blando, J.M.; Katkhuda, R.; Menter, D.; Sharma, P.; Allison, J.P.; Kopetz, S.; Maru, D.M.; Overman, M.J. Immunologic profiling of consensus molecular subtype (CMS) stratified colorectal cancer (CRC) primary and liver metastectomy specimens: Implications for immune targeting of proficient mismatch repair CRC. J. Clin. Oncol. 2016, 34, 3520. [Google Scholar] [CrossRef]
- Nusrat, M.; Roszik, J.; Katkhuda, R.; Menter, D.; Raghav, K.P.S.; Morris, V.K.; Sharma, P.; Allison, J.P.; Blando, J.M.; Maru, D.M.; et al. Association of PIK3CA mutations (mut) with immune engagement and clinical benefit from immunotherapy in microsatellite stable (MSS) colorectal cancer (CRC) patients (pts). J. Clin. Oncol. 2019, 37, 3604. [Google Scholar] [CrossRef]
- Guo, R.; Chen, M.; Ding, Y.; Yang, P.; Wang, M.; Zhang, H.; He, Y.; Ma, H. Polysaccharides as Potential Anti-tumor Biomacromolecules—A Review. Front. Nutr. 2022, 9, 838179. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Muhmood, A.; Akhter, M.; Gao, X.; Sun, J.; Du, Z.B.; Wei, Y.X.; Zhang, T.; Wei, Y.L. Characterization, health benefits, and food applications of enzymatic digestion- resistant dextrin: A review. Int. J. Biol. Macromol. 2023, 253, 126970. [Google Scholar] [CrossRef]
- He, R.; Lao, Y.F.; Yu, W.Y.; Zhang, X.H.; Jiang, M.; Zhu, C.R. Progress in the Application of Immune Checkpoint Inhibitor-Based Immunotherapy for Targeting Different Types of Colorectal Cancer. Front. Oncol. 2021, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.F.; Zhu, M.S.; Wang, D.L.; Tao, W.Y.; Liu, D.H.; Zhang, F.M.; Linhardt, R.J.; Ye, X.Q.; Chen, S.G. Oral Administration of Fucosylated Chondroitin Sulfate Oligomers in Gastro-Resistant Microcapsules Exhibits a Safe Antithrombotic Activity. Thromb. Haemost. 2021, 121, 15–26. [Google Scholar] [CrossRef]
- Cheong, K.L.; Xia, L.X.; Liu, Y. Isolation and Characterization of Polysaccharides from Oysters (Crassostrea gigas) with Anti-Tumor Activities Using an Aqueous Two-Phase System. Mar. Drugs 2017, 15, 338. [Google Scholar] [CrossRef]
- Zhang, R.J.; Shi, Y.; Zheng, J.; Mao, X.M.; Liu, Z.Y.; Chen, Q.X.; Wang, Q. Effects of polysaccharides from abalone viscera (Haliotis discus hannai Ino) on MGC 803 cells proliferation. Int. J. Biol. Macromol. 2018, 106, 587–595. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wang, J.; Jin, W.H.; Zhang, Q.B. The antioxidant activities and neuroprotective effect of polysaccharides from the starfish Asterias rollestoni. Carbohydr. Polym. 2013, 95, 9–15. [Google Scholar] [CrossRef]
- Lv, M.B.; Liu, M.Y.; Zou, S.C.; Yin, D.L.; Lv, C.H.; Li, F.; Wei, Y.X. Immune Enhancement of Clam Peptides on Immunosuppressed Mice Induced by Hydrocortisone. Molecules 2023, 28, 5709. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, F.J.; Wang, X.M.; Zhao, G.H.; Cai, D.; Yu, J.H.; Yin, F.W.; Zhou, D.Y. Preparation and Hepatoprotective Activities of Peptides Derived from Mussels (Mytilus edulis) and Clams (Ruditapes philippinarum). Mar. Drugs 2022, 20, 719. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, H.S.; Shi, R.; Fan, F.J.; Cheng, W.J. Unlocking the Therapeutic Potential of a Manila Clam-Derived Antioxidant Peptide: Insights into Mechanisms of Action and Cytoprotective Effects against Oxidative Stress. Foods 2024, 13, 1160. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, F.; Feng, S.; Guo, J.; Yu, J.; Zou, S.; Gao, X.; Wei, Y. Evaluation of Anticancer and Immunomodulatory Effects of Microwave-Extracted Polysaccharide from Ruditapes philippinarum. Foods 2024, 13, 3552. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.T.; Elson, D.J.; Greenwood, J.A.; Tanguay, R.L.; Kolluri, S.K. The Zebrafish Xenograft Models for Investigating Cancer and Cancer Therapeutics. Biology 2021, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- White, R.; Rose, K.; Zon, L. Zebrafish cancer: The state of the art and the path forward. Nat. Rev. Cancer 2013, 13, 624–636. