A Comprehensive Review on the Valorization of Bioactives from Marine Animal By-Products for Health-Promoting, Biofunctional Cosmetics
Abstract
1. Introduction
1.1. Mollusks and Mollusk By-Products
1.2. Crustaceans and Crustacean By-Products
1.3. Fish and Fish By-Products
2. Extraction Techniques of Bioactive Compounds
2.1. Conventional Extraction Techniques
2.2. Non-Conventional Extraction Techniques
2.2.1. Enzyme-Assisted Extraction (EAE)
2.2.2. Microwave-Assisted Extraction (MAE)
2.2.3. Subcritical Water Extraction (SWE)
2.2.4. Supercritical Fluid Extraction (SFE)
2.2.5. Ultrasound-Assisted Extraction (UAE)
3. Bioactive Compounds from Marine By-Products
3.1. Bioactive Compounds and Structural Components of Mollusks
3.1.1. Shell Structure of Mollusks
3.1.2. Terpenes and Other Bioactive Metabolites in Mollusks
3.1.3. Cephalopods: Protein-Rich Tissues and Bioactive Ink
3.1.4. Gastropods and Bivalves: Minerals and Metabolites
3.1.5. Oysters: Peptides, Minerals, and Shell Components
3.2. Bioactive Compounds and Structural Components of Crustaceans
3.2.1. Shell Structure of Crustaceans
3.2.2. Chitin and Its Derivatives
3.2.3. Lipids and Pigments
Astaxanthin
3.2.4. Proteins and Protein Hydrolysates
3.2.5. Minerals and Metals
3.3. Fish Bioactive Compounds: Composition, Types, and Applications
3.3.1. General Composition and Nutritional Value of Fish and Its By-Products
3.3.2. Protein-Based Bioactive Compounds
3.3.3. Fatty Acid (Lipid) Content
3.3.4. Fish Polar Lipids
3.3.5. Vitamin and Mineral Content
3.3.6. Pigments and Carotenoids
3.3.7. Collagen and Gelatin
3.3.8. Glycosaminoglycans: Chondroitin, Glucosamine, and Hyaluronic Acid
4. Cosmetic Applications of Marine By-Products
4.1. Marine Polysaccharides as Cosmetic Ingredients
4.1.1. Marine Chitin and Its Derivatives as Cosmetic Ingredients
Chitosan as an Anti-Aging and Moisturizing Agent
Chitosan as a UV-Radiation-Protective Agent
Chitosan as a Skin-Cleansing Agent
Chitosan as an Antibacterial Agent
Chitosan as a Nail-Care Agent
Chitosan as a Hair-Care Agent
Chitosan as an Oral-Cavity-Protective Agent
4.1.2. Marine Hyaluronic Acid, Glycosaminoglycans (GAGs), Carrageenan, and Fucoidans as Cosmetic Ingredients
4.2. Marine Fatty Acids (ω-3 PUFAs) as Cosmetic Ingredients
4.2.1. Fatty Acids as Anti-Photo-Aging Cosmetic Agents
4.2.2. Fatty Acids as Anti-Hyperpigmentation Cosmetic Agents
4.2.3. Fatty Acids as Anti-Skin-Cancer Cosmetic Agents
4.2.4. Fatty Acids as Anti-Dermatitis and Anti-Erythema Cosmetic Agents
4.2.5. Fatty Acid Agents Against Psoriasis and Acne Vulgaris
4.2.6. Fatty Acids as Anti-Inflammatory Cosmetic Agents
4.3. Marine Polar Lipids and Lipid Vitamins as Cosmetic Ingredients
4.4. Marine Amino Acids as Cosmetic Ingredients
Mycosporine-like Amino Acids (MAAs)
4.5. Predominant Marine Proteins as Cosmetic Ingredients
4.5.1. Marine Collagen as a Cosmetic Agent
Collagen as a Moisturizing Agent
Collagen as an Anti-Aging Agent
Collagen as a Wound-Healing Agent
4.5.2. Marine Gelatin as a Cosmetic Agent
4.6. Peptides as Cosmetic Ingredients
4.7. Marine Pigments as Cosmetic Ingredients
4.7.1. Astaxanthin as a Cosmetic Agent
4.7.2. Melanin as a Cosmetic Agent
4.8. Phenolic Compounds as Cosmetic Ingredients
4.9. Marine Substrates and Minerals as Cosmetic Ingredients
4.10. Marine Bioactives as Potential Cosmetic Ingredients
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harinisri Ram, K.; Thamarai Selvi, B. Inventive Applications of Marine Resources in Cosmetic Production: A Review. In Multidisciplinary Applications of Marine Resources; Rafatullah, M., Siddiqui, M.R., Khan, M.A., Kapoor, R.T., Eds.; Springer Nature: Singapore, 2024; pp. 407–441. ISBN 978-981-9750-56-6. [Google Scholar]
- Fonseca, S.; Amaral, M.N.; Reis, C.P.; Custódio, L. Marine Natural Products as Innovative Cosmetic Ingredients. Mar. Drugs 2023, 21, 170. [Google Scholar] [CrossRef]
- Alves, A.; Sousa, E.; Kijjoa, A.; Pinto, M. Marine-Derived Compounds with Potential Use as Cosmeceuticals and Nutricosmetics. Molecules 2020, 25, 2536. [Google Scholar] [CrossRef]
- Ghareeb, M.A.; Tammam, M.A.; El-Demerdash, A.; Atanasov, A.G. Insights about Clinically Approved and Preclinically Investigated Marine Natural Products. Curr. Res. Biotechnol. 2020, 2, 88–102. [Google Scholar] [CrossRef]
- Lu, W.-Y.; Li, H.-J.; Li, Q.-Y.; Wu, Y.-C. Application of Marine Natural Products in Drug Research. Bioorg. Med. Chem. 2021, 35, 116058. [Google Scholar] [CrossRef]
- Oliver, J.M.; Ross, E.L.; Frank, E.K. The Discovery of Marine Natural Products with Therapeutic Potential. In Discovery of Novel Natural Products with Therapeutic Potential; Elsevier: Amsterdam, The Netherlands, 1994; pp. 109–174. ISBN 978-0-7506-9003-4. [Google Scholar]
- Papikinou, M.-A.; Pavlidis, K.; Cholidis, P.; Kranas, D.; Adamantidi, T.; Anastasiadou, C.; Tsoupras, A. Marine Fungi Bioactives with Anti-Inflammatory, Antithrombotic and Antioxidant Health-Promoting Properties Against Inflammation-Related Chronic Diseases. Mar. Drugs 2024, 22, 520. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Lefranc, F.; Vieira, L.M.; Kiss, R.; Carbone, M.; Van Otterlo, W.A.L.; Lopanik, N.B.; Waeschenbach, A. The Phylum Bryozoa: From Biology to Biomedical Potential. Mar. Drugs 2020, 18, 200. [Google Scholar] [CrossRef]
- Bergmann, W.; Feeney, R.J. The isolation of a new thymine pentoside from sponges. J. Am. Chem. Soc. 1950, 72, 2809–2810. [Google Scholar] [CrossRef]
- Nash, K.L.; Van Putten, I.; Alexander, K.A.; Bettiol, S.; Cvitanovic, C.; Farmery, A.K.; Flies, E.J.; Ison, S.; Kelly, R.; Mackay, M.; et al. Oceans and Society: Feedbacks between Ocean and Human Health. Rev. Fish Biol. Fish. 2022, 32, 161–187. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.G.; Burgess, J.G. The Promise of Marine Molecules as Cosmetic Active Ingredients. Int. J. Cosmet. Sci. 2018, 40, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Abid, F.; Zahid, M.A.; Abedin, Z.U.; Nizami, S.B.; Abid, M.J.; Kazmi, S.Z.H.; Khan, S.U.; Hasan, H.; Ali, M.; Gul, A. Omics Approaches in Marine Biotechnology. In Omics Technologies and Bio-Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 47–61. ISBN 978-0-12-804659-3. [Google Scholar]
- Ngandjui, Y.A.T.; Kereeditse, T.T.; Kamika, I.; Madikizela, L.M.; Msagati, T.A.M. Nutraceutical and Medicinal Importance of Marine Molluscs. Mar. Drugs 2024, 22, 201. [Google Scholar] [CrossRef] [PubMed]
- Mititelu, M.; Stanciu, G.; Drăgănescu, D.; Ioniță, A.C.; Neacșu, S.M.; Dinu, M.; Stefan-van Staden, R.-I.; Moroșan, E. Mussel Shells, a Valuable Calcium Resource for the Pharmaceutical Industry. Mar. Drugs 2021, 20, 25. [Google Scholar] [CrossRef]
- Oys, S.; Prince, U. Utilization of mollusk shell in mushroom cultivation. In Proceedings of the 2nd International Cankaya Scientific Studies Congress, Ankara, Turkey, 28–29 September 2023. [Google Scholar]
- Azarian, M.H.; Sutapun, W. Biogenic Calcium Carbonate Derived from Waste Shells for Advanced Material Applications: A Review. Front. Mater. 2022, 9, 1024977. [Google Scholar] [CrossRef]
- Guo, L.; Li, W.; Gu, Z.; Wang, L.; Guo, L.; Ma, S.; Li, C.; Sun, J.; Han, B.; Chang, J. Recent Advances and Progress on Melanin: From Source to Application. Int. J. Mol. Sci. 2023, 24, 4360. [Google Scholar] [CrossRef] [PubMed]
- Prajaputra, V.; Isnaini, N.; Maryam, S.; Ernawati, E.; Deliana, F.; Haridhi, H.A.; Fadli, N.; Karina, S.; Agustina, S.; Nurfadillah, N.; et al. Exploring Marine Collagen: Sustainable Sourcing, Extraction Methods, and Cosmetic Applications. S. Afr. J. Chem. Eng. 2024, 47, 197–211. [Google Scholar] [CrossRef]
- Eghianruwa, Q.A.; Osoniyi, O.R.; Maina, N.; Wachira, S. Bioactive Peptides from Marine Molluscs—A Review. Int. J. Biochem. Res. Rev. 2019, 27, 1–12. [Google Scholar] [CrossRef]
- Benkendorff, K. Molluscan Biological and Chemical Diversity: Secondary Metabolites and Medicinal Resources Produced by Marine Molluscs. Biol. Rev. 2010, 85, 757–775. [Google Scholar] [CrossRef]
- Haszprunar, G.; Wanninger, A. Molluscs. Curr. Biol. 2012, 22, R510–R514. [Google Scholar] [CrossRef]
- Pyron, M.; Brown, K.M. Introduction to Mollusca and the Class Gastropoda. In Thorp and Covich’s Freshwater Invertebrates; Elsevier: Amsterdam, The Netherlands, 2015; pp. 383–421. ISBN 978-0-12-385026-3. [Google Scholar]
- Amodio, P.; Boeckle, M.; Schnell, A.K.; Ostojíc, L.; Fiorito, G.; Clayton, N.S. Grow Smart and Die Young: Why Did Cephalopods Evolve Intelligence? Trends Ecol. Evol. 2019, 34, 45–56. [Google Scholar] [CrossRef]
- Simões-Costa, M.S.; Vasconcelos, M.; Sampaio, A.C.; Cravo, R.M.; Linhares, V.L.; Hochgreb, T.; Yan, C.Y.I.; Davidson, B.; Xavier-Neto, J. The Evolutionary Origin of Cardiac Chambers. Dev. Biol. 2005, 277, 1–15. [Google Scholar] [CrossRef]
- Lopes-Lima, M.; Freitas, S.; Pereira, L.; Gouveia, E.; Hinzmann, M.; Checa, A.; Machado, J. Ionic Regulation and Shell Mineralization in the Bivalve Anodonta cygnea (Swan Mussel) Following Heavy-Metal Exposure. Can. J. Zool. 2012, 90, 267–283. [Google Scholar] [CrossRef]
- Castillo, M.G.; Salazar, K.A.; Joffe, N.R. The Immune Response of Cephalopods from Head to Foot. Fish Shellfish Immunol. 2015, 46, 145–160. [Google Scholar] [CrossRef]
- Aldairi, A.F.; Ogundipe, O.D.; Pye, D.A. Antiproliferative Activity of Glycosaminoglycan-Like Polysaccharides Derived from Marine Molluscs. Mar. Drugs 2018, 16, 63. [Google Scholar] [CrossRef]
- Siahaan, E.A.; Agusman, E.A.; Pangestuti, R.; Shin, K.-H.; Kim, S.-K. Potential Cosmetic Active Ingredients Derived from Marine By-Products. Mar. Drugs 2022, 20, 734. [Google Scholar] [CrossRef]
- Eduardo Rebolledo Ranz, R. (Ed.) Arthropods: Are They Beneficial for Mankind? IntechOpen: London, UK, 2021; ISBN 978-1-78984-165-7. [Google Scholar]
- Covich, A.P.; Thorp, J.H.; Rogers, D.C. Introduction to the Subphylum Crustacea. In Ecology and Classification of North American Freshwater Invertebrates; Elsevier: Amsterdam, The Netherlands, 2010; pp. 695–723. ISBN 978-0-12-374855-3. [Google Scholar]
- Nugroho Susanto, G. Crustacea: The Increasing Economic Importance of Crustaceans to Humans. In Arthropods—Are They Beneficial for Mankind? Eduardo Rebolledo Ranz, R., Ed.; IntechOpen: London, UK, 2021; ISBN 978-1-78984-165-7. [Google Scholar]
- Mahmood Ghafor, I. Crustacean. In Crustacea; Diarte-Plata, G., Escamilla-Montes, R., Eds.; IntechOpen: London, UK, 2020; ISBN 978-1-78985-629-3. [Google Scholar]
- Piscart, C.; Camacho, A.I.; Coineau, N.; Christodoulou, M.; Messana, G.; Wittmann, K.J. Class Malacostraca (Subclass Eumalacostraca). In Identification and Ecology of Freshwater Arthropods in the Mediterranean Basin; Elsevier: Amsterdam, The Netherlands, 2024; pp. 157–223. ISBN 978-0-12-821844-0. [Google Scholar]
- Kenny, N.; Sin, Y.; Shen, X.; Zhe, Q.; Wang, W.; Chan, T.; Tobe, S.; Shimeld, S.; Chu, K.; Hui, J. Genomic Sequence and Experimental Tractability of a New Decapod Shrimp Model, Neocaridina denticulata. Mar. Drugs 2014, 12, 1419–1437. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.; Heras Caballero, A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef]
- Cholidis, P.; Kranas, D.; Chira, A.; Galouni, E.A.; Adamantidi, T.; Anastasiadou, C.; Tsoupras, A. Shrimp Lipid Bioactives with Anti-Inflammatory, Antithrombotic, and Antioxidant Health-Promoting Properties for Cardio-Protection. Mar. Drugs 2024, 22, 554. [Google Scholar] [CrossRef]
- Caruso, G.; Floris, R.; Serangeli, C.; Di Paola, L. Fishery Wastes as a Yet Undiscovered Treasure from the Sea: Biomolecules Sources, Extraction Methods and Valorization. Mar. Drugs 2020, 18, 622. [Google Scholar] [CrossRef] [PubMed]
- Suuronen, P.; Gilman, E. Monitoring and Managing Fisheries Discards: New Technologies and Approaches. Mar. Policy 2020, 116, 103554. [Google Scholar] [CrossRef]
- Teixeira, C.M.; Coelho, M.P.; Gil, M.M.; Pita, C.; Silva, P.M. Changing the Way We Look to Fisheries’ Discards. Reg. Stud. Mar. Sci. 2024, 71, 103434. [Google Scholar] [CrossRef]
- Honrado, A.; Rubio, S.; Beltrán, J.A.; Calanche, J. Fish By-Product Valorization as Source of Bioactive Compounds for Food Enrichment: Characterization, Suitability and Shelf Life. Foods 2022, 11, 3656. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Meduíña, A.; Durán, A.I.; Nogueira, M.; Fernández-Compás, A.; Pérez-Martín, R.I.; Rodríguez-Amado, I. Production of Valuable Compounds and Bioactive Metabolites from By-Products of Fish Discards Using Chemical Processing, Enzymatic Hydrolysis, and Bacterial Fermentation. Mar. Drugs 2019, 17, 139. [Google Scholar] [CrossRef]
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; De Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Kandyliari, A.; Mallouchos, A.; Papandroulakis, N.; Golla, J.P.; Lam, T.T.; Sakellari, A.; Karavoltsos, S.; Vasiliou, V.; Kapsokefalou, M. Nutrient Composition and Fatty Acid and Protein Profiles of Selected Fish By-Products. Foods 2020, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Champi, D.; Romero-Orejon, F.L.; Muñoz, A.M.; Ramos-Escudero, F. The Revalorization of Fishery By-Products: Types, Bioactive Compounds, and Food Applications. Int. J. Food Sci. 2024, 2024, 6624083. [Google Scholar] [CrossRef] [PubMed]
- Ghalamara, S.; Brazinha, C.; Silva, S.; Pintado, M. Valorization of Fish Processing By-Products: Biological and Functional Properties of Bioactive Peptides. Curr. Food Sci. Technol. Rep. 2024, 2, 393–409. [Google Scholar] [CrossRef]
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic Potential of Marine Fish Skin Collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Rajabimashhadi, Z.; Gallo, N.; Salvatore, L.; Lionetto, F. Collagen Derived from Fish Industry Waste: Progresses and Challenges. Polymers 2023, 15, 544. [Google Scholar] [CrossRef]
- Racioppo, A.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R.; Bevilacqua, A. Fish Loss/Waste and Low-Value Fish Challenges: State of Art, Advances, and Perspectives. Foods 2021, 10, 2725. [Google Scholar] [CrossRef]
- Samarajeewa, U. Safety, Processing, and Utilization of Fishery Products. Fishes 2024, 9, 146. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A Review of Modern and Conventional Extraction Techniques and Their Applications for Extracting Phytochemicals from Plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P. Alternative and Efficient Extraction Methods for Marine-Derived Compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaq, I.; Al Jbawi, E.; Saewan, S.A. Traditional and Innovative Approaches for the Extraction of Bioactive Compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of Novel Extraction Technologies for Bioactives from Marine Algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef] [PubMed]
- Aitta, E.; Marsol-Vall, A.; Damerau, A.; Yang, B. Enzyme-Assisted Extraction of Fish Oil from Whole Fish and by-Products of Baltic Herring (Clupea harengus membras). Foods 2021, 10, 1811. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef]
- Getachew, A.T.; Jacobsen, C.; Holdt, S.L. Emerging Technologies for the Extraction of Marine Phenolics: Opportunities and Challenges. Mar. Drugs 2020, 18, 389. [Google Scholar] [CrossRef]
- Pinela, J.; Fuente, B.D.L.; Rodrigues, M.; Pires, T.C.S.P.; Mandim, F.; Almeida, A.; Dias, M.I.; Caleja, C.; Barros, L. Upcycling Fish By-Products into Bioactive Fish Oil: The Suitability of Microwave-Assisted Extraction. Biomolecules 2022, 13, 1. [Google Scholar] [CrossRef]
- Getachew, A.T.; Lee, H.J.; Cho, Y.J.; Chae, S.J.; Chun, B.S. Optimization of Polysaccharides Extraction from Pacific Oyster (Crassostrea gigas) Using Subcritical Water: Structural Characterization and Biological Activities. Int. J. Biol. Macromol. 2019, 121, 852–861. [Google Scholar] [CrossRef]
- Cheng, Y.; Xue, F.; Yu, S.; Du, S.; Yang, Y. Subcritical Water Extraction of Natural Products. Molecules 2021, 26, 4004. [Google Scholar] [CrossRef]
- Zhuang, B.; Ramanauskaite, G.; Koa, Z.Y.; Wang, Z.-G. Like Dissolves like: A First-Principles Theory for Predicting Liquid Miscibility and Mixture Dielectric Constant. Sci. Adv. 2021, 7, eabe7275. [Google Scholar] [CrossRef] [PubMed]
- Uwineza, P.A.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Benchikh, Y.; Bachir-bey, M.; Chaalal, M.; Ydjedd, S.; Kati, D.E. Extraction of Phenolic Compounds. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2023; pp. 329–354. ISBN 978-0-323-95156-2. [Google Scholar]
- Koutra, E.; Papavasileiou, P.; Andriopoulos, V.; Mastropetros, S.G.; Kornaros, M. Bioactive Compounds from Microalgae Cultivated in Wastewaters. In An Integration of Phycoremediation Processes in Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 177–202. ISBN 978-0-12-823499-0. [Google Scholar]
- Romanik, G.; Gilgenast, E.; Przyjazny, A.; Kamiński, M. Techniques of Preparing Plant Material for Chromatographic Separation and Analysis. J. Biochem. Biophys. Methods 2007, 70, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Šojić, B.; Putnik, P.; Danilović, B.; Teslić, N.; Bursać Kovačević, D.; Pavlić, B. Lipid Extracts Obtained by Supercritical Fluid Extraction and Their Application in Meat Products. Antioxidants 2022, 11, 716. [Google Scholar] [CrossRef]
- Badfar, N.; Jafarpour, A.; Casanova, F.; Sales Queiroz, L.; Tilahun Getachew, A.; Jacobsen, C.; Jessen, F.; Gringer, N. Influence of Supercritical Fluid Extraction Process on Techno-Functionality of Enzymatically Derived Peptides from Filter-Pressed Shrimp Waste. Mar. Drugs 2025, 23, 122. [Google Scholar] [CrossRef]
- Vázquez-Rodríguez, B.; Gutiérrez-Uribe, J.A.; Antunes-Ricardo, M.; Santos-Zea, L.; Cruz-Suárez, L.E. Ultrasound-Assisted Extraction of Phlorotannins and Polysaccharides from Silvetia compressa (Phaeophyceae). J. Appl. Phycol. 2020, 32, 1441–1453. [Google Scholar] [CrossRef]
- Dang, T.T.; Van Vuong, Q.; Schreider, M.J.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimisation of Ultrasound-Assisted Extraction Conditions for Phenolic Content and Antioxidant Activities of the Alga Hormosira banksii Using Response Surface Methodology. J. Appl. Phycol. 2017, 29, 3161–3173. [Google Scholar] [CrossRef]
- Carreira-Casais, A.; Otero, P.; Garcia-Perez, P.; Garcia-Oliveira, P.; Pereira, A.G.; Carpena, M.; Soria-Lopez, A.; Simal-Gandara, J.; Prieto, M.A. Benefits and Drawbacks of Ultrasound-Assisted Extraction for the Recovery of Bioactive Compounds from Marine Algae. Int. J. Environ. Res. Public Health 2021, 18, 9153. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A Clean, Green Extraction Technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Ko, S.-C.; Lee, S.-H.; Ahn, G.; Kim, K.-N.; Cha, S.-H.; Kim, S.-K.; Jeon, B.-T.; Park, P.-J.; Lee, K.-W.; Jeon, Y.-J. Effect of Enzyme-Assisted Extract of Sargassum Coreanum on Induction of Apoptosis in HL-60 Tumor Cells. J. Appl. Phycol. 2012, 24, 675–684. [Google Scholar] [CrossRef]
- Heng, M.Y.; Tan, S.N.; Yong, J.W.H.; Ong, E.S. Emerging Green Technologies for the Chemical Standardization of Botanicals and Herbal Preparations. TrAC Trends Anal. Chem. 2013, 50, 1–10. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Nobre, B.P.; Ertekin, F.; Hayes, M.; Garciá-Vaquero, M.; Vieira, F.; Koc, M.; Gouveia, L.; Aires-Barros, M.R.; Palavra, A.M.F. Extraction of Value-Added Compounds from Microalgae. In Microalgae-Based Biofuels and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2017; pp. 461–483. ISBN 978-0-08-101023-5. [Google Scholar]
- Costa, J.M.; Strieder, M.M.; Saldaña, M.D.A.; Rostagno, M.A.; Forster-Carneiro, T. Recent Advances in the Processing of Agri-Food By-Products by Subcritical Water. Food Bioprocess Technol. 2023, 16, 2705–2724. [Google Scholar] [CrossRef]
- Al Khawli, F.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Gullón, P.; Kousoulaki, K.; Ferrer, E.; Berrada, H.; Barba, F.J. Innovative Green Technologies of Intensification for Valorization of Seafood and Their By-Products. Mar. Drugs 2019, 17, 689. [Google Scholar] [CrossRef]
- Linares, G.; Rojas, M.L. Ultrasound-Assisted Extraction of Natural Pigments From Food Processing By-Products: A Review. Front. Nutr. 2022, 9, 891462. [Google Scholar] [CrossRef] [PubMed]
- Rotter, A.; Varamogianni-Mamatsi, D.; Zvonar Pobirk, A.; Gosenca Matjaž, M.; Cueto, M.; Díaz-Marrero, A.R.; Jónsdóttir, R.; Sveinsdóttir, K.; Catalá, T.S.; Romano, G.; et al. Marine Cosmetics and the Blue Bioeconomy: From Sourcing to Success Stories. iScience 2024, 27, 111339. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; Resende, D.I.S.P.; Lobo, J.M.S.; Sousa, E.; Almeida, I.F. Marine Ingredients for Sensitive Skin: Market Overview. Mar. Drugs 2021, 19, 464. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Liu, Z.; Wang, L.; Song, L. Recent Advances of Shell Matrix Proteins and Cellular Orchestration in Marine Molluscan Shell Biomineralization. Front. Mar. Sci. 2019, 6, 41. [Google Scholar] [CrossRef]
- Saruwatari, K.; Matsui, T.; Mukai, H.; Nagasawa, H.; Kogure, T. Nucleation and Growth of Aragonite Crystals at the Growth Front of Nacres in Pearl Oyster, Pinctada fucata. Biomaterials 2009, 30, 3028–3034. [Google Scholar] [CrossRef]