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, J. siRNA against TSG101 reduces proliferation and induces G0/G1 arrest in renal cell carcinoma—Involvement of c-myc, cyclin E1, and CDK2. Cell. Mol. Biol. Lett. 2019, 24, 7. [Google Scholar] [CrossRef]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Bobadilla, A.V.P.; Arévalo, J.; Sarró, E.; Byrne, H.M.; Maini, P.K.; Carraro, T.; Balocco, S.; Meseguer, A.; Alarcón, T. In vitro cell migration quantification method for scratch assays. J. R. Soc. Interface 2019, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, W.S.; Han, B.Q.; Sun, J.P.; Wang, D.F. Isolation and characterization of antitumor polysaccharides from the marine mollusk Ruditapes philippinarum. Eur. Food Res. Technol. 2008, 227, 103–110. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Shimizu, S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 2007, 12, 835–840. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kroemer, G. Mitochondrial apoptosis without VDAC. Nat. Cell. Biol. 2007, 9, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.C.; Fang, F.; Shao, T.L.; Li, P.; Hu, W.; Zhou, Y.Y.; Wang, G.D.; Han, J.; Chen, K.S. Structure and Anti-Tumor Activities of Exopolysaccharides from Alternaria mali Roberts. Molecules 2019, 24, 1345. [Google Scholar] [CrossRef]
- Lin, H.C.; Lin, J.Y. GSF3, a polysaccharide from guava (Psidium guajava L.) seeds, inhibits MCF-7 breast cancer cell growth via increasing Bax/Bcl-2 ratio or Fas mRNA expression levels. Int. J. Biol. Macromol. 2020, 161, 1261–1271. [Google Scholar] [CrossRef]
- Chen, P.; Liu, H.P.; Ji, H.H.; Sun, N.X.; Feng, Y.Y. A cold-water soluble polysaccharide isolated from Grifola frondosa induces the apoptosis of HepG2 cells through mitochondrial passway. Int. J. Biol. Macromol. 2019, 125, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Norton, K.A.; Popel, A.S. Effects of endothelial cell proliferation and migration rates in a computational model of sprouting angiogenesis. Sci. Rep. 2016, 6, 36992. [Google Scholar] [CrossRef]
- Kang, Y.; Li, H.; Liu, Y.; Li, Z. Regulation of VEGF-A expression and VEGF-A-targeted therapy in malignant tumors. J. Cancer Res. Clin. Oncol. 2024, 150, 221. [Google Scholar] [CrossRef]
- Shen, S.G.; Jia, S.R.; Wu, Y.K.; Yan, R.R.; Lin, Y.H.; Zhao, D.X.; Han, P.P. Effect of culture conditions on the physicochemical properties and antioxidant activities of polysaccharides from Nostoc flagelliforme. Carbohydr. Polym. 2018, 198, 426–433. [Google Scholar] [CrossRef]
- Feng, L.J.; Qian, T.T.; Yang, G.F.; Mu, J. Characteristics of exopolysaccharides produced by isolates from natural bioflocculant of Ruditapes philippinarum conglutination mud. Front. Microbiol. 2023, 13, 1068922. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.M.; Pan, L.C.; Zhang, L.J.; Yin, Y.; Zhu, Z.Y.; Sun, H.Q.; Liu, C.Y. Chemical structure and antioxidant activity of a polysaccharide from Siraitia grosvenorii. Int. J. Biol. Macromol. 2020, 165, 1900–1910. [Google Scholar] [CrossRef]
- Öztürk, M.; Aydogmus-Öztürk, F.; Duru, M.E.; Topcu, G. Antioxidant activity of stem and root extracts of Rhubarb (Rheum ribes): An edible medicinal plant. Food Chem. 2007, 103, 623–630. [Google Scholar] [CrossRef]
- Yarley, O.P.N.; Kojo, A.B.; Zhou, C.; Yu, X.; Gideon, A.; Kwadwo, H.H.; Richard, O. Reviews on mechanisms of in vitro antioxidant, antibacterial and anticancer activities of water-soluble plant polysaccharides. Int. J. Biol. Macromol. 2021, 183, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Bhuyan, P.P.; Ki, J.S. Immunomodulatory, Antioxidant, Anticancer, and Pharmacokinetic Activity of Ulvan, a Seaweed-Derived Sulfated Polysaccharide: An Updated Comprehensive Review. Mar. Drugs 2023, 21, 300. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Xu, J.; Yu, Y.; Chen, K.; Wang, Y.; Zhu, Y.; Zou, X.; Xu, X.; Jiang, Y. Astragalus polysaccharides ameliorate osteoarthritis via inhibiting apoptosis by regulating ROS-mediated ASK1/p38 MAPK signaling pathway targeting on TXN. Int. J. Biol. Macromol. 2024, 258, 129004. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Xue, Y.T. Optimization of microwave assisted extraction, chemical characterization and antitumor activities of polysaccharides from porphyra haitanensis. Carbohydr. Polym. 2019, 206, 179–186. [Google Scholar] [CrossRef]