- Marie, B.; Joubert, C.; Tayalé, A.; Zanella-Cléon, I.; Belliard, C.; Piquemal, D.; Cochennec-Laureau, N.; Marin, F.; Gueguen, Y.; Montagnani, C. Different Secretory Repertoires Control the Biomineralization Processes of Prism and Nacre Deposition of the Pearl Oyster Shell. Proc. Natl. Acad. Sci. USA 2012, 109, 20986–20991. [Google Scholar] [CrossRef]
- Marin, F. The Formation and Mineralization of Mollusk Shell. Front. Biosci. 2012, S4, 1099–1125. [Google Scholar] [CrossRef]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk Shell Formation: A Source of New Concepts for Understanding Biomineralization Processes. Chem.—Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan Shell Proteins: Primary Structure, Origin, and Evolution. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2007; Volume 80, pp. 209–276. ISBN 978-0-12-373914-8. [Google Scholar]
- Avila, C. Terpenoids in Marine Heterobranch Molluscs. Mar. Drugs 2020, 18, 162. [Google Scholar] [CrossRef]
- Mudianta, I.W.; White, A.M.; Suciati; Katavic, P.L.; Krishnaraj, R.R.; Winters, A.E.; Mollo, E.; Cheney, K.L.; Garson, M.J. Chemoecological Studies on Marine Natural Products: Terpene Chemistry from Marine Mollusks. Pure Appl. Chem. 2014, 86, 995–1002. [Google Scholar] [CrossRef]
- Pérez-Polo, S.; Imran, M.A.S.; Dios, S.; Pérez, J.; Barros, L.; Carrera, M.; Gestal, C. Identifying Natural Bioactive Peptides from the Common Octopus (Octopus vulgaris Cuvier, 1797) Skin Mucus By-Products Using Proteogenomic Analysis. Int. J. Mol. Sci. 2023, 24, 7145. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Boo, S.; Gervais, O.; Prado-Alvarez, M.; Chollet, B.; Claverol, S.; Lecadet, C.; Dubreuil, C.; Arzul, I. Is Pallial Mucus Involved in Ostrea edulis Defenses against the Parasite Bonamia ostreae? J. Invertebr. Pathol. 2020, 169, 107259. [Google Scholar] [CrossRef]
- Madan, J.J.; Wells, M.J. Cutaneous Respiration In Octopus vulgaris. J. Exp. Biol. 1996, 199, 2477–2483. [Google Scholar] [CrossRef]
- Derby, C. Cephalopod Ink: Production, Chemistry, Functions and Applications. Mar. Drugs 2014, 12, 2700–2730. [Google Scholar] [CrossRef] [PubMed]
- Ghattavi, K.; Homaei, A.; Kamrani, E.; Kim, S.-K. Melanin Pigment Derived from Marine Organisms and Its Industrial Applications. Dyes Pigments 2022, 201, 110214. [Google Scholar] [CrossRef]
- Mikheil, D.; Prabhakar, K.; Jayanthy, A.-S.; Setaluri, V. Coat Color Mutations, Animals. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; p. B9780128096338062324. ISBN 978-0-12-809633-8. [Google Scholar]
- Lucero, M.T.; Farrington, H.; Gilly, W.F. Quantification of L-Dopa and Dopamine in Squid Ink: Implications for Chemoreception. Biol. Bull. 1994, 187, 55–63. [Google Scholar] [CrossRef]
- Russo, G.L.; De Nisco, E.; Fiore, G.; Di Donato, P.; d’Ischia, M.; Palumbo, A. Toxicity of Melanin-Free Ink of Sepia Officinalis to Transformed Cell Lines: Identification of the Active Factor as Tyrosinase. Biochem. Biophys. Res. Commun. 2003, 308, 293–299. [Google Scholar] [CrossRef]
- Palumbo, A. Melanogenesis in the Ink Gland of Sepia officinalis. Pigment Cell Res. 2003, 16, 517–522. [Google Scholar] [CrossRef]
- Kicklighter, C.E.; Shabani, S.; Johnson, P.M.; Derby, C.D. Sea Hares Use Novel Antipredatory Chemical Defenses. Curr. Biol. 2005, 15, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, R.; Riba, J.; Ruíz-Capillas, C.; González, A.V.; Baeta, M. Amino Acid Composition of Early Stages of Cephalopods and Effect of Amino Acid Dietary Treatments on Octopus vulgaris Paralarvae. Aquaculture 2004, 242, 455–478. [Google Scholar] [CrossRef]
- Derby, C.D.; Kicklighter, C.E.; Johnson, P.M.; Zhang, X. Chemical Composition of Inks of Diverse Marine Molluscs Suggests Convergent Chemical Defenses. J. Chem. Ecol. 2007, 33, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Malek, A.R.; Moustafa, A.Y.; Salem, S.H. Antimicrobial and Cytotoxic Activities of Flavonoid and Phenolics Extracted from Sepia Pharaonis Ink (Mollusca: Cephalopoda). BMC Biotechnol. 2024, 24, 54. [Google Scholar] [CrossRef]
- Ab Lah, R.; Smith, J.; Savins, D.; Dowell, A.; Bucher, D.; Benkendorff, K. Investigation of Nutritional Properties of Three Species of Marine Turban Snails for Human Consumption. Food Sci. Nutr. 2017, 5, 14–30. [Google Scholar] [CrossRef]
- Durazzo, A.; Di Lena, G.; Gabrielli, P.; Santini, A.; Lombardi-Boccia, G.; Lucarini, M. Nutrients and Bioactive Compounds in Seafood: Quantitative Literature Research Analysis. Fishes 2022, 7, 132. [Google Scholar] [CrossRef]
- Astorga España, M.S.; Rodríguez Rodríguez, E.M.; Díaz Romero, C. Comparison of Mineral and Trace Element Concentrations in Two Molluscs from the Strait of Magellan (Chile). J. Food Compos. Anal. 2007, 20, 273–279. [Google Scholar] [CrossRef]
- Khanna, S.; Parinandi, N.L.; Kotha, S.R.; Roy, S.; Rink, C.; Bibus, D.; Sen, C.K. Nanomolar Vitamin E A-tocotrienol Inhibits Glutamate-induced Activation of Phospholipase A2 and Causes Neuroprotection. J. Neurochem. 2010, 112, 1249–1260. [Google Scholar] [CrossRef]
- Sen, C.K.; Khanna, S.; Roy, S. Tocotrienols in Health and Disease: The Other Half of the Natural Vitamin E Family. Mol. Aspects Med. 2007, 28, 692–728. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids in Marine Animals. Mar. Drugs 2011, 9, 278–293. [Google Scholar] [CrossRef]
- Xie, C.-L.; Kang, S.S.; Lu, C.; Choi, Y.J. Quantification of Multifunctional Dipeptide YA from Oyster Hydrolysate for Quality Control and Efficacy Evaluation. BioMed Res. Int. 2018, 2018, 8437379. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhao, F.; Chen, H.; Tu, M.; Tao, S.; Wang, Z.; Wu, C.; He, S.; Du, M. Heat Treatments of Peptides from Oyster (Crassostrea gigas) and the Impact on Their Digestibility and Angiotensin I Converting Enzyme Inhibitory Activity. Food Sci. Biotechnol. 2020, 29, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Xing, R.; Liu, S.; Chen, X.; Li, P. Optimization of Oyster (Crassostrea talienwhanensis) Protein Hydrolysates Using Response Surface Methodology. Molecules 2020, 25, 2844. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gong, C.; Wang, Z.; Gao, R.; Ren, J.; Zhou, X.; Wang, H.; Xu, H.; Xiao, F.; Cao, Y.; et al. Oyster-Derived Zinc-Binding Peptide Modified by Plastein Reaction via Zinc Chelation Promotes the Intestinal Absorption of Zinc. Mar. Drugs 2019, 17, 341. [Google Scholar] [CrossRef]
- Upadhyay, A.; Thiyagarajan, V.; Tong, Y. Proteomic Characterization of Oyster Shell Organic Matrix Proteins (OMP). Bioinformation 2016, 12, 266–278. [Google Scholar] [CrossRef][Green Version]
- Ulagesan, S.; Krishnan, S.; Nam, T.-J.; Choi, Y.-H. A Review of Bioactive Compounds in Oyster Shell and Tissues. Front. Bioeng. Biotechnol. 2022, 10, 913839. [Google Scholar] [CrossRef]
- Šimat, V.; Rathod, N.; Čagalj, M.; Hamed, I.; Generalić Mekinić, I. Astaxanthin from Crustaceans and Their Byproducts: A Bioactive Metabolite Candidate for Therapeutic Application. Mar. Drugs 2022, 20, 206. [Google Scholar] [CrossRef]
- Hossain, A.; Shahidi, F. Upcycling Shellfish Waste: Distribution of Amino Acids, Minerals, and Carotenoids in Body Parts of North Atlantic Crab and Shrimp. Foods 2024, 13, 2700. [Google Scholar] [CrossRef]
- Yan, N.; Chen, X. Sustainability: Don’t Waste Seafood Waste. Nature 2015, 524, 155–157. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of Chitosan in Food, Pharmaceuticals, Medicine, Cosmetics, Agriculture, Textiles, Pulp and Paper, Biotechnology, and Environmental Chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Casadidio, C.; Peregrina, D.V.; Gigliobianco, M.R.; Deng, S.; Censi, R.; Di Martino, P. Chitin and Chitosans: Characteristics, Eco-Friendly Processes, and Applications in Cosmetic Science. Mar. Drugs 2019, 17, 369. [Google Scholar] [CrossRef] [PubMed]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Terkula Iber, B.; Azman Kasan, N.; Torsabo, D.; Wese Omuwa, J. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2022, 10, 1097–1123. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Advances in Chitin-Based Nanoparticle Use in Biodegradable Polymers: A Review. Carbohydr. Polym. 2023, 312, 120789. [Google Scholar] [CrossRef]
- Özogul, F.; Hamed, I.; Özogul, Y.; Regenstein, J.M. Crustacean By-Products. In Encyclopedia of Food Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 33–38. ISBN 978-0-12-814045-1. [Google Scholar]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Cheung, R.; Ng, T.; Wong, J.; Chan, W. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Klinger, C.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Petrenko, I.; Machałowski, T.; Ereskovsky, A.; Martinović, R.; Muzychka, L.; et al. Express Method for Isolation of Ready-to-Use 3D Chitin Scaffolds from Aplysina Archeri (Aplysineidae: Verongiida) Demosponge. Mar. Drugs 2019, 17, 131. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G.H. Production of Chitooligosaccharides and Their Potential Applications in Medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Guan, F.; Han, Y.; Yan, K.; Zhang, Y.; Zhang, Z.; Wu, N.; Tian, J. Highly Efficient Production of Chitooligosaccharides by Enzymes Mined Directly from the Marine Metagenome. Carbohydr. Polym. 2020, 234, 115909. [Google Scholar] [CrossRef]
- Díaz-Santana-Iturrios, M.; Zepeda-Benitez, V.; Pacheco-Ovando, R.; Cornejo, C.F.; Cerda, J.M.; Salinas-Zavala, C.A.; Granados-Amores, J. Northeastern Pacific Octopus Beak Shape for Species-Level Detection. Malacologia 2022, 64, 169–184. [Google Scholar] [CrossRef]
- Hatchett, C. XVIII. Experiments and Observations on Shell and Bone. Philos. Trans. R. Soc. Lond. 1799, 89, 315–334. [Google Scholar] [CrossRef]
- Jia, M.; Li, Y.; Yang, X.; Huang, Y.; Wu, H.; Huang, Y.; Lin, J.; Li, Y.; Hou, Z.; Zhang, Q. Development of Both Methotrexate and Mitomycin C Loaded PEGylated Chitosan Nanoparticles for Targeted Drug Codelivery and Synergistic Anticancer Effect. ACS Appl. Mater. Interfaces 2014, 6, 11413–11423. [Google Scholar] [CrossRef]
- López-Barrera, L.D.; Díaz-Torres, R.; Martínez-Rosas, J.R.; Salazar, A.M.; Rosales, C.; Ramírez-Noguera, P. Modification of Proliferation and Apoptosis in Breast Cancer Cells by Exposure of Antioxidant Nanoparticles Due to Modulation of the Cellular Redox State Induced by Doxorubicin Exposure. Pharmaceutics 2021, 13, 1251. [Google Scholar] [CrossRef]
- Tang, L.; Li, J.; Zhao, Q.; Pan, T.; Zhong, H.; Wang, W. Advanced and Innovative Nano-Systems for Anticancer Targeted Drug Delivery. Pharmaceutics 2021, 13, 1151. [Google Scholar] [CrossRef]
- Park, J.K.; Chung, M.J.; Choi, H.N.; Park, Y.I. Effects of the Molecular Weight and the Degree of Deacetylation of Chitosan Oligosaccharides on Antitumor Activity. Int. J. Mol. Sci. 2011, 12, 266–277. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Mehwish, H.M.; Wu, Y.; Zhao, L.; Arfat, Y.; Majeed, K.; Anwaar, S. Chitin/Chitosan Derivatives and Their Interactions with Microorganisms: A Comprehensive Review and Future Perspectives. Crit. Rev. Biotechnol. 2020, 40, 365–379. [Google Scholar] [CrossRef]
- Khattak, S.; Wahid, F.; Liu, L.-P.; Jia, S.-R.; Chu, L.-Q.; Xie, Y.-Y.; Li, Z.-X.; Zhong, C. Applications of Cellulose and Chitin/Chitosan Derivatives and Composites as Antibacterial Materials: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 1989–2006. [Google Scholar] [CrossRef]
- Ahmad, S.I.; Ahmad, R.; Khan, M.S.; Kant, R.; Shahid, S.; Gautam, L.; Hasan, G.M.; Hassan, M.I. Chitin and Its Derivatives: Structural Properties and Biomedical Applications. Int. J. Biol. Macromol. 2020, 164, 526–539. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Asan-Ozusaglam, M.; Cakmak, Y.S.; Tozak, K.O.; Mol, A.; Mentes, A.; Sezen, G. Extraction and Characterization of Chitin and Chitosan with Antimicrobial and Antioxidant Activities from Cosmopolitan Orthoptera Species (Insecta). Biotechnol. Bioprocess Eng. 2015, 20, 168–179. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Kim, S.-K. Antioxidant Effects of Chitin, Chitosan, and Their Derivatives. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 73, pp. 15–31. ISBN 978-0-12-800268-1. [Google Scholar]
- Je, J.; Park, P.; Kim, B.; Kim, S. Antihypertensive Activity of Chitin Derivatives. Biopolymers 2006, 83, 250–254. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef]
- Tzeng, H.-P.; Liu, S.-H.; Chiang, M.-T. Antidiabetic Properties of Chitosan and Its Derivatives. Mar. Drugs 2022, 20, 784. [Google Scholar] [CrossRef]
- Priyanka, D.N.; Prashanth, K.V.H.; Tharanathan, R.N. A Review on Potential Anti-Diabetic Mechanisms of Chitosan and Its Derivatives. Carbohydr. Polym. Technol. Appl. 2022, 3, 100188. [Google Scholar] [CrossRef]
- Kalita, P.; Ahmed, A.B.; Sen, S.; Chakraborty, R. A Comprehensive Review on Polysaccharides with Hypolipidemic Activity: Occurrence, Chemistry and Molecular Mechanism. Int. J. Biol. Macromol. 2022, 206, 681–698. [Google Scholar] [CrossRef]
- Silva, I.M.V.; Machado, F.; Moreno, M.J.; Nunes, C.; Coimbra, M.A.; Coreta-Gomes, F. Polysaccharide Structures and Their Hypocholesterolemic Potential. Molecules 2021, 26, 4559. [Google Scholar] [CrossRef]
- Sajith, M.P.; Pitchai, A.; Ramasamy, P. Anticoagulant Protective Effects of Sulfated Chitosan Derived From the Internal Bone of Spineless Cuttlefish (Sepiella inermis). Cureus 2024, 16, e64558. [Google Scholar] [CrossRef]
- Tsoupras, A.; Cholidis, P.; Kranas, D.; Galouni, E.A.; Ofrydopoulou, A.; Efthymiopoulos, P.; Shiels, K.; Saha, S.K.; Kyzas, G.Z.; Anastasiadou, C. Anti-Inflammatory, Antithrombotic, and Antioxidant Properties of Amphiphilic Lipid Bioactives from Shrimp. Pharmaceuticals 2024, 18, 25. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, Z.; Song, L.; Farag, M.A. Maximizing Crustaceans (Shrimp, Crab, and Lobster) by-Products Value for Optimum Valorization Practices: A Comparative Review of Their Active Ingredients, Extraction, Bioprocesses and Applications. J. Adv. Res. 2024, 57, 59–76. [Google Scholar] [CrossRef]
- Ahmadkelayeh, S.; Hawboldt, K. Extraction of Lipids and Astaxanthin from Crustacean By-Products: A Review on Supercritical CO2 Extraction. Trends Food Sci. Technol. 2020, 103, 94–108. [Google Scholar] [CrossRef]
- Albalat, A.; Collard, A.; Brucem, C.; Coates, C.J.; Fox, C.J. Physiological Condition, Short-Term Survival, and Predator Avoidance Behavior of Discarded Norway Lobsters (Nephrops norvegicus). J. Shellfish Res. 2016, 35, 1053–1065. [Google Scholar] [CrossRef]
- Balzano, M.; Pacetti, D.; Lucci, P.; Fiorini, D.; Frega, N.G. Bioactive Fatty Acids in Mantis Shrimp, Crab and Caramote Prawn: Their Content and Distribution among the Main Lipid Classes. J. Food Compos. Anal. 2017, 59, 88–94. [Google Scholar] [CrossRef]
- Rivera-Madrid, R.; Carballo-Uicab, V.M.; Cárdenas-Conejo, Y.; Aguilar-Espinosa, M.; Siva, R. Overview of Carotenoids and Beneficial Effects on Human Health. In Carotenoids: Properties, Processing and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–40. ISBN 978-0-12-817067-0. [Google Scholar]
- Yabuzaki, J. Carotenoids Database: Structures, Chemical Fingerprints and Distribution among Organisms. Database 2017, 2017, bax004. [Google Scholar] [CrossRef]
- Viera, I.; Pérez-Gálvez, A.; Roca, M. Bioaccessibility of Marine Carotenoids. Mar. Drugs 2018, 16, 397. [Google Scholar] [CrossRef]
- Riaz, M.; Zia-Ul-Haq, M.; Dou, D. Chemistry of Carotenoids. In Carotenoids: Structure and Function in the Human Body; Zia-Ul-Haq, M., Dewanjee, S., Riaz, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 43–76. ISBN 978-3-030-46458-5. [Google Scholar]
- Steven, R.; Humaira, Z.; Natanael, Y.; Dwivany, F.M.; Trinugroho, J.P.; Dwijayanti, A.; Kristianti, T.; Tallei, T.E.; Emran, T.B.; Jeon, H.; et al. Marine Microbial-Derived Resource Exploration: Uncovering the Hidden Potential of Marine Carotenoids. Mar. Drugs 2022, 20, 352. [Google Scholar] [CrossRef]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A Mechanistic Review on Its Biological Activities and Health Benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Yang, J.; Hua, S.; Huang, Z.; Gu, Z.; Cheng, L.; Hong, Y. Comparison of Bioaccessibility of Astaxanthin Encapsulated in Starch-Based Double Emulsion with Different Structures. Carbohydr. Polym. 2021, 272, 118475. [Google Scholar] [CrossRef]
- Brotosudarmo, T.H.P.; Limantara, L.; Setiyono, E.; Heriyanto. Structures of Astaxanthin and Their Consequences for Therapeutic Application. Int. J. Food Sci. 2020, 2020, 2156582. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, X.; Gu, J.; Li, X.; Cao, Y.; Xu, J.; Xue, C. Influence of Molecular Structure of Astaxanthin Esters on Their Stability and Bioavailability. Food Chem. 2021, 343, 128497. [Google Scholar] [CrossRef]
- Su, F.; Huang, B.; Liu, J. The Carotenoids of Shrimps (Decapoda: Caridea and Dendrobranchiata) Cultured in China. J. Crustac. Biol. 2018, 38, 523–530. [Google Scholar] [CrossRef]
- Ambati, R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Snell, T.W.; Carberry, J. Astaxanthin Bioactivity Is Determined by Stereoisomer Composition and Extraction Method. Nutrients 2022, 14, 1522. [Google Scholar] [CrossRef]
- Qiu, D.; Wu, Y.; Zhu, W.; Yin, H.; Yi, L. Identification of Geometrical Isomers and Comparison of Different Isomeric Samples of Astaxanthin. J. Food Sci. 2012, 77, C934–C940. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from Fruits and Vegetables: Chemistry, Analysis, Occurrence, Bioavailability and Biological Activities. Food Res. Int. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Liu, X.; Osawa, T. Cis Astaxanthin and Especially 9-Cis Astaxanthin Exhibits a Higher Antioxidant Activity in Vitro Compared to the All-Trans Isomer. Biochem. Biophys. Res. Commun. 2007, 357, 187–193. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Félix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A Review of Its Chemistry and Applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Nishida, Y.; Berg, P.; Shakersain, B.; Hecht, K.; Takikawa, A.; Tao, R.; Kakuta, Y.; Uragami, C.; Hashimoto, H.; Misawa, N.; et al. Astaxanthin: Past, Present, and Future. Mar. Drugs 2023, 21, 514. [Google Scholar] [CrossRef]
- Munaro, D.; Nunes, A.; Schmitz, C.; Bauer, C.; Coelho, D.S.; Oliveira, E.R.; Yunes, R.A.; Moura, S.; Maraschin, M. Metabolites Produced by Macro- and Microalgae as Plant Biostimulants. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; Volume 71, pp. 87–120. ISBN 978-0-323-91095-8. [Google Scholar]
- Camargo, T.R.; Mantoan, P.; Ramos, P.; Monserrat, J.M.; Prentice, C.; Fernandes, C.C.; Zambuzzi, W.F.; Valenti, W.C. Bioactivity of the Protein Hydrolysates Obtained from the Most Abundant Crustacean Bycatch. Mar. Biotechnol. 2021, 23, 881–891. [Google Scholar] [CrossRef]
- Andriamanalina, T.M.; Andrianandrasana, M.D.; Ravonizafy, C.; Andrianandrasana, E.R. Calcium Salts from the Demineralization of Crab (Scylla serrata) Wastes. Am. J. Innov. Sci. Eng. 2023, 2, 25–29. [Google Scholar] [CrossRef]
- Londono-Zuluaga, C.; Jameel, H.; Gonzalez, R.W.; Lucia, L. Crustacean Shell-Based Biosorption Water Remediation Platforms: Status and Perspectives. J. Environ. Manag. 2019, 231, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Gbenebor, O.P.; Adeosun, S.O.; Lawal, G.I.; Jun, S. Role of CaCO3 in the Physicochemical Properties of Crustacean-Sourced Structural Polysaccharides. Mater. Chem. Phys. 2016, 184, 203–209. [Google Scholar] [CrossRef]
- Atef, M.; Mahdi Ojagh, S. Health Benefits and Food Applications of Bioactive Compounds from Fish Byproducts: A Review. J. Funct. Foods 2017, 35, 673–681. [Google Scholar] [CrossRef]
- Wang, X. Natural Bioactive Compounds from Fish. In Natural Bioactive Compounds; Elsevier: Amsterdam, The Netherlands, 2021; pp. 393–408. ISBN 978-0-12-820655-3. [Google Scholar]
- Awuchi, C.G.; Chukwu, C.N.; Iyiola, A.O.; Noreen, S.; Morya, S.; Adeleye, A.O.; Twinomuhwezi, H.; Leicht, K.; Mitaki, N.B.; Okpala, C.O.R. Bioactive Compounds and Therapeutics from Fish: Revisiting Their Suitability in Functional Foods to Enhance Human Wellbeing. BioMed Res. Int. 2022, 2022, 3661866. [Google Scholar] [CrossRef]
- Sousa, R.O.; Alves, A.L.; Carvalho, D.N.; Martins, E.; Oliveira, C.; Silva, T.H.; Reis, R.L. Acid and Enzymatic Extraction of Collagen from Atlantic Cod (Gadus morhua) Swim Bladders Envisaging Health-Related Applications. J. Biomater. Sci. Polym. Ed. 2020, 31, 20–37. [Google Scholar] [CrossRef]
- Al Khawli, F.; Martí-Quijal, F.J.; Ferrer, E.; Ruiz, M.-J.; Berrada, H.; Gavahian, M.; Barba, F.J.; De La Fuente, B. Aquaculture and Its By-Products as a Source of Nutrients and Bioactive Compounds. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2020; Volume 92, pp. 1–33. ISBN 978-0-12-820216-6. [Google Scholar]
- Afreen, M.; Ucak, I. Fish Processing Wastes Used as Feed Ingredient for Animal Feed and Aquaculture Feed. J. Surv. Fish. Sci. 2020, 6, 55–64. [Google Scholar] [CrossRef]
- Proximate Composition of New Zealand Marine Finfish and Shellfish. Available online: https://www.fao.org/4/ae581e/ae581e09.htm#bm9 (accessed on 20 June 2025).