- Wu, J.; Gao, W.P.; Song, Z.Y.; Xiong, Q.P.; Xu, Y.T.; Han, Y.; Yuan, J.; Zhang, R.; Cheng, Y.B.; Fang, J.S.; et al. Anticancer activity of polysaccharide from Glehnia littoralis on human lung cancer cell line A549. Int. J. Biol. Macromol. 2018, 106, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Abu-Qare, A.W.; Abou-Donia, M.B. Biomarkers of apoptosis: Release of cytochrome c, activation of caspase-3, induction of 8-hydroxy-2′-deoxyguanosine, increased 3-nitrotyrosine, and alteration of p53 gene. J. Toxicol. Environ. Health Part B Crit. Rev. 2001, 4, 313–332. [Google Scholar] [CrossRef]
- Lee, K.S.; Shin, J.S.; Nam, K.S. Inhibitory effect of starfish polysaccharides on metastasis in HT-29 human colorectal adenocarcinoma. Biotechnol. Bioprocess Eng. 2012, 17, 764–769. [Google Scholar] [CrossRef]
- Hata, A.N.; Engelman, J.A.; Faber, A.C. The BCL2 Family: Key Mediators of the Apoptotic Response to Targeted Anticancer Therapeutics. Cancer Discov. 2015, 5, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Sahayanathan, G.J.; Padmanaban, D.; Raja, K.; Chinnasamy, A. Anticancer effect of purified polysaccharide from marine clam Donax variabilis on A549 cells. J. Food Biochem. 2020, 44, e13486. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Yi, T.; Li, H.; Tang, X.; Liu, D.; Wu, D.; Li, Y. Decoding tumor angiogenesis: Pathways, mechanisms, and future directions in anti-cancer strategies. Biomark. Res. 2025, 13, 62. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chen, H.H.; Zheng, L.L.; Sun, L.P.; Shi, L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct. Target. Ther. 2023, 8, 198. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, Y.; Yang, Z.; Yang, M.; Wong, C.Y. NIR-II-driven and glutathione depletion-enhanced hypoxia-irrelevant free radical nanogenerator for combined cancer therapy. J. Nanobiotechnology 2021, 19, 265. [Google Scholar] [CrossRef]
- Li, F.; Jiao, X.; Zhao, J.; Liao, X.; Wei, Y.; Li, Q. Antitumor mechanisms of an exopolysaccharide from Lactobacillus fermentum on HT-29 cells and HT-29 tumor-bearing mice. Int. J. Biol. Macromol. 2022, 209, 552–562. [Google Scholar] [CrossRef]





| Concentration (μg/mL) | Number of Zebrafish Fatalities | Mortality Rate (%) | Phenotype of Zebrafish |
|---|---|---|---|
| 15.6 | 0 | 0 | Consistent with the model control group |
| 31.2 | 0 | 0 | |
| 61.5 | 0 | 0 | |
| 125 | 0 | 0 | |
| 250 | 16 | 63 | — |
| Gene | Primer Sequence |
|---|---|
| Bcl-2 | F: ATCGCCCTGTGGATGACTGAGT R: GCCAGGAGAAATCAAACAGAGGC |
| CASP-3 | F: AGAGGGGATCGTTGTAGAAGTC R: ACAGTCCAGTTCTGTACCACG |
| CYCS | F: CTTTGGGCGGAAGACAGGTC R: TTATTGGCGGCTGTGTAAGAG |
| Bax | F: GACTTGGGAGCTGCACTTCT R: TCCGATCTGCTGCAAACACT |
| vegfaa | F: TCCCGACAGAGACACGAAAC R: CATCTTGGCTTTTCACATCTTTCT |
| β-actin | F: TCGAGCAGGAGATGGGAACC R: CTCGTGGATACCGCAAGATTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Wang, W.; Wang, H.; Zhao, S.; Yin, D.; Zhang, H.; Zou, C.; Zou, S.; Yu, J.; Wei, Y. Antitumor Activity of Ruditapes philippinarum Polysaccharides Through Mitochondrial Apoptosis in Cellular and Zebrafish Models. Mar. Drugs 2025, 23, 304. https://doi.org/10.3390/md23080304
Liu M, Wang W, Wang H, Zhao S, Yin D, Zhang H, Zou C, Zou S, Yu J, Wei Y. Antitumor Activity of Ruditapes philippinarum Polysaccharides Through Mitochondrial Apoptosis in Cellular and Zebrafish Models. Marine Drugs. 2025; 23(8):304. https://doi.org/10.3390/md23080304
Chicago/Turabian StyleLiu, Mengyue, Weixia Wang, Haoran Wang, Shuang Zhao, Dongli Yin, Haijun Zhang, Chunze Zou, Shengcan Zou, Jia Yu, and Yuxi Wei. 2025. "Antitumor Activity of Ruditapes philippinarum Polysaccharides Through Mitochondrial Apoptosis in Cellular and Zebrafish Models" Marine Drugs 23, no. 8: 304. https://doi.org/10.3390/md23080304
APA StyleLiu, M., Wang, W., Wang, H., Zhao, S., Yin, D., Zhang, H., Zou, C., Zou, S., Yu, J., & Wei, Y. (2025). Antitumor Activity of Ruditapes philippinarum Polysaccharides Through Mitochondrial Apoptosis in Cellular and Zebrafish Models. Marine Drugs, 23(8), 304. https://doi.org/10.3390/md23080304