- Nerhus, I.; Wik Markhus, M.; Nilsen, B.M.; Øyen, J.; Maage, A.; Ødegård, E.R.; Midtbø, L.K.; Frantzen, S.; Kögel, T.; Graff, I.E.; et al. Iodine Content of Six Fish Species, Norwegian Dairy Products and Hen’s Egg. Food Nutr. Res. 2018, 62, 10–29219. [Google Scholar] [CrossRef]
- Marques, I.; Botelho, G.; Guiné, R. Comparative Study on Nutritional Composition of Fish Available in Portugal. Nutr. Food Sci. 2019, 49, 925–941. [Google Scholar] [CrossRef]
- Suleria, H.; Osborne, S.; Masci, P.; Gobe, G. Marine-Based Nutraceuticals: An Innovative Trend in the Food and Supplement Industries. Mar. Drugs 2015, 13, 6336–6351. [Google Scholar] [CrossRef]
- Lall, S.P.; Kaushik, S.J. Nutrition and Metabolism of Minerals in Fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef]
- Öz, M.; Ucak, İ.; Nayik, G.A. PUFA and MUFA. In Nutraceuticals and Health Care; Elsevier: Amsterdam, The Netherlands, 2022; pp. 199–215. ISBN 978-0-323-89779-2. [Google Scholar]
- Mata-Sotres, J.A.; Marques, V.H.; Barba, D.; Braga, A.; Araújo, B.; Viana, M.T.; Rombenso, A.N. Increasing Dietary SFA:MUFA Ratio with Low Levels of LC-PUFA Affected Lipid Metabolism, Tissue Fatty Acid Profile and Growth of Juvenile California Yellowtail (Seriola dorsalis). Aquaculture 2021, 543, 737011. [Google Scholar] [CrossRef]
- Grundy, S.M. CHOLESTEROL|Factors Determining Blood Cholesterol Levels. In Encyclopedia of Food Sciences and Nutrition; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1237–1243. ISBN 978-0-12-227055-0. [Google Scholar]
- Hossain, A.; Dave, D.; Shahidi, F. Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector. Mar. Drugs 2020, 18, 274. [Google Scholar] [CrossRef] [PubMed]
- Senadheera, T.R.L.; Hossain, A.; Shahidi, F. Marine Bioactives and Their Application in the Food Industry: A Review. Appl. Sci. 2023, 13, 12088. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Fitzgerald, R.J. Bioactive Proteins and Peptides from Macroalgae, Fish, Shellfish and Marine Processing Waste. In Marine Proteins and Peptides; Kim, S., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 5–39. ISBN 978-1-118-37506-8. [Google Scholar]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from Fish By-Product Protein Hydrolysates and Its Functional Properties: An Overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Adnan, M.; Patel, M.; Siddiqui, A.J.; Sachidanandan, M.; Snoussi, M.; Hadi, S. Fish-Based Bioactives as Potent Nutraceuticals: Exploring the Therapeutic Perspective of Sustainable Food from the Sea. Mar. Drugs 2020, 18, 265. [Google Scholar] [CrossRef]
- Kundam, D.N.; Acham, I.O.; Girgih, A.T. Bioactive Compounds in Fish and Their Health Benefits. Asian Food Sci. J. 2018, 4, 1–14. [Google Scholar] [CrossRef]
- Ozogul, F.; Cagalj, M.; Šimat, V.; Ozogul, Y.; Tkaczewska, J.; Hassoun, A.; Kaddour, A.A.; Kuley, E.; Rathod, N.B.; Phadke, G.G. Recent Developments in Valorisation of Bioactive Ingredients in Discard/Seafood Processing by-Products. Trends Food Sci. Technol. 2021, 116, 559–582. [Google Scholar] [CrossRef]
- Taşbozan, O.; Gökçe, M.A. Fatty Acids in Fish. In Fatty Acids; Catala, A., Ed.; InTech: London, UK, 2017; ISBN 978-953-51-3301-8. [Google Scholar]
- Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Mar. Drugs 2022, 20, 187. [Google Scholar] [CrossRef]
- Inflammation and Cardiovascular Disease: Are Marine Phospholipids the Answer?—Food & Function (RSC Publishing). Available online: https://pubs.rsc.org/en/content/articlelanding/2020/fo/c9fo01742a/unauth (accessed on 24 June 2025).
- Tsoupras, A.; O’Keeffe, E.; Lordan, R.; Redfern, S.; Zabetakis, I. Bioprospecting for Antithrombotic Polar Lipids from Salmon, Herring, and Boarfish By-Products. Foods 2019, 8, 416. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Lordan, R.; Demuru, M.; Shiels, K.; Saha, S.; Nasopoulou, C.; Zabetakis, I. Structural Elucidation of Irish Organic Farmed Salmon (Salmo salar) Polar Lipids with Antithrombotic Activities. Mar. Drugs 2018, 16, 176. [Google Scholar] [CrossRef] [PubMed]
- Tsoupras, A.; Lordan, R.; Shiels, K.; Saha, S.K.; Nasopoulou, C.; Zabetakis, I. In Vitro Antithrombotic Properties of Salmon (Salmo salar) Phospholipids in a Novel Food-Grade Extract. Mar. Drugs 2019, 17, 62. [Google Scholar] [CrossRef]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine Omega-3 Phospholipids: Metabolism and Biological Activities. Int. J. Mol. Sci. 2012, 13, 15401–15419. [Google Scholar] [CrossRef] [PubMed]
- Cheng-Sánchez, I.; Sarabia, F. Chemistry and Biology of Bioactive Glycolipids of Marine Origin. Mar. Drugs 2018, 16, 294. [Google Scholar] [CrossRef]
- Shiels, K.; Tsoupras, A.; Lordan, R.; Nasopoulou, C.; Zabetakis, I.; Murray, P.; Saha, S.K. Bioactive Lipids of Marine Microalga Chlorococcum Sp. SABC 012504 with Anti-Inflammatory and Anti-Thrombotic Activities. Mar. Drugs 2021, 19, 28. [Google Scholar] [CrossRef]
- Koukouraki, P.; Tsoupras, A.; Sotiroudis, G.; Demopoulos, C.A.; Sotiroudis, T.G. Antithrombotic Properties of Spirulina Extracts against Platelet-Activating Factor and Thrombin. Food Biosci. 2020, 37, 100686. [Google Scholar] [CrossRef]
- Nasopoulou, C.; Tsoupras, A.B.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Fish Polar Lipids Retard Atherosclerosis in Rabbits by Down-Regulating PAF Biosynthesis and up-Regulating PAF Catabolism. Lipids Health Dis. 2011, 10, 213. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Zabetakis, I. Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties. Molecules 2017, 22, 1964. [Google Scholar] [CrossRef]
- Chouinard-Watkins, R.; Lacombe, R.J.S.; Metherel, A.H.; Masoodi, M.; Bazinet, R.P. DHA Esterified to Phosphatidylserine or Phosphatidylcholine Is More Efficient at Targeting the Brain than DHA Esterified to Triacylglycerol. Mol. Nutr. Food Res. 2019, 63, 1801224. [Google Scholar] [CrossRef]
- Redfern, S.; Dermiki, M.; Fox, S.; Lordan, R.; Shiels, K.; Kumar Saha, S.; Tsoupras, A.; Zabetakis, I. The Effects of Cooking Salmon Sous-Vide on Its Antithrombotic Properties, Lipid Profile and Sensory Characteristics. Food Res. Int. 2021, 139, 109976. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Fragopoulou, E.; Nomikos, T.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Characterization of the De Novo Biosynthetic Enzyme of Platelet Activating Factor, DDT-Insensitive Cholinephosphotransferase, of Human Mesangial Cells. Mediators Inflamm. 2007, 2007, 27683. [Google Scholar] [CrossRef] [PubMed]
- Kazuo, M. Prevention of Fish Oil Oxidation. J. Oleo Sci. 2019, 68, 1–11. [Google Scholar] [CrossRef]
- Scholey, A.B.; Camfield, D.A.; Hughes, M.E.; Woods, W.; Stough, C.K.K.; White, D.J.; Gondalia, S.V.; Frederiksen, P.D. A Randomized Controlled Trial Investigating the Neurocognitive Effects of Lacprodan® PL-20, a Phospholipid-Rich Milk Protein Concentrate, in Elderly Participants with Age-Associated Memory Impairment: The Phospholipid Intervention for Cognitive Ageing Reversal (PLICAR): Study Protocol for a Randomized Controlled Trial. Trials 2013, 14, 404. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.-C.; Shibasaki, K.; Yoshida, R.; Sato, M.; Imaizumi, K. Learning Behaviour and Cerebral Protein Kinase C, Antioxidant Status, Lipid Composition in Senescence-Accelerated Mouse: Influence of a Phosphatidylcholine–Vitamin B12 Diet. Br. J. Nutr. 2001, 86, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Karantonis, H.C.; Perrea, D.N.; Theocharis, S.E.; Iliopoulos, D.G.; Demopoulos, C.A.; Zabetakis, I. In Vivo Anti-Atherogenic Properties of Cultured Gilthead Sea Bream (Sparus aurata) Polar Lipid Extracts in Hypercholesterolaemic Rabbits. Food Chem. 2010, 120, 831–836. [Google Scholar] [CrossRef]
- Nasopoulou, C.; Nomikos, T.; Demopoulos, C.A.; Zabetakis, I. Comparison of Antiatherogenic Properties of Lipids Obtained from Wild and Cultured Sea Bass (Dicentrarchus labrax) and Gilthead Sea Bream (Sparus aurata). Food Chem. 2007, 100, 560–567. [Google Scholar] [CrossRef]
- Rementzis, J.; Antonopoulou, S.; Argyropoulos, D.; Demopoulos, C.A. Biologically Active Lipids from S. scombrus. In Platelet-Activating Factor and Related Lipid Mediators 2; Advances in Experimental Medicine and Biology; Nigam, S., Kunkel, G., Prescott, S.M., Eds.; Springer: Boston, MA, USA, 1996; Volume 416, pp. 65–72. ISBN 978-1-4899-0181-1. [Google Scholar]
- Nasopoulou, C.; Psani, E.; Sioriki, E.; Demopoulos, C.A.; Zabetakis, I. Evaluation of Sensory and In Vitro Cardio Protective Properties of Sardine (Sardina pilchardus): The Effect of Grilling and Brining. Food Nutr. Sci. 2013, 04, 940–949. [Google Scholar] [CrossRef]
- Panayiotou, A.; Samartzis, D.; Nomikos, T.; Fragopoulou, E.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Lipid Fractions with Aggregatory and Antiaggregatory Activity toward Platelets in Fresh and Fried Cod (Gadus morhua): Correlation with Platelet-Activating Factor and Atherogenesis. J. Agric. Food Chem. 2000, 48, 6372–6379. [Google Scholar] [CrossRef]
- Balami, S.; Sharma, A.; Karn, R. Significance of Nutritional Value of Fish for Human Health. Malays. J. Halal Res. 2019, 2, 32–34. [Google Scholar] [CrossRef]
- Masamba, W.R.; Mosepele, K.; Mogobe, O. Essential Mineral Content of Common Fish Species in Chanoga, Okavango Delta, Botswana. Afr. J. Food Sci. 2015, 9, 480–486. [Google Scholar] [CrossRef][Green Version]
- Jiang, D.; Hu, Z.; Liu, F.; Zhang, R.; Duo, B.; Fu, J.; Cui, Y.; Li, M. Heavy Metals Levels in Fish from Aquaculture Farms and Risk Assessment in Lhasa, Tibetan Autonomous Region of China. Ecotoxicology 2014, 23, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Use of Natural Carotenoids for Pigmentation in Fishes. Available online: https://www.researchgate.net/publication/286483304_Use_of_natural_carotenoids_for_pigmentation_in_fishes (accessed on 20 June 2025).
- Martins, E.; Reis, R.L.; Silva, T.H. In Vivo Skin Hydrating Efficacy of Fish Collagen from Greenland Halibut as a High-Value Active Ingredient for Cosmetic Applications. Mar. Drugs 2023, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, A.D.T.; Balbinot, E.; Weber, C.I.; Tonial, I.B.; Machado-Lunkes, A. Fish Gelatin: Characteristics, Functional Properties, Applications and Future Potentials. Food Eng. Rev. 2015, 7, 33–44. [Google Scholar] [CrossRef]
- Regenstein, J.M.; Zhou, P. Collagen and Gelatin from Marine By-Products. In Maximising the Value of Marine By-Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 279–303. ISBN 978-1-84569-013-7. [Google Scholar]
- Nurilmala, M.; Suryamarevita, H.; Husein Hizbullah, H.; Jacoeb, A.M.; Ochiai, Y. Fish Skin as a Biomaterial for Halal Collagen and Gelatin. Saudi J. Biol. Sci. 2022, 29, 1100–1110. [Google Scholar] [CrossRef]
- Raman, M.; Gopakumar, K. Fish Collagen and Its Applications in Food and Pharmaceutical Industry: A Review. EC Nutr. 2018, 13, 752–767. [Google Scholar]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A Review on Recent Advances and Applications of Fish Collagen. Crit. Rev. Food Sci. Nutr. 2021, 61, 1027–1037. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Morawska, M.; Kulawik, P.; Zając, M. Characterization of Carp (Cyprinus carpio) Skin Gelatin Extracted Using Different Pretreatments Method. Food Hydrocoll. 2018, 81, 169–179. [Google Scholar] [CrossRef]
- Joy, J.M.; Padmaprakashan, A.; Pradeep, A.; Paul, P.T.; Mannuthy, R.J.; Mathew, S. A Review on Fish Skin-Derived Gelatin: Elucidating the Gelatin Peptides—Preparation, Bioactivity, Mechanistic Insights, and Strategies for Stability Improvement. Foods 2024, 13, 2793. [Google Scholar] [CrossRef]
- Valcarcel, J.; Hermida-Merino, C.; Piñeiro, M.M.; Hermida-Merino, D.; Vázquez, J.A. Extraction and Characterization of Gelatin from Skin By-Products of Seabream, Seabass and Rainbow Trout Reared in Aquaculture. Int. J. Mol. Sci. 2021, 22, 12104. [Google Scholar] [CrossRef]
- He, J.; Zhang, J.; Xu, Y.; Ma, Y.; Guo, X. The Structural and Functional Differences between Three Species of Fish Scale Gelatin and Pigskin Gelatin. Foods 2022, 11, 3960. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Trovato, F.; Loreto, C.; Nsir, H.; Szychlinska, M.; Musumeci, G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef]
- Agustin, T.; Nursyam, H.; Firdaus, M.; Rifa’i, M.; Ekaputri, I.D.; Mahmiah. The Characterization of Blue Shark (Prionace glauca) Cartilage Potential as Nature-Derived Drug Material. IOP Conf. Ser. Earth Environ. Sci. 2025, 1473, 012049. [Google Scholar] [CrossRef]
- López-Álvarez, M.; González, P.; Serra, J.; Fraguas, J.; Valcarcel, J.; Vázquez, J.A. Chondroitin Sulfate and Hydroxyapatite from Prionace glauca Shark Jaw: Physicochemical and Structural Characterization. Int. J. Biol. Macromol. 2020, 156, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Golovach, I.; Rekalov, D.; Akimov, O.Y.; Kostenko, H.; Kostenko, V.; Mishchenko, A.; Solovyova, N.; Kostenko, V. Molecular Mechanisms and Potential Applications of Chondroitin Sulphate in Managing Post-Traumatic Osteoarthritis. Rheumatology 2023, 61, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Paththuwe Arachchi, M.J.; Subash, A.; Bamigbade, G.B.; Abdin, M.; Ulla, N.; Ayyash, M. Fish Byproducts as a Sustainable Source of Glycosaminoglycans: Extraction Processes, Food Applications, Nutraceutical Advancements, and Challenges. Trends Food Sci. Technol. 2025, 159, 104963. [Google Scholar] [CrossRef]
- Medeiros, L.H.C.; Vasconcelos, B.M.F.; Silva, M.B.; Souza-Junior, A.A.; Chavante, S.F.; Andrade, G.P.V. Chondroitin Sulfate from Fish Waste Exhibits Strong Intracellular Antioxidant Potential. Braz. J. Med. Biol. Res. 2021, 54, e10730. [Google Scholar] [CrossRef]
- Urbi, Z.; Azmi, N.S.; Ming, L.C.; Hossain, M.S. A Concise Review of Extraction and Characterization of Chondroitin Sulphate from Fish and Fish Wastes for Pharmacological Application. Curr. Issues Mol. Biol. 2022, 44, 3905–3922. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Fernández, N.; Matias, A.A.; Bronze, M.D.R. Hyaluronic Acid and Chondroitin Sulfate from Marine and Terrestrial Sources: Extraction and Purification Methods. Carbohydr. Polym. 2020, 243, 116441. [Google Scholar] [CrossRef]
- Jerosch, J. Effects of Glucosamine and Chondroitin Sulfate on Cartilage Metabolism in OA: Outlook on Other Nutrient Partners Especially Omega-3 Fatty Acids. Int. J. Rheumatol. 2011, 2011, 969012. [Google Scholar] [CrossRef]
- Elhiss, S.; Hamdi, A.; Chahed, L.; Boisson-Vidal, C.; Majdoub, H.; Bouchemal, N.; Laschet, J.; Kraiem, J.; Le Cerf, D.; Maaroufi, R.M.; et al. Hyaluronic Acid from Bluefin Tuna By-Product: Structural Analysis and Pharmacological Activities. Int. J. Biol. Macromol. 2024, 264, 130424. [Google Scholar] [CrossRef] [PubMed]
- Suresh, P.V.; Kudre, T.G.; Johny, L.C. Sustainable Valorization of Seafood Processing By-Product/Discard. In Waste to Wealth; Energy, Environment, and Sustainability; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K., Eds.; Springer: Singapore, 2018; pp. 111–139. ISBN 978-981-10-7430-1. [Google Scholar]
- Molina-Ramírez, C.; Mazo, P.; Zuluaga, R.; Gañán, P.; Álvarez-Caballero, J. Characterization of Chitosan Extracted from Fish Scales of the Colombian Endemic Species Prochilodus Magdalenae as a Novel Source for Antibacterial Starch-Based Films. Polymers 2021, 13, 2079. [Google Scholar] [CrossRef] [PubMed]
- Prihanto, A.A.; Nurdiani, R.; Bagus, A.D. Production and Characteristics of Fish Protein Hydrolysate from Parrotfish (Chlorurus sordidus) Head. PeerJ 2019, 7, e8297. [Google Scholar] [CrossRef]
- Kuang, C.Y.; Mohtar, N.F. Effects of Different Soaking Time on the Extraction of Gelatin from Shortfin Scad (Decapterus macrosoma) Heads. J. Environ. Biol. 2018, 39, 888–894. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Liu, S.; Wei, S.; Xia, Q.; Ji, H.; Deng, C.; Hao, J. Extraction of Fish Oil from Fish Heads Using Ultra-High Pressure Pre-Treatment Prior to Enzymatic Hydrolysis. Innov. Food Sci. Emerg. Technol. 2021, 70, 102670. [Google Scholar] [CrossRef]
- Wang, C.-H.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.-L. Reclamation of Fishery Processing Waste: A Mini-Review. Molecules 2019, 24, 2234. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Wu, S.; Chen, J.; Lin, H. Structural, Functional, Rheological, and Biological Properties of the Swim Bladder Collagen Extracted from Grass Carp (Ctenopharyngodon idella). LWT 2022, 153, 112518. [Google Scholar] [CrossRef]
- Rocha, M.S.; Marques, C.F.; Carvalho, A.C.; Martins, E.; Ereskovsky, A.; Reis, R.L.; Silva, T.H. The Characterization and Cytotoxic Evaluation of Chondrosia Reniformis Collagen Isolated from Different Body Parts (Ectosome and Choanosome) Envisaging the Development of Biomaterials. Mar. Drugs 2024, 22, 55. [Google Scholar] [CrossRef]
- Badilli, U.; Inal, O. Current Approaches in Cosmeceuticals: Peptides, Biotics and Marine Biopolymers. Polymers 2025, 17, 798. [Google Scholar] [CrossRef]
- Sotelo, C.G.; Blanco, M.; Ramos, P.; Vázquez, J.A.; Perez-Martin, R.I. Sustainable Sources from Aquatic Organisms for Cosmeceuticals Ingredients. Cosmetics 2021, 8, 48. [Google Scholar] [CrossRef]
- Premium High-Purity Marine Collagen Peptides|Thala. Available online: https://www.thalaingredients.com/thalacol/ (accessed on 24 June 2025).
- NUTRICOLL NATURAL MARINE COLLAGEN POWDER—Chitinor. Available online: https://cosmetics.specialchem.com/product/i-chitinor-seagarden-nutricoll-natural-marine-collagen-powder (accessed on 24 June 2025).
- Cropure Orange Roughy—Croda. Available online: https://cosmetics.specialchem.com/product/i-croda-cropure-orange-roughy (accessed on 24 June 2025).
- Zellulin® ZelluGEN 10% Solution—Avant. Available online: https://cosmetics.specialchem.com/product/i-avant-zellulin-zellugen-10-solution (accessed on 24 June 2025).
- Hydamer HCMF—Chitinor. Available online: https://chitinor.com/hydamer-hcmf/ (accessed on 24 June 2025).
- InstantPeelTM Exfoliant. Available online: https://earthenskincare.com/products/instant-peel-exfoliant (accessed on 24 June 2025).
- Zou, Y.; Heyndrickx, M.; Debode, J.; Raes, K.; De Pascale, D.; Behan, P.; Giltrap, M.; O’Connor, C.; Solstad, R.G.; Lian, K.; et al. Valorisation of Crustacean and Bivalve Processing Side Streams for Industrial Fast Time-to-Market Products: A Review from the European Union Regulation Perspective. Front. Mar. Sci. 2023, 10, 1068151. [Google Scholar] [CrossRef]
- Astaxanthin Collagen All-in-One Gel. Available online: https://www.dhccare.com/astaxanthin-collagen-all-in-one-gel.html (accessed on 24 June 2025).
- Marin Skincare. Available online: https://www.marinskincare.com/ (accessed on 24 June 2025).
- Akoshine Oyster Shell Pearl Powder—Akott. Available online: https://cosmetics.specialchem.com/product/i-akott-akoshine-oyster-shell-pearl-powder (accessed on 24 June 2025).
- Oligoceane PH—Croda. Available online: https://cosmetics.specialchem.com/product/i-croda-oligoceane-ph (accessed on 24 June 2025).
- Huzhou Pearl Powder—Huzhou Nanxun Shengtao Botanical. Available online: https://cosmetics.specialchem.com/product/i-huzhou-shengtao-biotech-huzhou-pearl-powder (accessed on 24 June 2025).
- Carrasqueira, J.; Bernardino, S.; Bernardino, R.; Afonso, C. Marine-Derived Polysaccharides and Their Potential Health Benefits in Nutraceutical Applications. Mar. Drugs 2025, 23, 60. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Cheng, N.; Fang, D.; Wang, H.; Rahman, F.-U.; Hao, H.; Zhang, Y. Recent Advances on Application of Polysaccharides in Cosmetics. J. Dermatol. Sci. Cosmet. Technol. 2024, 1, 100004. [Google Scholar] [CrossRef]
- CosIng—Cosmetics—GROWTH—European Commission. Available online: https://ec.europa.eu/growth/tools-databases/cosing/details/74131 (accessed on 9 January 2025).
- Hesse, G.W. Chronic Zinc Deficiency Alters Neuronal Function of Hippocampal Mossy Fibers. Science 1979, 205, 1005–1007. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, M.; Tishchenko, G.; Yudin, V.; Guarneri, F.; Cardillo, M.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Fabrizi, G. Chitin-Hyaluronan Nanoparticles: A Multifunctional Carrier to Deliver Anti-Aging Active Ingredients through the Skin. Cosmetics 2014, 1, 140–158. [Google Scholar] [CrossRef]
- Morganti, P.; Palombo, M.; Carezzi, F.; Nunziata, M.; Morganti, G.; Cardillo, M.; Chianese, A. Green Nanotechnology Serving the Bioeconomy: Natural Beauty Masks to Save the Environment. Cosmetics 2016, 3, 41. [Google Scholar] [CrossRef]
- Afonso, C.R.; Hirano, R.S.; Gaspar, A.L.; Chagas, E.G.L.; Carvalho, R.A.; Silva, F.V.; Leonardi, G.R.; Lopes, P.S.; Silva, C.F.; Yoshida, C.M.P. Biodegradable Antioxidant Chitosan Films Useful as an Anti-Aging Skin Mask. Int. J. Biol. Macromol. 2019, 132, 1262–1273. [Google Scholar] [CrossRef]
- Chaiwong, N.; Leelapornpisid, P.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sakdatorn, V.; Leksawasdi, N.; Phimolsiripol, Y. Antioxidant and Moisturizing Properties of Carboxymethyl Chitosan with Different Molecular Weights. Polymers 2020, 12, 1445. [Google Scholar] [CrossRef]
- Chen, K.; Guo, B.; Luo, J. Quaternized Carboxymethyl Chitosan/Organic Montmorillonite Nanocomposite as a Novel Cosmetic Ingredient against Skin Aging. Carbohydr. Polym. 2017, 173, 100–106. [Google Scholar] [CrossRef]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The Impact of Ultraviolet Radiation on Skin Photoaging—Review of in Vitro Studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef]
- Valorization of Chitosan from Squid Pens and Further Use on the Development of Scaffolds for Biomedical Applications. Available online: https://www.researchgate.net/publication/255564499_Valorization_of_Chitosan_from_Squid_Pens_and_Further_Use_on_the_Development_of_Scaffolds_for_Biomedical_Applications (accessed on 15 July 2025).
- Silvestre, J.; Delattre, C.; Michaud, P.; De Baynast, H. Optimization of Chitosan Properties with the Aim of a Water Resistant Adhesive Development. Polymers 2021, 13, 4031. [Google Scholar] [CrossRef]
- Nitulescu, G.; Lupuliasa, D.; Adam-Dima, I.; Nitulescu, G.M. Ultraviolet Filters for Cosmetic Applications. Cosmetics 2023, 10, 101. [Google Scholar] [CrossRef]
- Ntohogian, S.; Gavriliadou, V.; Christodoulou, E.; Nanaki, S.; Lykidou, S.; Naidis, P.; Mischopoulou, L.; Barmpalexis, P.; Nikolaidis, N.; Bikiaris, D.N. Chitosan Nanoparticles with Encapsulated Natural and UF-Purified Annatto and Saffron for the Preparation of UV Protective Cosmetic Emulsions. Molecules 2018, 23, 2107. [Google Scholar] [CrossRef]
- Morsy, R.; Ali, S.S.; El-Shetehy, M. Development of Hydroxyapatite-Chitosan Gel Sunscreen Combating Clinical Multidrug-Resistant Bacteria. J. Mol. Struct. 2017, 1143, 251–258. [Google Scholar] [CrossRef]
- Tunku Mahmud, T.H.; Abdul-Aziz, A.; Muda, R. A Review on the Potential Use of Chitosan-Based Delivery System in Mild Facial Cleansing Formulation. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 432–437. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Chitosan: A Promising Multifunctional Cosmetic Ingredient for Skin and Hair Care. Cosmetics 2022, 9, 99. [Google Scholar] [CrossRef]
- Tangkijngamvong, N.; Phaiyarin, P.; Wanichwecharungruang, S.; Kumtornrut, C. The Anti-sebum Property of Chitosan Particles. J. Cosmet. Dermatol. 2020, 19, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Theerawattanawit, C.; Phaiyarin, P.; Wanichwecharungruang, S.; Noppakun, N.; Asawanonda, P.; Kumtornrut, C. The Efficacy and Safety of Chitosan on Facial Skin Sebum. Skin Pharmacol. Physiol. 2022, 35, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Choi, G.; Choy, J.-H. Chitosan Hybrids for Cosmeceutical Applications in Skin, Hair and Dental Care: An Update. Emergent Mater. 2021, 4, 1125–1142. [Google Scholar] [CrossRef]
- Verma, M.; Gahlot, N.; Singh, S.S.J.; Rose, N.M. UV Protection and Antibacterial Treatment of Cellulosic Fibre (Cotton) Using Chitosan and Onion Skin Dye. Carbohydr. Polym. 2021, 257, 117612. [Google Scholar] [CrossRef]
- Deng, L.; Wang, B.; Li, W.; Han, Z.; Chen, S.; Wang, H. Bacterial Cellulose Reinforced Chitosan-Based Hydrogel with Highly Efficient Self-Healing and Enhanced Antibacterial Activity for Wound Healing. Int. J. Biol. Macromol. 2022, 217, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mao, J.; Guo, Z.; Hu, Y.; Wang, S. Polyvinyl Alcohol/Carboxymethyl Chitosan Hydrogel Loaded with Silver Nanoparticles Exhibited Antibacterial and Self-Healing Properties. Int. J. Biol. Macromol. 2022, 220, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Lai-Cheong, J.E.; McGrath, J.A. Structure and Function of Skin, Hair and Nails. Medicine 2021, 49, 337–342. [Google Scholar] [CrossRef]
- Maskan Bermudez, N.; Rodríguez-Tamez, G.; Perez, S.; Tosti, A. Onychomycosis: Old and New. J. Fungi 2023, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Falotico, J.M.; Lipner, S.R. Updated Perspectives on the Diagnosis and Management of Onychomycosis. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1933–1957. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Tampucci, S.; Paganini, V.; Burgalassi, S.; Chetoni, P.; Galván, J.; Celandroni, F.; Ghelardi, E. Ciclopirox Hydroxypropyl Chitosan (CPX-HPCH) Nail Lacquer and Breathable Cosmetic Nail Polish: In Vitro Evaluation of Drug Transungual Permeation Following the Combined Application. Life 2022, 12, 801. [Google Scholar] [CrossRef]
- Palmieri, R.; Cantoresi, F.; Caserini, M.; Bidoli, A.; Maggio, F.; Marino, R.; Carnevale, C.; Sorgi, P. Randomized Controlled Trial of a Water-Soluble Nail Lacquer Based on Hydroxypropyl-Chitosan (HPCH), in the Management of Nail Psoriasis. Clin. Cosmet. Investig. Dermatol. 2014, 7, 185–190. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Long, L.; Isham, N.; Bulgheroni, A.; Setaro, M.; Caserini, M.; Palmieri, R.; Mailland, F. Ability of Hydroxypropyl Chitosan Nail Lacquer To Protect against Dermatophyte Nail Infection. Antimicrob. Agents Chemother. 2015, 59, 1844–1848. [Google Scholar] [CrossRef]
- Csuka, D.A.; Csuka, E.A.; Juhász, M.L.W.; Sharma, A.N.; Mesinkovska, N.A. A Systematic Review on the Lipid Composition of Human Hair. Int. J. Dermatol. 2023, 62, 404–415. [Google Scholar] [CrossRef]
- Morganti, P.; Morganti, G.; Coltelli, M.-B. Smart and Sustainable Hair Products Based on Chitin-Derived Compounds. Cosmetics 2021, 8, 20. [Google Scholar] [CrossRef]
- Sionkowska, A.; Kaczmarek, B.; Michalska, M.; Lewandowska, K.; Grabska, S. Preparation and Characterization of Collagen/Chitosan/Hyaluronic Acid Thin Films for Application in Hair Care Cosmetics. Pure Appl. Chem. 2017, 89, 1829–1839. [Google Scholar] [CrossRef]
- Liu, Y.; Shang, J.; Chen, Y.; Feng, X. Potential Applications of Chitosan in Seborrheic Dermatitis and Other Skin Diseases: A Comprehensive Review. Clin. Cosmet. Investig. Dermatol. 2025, 18, 533–542. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Elgammal, W.E.; Ibrahim, A.G.; Dawaba, H.M.; Nossier, E.S.; Dawaba, A.M. Thiadiazole Chitosan Conjugates as a Novel Cosmetic Ingredient for Rinse-off Hair Conditioners: Design, Formulation, Characterization and in Silico-Molecular Docking Studies. BMC Chem. 2025, 19, 104. [Google Scholar] [CrossRef]
- Akanno, A.; Guzmán, E.; Ortega, F.; Rubio, R.G. Behavior of the Water/Vapor Interface of Chitosan Solutions with an Anionic Surfactant: Effect of Polymer–Surfactant Interactions. Phys. Chem. Chem. Phys. 2020, 22, 23360–23373. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Peña, L.; Guzmán, E. Physicochemical Aspects of the Performance of Hair-Conditioning Formulations. Cosmetics 2020, 7, 26. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural Modification, Biological Activity and Application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef] [PubMed]
- Martins, E.; Castro, P.; Ribeiro, A.B.; Pereira, C.F.; Casanova, F.; Vilarinho, R.; Moreira, J.; Ramos, Ó.L. Bleached Hair as Standard Template to Insight the Performance of Commercial Hair Repair Products. Cosmetics 2024, 11, 150. [Google Scholar] [CrossRef]
- Gao, H.; Wu, N.; Wang, N.; Li, J.; Sun, J.; Peng, Q. Chitosan-Based Therapeutic Systems and Their Potentials in Treatment of Oral Diseases. Int. J. Biol. Macromol. 2022, 222, 3178–3194. [Google Scholar] [CrossRef]
- Zúñiga-López, C.-M.; Márquez-Pérez, K.; Argueta-Figueroa, L.; Bautista-Hernández, M.-A.; Torres-Rosas, R. Chitosan for the Treatment of Inflammation of the Oral Mucosa: A Systematic Review. Med. Oral Patol. Oral Cirugia Bucal 2024, 29, e9–e17. [Google Scholar] [CrossRef]
- Ramadhany, Y.F.; Achmad, H.; Khairunnisa, P.; Mardiana, M. The Efficacy of Chitosan Toothpaste Based White Shrimp (Litopenaeus vannamei) to Decrease Streptococcus Mutans Colonies in Children with Early Childhood Caries. In Proceedings of the 11th International Dentistry Scientific Meeting (IDSM 2017), Central Jakarta, Indonesia, 16–17 September 2017; Atlantis Press: Jakarta, Indonesia, 2018. [Google Scholar]
- Ganguly, A.; Ian, C.K.; Sheshala, R.; Sahu, P.S.; Al-Waeli, H.; Meka, V.S. Application of Diverse Natural Polymers in the Design of Oral Gels for the Treatment of Periodontal Diseases. J. Mater. Sci. Mater. Med. 2017, 28, 39. [Google Scholar] [CrossRef]
- Nguyen, S.; Escudero, C.; Sediqi, N.; Smistad, G.; Hiorth, M. Fluoride Loaded Polymeric Nanoparticles for Dental Delivery. Eur. J. Pharm. Sci. 2017, 104, 326–334. [Google Scholar] [CrossRef]
- Samiraninezhad, N.; Asadi, K.; Rezazadeh, H.; Gholami, A. Using Chitosan, Hyaluronic Acid, Alginate, and Gelatin-Based Smart Biological Hydrogels for Drug Delivery in Oral Mucosal Lesions: A Review. Int. J. Biol. Macromol. 2023, 252, 126573. [Google Scholar] [CrossRef] [PubMed]
- Murado, M.A.; Montemayor, M.I.; Cabo, M.L.; Vázquez, J.A.; González, M.P. Optimization of Extraction and Purification Process of Hyaluronic Acid from Fish Eyeball. Food Bioprod. Process. 2012, 90, 491–498. [Google Scholar] [CrossRef]
- Salbach, J.; Rachner, T.D.; Rauner, M.; Hempel, U.; Anderegg, U.; Franz, S.; Simon, J.-C.; Hofbauer, L.C. Regenerative Potential of Glycosaminoglycans for Skin and Bone. J. Mol. Med. 2012, 90, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.; Pomin, V. The Sea as a Rich Source of Structurally Unique Glycosaminoglycans and Mimetics. Microorganisms 2017, 5, 51. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, M.; Costa, R.; Mano, J. Marine Origin Polysaccharides in Drug Delivery Systems. Mar. Drugs 2016, 14, 34. [Google Scholar] [CrossRef]
- Irianto, H.E.; Giyatmi. Marine Chondroitin Sulfate and Its Potential Applications. In Marine Biochemistry; CRC Press: Boca Raton, FL, USA, 2022; pp. 247–269. ISBN 978-1-00-330391-6. [Google Scholar]
- Vázquez, J.; Rodríguez-Amado, I.; Montemayor, M.; Fraguas, J.; González, M.; Murado, M. Chondroitin Sulfate, Hyaluronic Acid and Chitin/Chitosan Production Using Marine Waste Sources: Characteristics, Applications and Eco-Friendly Processes: A Review. Mar. Drugs 2013, 11, 747–774. [Google Scholar] [CrossRef]
- Yuan, Q.; Shi, X.; Ma, H.; Yao, Y.; Zhang, B.; Zhao, L. Recent Progress in Marine Chondroitin Sulfate, Dermatan Sulfate, and Chondroitin Sulfate/Dermatan Sulfate Hybrid Chains as Potential Functional Foods and Therapeutic Agents. Int. J. Biol. Macromol. 2024, 262, 129969. [Google Scholar] [CrossRef]
- Wauquier, F.; Boutin-Wittrant, L.; Bouvret, E.; Le Faouder, J.; Roux, V.; Macian, N.; Pickering, G.; Wittrant, Y. Benefits of Circulating Human Metabolites from Fish Cartilage Hydrolysate on Primary Human Dermal Fibroblasts, an Ex Vivo Clinical Investigation for Skin Health Applications. Nutrients 2022, 14, 5027. [Google Scholar] [CrossRef]
- Mourão, P.A.; Vilanova, E.; Soares, P.A. Unveiling the Structure of Sulfated Fucose-Rich Polysaccharides via Nuclear Magnetic Resonance Spectroscopy. Curr. Opin. Struct. Biol. 2018, 50, 33–41. [Google Scholar] [CrossRef]
- Wang, L.; Jayawardena, T.U.; Hyun, J.; Wang, K.; Fu, X.; Xu, J.; Gao, X.; Park, Y.; Jeon, Y.-J. Antioxidant and Anti-Photoaging Effects of a Fucoidan Isolated from Turbinaria ornata. Int. J. Biol. Macromol. 2023, 225, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Hyun, S.H.; Kim, H.; Park, S.; Lee, J.; Lee, I.; Bae, J. The Effects of Fucoidan-rich Polysaccharides Extracted from Sargassum horneri on Enhancing Collagen-related Skin Barrier Function as a Potential Cosmetic Product. J. Cosmet. Dermatol. 2024, 23, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-H.; Wang, P.-W.; Yang, S.-C.; Chou, W.-L.; Fang, J.-Y. Cosmetic and Therapeutic Applications of Fish Oil’s Fatty Acids on the Skin. Mar. Drugs 2018, 16, 256. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, M.; Song, L. A Review of Fatty Acids Influencing Skin Condition. J. Cosmet. Dermatol. 2020, 19, 3199–3204. [Google Scholar] [CrossRef]
- Ansary, T.M.; Hossain, M.R.; Kamiya, K.; Komine, M.; Ohtsuki, M. Inflammatory Molecules Associated with Ultraviolet Radiation-Mediated Skin Aging. Int. J. Mol. Sci. 2021, 22, 3974. [Google Scholar] [CrossRef]
- Calder, P.C. Mechanisms of Action of (n-3) Fatty Acids. J. Nutr. 2012, 142, 592S–599S. [Google Scholar] [CrossRef]
- Desai, S.J.; Prickril, B.; Rasooly, A. Mechanisms of Phytonutrient Modulation of Cyclooxygenase-2 (COX-2) and Inflammation Related to Cancer. Nutr. Cancer 2018, 70, 350–375. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Watson, R.E.B.; Nicolaou, A.; Rhodes, L.E. Omega-3 Polyunsaturated Fatty Acids: Photoprotective Macronutrients. Exp. Dermatol. 2011, 20, 537–543. [Google Scholar] [CrossRef]
- Reilly, D.M.; Lozano, J. Skin Collagen through the Lifestages: Importance for Skin Health and Beauty. Plast. Aesthetic Res. 2021, 8, 2. [Google Scholar] [CrossRef]
- Wang, X.; Wu, J. Modulating Effect of Fatty Acids and Sterols on Skin Aging. J. Funct. Foods 2019, 57, 135–140. [Google Scholar] [CrossRef]
- Latreille, J.; Kesse-Guyot, E.; Malvy, D.; Andreeva, V.; Galan, P.; Tschachler, E.; Hercberg, S.; Guinot, C.; Ezzedine, K. Association between Dietary Intake of N-3 Polyunsaturated Fatty Acids and Severity of Skin Photoaging in a Middle-Aged Caucasian Population. J. Dermatol. Sci. 2013, 72, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Jakus, J.; Portillo, M.; Gvirtz, R.; Ogen-Shtern, N.; Silberstein, E.; Ayzenberg, T.; Rozenblat, S. In Vitro, Ex Vivo, and Clinical Evaluation of Anti-aging Gel Containing EPA and CBD. J. Cosmet. Dermatol. 2023, 22, 3047–3057. [Google Scholar] [CrossRef] [PubMed]
- Rathee, P.; Kumar, S.; Kumar, D.; Kumari, B.; Yadav, S.S. Skin Hyperpigmentation and Its Treatment with Herbs: An Alternative Method. Future J. Pharm. Sci. 2021, 7, 132. [Google Scholar] [CrossRef]
- Yuan, X.H.; Jin, Z.H. Paracrine Regulation of Melanogenesis. Br. J. Dermatol. 2018, 178, 632–639. [Google Scholar] [CrossRef]
- Balcos, M.C.; Kim, S.Y.; Jeong, H.; Yun, H.; Baek, K.J.; Kwon, N.S.; Park, K.; Kim, D. Docosahexaenoic Acid Inhibits Melanin Synthesis in Murine Melanoma Cells in Vitro through Increasing Tyrosinase Degradation. Acta Pharmacol. Sin. 2014, 35, 489–495. [Google Scholar] [CrossRef]
- Makgobole, M.U.; Onwubu, S.; Khathi, A.; Mpofana, N.; Mkhwanazi, B. Cosmeceuticals from Marine: The Prospect of Marine Products in Skin Rejuvenation and Care. Egypt. J. Basic Appl. Sci. 2024, 11, 297–317. [Google Scholar] [CrossRef]
- Shin, J.W.; Park, K.C. Current Clinical Use of Depigmenting Agents. Dermatol. Sin. 2014, 32, 205–210. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, J.; Quan, Z.; Qian, M.; Liu, W.; Zheng, W.; Yin, F.; Du, J.; Zhi, Y.; Song, N. Klotho Protein Protects Human Keratinocytes from UVB-Induced Damage Possibly by Reducing Expression and Nuclear Translocation of NF-κB. Med. Sci. Monit. 2018, 24, 8583–8591. [Google Scholar] [CrossRef]
- Bernerd, F.; Passeron, T.; Castiel, I.; Marionnet, C. The Damaging Effects of Long UVA (UVA1) Rays: A Major Challenge to Preserve Skin Health and Integrity. Int. J. Mol. Sci. 2022, 23, 8243. [Google Scholar] [CrossRef]
- Ciążyńska, M.; Olejniczak-Staruch, I.; Sobolewska-Sztychny, D.; Narbutt, J.; Skibińska, M.; Lesiak, A. Ultraviolet Radiation and Chronic Inflammation—Molecules and Mechanisms Involved in Skin Carcinogenesis: A Narrative Review. Life 2021, 11, 326. [Google Scholar] [CrossRef]
- Yamada, H.; Hakozaki, M.; Uemura, A.; Yamashita, T. Effect of Fatty Acids on Melanogenesis and Tumor Cell Growth in Melanoma Cells. J. Lipid Res. 2019, 60, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, Z.; Shaikh, M.H.; Dehlawi, H.; Michael-Titus, A.T.; Parkinson, E.K. The Induction of Apoptosis in Pre-Malignant Keratinocytes by Omega-3 Polyunsaturated Fatty Acids Docosahexaenoic Acid (DHA) and Eicosapentaenoic Acid (EPA) Is Inhibited by Albumin. Toxicol. Lett. 2013, 218, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, M.; Zolghadr, L.; Nezhad, N.S.; Ahmadpour Yazdi, H.; Esfahani, A.J.; Gheibi, N. Investigating the Effects of Quercetin Fatty Acid Esters on Apoptosis, Mechanical Properties, and Expression of ERK in Melanoma Cell Line (A375). Life Sci. 2022, 310, 121007. [Google Scholar] [CrossRef] [PubMed]
- Sroka-Tomaszewska, J.; Trzeciak, M. Molecular Mechanisms of Atopic Dermatitis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4130. [Google Scholar] [CrossRef]
- Avena-Woods, C. Overview of Atopic Dermatitis. Am. J. Manag. Care 2017, 23, S115–S123. [Google Scholar]
- Danby, S.G.; Andrew, P.V.; Taylor, R.N.; Kay, L.J.; Chittock, J.; Pinnock, A.; Ulhaq, I.; Fasth, A.; Carlander, K.; Holm, T.; et al. Different Types of Emollient Cream Exhibit Diverse Physiological Effects on the Skin Barrier in Adults with Atopic Dermatitis. Clin. Exp. Dermatol. 2022, 47, 1154–1164. [Google Scholar] [CrossRef]
- Ivic, M.; Slugan, A.; Leskur, D.; Rusic, D.; Seselja Perisin, A.; Modun, D.; Durdov, T.; Bozic, J.; Vukovic, D.; Bukic, J. Efficacy and Safety Assessment of Topical Omega Fatty Acid Product in Experimental Model of Irritant Contact Dermatitis: Randomized Controlled Trial. Appl. Sci. 2024, 14, 6423. [Google Scholar] [CrossRef]
- Nip, J.; Hermanson, K.; Lee, J. N-3 PUFAs Docosahexaenoic Acid and Eicosapentaenoic Acid Are Effective Natural Pro-resolution Ingredients for Topical Skin Applications. Int. J. Cosmet. Sci. 2025; early view. [Google Scholar] [CrossRef]
- Saretzky, I.; Cassini, M. Enhancing Skin Cicatrization with Natural Sources—The Role of Polyunsaturated Fatty Acids (PUFAs) and Beeswax. In Cosmetic Products and Industry—New Advances and Applications; Ahmad, U., Akhtar, J., Eds.; IntechOpen: London, UK, 2023; ISBN 978-1-83768-622-3. [Google Scholar]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and Comorbid Diseases. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef]
- Raharja, A.; Mahil, S.K.; Barker, J.N. Psoriasis: A Brief Overview. Clin. Med. 2021, 21, 170–173. [Google Scholar] [CrossRef]
- Barrea, L.; Balato, N.; Di Somma, C.; Macchia, P.; Napolitano, M.; Savanelli, M.; Esposito, K.; Colao, A.; Savastano, S. Nutrition and Psoriasis: Is There Any Association between the Severity of the Disease and Adherence to the Mediterranean Diet? J. Transl. Med. 2015, 13, 18. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 Versus Omega-6 Polyunsaturated Fatty Acids in the Prevention and Treatment of Inflammatory Skin Diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, C.; Zou, B.; Fu, L.; Ren, S.; Zhang, X. Design and Evaluation of Tretinoin Fatty Acid Vesicles for the Topical Treatment of Psoriasis. Molecules 2023, 28, 7868. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Balbás, G.; Sánchez-Regaña, M.; Millet, U. Study on the Use of Omega-3 Fatty Acids as a Therapeutic Supplement in Treatment of Psoriasis. Clin. Cosmet. Investig. Dermatol. 2011, 4, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Vasam, M.; Korutla, S.; Bohara, R.A. Acne Vulgaris: A Review of the Pathophysiology, Treatment, and Recent Nanotechnology Based Advances. Biochem. Biophys. Rep. 2023, 36, 101578. [Google Scholar] [CrossRef]
- Leung, A.K.; Barankin, B.; Lam, J.M.; Leong, K.F.; Hon, K.L. Dermatology: How to Manage Acne Vulgaris. Drugs Context 2021, 10, 1–18. [Google Scholar] [CrossRef]
- Jung, J.Y.; Yoon, M.Y.; Min, S.U.; Hong, J.S.; Choi, Y.S.; Suh, D.H. The Influence of Dietary Patterns on Acne Vulgaris in Koreans. Eur. J. Dermatol. EJD 2010, 20, 768–772. [Google Scholar]
- Di Landro, A.; Cazzaniga, S.; Parazzini, F.; Ingordo, V.; Cusano, F.; Atzori, L.; Cutrì, F.T.; Musumeci, M.L.; Zinetti, C.; Pezzarossa, E.; et al. Family History, Body Mass Index, Selected Dietary Factors, Menstrual History, and Risk of Moderate to Severe Acne in Adolescents and Young Adults. J. Am. Acad. Dermatol. 2012, 67, 1129–1135. [Google Scholar] [CrossRef]
- Das, P.; Dutta, A.; Panchali, T.; Khatun, A.; Kar, R.; Das, T.K.; Phoujdar, M.; Chakrabarti, S.; Ghosh, K.; Pradhan, S. Advances in Therapeutic Applications of Fish Oil: A Review. Meas. Food 2024, 13, 100142. [Google Scholar] [CrossRef]
- Balboa, E.M.; Li, Y.-X.; Ahn, B.-N.; Eom, S.-H.; Domínguez, H.; Jiménez, C.; Rodríguez, J. Photodamage Attenuation Effect by a Tetraprenyltoluquinol Chromane Meroterpenoid Isolated from Sargassum muticum. J. Photochem. Photobiol. B 2015, 148, 51–58. [Google Scholar] [CrossRef]
- Januário, A.P.; Félix, C.; Félix, R.; Shiels, K.; Murray, P.; Valentão, P.; Lemos, M.F.L. Exploring the Therapeutical Potential of Asparagopsis armata Biomass: A Novel Approach for Acne Vulgaris Treatment. Mar. Drugs 2024, 22, 489. [Google Scholar] [CrossRef]
- Parolini, C. The Role of Marine N-3 Polyunsaturated Fatty Acids in Inflammatory-Based Disease: The Case of Rheumatoid Arthritis. Mar. Drugs 2023, 22, 17. [Google Scholar] [CrossRef]
- Calder, P.C. Marine Omega-3 Fatty Acids and Inflammatory Processes: Effects, Mechanisms and Clinical Relevance. Biochim. Biophys. Acta BBA—Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C. Marine N-3 Polyunsaturated Fatty Acids: Efficacy on Inflammatory-Based Disorders. Life Sci. 2020, 263, 118591. [Google Scholar] [CrossRef]
- Loftsson, T.; Ilievska, B.; Asgrimsdottir, G.M.; Ormarsson, O.T.; Stefansson, E. Fatty Acids from Marine Lipids: Biological Activity, Formulation and Stability. J. Drug Deliv. Sci. Technol. 2016, 34, 71–75. [Google Scholar] [CrossRef]
- Lopes, D.; Rey, F.; Leal, M.C.; Lillebø, A.I.; Calado, R.; Domingues, M.R. Bioactivities of Lipid Extracts and Complex Lipids from Seaweeds: Current Knowledge and Future Prospects. Mar. Drugs 2021, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, C.; Leamy, A.; O’Sullivan, E.; Zabetakis, I.; Lordan, R.; Nasopoulou, C. Cardiovascular Diseases and Marine Oils: A Focus on Omega-3 Polyunsaturated Fatty Acids and Polar Lipids. Mar. Drugs 2023, 21, 549. [Google Scholar] [CrossRef]
- Haq, M.; Suraiya, S.; Ahmed, S.; Chun, B.-S. Phospholipids from Marine Source: Extractions and Forthcoming Industrial Applications. J. Funct. Foods 2021, 80, 104448. [Google Scholar] [CrossRef]
- Fu, D.; Li, J.; Dai, D.; Zhou, D.; Zhu, B.; Song, L. Development and Characterization of Self-Emulsifying High Internal Phase Emulsions Using Endogenous Phospholipids from Antarctic Krill Oil. Food Chem. 2023, 428, 136765. [Google Scholar] [CrossRef]
- Lens, M. Phospholipid-Based Vesicular Systems as Carriers for the Delivery of Active Cosmeceutical Ingredients. Int. J. Mol. Sci. 2025, 26, 2484. [Google Scholar] [CrossRef]
- Alfutaimani, A.S.; Alharbi, N.K.; Alahmari, A.S.; Alqabbani, A.A.; Aldayel, A.M. Exploring the Landscape of Lipid Nanoparticles (LNPs): A Comprehensive Review of LNPs Types and Biological Sources of Lipids. Int. J. Pharm. X 2024, 8, 100305. [Google Scholar] [CrossRef]
- Van Hoogevest, P.; Fahr, A. Phospholipids in Cosmetic Carriers. In Nanocosmetics; Cornier, J., Keck, C.M., Van De Voorde, M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 95–140. ISBN 978-3-030-16572-7. [Google Scholar]
- Topuz, O.K.; Aygün, T.; Alp, A.C.; Yatmaz, H.A.; Torun, M.; Yerlikaya, P. Characterization and Emulsifying Properties of Aquatic Lecithins Isolated from Processing Discard of Rainbow Trout Fish and Its Eggs. Food Chem. 2021, 339, 128103. [Google Scholar] [CrossRef]
- Thy, L.T.M.; Duy, H.K.; Dat, N.M. Applications of Lecithin in Emulsion Stabilization and Advanced Delivery Systems in Cosmetics: A Mini-Review. Results Surf. Interfaces 2025, 19, 100543. [Google Scholar] [CrossRef]
- Adamantidi, T.; Lafara, M.-P.; Venetikidou, M.; Likartsi, E.; Toganidou, I.; Tsoupras, A. Utilization and Bio-Efficacy of Carotenoids, Vitamin A and Its Vitaminoids in Nutricosmetics, Cosmeceuticals, and Cosmetics’ Applications with Skin-Health Promoting Properties. Appl. Sci. 2025, 15, 1657. [Google Scholar] [CrossRef]
- Akbaş, A.; Kılınç, F.; Şener, S.; Hayran, Y. Vitamin D Levels in Patients with Seborrheic Dermatitis. Rev. Assoc. Médica Bras. 2023, 69, e20230022. [Google Scholar] [CrossRef] [PubMed]
- Zasada, M.; Budzisz, E. Retinoids: Active Molecules Influencing Skin Structure Formation in Cosmetic and Dermatological Treatments. Adv. Dermatol. Allergol. 2019, 36, 392–397. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Papadopoulou, S.N.A.; Anastasiou, E.A.; Adamantidi, T.; Ofrydopoulou, A.; Letsiou, S.; Tsoupras, A. A Comprehensive Review on the Beneficial Roles of Vitamin D in Skin Health as a Bio-Functional Ingredient in Nutricosmetic, Cosmeceutical, and Cosmetic Applications. Appl. Sci. 2025, 15, 796. [Google Scholar] [CrossRef]
- Formisano, E.; Proietti, E.; Borgarelli, C.; Pisciotta, L. Psoriasis and Vitamin D: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3387. [Google Scholar] [CrossRef]
- Yang, Y.; McClements, D.J. Vitamin E Bioaccessibility: Influence of Carrier Oil Type on Digestion and Release of Emulsified α-Tocopherol Acetate. Food Chem. 2013, 141, 473–481. [Google Scholar] [CrossRef]
- Shearer, M.J.; Newman, P. Recent Trends in the Metabolism and Cell Biology of Vitamin K with Special Reference to Vitamin K Cycling and MK-4 Biosynthesis. J. Lipid Res. 2014, 55, 345–362. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, T.; O’Connor, C.; Barlow, J.W.; Walsh, J.; Scalabrino, G.; Xu, F.; Sheridan, H. The Biological Responses of Vitamin K2: A Comprehensive Review. Food Sci. Nutr. 2023, 11, 1634–1656. [Google Scholar] [CrossRef]
- Solano, F. Metabolism and Functions of Amino Acids in the Skin. In Amino Acids in Nutrition and Health; Advances in Experimental Medicine and Biology; Wu, G., Ed.; Springer International Publishing: Cham, Switzerland, 2020; Volume 1265, pp. 187–199. ISBN 978-3-030-45327-5. [Google Scholar]
- Cadar, E.; Pesterau, A.-M.; Prasacu, I.; Ionescu, A.-M.; Pascale, C.; Dragan, A.-M.L.; Sirbu, R.; Tomescu, C.L. Marine Antioxidants from Marine Collagen and Collagen Peptides with Nutraceuticals Applications: A Review. Antioxidants 2024, 13, 919. [Google Scholar] [CrossRef]
- Al Hajj, W.; Salla, M.; Krayem, M.; Khaled, S.; Hassan, H.F.; El Khatib, S. Hydrolyzed Collagen: Exploring Its Applications in the Food and Beverage Industries and Assessing Its Impact on Human Health—A Comprehensive Review. Heliyon 2024, 10, e36433. [Google Scholar] [CrossRef]
- Murakami, H.; Shimbo, K.; Inoue, Y.; Takino, Y.; Kobayashi, H. Importance of Amino Acid Composition to Improve Skin Collagen Protein Synthesis Rates in UV-Irradiated Mice. Amino Acids 2012, 42, 2481–2489. [Google Scholar] [CrossRef]
- Kalyani, A.; Khajure, P.B.R. Marine-Derived Products for Cosmeceuticals: A Comprehensive Review. Int. J. Sci. Res. Technol. 2024, 1, 1–12. [Google Scholar] [CrossRef]
- Beaumont, M.; Blachier, F. Amino Acids in Intestinal Physiology and Health. In Amino Acids in Nutrition and Health; Advances in Experimental Medicine and Biology; Wu, G., Ed.; Springer International Publishing: Cham, Switzerland, 2020; Volume 1265, pp. 1–20. ISBN 978-3-030-45327-5. [Google Scholar]
- Yoshimura, T.; Manabe, C.; Nagumo, J.-I.; Nagahama, T.; Sato, T.; Murakami, S. Taurine Accelerates the Synthesis of Ceramides and Hyaluronic Acid in Cultured Epidermis and Dermal Fibroblasts. Exp. Ther. Med. 2023, 26, 512. [Google Scholar] [CrossRef] [PubMed]
- Rosic, N. Mycosporine-Like Amino Acids: Making the Foundation for Organic Personalised Sunscreens. Mar. Drugs 2019, 17, 638. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Zhang, Q.; Matsunaga, S.; Fujita, M.J.; Sakai, R. Two New Mycosporine-like Amino Acids LC-343 and Mycosporine-Ethanolamine from the Micronesian Marine Sponge Lendenfeldia chondrodes. Chem. Lett. 2017, 46, 1272–1274. [Google Scholar] [CrossRef]
- Eckes, M.; Dove, S.; Siebeck, U.E.; Grutter, A.S. Fish Mucus versus Parasitic Gnathiid Isopods as Sources of Energy and Sunscreens for a Cleaner Fish. Coral Reefs 2015, 34, 823–833. [Google Scholar] [CrossRef]
- Brotherton, C.A.; Balskus, E.P. Shedding Light on Sunscreen Biosynthesis in Zebrafish. eLife 2015, 4, e07961. [Google Scholar] [CrossRef]
- Urrea-Victoria, V.; Hernández, A.R.; Castellanos, L.; Alves, I.A.; Novoa, D.M.A. The Role of Mycosporine-like Amino Acids in Skin Care Formulations: A Patent Review (2014–2024). Photochem. Photobiol. Sci. 2025, 24, 847–861. [Google Scholar] [CrossRef]
- Chavda, V.P.; Acharya, D.; Hala, V.; Daware, S.; Vora, L.K. Sunscreens: A Comprehensive Review with the Application of Nanotechnology. J. Drug Deliv. Sci. Technol. 2023, 86, 104720. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like Amino Acids and Their Derivatives as Natural Antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.P.; Gacesa, R.; Long, P.F.; Young, A.R. Molecular Photoprotection of Human Keratinocytes in Vitro by the Naturally Occurring Mycosporine-like Amino Acid Palythine. Br. J. Dermatol. 2018, 178, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Gacesa, R.; Lawrence, K.P.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.J.; Wells, G.; Young, A.R.; Long, P.F. The Mycosporine-like Amino Acids Porphyra-334 and Shinorine Are Antioxidants and Direct Antagonists of Keap1-Nrf2 Binding. Biochimie 2018, 154, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Mar. Drugs 2019, 17, 222. [Google Scholar] [CrossRef] [PubMed]
- Waditee-Sirisattha, R.; Kageyama, H. Protective Effects of Mycosporine-like Amino Acid-Containing Emulsions on UV-Treated Mouse Ear Tissue from the Viewpoints of Antioxidation and Antiglycation. J. Photochem. Photobiol. B 2021, 223, 112296. [Google Scholar] [CrossRef]
- Claus, J.; Suess, E.; Meyer, I.; Bugdahn, N.; Lange, S.; Hein, C. Composition with Improved Water Resistance. WO2024027929A1, 8 February 2024. [Google Scholar]
- Claus, J.; Suess, E.; Meyer, I.; Bugdahn, N.; Lange, S.; Hein, C. Composition with Improved Spf and Uva Photoprotection. WO2024027928A1, 8 February 2024. [Google Scholar]
- Yang, Y. Biological Sun-Screening Cosmetic and Preparation Method Thereof. CN105342903A, 24 February 2016. [Google Scholar]
- Zhang, C.; Miao, Z.; Wu, H.; Chen, L.; Zhu, Y.; Chen, D.; Wu, W. Sunscreen cream with Multiple Effects and Application Thereof. CN103720625A, 16 April 2014. [Google Scholar]
- Lu, C. A Kind of Natural Radiation-Preventing Composition and Its Application. CN106937918A, 11 March 2017. [Google Scholar]
- Cayo, P.A.O.; Jerez, F.A.S. Nanoemulsión Para El Cuidado De La Piel Con Bioactivos A Base De Curcumina Y Micosporina (Nanoemulsion with Curcumin- and Mycosporine-Based Bioactive Compounds for Skin Care). 202400247, 26 January 2024.
- Claus, J.; Suess, E.; Meyer, I.; Bugdahn, N.; Lange, S. Composition Comprising a UV-Filter Stabilizer. WO2024027926A1, 8 February 2024. [Google Scholar]
- Wound Healing Composition for Skin External Application Comprising Mycosporine-like Amino Acid and Method for Preparing the Same. KR20170090690A, 5 March 2017.
- Yang, Y. Biological Frost Crack Prevention Hand Cream and Preparation Method Thereof. CN105310897A, 10 February 2016. [Google Scholar]
- Claus, J.; Suess, E.; Meyer, I.; Lange, S.; Nordzieke, S. Compositions Comprising an Antimicrobial Boosting Agent. WO2024027930A1, 8 February 2024. [Google Scholar]
- Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M. Collagen Based Materials in Cosmetic Applications: A Review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef]
- Sugibayashi, K.; Yusuf, E.; Todo, H.; Dahlizar, S.; Sakdiset, P.; Arce, F.J.; See, G.L. Halal Cosmetics: A Review on Ingredients, Production, and Testing Methods. Cosmetics 2019, 6, 37. [Google Scholar] [CrossRef]
- Venkatesan, J.; Anil, S.; Kim, S.-K.; Shim, M. Marine Fish Proteins and Peptides for Cosmeceuticals: A Review. Mar. Drugs 2017, 15, 143. [Google Scholar] [CrossRef]
- Allard, R.; Malak, N.A.; Huc, A. Collagen Product Containing Collagen of Marine Origin with a Low Odor and Preferably with Improved Mechanical Properties, and Its Use in the Form of Cosmetic or Pharmaceutical Compositions or Products. US6660280B1, 9 November 2019. [Google Scholar]
- Xhauflaire-Uhoda, E.; Fontaine, K.; Piérard, G.E. Kinetics of Moisturizing and Firming Effects of Cosmetic Formulations. Int. J. Cosmet. Sci. 2008, 30, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Al-Atif, H. Collagen Supplements for Aging and Wrinkles: A Paradigm Shift in the Field of Dermatology and Cosmetics. Dermatol. Pract. Concept. 2022, 12, e2022018. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, Y.; Wang, X.; Zhou, J.; Cui, H.; Xu, W.; He, Y.; Ma, H.; Gao, R. Effect of Oral Administration of Collagen Hydrolysates from Nile Tilapia on the Chronologically Aged Skin. J. Funct. Foods 2018, 44, 112–117. [Google Scholar] [CrossRef]
- Li, C.; Fu, Y.; Dai, H.; Wang, Q.; Gao, R.; Zhang, Y. Recent Progress in Preventive Effect of Collagen Peptides on Photoaging Skin and Action Mechanism. Food Sci. Hum. Wellness 2022, 11, 218–229. [Google Scholar] [CrossRef]
- Salvatore, L.; Gallo, N.; Natali, M.L.; Campa, L.; Lunetti, P.; Madaghiele, M.; Blasi, F.S.; Corallo, A.; Capobianco, L.; Sannino, A. Marine Collagen and Its Derivatives: Versatile and Sustainable Bio-Resources for Healthcare. Mater. Sci. Eng. C 2020, 113, 110963. [Google Scholar] [CrossRef]
- Klimaszewska, E.; Seweryn, A.; Ogorzałek, M.; Nizioł-Łukaszewska, Z.; Wasilewski, T. Reduction of Irritation Potential Caused by Anionic Surfactants in the Use of Various Forms of Collagen Derived from Marine Sources in Cosmetics for Children. Tenside Surfactants Deterg. 2019, 56, 180–187. [Google Scholar] [CrossRef]
- Jimbo, N.; Kawada, C.; Nomura, Y. Optimization of Dose of Collagen Hydrolysate to Prevent UVB-Irradiated Skin Damage. Biosci. Biotechnol. Biochem. 2016, 80, 356–359. [Google Scholar] [CrossRef]
- Hou, H.; Li, B.; Zhang, Z.; Xue, C.; Yu, G.; Wang, J.; Bao, Y.; Bu, L.; Sun, J.; Peng, Z.; et al. Moisture Absorption and Retention Properties, and Activity in Alleviating Skin Photodamage of Collagen Polypeptide from Marine Fish Skin. Food Chem. 2012, 135, 1432–1439. [Google Scholar] [CrossRef]
- Shibuya, S.; Ozawa, Y.; Toda, T.; Watanabe, K.; Tometsuka, C.; Ogura, T.; Koyama, Y.; Shimizu, T. Collagen Peptide and Vitamin C Additively Attenuate Age-Related Skin Atrophy in Sod1-Deficient Mice. Biosci. Biotechnol. Biochem. 2014, 78, 1212–1220. [Google Scholar] [CrossRef]
- Rahman, A.; Rehmani, R.; Pirvu, D.G.; Huang, S.M.; Puri, S.; Arcos, M. Unlocking the Therapeutic Potential of Marine Collagen: A Scientific Exploration for Delaying Skin Aging. Mar. Drugs 2024, 22, 159. [Google Scholar] [CrossRef]
- Elbialy, Z.I.; Atiba, A.; Abdelnaby, A.; Al-Hawary, I.I.; Elsheshtawy, A.; El-Serehy, H.A.; Abdel-Daim, M.M.; Fadl, S.E.; Assar, D.H. Collagen Extract Obtained from Nile Tilapia (Oreochromis niloticus L.) Skin Accelerates Wound Healing in Rat Model via up Regulating VEGF, bFGF, and α-SMA Genes Expression. BMC Vet. Res. 2020, 16, 352. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, M.; Agwa, M.; Saeed, H.; Khedr, S.M.; Morsy, O.; El-Demellawy, M.A. Fish Scale Collagen Preparation, Characterization and Its Application in Wound Healing. J. Polym. Environ. 2020, 28, 166–178. [Google Scholar] [CrossRef]
- Al-Nimry, S.; Dayah, A.A.; Hasan, I.; Daghmash, R. Cosmetic, Biomedical and Pharmaceutical Applications of Fish Gelatin/Hydrolysates. Mar. Drugs 2021, 19, 145. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hou, H. Protective Effect of Gelatin Polypeptides from Pacific Cod (Gadus macrocephalus) against UV Irradiation-Induced Damages by Inhibiting Inflammation and Improving Transforming Growth Factor-β/Smad Signaling Pathway. J. Photochem. Photobiol. B 2016, 162, 633–640. [Google Scholar] [CrossRef]
- Chen, T.; Hou, H.; Lu, J.; Zhang, K.; Li, B. Protective Effect of Gelatin and Gelatin Hydrolysate from Salmon Skin on UV Irradiation-Induced Photoaging of Mice Skin. J. Ocean Univ. China 2016, 15, 711–718. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Zhuang, Y. Antiphotoaging Effect and Purification of an Antioxidant Peptide from Tilapia (Oreochromis niloticus) Gelatin Peptides. J. Funct. Foods 2013, 5, 154–162. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sun, L. Preparation of Reactive Oxygen Scavenging Peptides from Tilapia (Oreochromis niloticus) Skin Gelatin: Optimization Using Response Surface Methodology. J. Food Sci. 2011, 76, C483–C489. [Google Scholar] [CrossRef]
- Lee, D.U.; Kim, S.-C.; Choi, D.Y.; Jung, W.-K.; Moon, M.J. Basic Amino Acid-Mediated Cationic Amphiphilic Surfaces for Antimicrobial pH Monitoring Sensor with Wound Healing Effects. Biomater. Res. 2023, 27, 14. [Google Scholar] [CrossRef]
- Park, J.I.; Lee, J.E.; Shin, H.J.; Song, S.; Lee, W.K.; Hwang, J.S. Oral Administration of Glycine and Leucine Dipeptides Improves Skin Hydration and Elasticity in UVB-Irradiated Hairless Mice. Biomol. Ther. 2017, 25, 528–534. [Google Scholar] [CrossRef]
- Lei, T.; Ye, L.; Pei, Y.; Sun, H.; Guo, C. Applications of Elastin in Cosmetics: Prospects and Challenges. Cosmetics 2025, 12, 18. [Google Scholar] [CrossRef]
- Waszkielewicz, A.M.; Mirosław, K. Peptides and Their Mechanisms of Action in the Skin. Appl. Sci. 2024, 14, 11495. [Google Scholar] [CrossRef]
- Bojarska, J. Amino Acids and Short Peptides as Anti-Aging “Superfood”. Int. J. Nutr. Sci. 2020, 5, 1039. [Google Scholar]
- Tałałaj, U.; Uścinowicz, P.; Bruzgo, I.; Surażyński, A.; Zaręba, I.; Markowska, A. The Effects of a Novel Series of KTTKS Analogues on Cytotoxicity and Proteolytic Activity. Molecules 2019, 24, 3698. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Nie, T.; Zhang, L.; Liu, X.; Deng, H. Peptides in Cosmetics: From Pharmaceutical Breakthroughs to Skincare Innovations. Cosmetics 2025, 12, 107. [Google Scholar] [CrossRef]
- Husein El Hadmed, H.; Castillo, R.F. Cosmeceuticals: Peptides, Proteins, and Growth Factors. J. Cosmet. Dermatol. 2016, 15, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.I.S.P.; Ferreira, M.S.; Sousa-Lobo, J.M.; Sousa, E.; Almeida, I.F. Usage of Synthetic Peptides in Cosmetics for Sensitive Skin. Pharmaceuticals 2021, 14, 702. [Google Scholar] [CrossRef]
- Pintea, A.; Manea, A.; Pintea, C.; Vlad, R.-A.; Bîrsan, M.; Antonoaea, P.; Rédai, E.M.; Ciurba, A. Peptides: Emerging Candidates for the Prevention and Treatment of Skin Senescence: A Review. Biomolecules 2025, 15, 88. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, L.; Zhang, C.; Xie, P.; Cheng, J.; Wang, X.; Liu, L. Skin-Care Functions of Peptides Prepared from Chinese Quince Seed Protein: Sequences Analysis, Tyrosinase Inhibition and Molecular Docking Study. Ind. Crops Prod. 2020, 148, 112331. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Ding, S.; Deng, Y.; Wang, X. Skin-Care Effects of Dandelion Leaf Extract and Stem Extract: Antioxidant Properties, Tyrosinase Inhibitory and Molecular Docking Simulations. Ind. Crops Prod. 2018, 111, 238–246. [Google Scholar] [CrossRef]
- Mota, S.; Rego, L.; Sousa, E.; Cruz, M.T.; Almeida, I.M.D. Usage Frequency and Ecotoxicity of Skin Depigmenting Agents. Pharmaceuticals 2025, 18, 368. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, N.; Liang, L.; Li, M.; Nie, X.; Wang, Y.; Liu, Q.; Zhou, Q.; Shu, P. Evaluation of the Anti-Aging Potential of Acetyl Tripeptide-30 Citrulline in Cosmetics. Int. J. Pharm. 2024, 663, 124557. [Google Scholar] [CrossRef] [PubMed]
- Henseler, H. Investigating the Effects of Argireline in a Skin Serum Containing Hyaluronic Acids on Skin Surface Wrinkles Using the Visia ® Complexion Analysis Camera System for Objective Skin Analysis. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2023, 12, Doc09. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Gao, J.; Su, W.; Cao, W.; Zhu, G.; Qin, X.; Zhang, C.; Qi, Y. Purification and Identification of Peptides from Oyster (Crassostrea hongkongensis) Protein Enzymatic Hydrolysates and Their Anti-Skin Photoaging Effects on UVB-Irradiated HaCaT Cells. Mar. Drugs 2022, 20, 749. [Google Scholar] [CrossRef]
- Xia, E.; Zhu, X.; Gao, X.; Ni, J.; Guo, H. Antiaging Potential of Peptides from Underused Marine Bioresources. Mar. Drugs 2021, 19, 513. [Google Scholar] [CrossRef]
- Hu, X.; Song, L.; Huang, L.; Zheng, Q.; Yu, R. Antitumor Effect of a Polypeptide Fraction from Arca subcrenata In Vitro and In Vivo. Mar. Drugs 2012, 10, 2782–2794. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Jayawardena, T.U.; Wu, J. Marine Proteins and Peptides: Production, Biological Activities, and Potential Applications. Food Innov. Adv. 2023, 2, 69–84. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, H.; Wu, S.; Mao, C.; Dai, Y.; Yan, H. Marine Bioactive Peptides: Anti-Photoaging Mechanisms and Potential Skin Protective Effects. Curr. Issues Mol. Biol. 2024, 46, 990–1009. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Q.; Zhang, B.; Zhao, Y.; Wang, N. Potential Active Marine Peptides as Anti-Aging Drugs or Drug Candidates. Mar. Drugs 2023, 21, 144. [Google Scholar] [CrossRef]
- Lu, J.; Hou, H.; Fan, Y.; Yang, T.; Li, B. Identification of MMP-1 Inhibitory Peptides from Cod Skin Gelatin Hydrolysates and the Inhibition Mechanism by MAPK Signaling Pathway. J. Funct. Foods 2017, 33, 251–260. [Google Scholar] [CrossRef]
- Gao, Q.; Shang, Y.; Zhou, W.; Deng, S.; Peng, C. Marine Collagen Peptides: A Novel Biomaterial for the Healing of Oral Mucosal Ulcers. Dent. Mater. J. 2022, 41, 850–859. [Google Scholar] [CrossRef]
- Fu, Y.; Li, C.; Wang, Q.; Gao, R.; Cai, X.; Wang, S.; Zhang, Y. The Protective Effect of Collagen Peptides from Bigeye Tuna (Thunnus Obesus) Skin and Bone to Attenuate UVB-Induced Photoaging via MAPK and TGF-β Signaling Pathways. J. Funct. Foods 2022, 93, 105101. [Google Scholar] [CrossRef]
- Maia Campos, P.M.B.G.; Franco, R.S.B.; Kakuda, L.; Cadioli, G.F.; Costa, G.M.D.; Bouvret, E. Oral Supplementation with Hydrolyzed Fish Cartilage Improves the Morphological and Structural Characteristics of the Skin: A Double-Blind, Placebo-Controlled Clinical Study. Mol. Basel Switz. 2021, 26, 4880. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jin, H.; Yang, F.; Jin, S.; Liu, C.; Zhang, L.; Huang, J.; Wang, S.; Yan, Z.; Cai, X.; et al. Physicochemical, Antioxidant Properties of Giant Croaker (Nibea japonica) Swim Bladders Collagen and Wound Healing Evaluation. Int. J. Biol. Macromol. 2019, 138, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Chotphruethipong, L.; Sukketsiri, W.; Aluko, R.E.; Sae-leaw, T.; Benjakul, S. Effect of Hydrolyzed Collagen from Defatted Asian Sea Bass (Lates calcarifer) Skin on Fibroblast Proliferation, Migration and Antioxidant Activities. J. Food Sci. Technol. 2021, 58, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, J.; Ding, Y.; Dai, X.; Li, Y. Oral Administration of Marine Collagen Peptides from Chum Salmon Skin Enhances Cutaneous Wound Healing and Angiogenesis in Rats. J. Sci. Food Agric. 2011, 91, 2173–2179. [Google Scholar] [CrossRef]
- Lønne, G.K.; Gammelsæter, R.; Haglerød, C. Composition Characterization and Clinical Efficacy Study of a Salmon Egg Extract. Int. J. Cosmet. Sci. 2013, 35, 515–522. [Google Scholar] [CrossRef]
- Kong, S.-Z.; Li, J.-C.; Li, S.-D.; Liao, M.-N.; Li, C.-P.; Zheng, P.-J.; Guo, M.-H.; Tan, W.-X.; Zheng, Z.-H.; Hu, Z. Anti-Aging Effect of Chitosan Oligosaccharide on d-Galactose-Induced Subacute Aging in Mice. Mar. Drugs 2018, 16, 181. [Google Scholar] [CrossRef]
- Ito, I.; Yoneda, T.; Omura, Y.; Osaki, T.; Ifuku, S.; Saimoto, H.; Azuma, K.; Imagawa, T.; Tsuka, T.; Murahata, Y.; et al. Protective Effect of Chitin Urocanate Nanofibers against Ultraviolet Radiation. Mar. Drugs 2015, 13, 7463–7475. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Abe, K.; Yoshioka, M.; Morimoto, M.; Saimoto, H.; Yano, H. Preparation of Chitin Nanofibers with a Uniform Width as α-Chitin from Crab Shells. Biomacromolecules 2009, 10, 1584–1588. [Google Scholar] [CrossRef]
- Wei, M.; Jeevithan, L.; Li, N.; Liu, L.; Xu, J.; Wu, W.; Elango, J. Stem-Cell-Regenerative and Protective Effects of Squid (Symplectoteuthis oualaniensis) Skin Collagen Peptides against H2O2-Induced Fibroblast Injury. Mar. Drugs 2024, 22, 255. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Vílchez, C.; Forján, E.; Cuaresma, M.; Bédmar, F.; Garbayo, I.; Vega, J.M. Marine Carotenoids: Biological Functions and Commercial Applications. Mar. Drugs 2011, 9, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.N.; Roda, A.; Gouveia, L.F.; Fernández, N.; Bronze, M.R.; Matias, A.A. Astaxanthin Extraction from Marine Crustacean Waste Streams: An Integrate Approach between Microwaves and Supercritical Fluids. ACS Sustain. Chem. Eng. 2021, 9, 3050–3059. [Google Scholar] [CrossRef]
- Eren, B.; Tuncay Tanrıverdi, S.; Aydın Köse, F.; Özer, Ö. Antioxidant Properties Evaluation of Topical Astaxanthin Formulations as Anti-Aging Products. J. Cosmet. Dermatol. 2019, 18, 242–250. [Google Scholar] [CrossRef]
- Imokawa, G. The Xanthophyll Carotenoid Astaxanthin Has Distinct Biological Effects to Prevent the Photoaging of the Skin Even by Its Postirradiation Treatment. Photochem. Photobiol. 2019, 95, 490–500. [Google Scholar] [CrossRef]
- Ikarashi, N.; Kon, R.; Nagoya, C.; Ishikura, A.; Sugiyama, Y.; Takahashi, J.; Sugiyama, K. Effect of Astaxanthin on the Expression and Activity of Aquaporin-3 in Skin in an In-Vitro Study. Life 2020, 10, 193. [Google Scholar] [CrossRef]
- Bose Subash Chandra Bose, V.; Balaganesan, V.; Govindaraj, G.; Veerichetty, V. Cellular Antioxidant and Cytotoxic Activity of Astaxanthin and Ellagic Acid on UV Irradiated Skin Melanoma Cells and Gel Formulation. Mater. Today Proc. 2023, S2214785323044784. [Google Scholar] [CrossRef]
- Veeruraj, A.; Liu, L.; Zheng, J.; Wu, J.; Arumugam, M. Evaluation of Astaxanthin Incorporated Collagen Film Developed from the Outer Skin Waste of Squid Doryteuthis Singhalensis for Wound Healing and Tissue Regenerative Applications. Mater. Sci. Eng. C 2019, 95, 29–42. [Google Scholar] [CrossRef]
- Nirmal, N.P.; Santivarangkna, C.; Rajput, M.S.; Benjakul, S. Trends in Shrimp Processing Waste Utilization: An Industrial Prospective. Trends Food Sci. Technol. 2020, 103, 20–35. [Google Scholar] [CrossRef]
- Lima, S.G.M.; Freire, M.C.L.C.; Oliveira, V.D.S.; Solisio, C.; Converti, A.; De Lima, Á.A.N. Astaxanthin Delivery Systems for Skin Application: A Review. Mar. Drugs 2021, 19, 511. [Google Scholar] [CrossRef]
- Chang, H.-I.; Shao, C.-W.; Huang, E.; Huang, K.-Y. Development of Astaxanthin-Loaded Nanosized Liposomal Formulation to Improve Bone Health. Pharmaceuticals 2022, 15, 490. [Google Scholar] [CrossRef]
- Rivera-Hernández, G.; Roether, J.A.; Aquino, C.; Boccaccini, A.R.; Sánchez, M.L. Delivery Systems for Astaxanthin: A Review on Approaches for in Situ Dosage in the Treatment of Inflammation Associated Diseases. Int. J. Pharm. 2025, 669, 125017. [Google Scholar] [CrossRef] [PubMed]
- Oninku, B.; Lomas, M.W.; Burr, G.; Aryee, A.N.A. Astaxanthin: An Overview of Its Sources, Extraction Methods, Encapsulation Techniques, Characterization, and Bioavailability. J. Agric. Food Res. 2025, 21, 101869. [Google Scholar] [CrossRef]
- Lee, H.S.; Sung, D.K.; Kim, S.H.; Choi, W.I.; Hwang, E.T.; Choi, D.J.; Chang, J.H. Controlled Release of Astaxanthin from Nanoporous Silicified-Phospholipids Assembled Boron Nitride Complex for Cosmetic Applications. Appl. Surf. Sci. 2017, 424, 15–19. [Google Scholar] [CrossRef]
- Zuluaga, M.; Barzegari, A.; Letourneur, D.; Gueguen, V.; Pavon-Djavid, G. Oxidative Stress Regulation on Endothelial Cells by Hydrophilic Astaxanthin Complex: Chemical, Biological, and Molecular Antioxidant Activity Evaluation. Oxid. Med. Cell. Longev. 2017, 2017, 8073798. [Google Scholar] [CrossRef]
- Yuan, C.; Du, L.; Jin, Z.; Xu, X. Storage Stability and Antioxidant Activity of Complex of Astaxanthin with Hydroxypropyl-β-Cyclodextrin. Carbohydr. Polym. 2013, 91, 385–389. [Google Scholar] [CrossRef]
- Niu, T.; Xuan, R.; Jiang, L.; Wu, W.; Zhen, Z.; Song, Y.; Hong, L.; Zheng, K.; Zhang, J.; Xu, Q.; et al. Astaxanthin Induces the Nrf2/HO-1 Antioxidant Pathway in Human Umbilical Vein Endothelial Cells by Generating Trace Amounts of ROS. J. Agric. Food Chem. 2018, 66, 1551–1559. [Google Scholar] [CrossRef]
- Komatsu, T.; Sasaki, S.; Manabe, Y.; Hirata, T.; Sugawara, T. Preventive Effect of Dietary Astaxanthin on UVA-Induced Skin Photoaging in Hairless Mice. PLoS ONE 2017, 12, e0171178. [Google Scholar] [CrossRef]
- Fang, Q.; Guo, S.; Zhou, H.; Han, R.; Wu, P.; Han, C. Astaxanthin Protects against Early Burn-Wound Progression in Rats by Attenuating Oxidative Stress-Induced Inflammation and Mitochondria-Related Apoptosis. Sci. Rep. 2017, 7, 41440. [Google Scholar] [CrossRef]
- Meephansan, J.; Rungjang, A.; Yingmema, W.; Deenonpoe, R.; Ponnikorn, S. Effect of Astaxanthin on Cutaneous Wound Healing. Clin. Cosmet. Investig. Dermatol. 2017, 10, 259–265. [Google Scholar] [CrossRef]
- Ito, N.; Seki, S.; Ueda, F. The Protective Role of Astaxanthin for UV-Induced Skin Deterioration in Healthy People—A Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2018, 10, 817. [Google Scholar] [CrossRef]
- Dutta, S.; Kumar, S.P.J.; Banerjee, R. A Comprehensive Review on Astaxanthin Sources, Structure, Biochemistry and Applications in the Cosmetic Industry. Algal Res. 2023, 74, 103168. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Patruno, C.; Napolitano, M.; Argenziano, G.; Peris, K.; Ortoncelli, M.; Girolomoni, G.; Offidani, A.; Ferrucci, S.M.; Amoruso, G.F.; Rossi, M.; et al. Dupilumab Therapy of Atopic Dermatitis of the Elderly: A Multicentre, Real-Life Study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yeo, I.J.; Han, J.H.; Suh, J.W.; Lee, H.P.; Hong, J.T. Anti-inflammatory Effect of Astaxanthin in Phthalic Anhydride-induced Atopic Dermatitis Animal Model. Exp. Dermatol. 2018, 27, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Yeo, I.J.; Jang, J.S.; Kim, K.C.; Park, M.H.; Lee, H.P.; Han, S.-B.; Hong, J.T. Combination Effect of Titrated Extract of Centella asiatica and Astaxanthin in a Mouse Model of Phthalic Anhydride-Induced Atopic Dermatitis. Allergy Asthma Immunol. Res. 2019, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Jeon, S.H.; Ham, H.J.; Lee, H.P.; Song, M.J.; Hong, J.T. Improved Anti-Inflammatory Effects of Liposomal Astaxanthin on a Phthalic Anhydride-Induced Atopic Dermatitis Model. Front. Immunol. 2020, 11, 565285. [Google Scholar] [CrossRef]
- Shanmugapriya, K.; Kim, H.; Saravana, P.S.; Chun, B.-S.; Kang, H.W. Astaxanthin-Alpha Tocopherol Nanoemulsion Formulation by Emulsification Methods: Investigation on Anticancer, Wound Healing, and Antibacterial Effects. Colloids Surf. B Biointerfaces 2018, 172, 170–179. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Fujishita, M.; Takahashi, Y.; Adachi, Y. Protective Effects of Astaxanthin on Skin Deterioration. J. Clin. Biochem. Nutr. 2017, 61, 33–39. [Google Scholar] [CrossRef]
- Chalyk, N.E.; Klochkov, V.A.; Bandaletova, T.Y.; Kyle, N.H.; Petyaev, I.M. Continuous Astaxanthin Intake Reduces Oxidative Stress and Reverses Age-Related Morphological Changes of Residual Skin Surface Components in Middle-Aged Volunteers. Nutr. Res. 2017, 48, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Liberti, D.; Alfieri, M.L.; Monti, D.M.; Panzella, L.; Napolitano, A. A Melanin-Related Phenolic Polymer with Potent Photoprotective and Antioxidant Activities for Dermo-Cosmetic Applications. Antioxidants 2020, 9, 270. [Google Scholar] [CrossRef] [PubMed]
- Creanatural Sepia Melanin—Cosmetics Innovations and Technologies. Available online: https://cosmetics.specialchem.com/product/i-the-innovation-company-creanatural-sepia-melanin (accessed on 22 June 2025).
- Boo, Y.C. Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants 2020, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Núñez-Pons, L.; Avila, C.; Romano, G.; Verde, C.; Giordano, D. UV-Protective Compounds in Marine Organisms from the Southern Ocean. Mar. Drugs 2018, 16, 336. [Google Scholar] [CrossRef]
- Neifar, A.; Ben Abdelmalek, I.; Bouajila, G.; Kolsi, R.; Bradai, M.N.; Abdelmouleh, A.; Gargouri, A.; Ayed, N. Purification and Incorporation of the Black Ink of Cuttlefish Sepia officinalis in Eye Cosmetic Products. Color. Technol. 2013, 129, 150–154. [Google Scholar] [CrossRef]
- Mateos, R.; Pérez-Correa, J.R.; Domínguez, H. Bioactive Properties of Marine Phenolics. Mar. Drugs 2020, 18, 501. [Google Scholar] [CrossRef]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.-K.; Rocha, J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef]
- Bidleman, T.F.; Andersson, A.; Brugel, S.; Ericson, L.; Haglund, P.; Kupryianchyk, D.; Lau, D.C.P.; Liljelind, P.; Lundin, L.; Tysklind, A.; et al. Bromoanisoles and Methoxylated Bromodiphenyl Ethers in Macroalgae from Nordic Coastal Regions. Environ. Sci. Process. Impacts 2019, 21, 881–892. [Google Scholar] [CrossRef]
- Malmvärn, A.; Marsh, G.; Kautsky, L.; Athanasiadou, M.; Bergman, Å.; Asplund, L. Hydroxylated and Methoxylated Brominated Diphenyl Ethers in the Red Algae Ceramium tenuicorne and Blue Mussels from the Baltic Sea. Environ. Sci. Technol. 2005, 39, 2990–2997. [Google Scholar] [CrossRef]
- Koch, C.; Sures, B. Environmental Concentrations and Toxicology of 2,4,6-Tribromophenol (TBP). Environ. Pollut. 2018, 233, 706–713. [Google Scholar] [CrossRef]
- Pereira, L.; Cotas, J. Therapeutic Potential of Polyphenols and Other Micronutrients of Marine Origin. Mar. Drugs 2023, 21, 323. [Google Scholar] [CrossRef] [PubMed]
- Franco, W.; Arazo, M.C.R.; Benavides, S. Recent Advances in the Encapsulation of Marine Phenolic Compounds. In Marine Phenolic Compounds; Elsevier: Amsterdam, The Netherlands, 2023; pp. 239–264. ISBN 978-0-12-823589-8. [Google Scholar]
- Carella, F.; Degli Esposti, L.; Adamiano, A.; Iafisco, M. The Use of Calcium Phosphates in Cosmetics, State of the Art and Future Perspectives. Materials 2021, 14, 6398. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Hoang, T.; Pham, T.; Truong, V.K.; Luo, X.; Qin, J.; Zhang, W. High Solubility and Bioavailability of Lobster Shell-Derived Calcium for Significantly Proliferating Bone and Skin Cells In Vitro. Mar. Drugs 2023, 21, 358. [Google Scholar] [CrossRef]
- Piccirillo, C.; Rocha, C.; Tobaldi, D.M.; Pullar, R.C.; Labrincha, J.A.; Ferreira, M.O.; Castro, P.M.L.; Pintado, M.M.E. A Hydroxyapatite–Fe2 O3 Based Material of Natural Origin as an Active Sunscreen Filter. J Mater Chem B 2014, 2, 5999–6009. [Google Scholar] [CrossRef] [PubMed]
- Helmi Rozaini, M.Z.; Hamzah, H.; Poh Wai, C.; Razali, M.H.; Osman, U.M.; Tuan Anuar, S.; Che Soh, S.K.; Binti Ghazali, S.R.; Ibrahim, N.H.; Fei, L.C.; et al. Calcium Hydroxyapatite-Based Marine Origin: Novel Sunscreen Materials for Cosmeceutical Treatments. Orient. J. Chem. 2018, 34, 2770–2776. [Google Scholar] [CrossRef]
- Kodali, D.; Hembrick-Holloman, V.; Gunturu, D.R.; Samuel, T.; Jeelani, S.; Rangari, V.K. Influence of Fish Scale-Based Hydroxyapatite on Forcespun Polycaprolactone Fiber Scaffolds. ACS Omega 2022, 7, 8323–8335. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Hsiao, S.-C.; Lu, Y.-Y.; Han, Y.-C.; Shen, Y.-C.; Venkatakrishnan, K.; Wang, C.-K. Efficacy of Protein Rich Pearl Powder on Antioxidant Status in a Randomized Placebo-Controlled Trial. J. Food Drug Anal. 2018, 26, 309–317. [Google Scholar] [CrossRef]
- Loh, X.J.; Young, D.J.; Guo, H.; Tang, L.; Wu, Y.; Zhang, G.; Tang, C.; Ruan, H. Pearl Powder—An Emerging Material for Biomedical Applications: A Review. Materials 2021, 14, 2797. [Google Scholar] [CrossRef]
- Zhou, X.; Cao, Q.; Orfila, C.; Zhao, J.; Zhang, L. Systematic Review and Meta-Analysis on the Effects of Astaxanthin on Human Skin Ageing. Nutrients 2021, 13, 2917. [Google Scholar] [CrossRef]
- Chintong, S.; Phatvej, W.; Rerk-Am, U.; Waiprib, Y.; Klaypradit, W. In Vitro Antioxidant, Antityrosinase, and Cytotoxic Activities of Astaxanthin from Shrimp Waste. Antioxidants 2019, 8, 128. [Google Scholar] [CrossRef]
- Trégarot, E.; D’Olivo, J.P.; Botelho, A.Z.; Cabrito, A.; Cardoso, G.O.; Casal, G.; Cornet, C.C.; Cragg, S.M.; Degia, A.K.; Fredriksen, S.; et al. Effects of Climate Change on Marine Coastal Ecosystems—A Review to Guide Research and Management. Biol. Conserv. 2024, 289, 110394. [Google Scholar] [CrossRef]
- Kumar, A.; Soratur, A.; Kumar, S.; Venmathi Maran, B.A. A Review of Marine Algae as a Sustainable Source of Antiviral and Anticancer Compounds. Macromol 2025, 5, 11. [Google Scholar] [CrossRef]
- Bermudez, G.; Terenzi, C.; Medri, F.; Andrisano, V.; Montanari, S. Extraction and Analytical Methods for the Characterization of Polyphenols in Marine Microalgae: A Review. Mar. Drugs 2024, 22, 538. [Google Scholar] [CrossRef] [PubMed]
- Amador, S.; Martins, A.; Matias, M.; Pedrosa, R.; Pinteus, S. Deep Eutectic Systems: A Game Changer for Marine Bioactives Recovery. Mar. Drugs 2025, 23, 211. [Google Scholar] [CrossRef]
- Odeleye, T.; White, W.L.; Lu, J. Extraction Techniques and Potential Health Benefits of Bioactive Compounds from Marine Molluscs: A Review. Food Funct. 2019, 10, 2278–2289. [Google Scholar] [CrossRef]
- Tsegay, Z.T.; Agriopoulou, S.; Chaari, M.; Smaoui, S.; Varzakas, T. Statistical Tools to Optimize the Recovery of Bioactive Compounds from Marine Byproducts. Mar. Drugs 2024, 22, 182. [Google Scholar] [CrossRef]
- Amiri, H.; Shabanpour, B.; Pourashouri, P.; Kashiri, M. Encapsulation of Marine Bioactive Compounds Using Liposome Technique: Evaluation of Physicochemical Properties and Oxidative Stability during Storage. Food Struct. 2023, 35, 100308. [Google Scholar] [CrossRef]
- Favas, R.; Almeida, H.; Peixoto, A.F.; Ferreira, D.; Silva, A.C. Advances in Encapsulating Marine Bioactive Compounds Using Nanostructured Lipid Carriers (NLCs) and Solid Lipid Nanoparticles (SLNs) for Health Applications. Pharmaceutics 2024, 16, 1517. [Google Scholar] [CrossRef]
- Masoomi Dezfooli, S.; Gutierrez-Maddox, N.; Alfaro, A.; Seyfoddin, A. Encapsulation for Delivering Bioactives in Aquaculture. Rev. Aquac. 2019, 11, 631–660. [Google Scholar] [CrossRef]
- Augusto, A.; Lemos, M.; Silva, S. Exploring Marine-Based Food Production: The Challenges for a Sustainable and Fast Biotechnology-Based Development. Appl. Sci. 2024, 14, 8255. [Google Scholar] [CrossRef]
- Mao, X.; Liu, Z.; Sun, J.; Lee, S.Y. Metabolic Engineering for the Microbial Production of Marine Bioactive Compounds. Biotechnol. Adv. 2017, 35, 1004–1021. [Google Scholar] [CrossRef]
- Schada Von Borzyskowski, L. Taking Synthetic Biology to the Seas: From Blue Chassis Organisms to Marine Aquaforming. ChemBioChem 2023, 24, e202200786. [Google Scholar] [CrossRef]
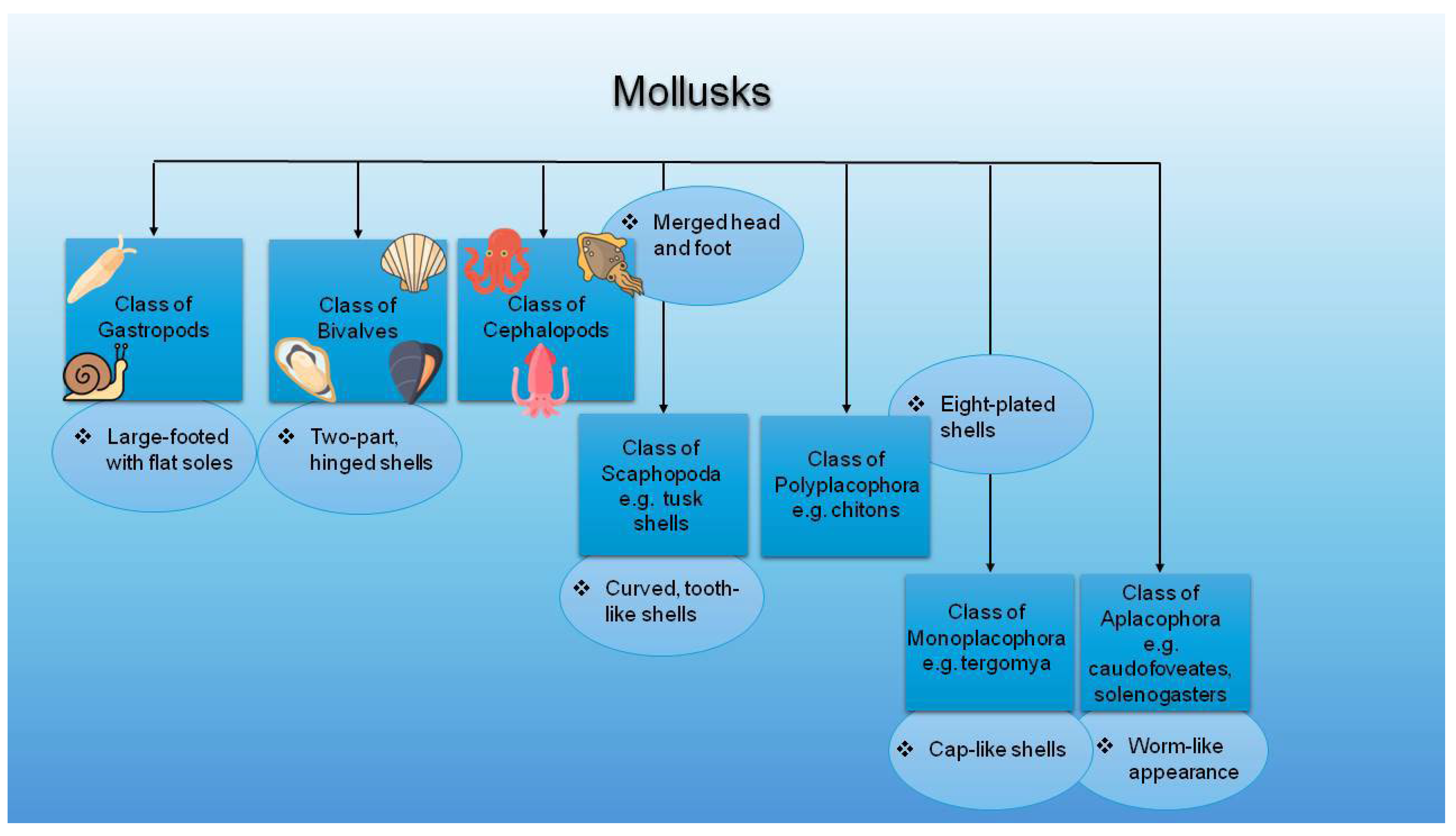
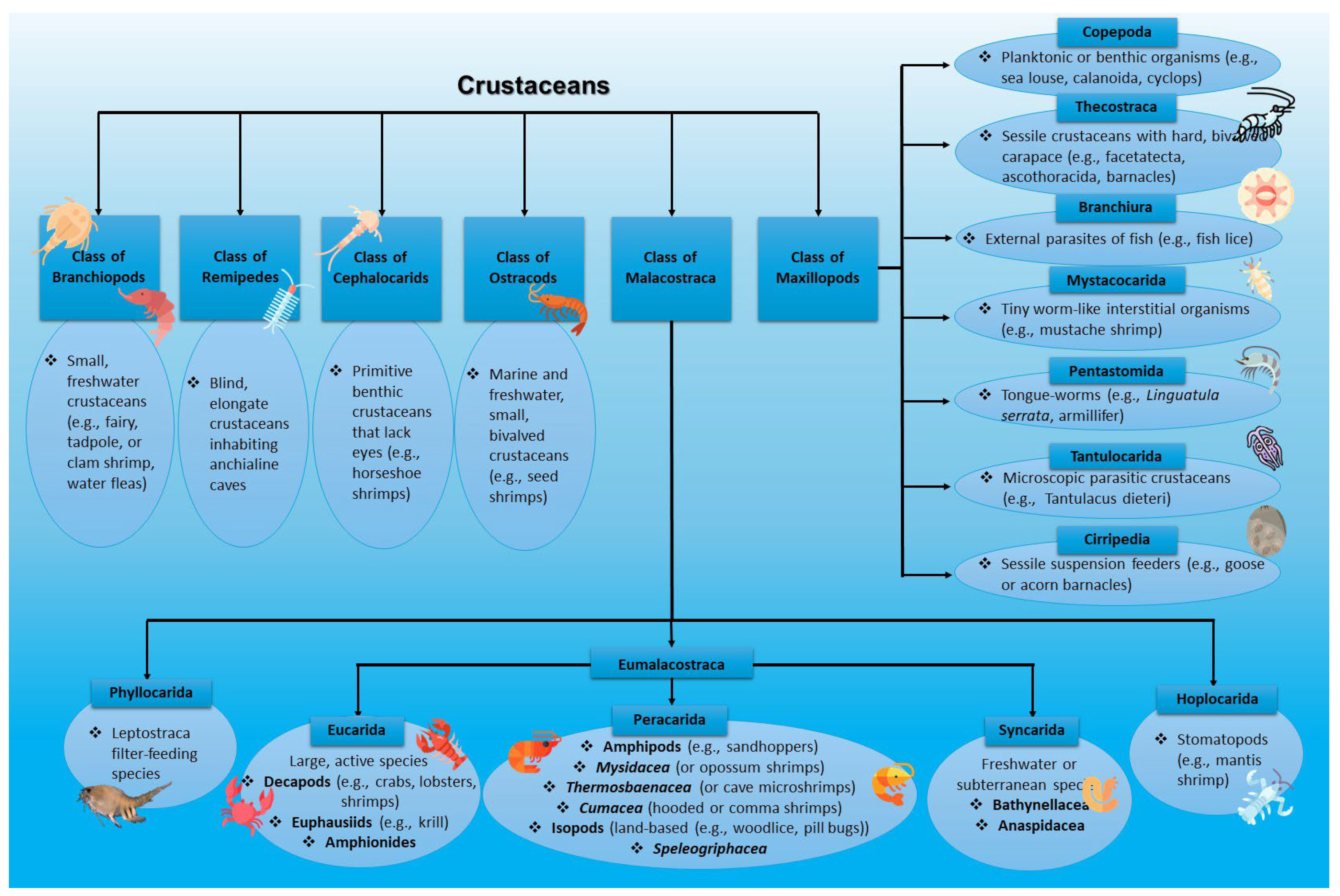
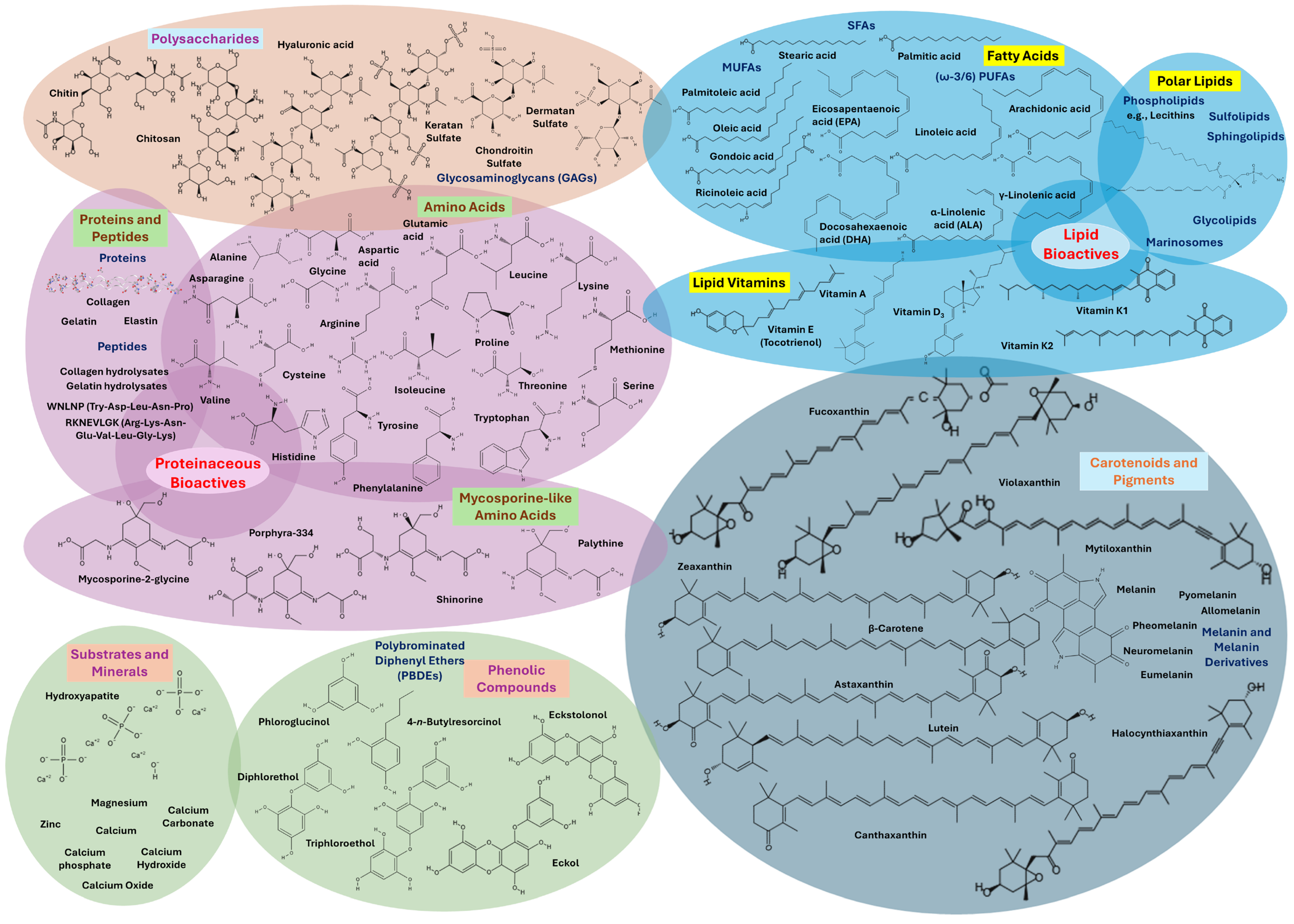
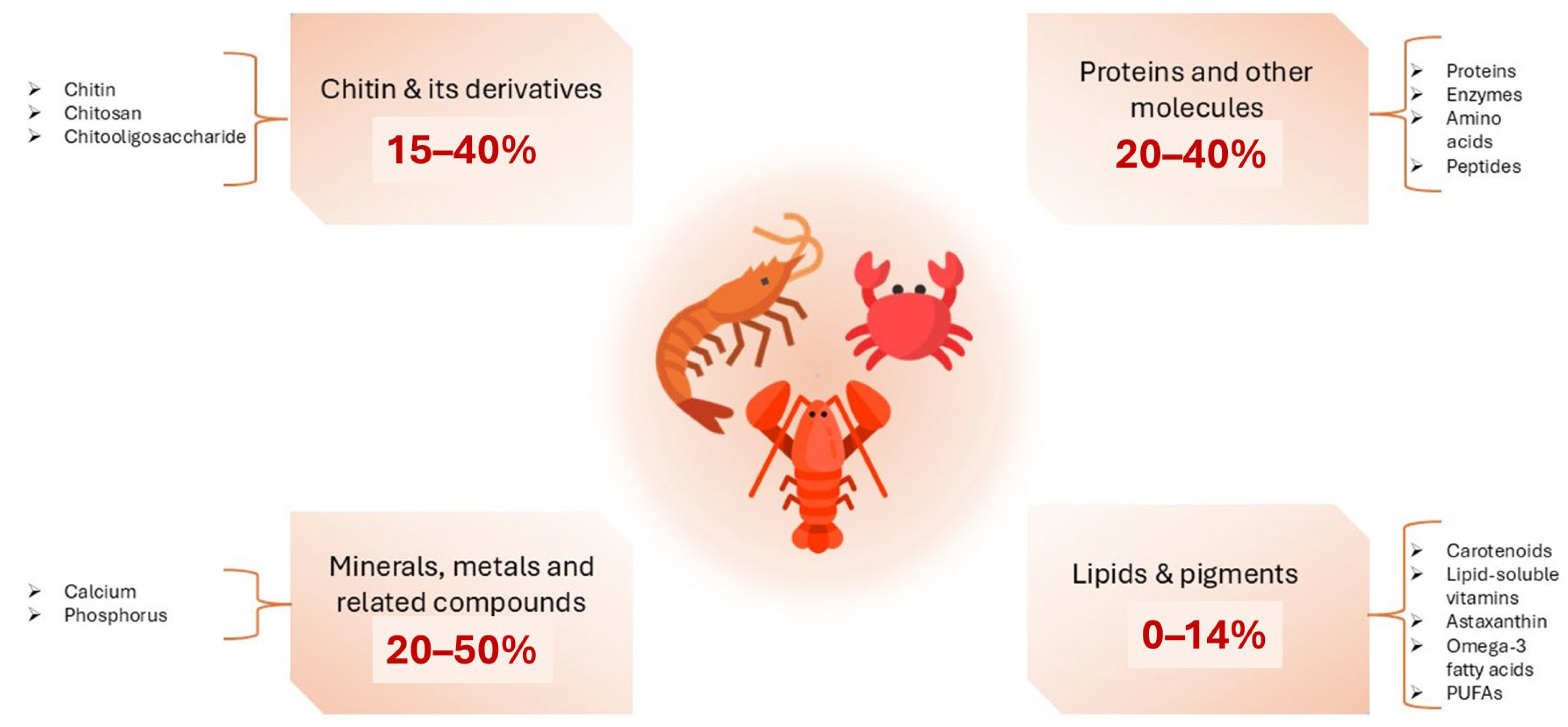
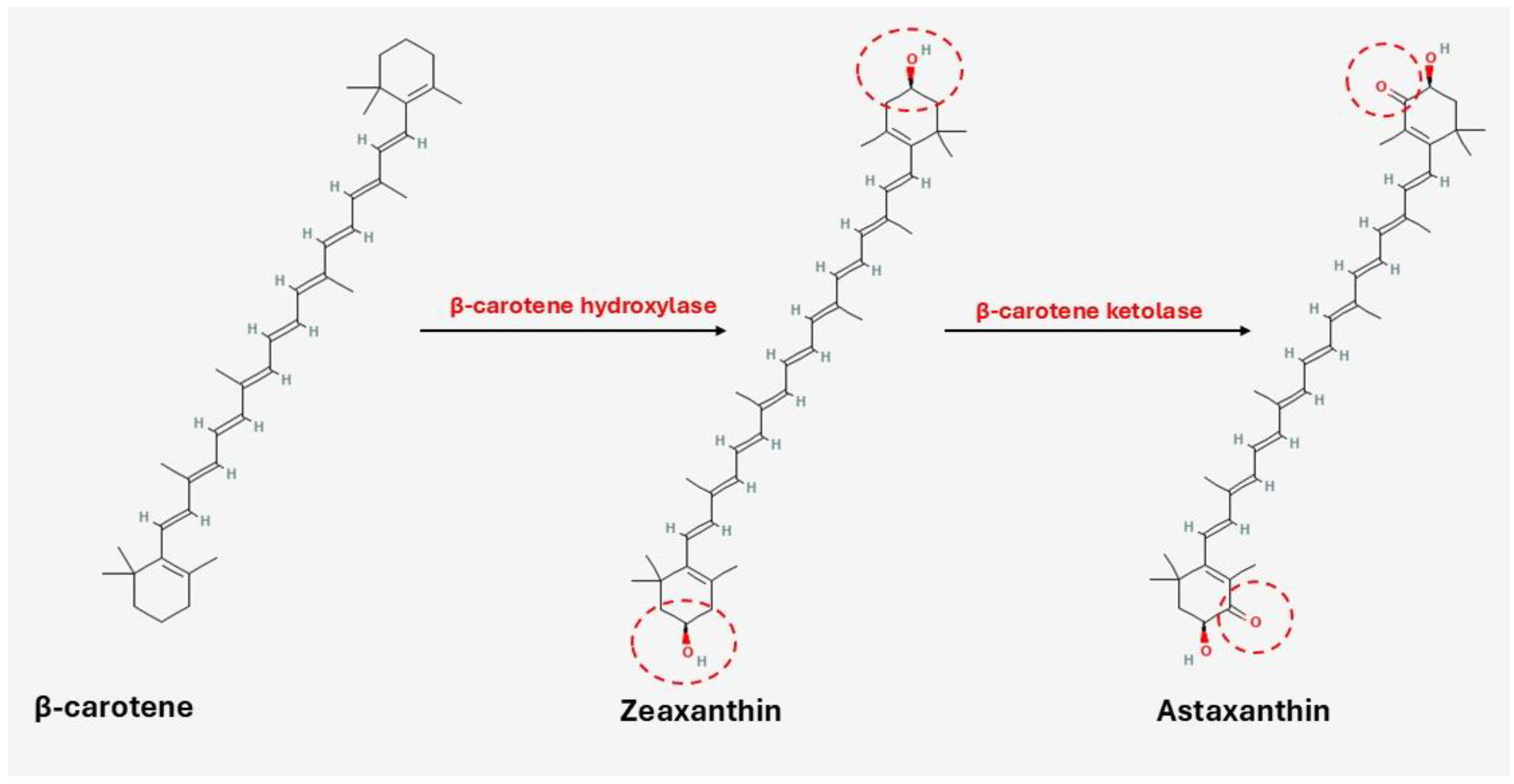
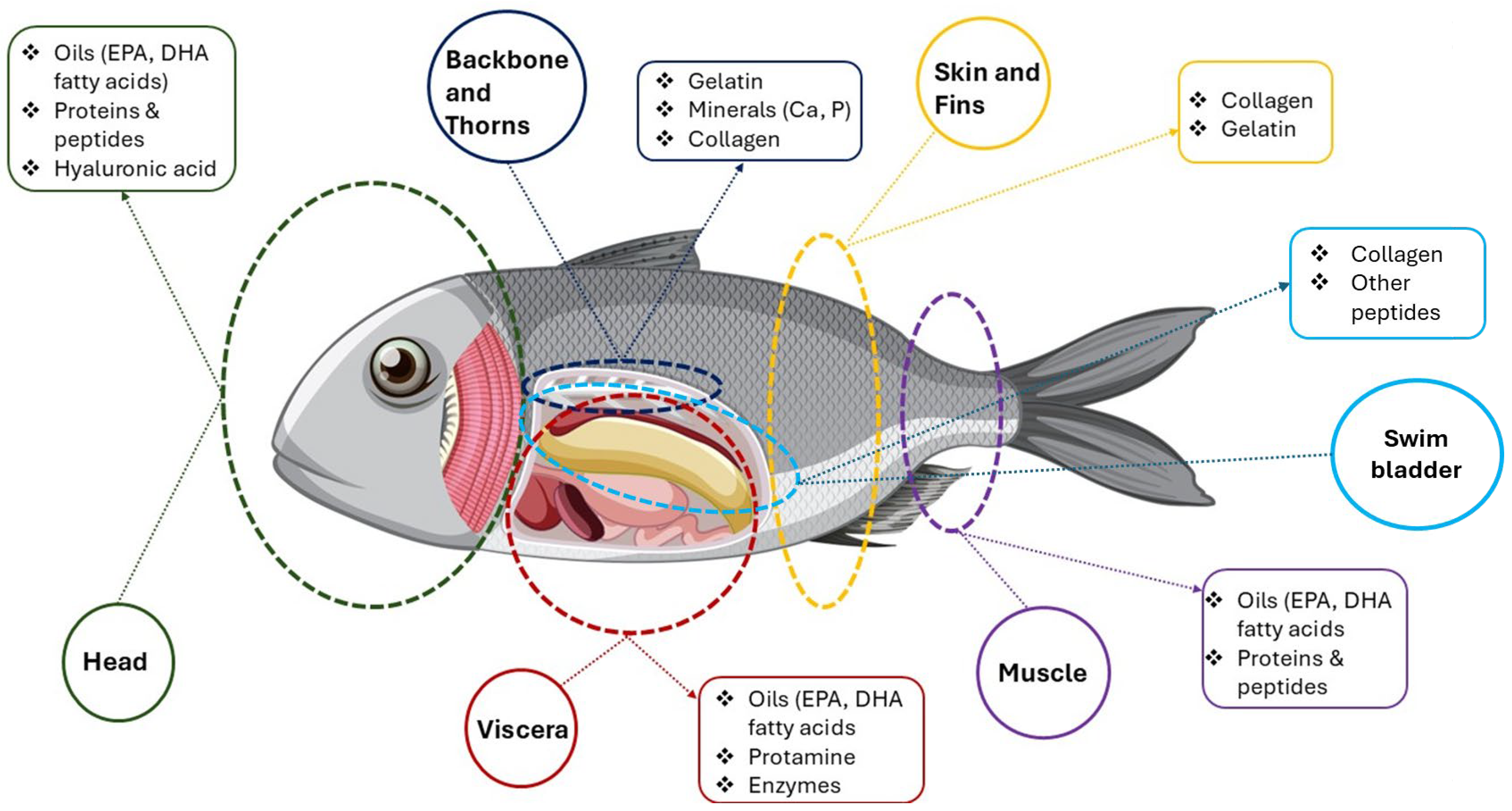
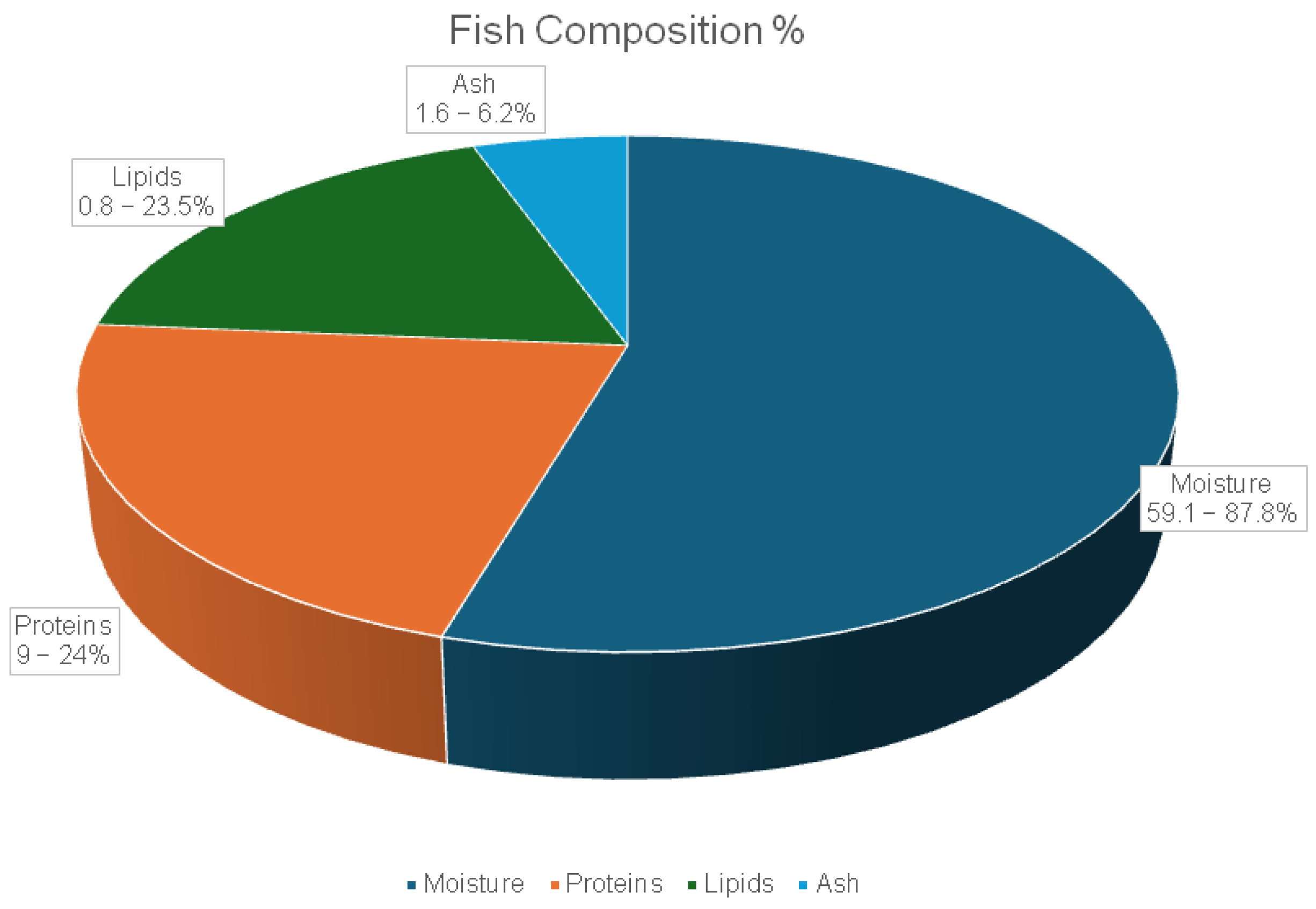
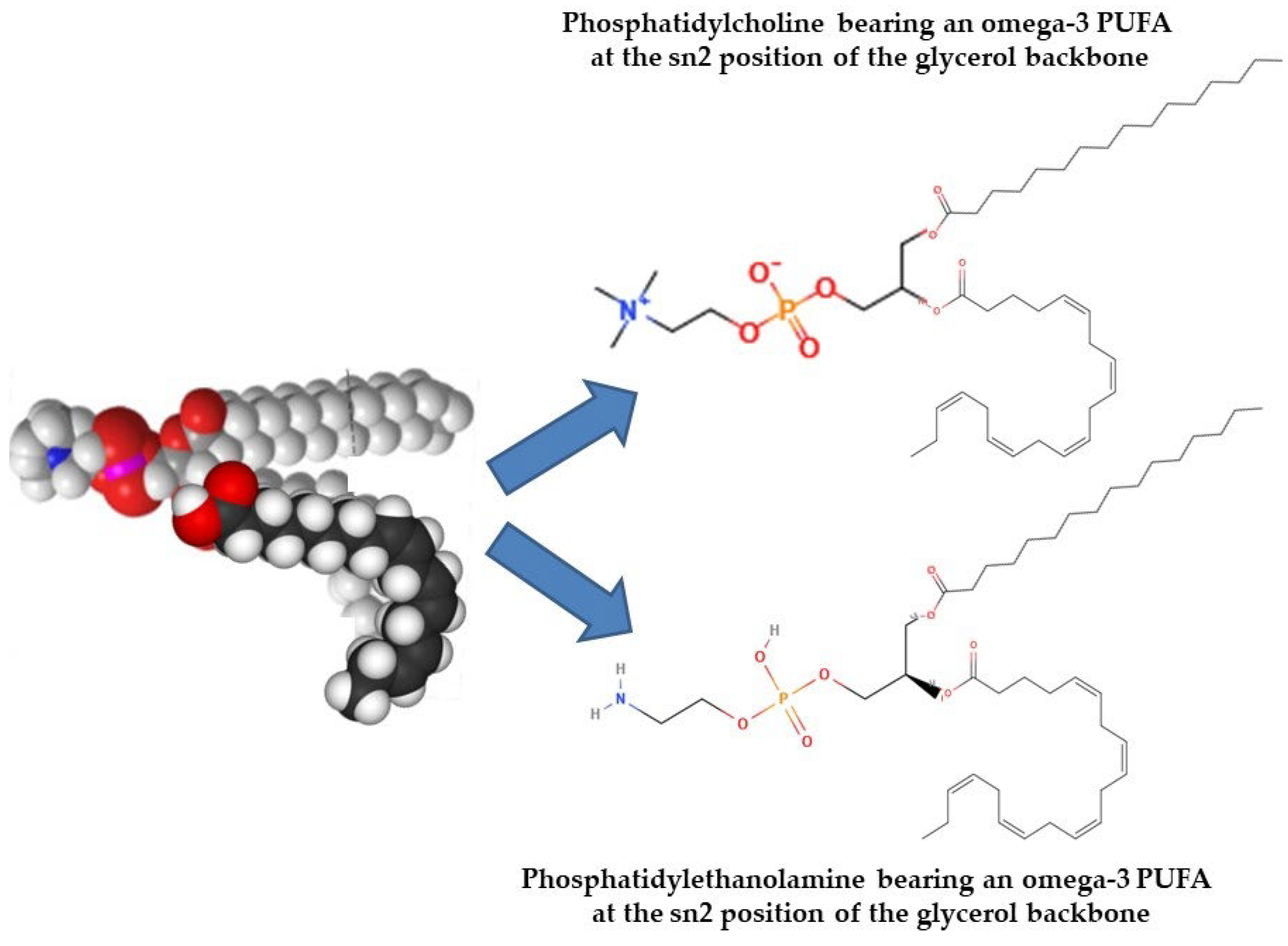
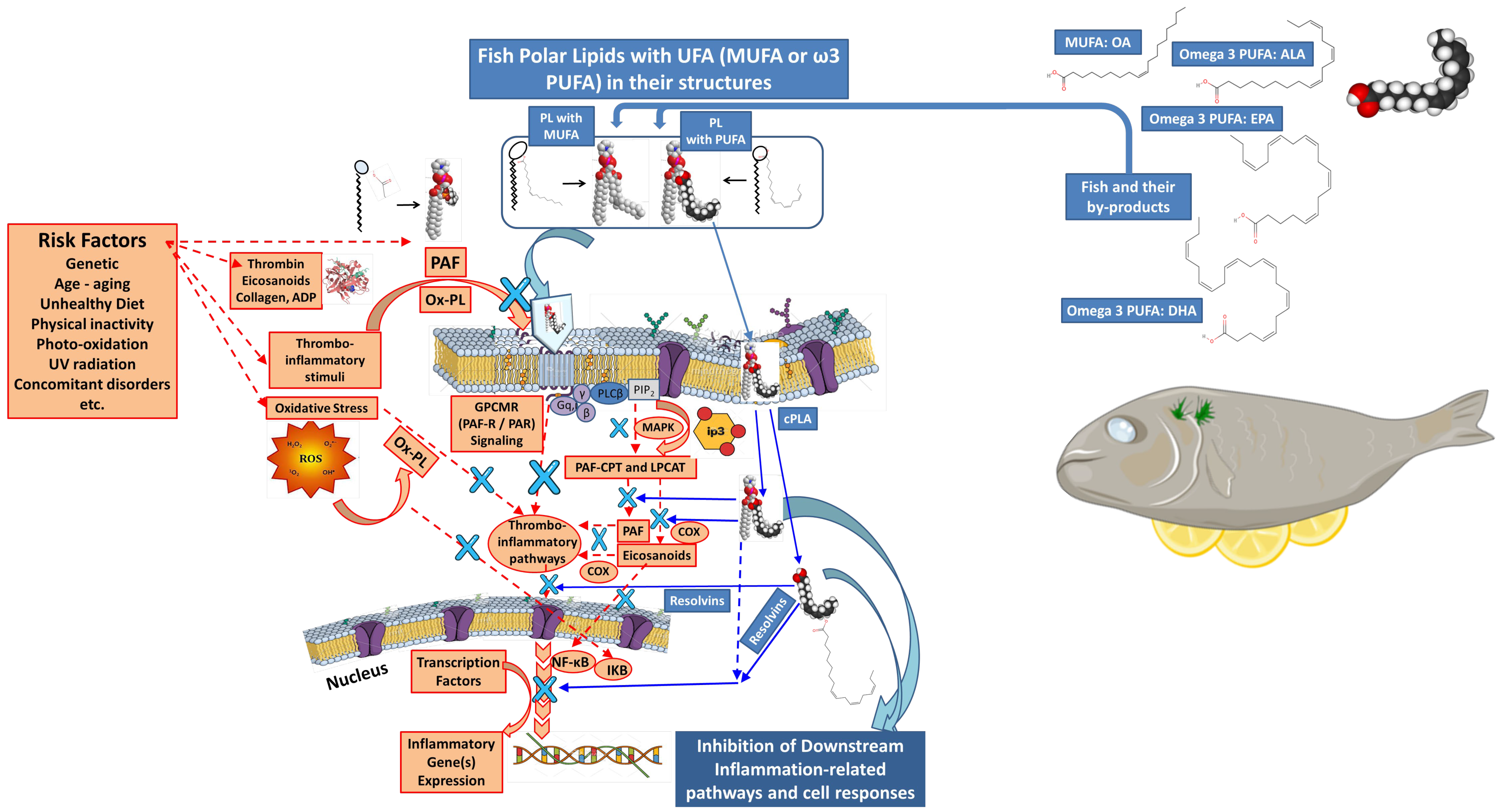
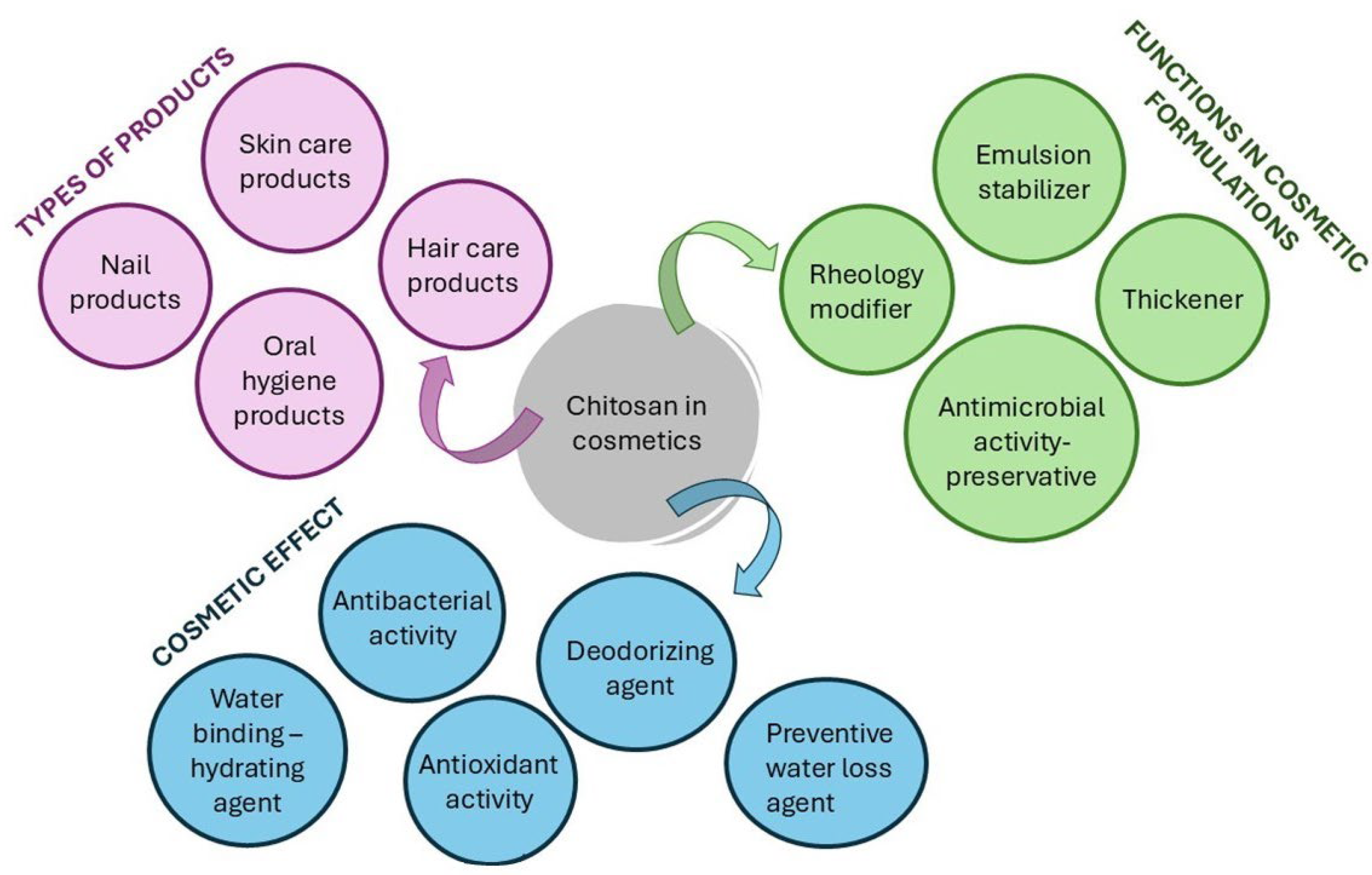
| Challenges | Benefits |
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Extraction Methods | Advantages | Disadvantages | References |
|---|---|---|---|
| Enzyme-assisted extraction (EAE) |
|
| [54,72] |
| Microwave- assisted extraction (MAE) |
|
| [73,74] |
| Subcritical water extraction (SWE) |
|
| [75] |
| Supercritical fluid extraction (SFE) |
|
| [76] |
| Ultrasound-assisted extraction (UAE) |
|
| [70,77] |
| Fish By-Product | Percentage (% w/w) | Valuable Compounds | References |
|---|---|---|---|
| Skin | 3.5 | Collagen, gelatin, antimicrobial compounds (e.g., cathelicidins, defensins), and ω-3 fatty acids | [37,174,192] |
| Bones | 10–15 | Calcium phosphate (hydroxyapatite), collagen, gelatin, ω-3 fatty acids, lipids, and P | [37,42,43,243] |
| Scales | 2 | Collagen, chitin, chitosan, minerals (e.g., Zn, Ca), and glycoproteins | [37,244] |
| Head | 10–15 | Protein hydrolysates, enzymes (e.g., pepsin, trypsin), bioactive lipids, gelatin, and bioactive peptides | [175,198,245,246,247] |
| Viscera | 12–18 | Fish oil (e.g., EPA, DHA), digestive enzymes (trypsin, lipase), probiotics, bile acids, and vitamin D | [37,175,248] |
| Swim bladder | 39.2 | Type I and II collagen, gelatin, GAGs, and elastin | [240,249,250] |
| Fins | ~1–2% (estimated *) | CS, HA, and collagen | [239,240] |
| Cartilage | - * | CS, GS, and protein–polysaccharide complexes | [240,241] |
| Liver | ~5–15% (estimated *) | Vitamins A and D3, ω-3 PUFAs, and antioxidant enzymes | [186,192,240] |
| Marine Source | Patent Name or Cosmetic Formulation | Marine Bioactives (Formulation Content) | Cosmetic-Associated Benefits and Commercial Use | References |
|---|---|---|---|---|
| Fish | Marine Hydrolyzed Collagen ImwTM (Ashland Global Holdings, Wilmington, DE, USA) | Marine collagen oligopeptides upcycled from fish skin |
| [251] |
| AffinisphereTM (BASF, Ludwigshafen, Germany) | Marine Atelocollagen microspheres from shark fins |
| [251] | |
| Marine Filling SpheresTM (BASF, Ludwigshafen, Germany) | Dehydrated microspheres of marine collagen and GAGs from shark fins |
| [251] | |
| COLLASURGETM (Croda Beauty, Yorkshire, UK) | Marine collagen amino acids from fish species |
| [251] | |
| ICHTYOCOLLAGENTM (Sederma by Croda Beauty, Snaith, UK) | pH-soluble marine collagen from fish skin extract |
| [251] | |
| Finn Canada (Canada) | FINN’s salmon skin collagen (Salmonollagen) |
| [28] | |
| PURE MARINE COLLAGEN (Kenney and Ross Limited, Port Saxon, Nova Scotia, Canada) | Hydrolyzed collagen from the skin of deep-sea, wild fish |
| [28] | |
| Collagen HMTM Sol, Elastin TMTM, Glycosann® Sol, and Protein M+TM (220 Rue du Petit Port, France) | Hydrolyzed marine collagen, elastin, chondroitin sulphate, and cartilage extract from fish by-products |
| [252] | |
| ThalaColTM (Thai Union Ingredients (Global), Bangkok, Thailand) | Contains 100% pure hydrolyzed marine collagen peptides from wild tuna skin |
| [253] | |
| NUTRICOLL NATURAL MARINE COLLAGEN POWDER (Chitinor, Seagarden), Norway) | Natural fish collagen peptide derived from wild cod |
| [254] | |
| CropureTM Orange Roughy (Croda Beauty, UK) | Orange roughy oil (Hoplostethus atlanticus) |
| [255] | |
| Zellulin® ZelluGENTM 10% Solution (Avant, Old Street, London) | Hydrolyzed minnow muscle cell lysate biopeptides |
| [256] | |
| Crustaceans | HYDAMERTM HCMFTM (ChitinorTM, Tromsoe, Norway) | Chitosan from crustacean chitin |
| [257] |
| EARTHEN InstantPeelTM Exfoliant (EARTHEN SkinCareTM, Pleasantville, New York) | Shrimp extract, fish oil, and hydrolyzed collagen from fish |
| [258] | |
| System4® R (Finland) | Chitosan from marine crustacean exoskeleton |
| [259] | |
| DHC Astaxanthin Power Cream (DHC, USA) | Astaxanthin is found in large amounts in the shells of crustaceans and salmon |
| [260] | |
| Marin Soothing Hydration Cream (Marin Skincare, ME, USA) | Marine lobster glycoproteins |
| [261] | |
| Mollusks | CRODAROM® BLACK PEARL (Croda Beauty, Yorkshire, UK) | Aragonite and conchiolin from Tahitian black pearls from the black-lip oyster (Pinctada margaritifera) |
| [251] |
| CreanaturalTM SEPIA MELANIN (The Innovation Company®, France) | Creanatural Sepia Melanin derived from squid Ink |
| [78] | |
| Akoshine® (Akott Evolution, Milan, Italy) | Oyster shell pearl powder |
| [262] | |
| OligoceaneTM PH (Croda Beauty, UK) | Oyster shell extract and sea salt extract |
| [263] | |
| Huzhou Pearl Powder (Huzhou Shengtao Biotech, Huzhou Zhejiang, China) | Amino acids and minerals from the jib clam of a natural naidinae animal or the wrinkle clam of a bivalve mollusk |
| [264] |
| Patent Name | Skincare Product | MAA Components/Formulation Content | Skin-Associated Benefits | References |
|---|---|---|---|---|
| WO2024027929A1 | Sunscreen |
|
| [400] |
| WO2024028510A1 | Sunscreen |
|
| [401] |
| CN105342903A | Biological sunscreen |
|
| [402] |
| CN103720625A | Sunscreen |
|
| [403] |
| CN106937918A | Cosmetic emulsion |
|
| [404] |
| WO2023004522A1 | Water-in-oil-water (W/O/W) nanoemulsion |
|
| [405] |
| WO2024027926A1 | Sunscreen |
|
| [406] |
| KR20170090690A | Wound-healing composition |
|
| [407] |
| CN105310897A | Anti-freeze hand cream |
|
| [408] |
| WO2024027930A1 | Antimicrobial boosting agent |
|
| [409] |
| Amino Acid Sequence (Small Peptide Chain) | Skin-Related Mechanism of Action | References |
|---|---|---|
| Valine–glycine–valine–alanine–proline–glycine (VGVAPG, Palmitoyl oligopeptide) |
| [382,433,434,435] |
| Lysine–threonine–threonine–lysine–serine (KTTKS and with palmitic acid: Palmitoyl Pentapeptide-4, Matrixyl®) |
| [382,435,436,437] |
| Tyrosine–tyrosine–arginine–alanine–aspartame–aspartame–alanine (YYRADDA) |
| [382,435,438] |
| Lysine–phenylalanine–lysine + elaidic acid (Lipospondine) (lysine–valine–lysine functions similarly + palmitic or bistrifluoroacetic acid) |
| [382,435,438] |
| Phenylalanine–valine–alanine–proline–phenylalanine–proline (Peptamide®-6) |
| [382,435,438,439] |
| Glycyl-L-histidyl-L-lysine |
| [382,435,438] |
| Heptapeptide (aspartic acid–glutamic acid–glutamic acid–threonine–glycine–glutamic acid–phenylalanine–DEETGEF-OH (Perfection Peptide P7TM) |
| [435,440] |
| Asparagine–tyrosine–arginine–arginine–glutamic acid (NYRRE) and arginine–histidine–alanine–lysine–phenylalanine (RHAKF) |
| [441,442] |
| Oligopeptide-68 (β-white, arginine–aspartic acid–glycine–glutamine–isoleucine–leucine–serine–threonine–tryptophan–tyrosine) |
| [435,443] |
| Acetyl tripeptide-30 citrulline |
| [444] |
| Pentapeptide 3 (Vialox®) |
| [435,438] |
| Pentapeptide-18 (Leuphasyl®) |
| [435,438] |
| Acetyl-glutamyl-glutamyl-methoxil-glutaminyl-arginyl-arginylamide (Argireline®) |
| [435,438,445] |
| Marine Type | Marine Species | Bioactive Peptides | Biological Activity | Cosmetic-Related Functions | References |
|---|---|---|---|---|---|
| Fish | Tilapia | Skin-collagen peptides | Oral mucosal ulcers |
| [454] |
| Fish | Tuna (Thunnus obesus) | Collagen peptide from skin (TSCP) and bone (TBCP) | UVB protection, photo-aging, and radical scavenging/antioxidant effects |
| [455] |
| Fish | Black pomfret (Parastromateus niger) | Peptides | Anti-aging potential |
| [448] |
| Fish | Hydrolyzed fish cartilage | Collagen peptides | Anti-aging potential |
| [456] |
| Fish | Giant croaker (Nibea japonica) | Swim bladder collagen peptides: acid- and pepsin-solubilized collagen (ASC and PSC) | Wound-healing and Antioxidant potential |
| [457] |
| Fish | Sea bass (Late calcarifer) | Hydrolyzed collagen from the defatted Asian sea bass (Asbs-HC) | Antioxidant, skin nourishment, and wound-healing potential |
| [458] |
| Fish | Crimson snapper | Crimson snapper scale peptides (CSSPs) | Antioxidant and anti-aging potential |
| [452] |
| Fish | Salmon and codfish skin | Collagen peptides | Anti-aging and skin-related health potential |
| [28] |
| Fish | Chum salmon skin | Marine collagen peptides (MCPs) | Wound-healing and anti-angiogenesis potential |
| [459] |
| Fish | Salmon skin | Gelatin and hydrolysates | Anti-aging, anti-UV, and anti-photo-aging potential |
| [428] |
| Fish | Atlantic salmon egg extract (Salmo salar) | Serine endoproteases, oleic and linoleic acid | Anti-wrinkle, anti-aging, and skin-health-promoting |
| [460] |
| Crustacean | Shrimp shell | Chitosan oligosaccharide | Anti-aging and antioxidant potential |
| [461] |
| Crustacean | Crab (Portunus sanguinolentus) | Crab chitin nanofibrils | Anti-UVB and photoprotective potential |
| [462,463] |
| Mollusk | Oyster (Crassostrea hongkongensis) | Protein enzymatic hydrolysates (WNLNP and RKNEVLGK) | Anti-photo-aging skin potential |
| [447] |
| Mollusk | Squid (Symplectoteuthis oualaniensis) | Acid- and pepsin-solubilized collagen peptides (ASC and PSC) | Stem-cell regenerative and skin-cell protective effects |
| [464] |
| Study Hypothesis | Administration Pattern | Cosmetic and Cosmeceutical Benefits | References |
|---|---|---|---|
| Randomized and double-blind study of 65 healthy women exposed to UVB radiation | Marine-derived AST capsules at 6 or 12 mg/day for 16 weeks or a placebo |
| [493] |
| Randomized and double-blind study of 23 healthy Japanese participants exposed to UVB radiation | Algal-derived AST capsules at 4 mg for 9 weeks or a placebo |
| [485] |
| Morphological analysis in residual skin surface components (RSSCs) for monitoring oxidative stress and skin aging in 31 middle-aged volunteers | AST capsules at 4 mg for 4 weeks (results before and after the administration) |
| [494] |
| Family of Compounds | Bioactive Compounds | Skin-Health-Related Function | Marine Organism | References |
|---|---|---|---|---|
| Polysaccharides | Chitin, chitosan, and derivatives, hyaluronic acid, GAGs, and fucoidans | Anti-pigmentation, antibacterial, moisturizing, antioxidant, anti-aging, MMP-inhibitory, anti-cancer, anti-inflammatory, antifungal, UV-protection, skin-cleansing, nail-care, hair-care, oral-cavity-care, gel-forming, skin-rejuvenant, and wound-healing properties | Exoskeleton of crustaceans, marine gastropods, fish, fish mucus, and mollusk tissues | [11,28,37,78,117,265,266] |
| Fatty acids | SFAs, MUFAs, and mainly PUFAs (e.g., LA, DHA, EPA, ALA, arachidonic acid) | Collagen stimulation, emollient, anti-inflammatory, anti-inflammatory, antioxidant, hydrating, anti-photo-aging, anti-melanogenesis, dermatitis-protective, anti-cancer, wound-healing, anti-hyperpigmentation, anti-erythema, anti-psoriasis, and acne vulgaris-protective properties | Fish head, oil, frame, trimming, viscera, skin of salmon and tuna, several fatty fish, and crustaceans like crab, mussels, and oysters | [11,28,37,78] |
| Lipid bioactives | Polar lipids like phospholipids, glycolipids, sphingolipids, and sulfolipids (e.g., marinosomes, lecithin) and lipid vitamins (e.g., A, B, D, E, or K) | Anti-inflammatory, emollient, hydrating, wound-healing, barrier-repairing, delivery-enhancing, photoprotective, restructuring of cell membranes, antioxidant, anti-aging, emulsifying, collagen-stimulating, and anti-skin-related diseases properties | Marine organisms, including microalgae, krill, sea cucumbers, mollusks, crustaceans, and fish by-products like oil, roe, liver, viscera, or skin | [11,78,147,320,364,365,366,373,374,375] |
| Amino acids | All 20 standard residues and mostly MAAs | Moisturizing, smoothing, youthful-looking skin-promoting, anti-aging, anti-allergic, photoprotective, wound-healing, collagen-stimulating, skin elasticity and firmness-enhancing, emulsifying, UV-protective, antimicrobial, antioxidant, anti-inflammatory, anti-wrinkle, exfoliation efficacy, anti-melanogenic, skin-soothing, and anti-irritating properties | Marine and freshwater organisms like cyanobacteria, fungi, and algae and higher-order creatures like cnidaria, fish, arthropods, tunicates, sponges, echinoderms, and mollusks | [11,78,383,389,390,391,392,393,394,498] |
| Proteins | Collagen, elastin, and gelatin | Antioxidant, anti-aging, anti-wrinkle, moisturizing, structural support, skin-whitening, UV-protection, collagen-stimulating, hydrating, water-binding, anti-melanogenic, skin-soothing, and anti-inflammatory properties | Fish bones, skin, scale, and swim bladder, mollusks, and crustaceans | [11,18,28,37,78,192,410,415,417,418,419,459] |
| Peptides | Small amino acid chains, collagen and gelatin hydrolysates | Anti-aging, antioxidant, anti-inflammatory, oxygen synthesis stimulation, elasticity restoration, collagen-stimulating, anti-wrinkle, skin firmness-enhancing, texture-improving, UV-protective, photoprotective, radical-scavenging, tyrosinase-inhibitory, skin-whitening, moisturizing, MMP-inhibitory, immunostimulant, antibacterial, and anti-cancer properties | Fish muscle, skin, heads, and trimming waste, hydrolyzed fish cartilage, shrimp shells, crabs, oysters, and squids | [11,37,78,382,435,438] |
| Carotenoids and pigments | Astaxanthin, melanin (allomelanin, neuromelanin, eumelanin, pheomelanin, and pyomelanin), lutein, β-carotene, halocynthiaxanthin, and fucoxanthin | Antioxidant, anti-inflammatory, photo-protective, skin pigmentation-regulating, cosmetic preservative, antioxidant, anti-UV, radical-scavenging, moisturizing, anti-wrinkle, UV-ray-protecting, anti-tumor, anti-eczema, and wound-healing properties | Crustaceans, shrimp head, shell, and tail, freshwater fish or red fish, Japanese mackerel and amberjack (yellowtail), rainbow trout, cephalopod ink, and mollusks | [11,28,78,192,473,495,496,515,516] |
| Phenolic compounds | Polyphenols, PBDEs, and phlorotannins (e.g., diphlorethol, triphloroethol, trifuhalol, tetrafuhalol, phloroglucinol, eckol, and eckstolonol) | Antioxidant, anti-inflammatory, anti-cancer, UV-protective, radical-scavenging, hydrating, anti-aging, anti-tumor, anti-eczema, wound-healing, anti-melanogenesis, and anti-hyperpigmentation properties | Fish, crustaceans, bivalves, shrimps, clams, oysters, mussels, red and brown algae | [78,192,502,503,504,505,506] |
| Substrates and minerals | Hydroxyapatite, CaPs, minerals like Ca, Mg, and Zn, and powdered pearl shells or nacreous shell layers | UV-protective, skin and teeth remineralization, wound-healing carrier, skin rejuvenation, skin cell proliferation, anti-inflammatory effects, anti-acne, sebum-modulating, anti-age-related degenerative disorders, skin tone-enhancing, skin fibroblast-regulating, moisturizing, collagen-rebuilding, angiogenesis-promoting, wound-healing, whitening, deodorizing, and antibacterial cosmetic properties against hyperpigmentation, atopic dermatitis, deep-burn porcine skin, burn scars, and skin cancer incidence | Fish bones, teeth of vertebrates, shells from crustaceans, mollusks, and pearls from oysters and mussels | [28,37,39,78,79,192,509,510,511,512,513,514] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulou, S.N.A.; Adamantidi, T.; Kranas, D.; Cholidis, P.; Anastasiadou, C.; Tsoupras, A. A Comprehensive Review on the Valorization of Bioactives from Marine Animal By-Products for Health-Promoting, Biofunctional Cosmetics. Mar. Drugs 2025, 23, 299. https://doi.org/10.3390/md23080299
Papadopoulou SNA, Adamantidi T, Kranas D, Cholidis P, Anastasiadou C, Tsoupras A. A Comprehensive Review on the Valorization of Bioactives from Marine Animal By-Products for Health-Promoting, Biofunctional Cosmetics. Marine Drugs. 2025; 23(8):299. https://doi.org/10.3390/md23080299
Chicago/Turabian StylePapadopoulou, Sofia Neonilli A., Theodora Adamantidi, Dimitrios Kranas, Paschalis Cholidis, Chryssa Anastasiadou, and Alexandros Tsoupras. 2025. "A Comprehensive Review on the Valorization of Bioactives from Marine Animal By-Products for Health-Promoting, Biofunctional Cosmetics" Marine Drugs 23, no. 8: 299. https://doi.org/10.3390/md23080299
APA StylePapadopoulou, S. N. A., Adamantidi, T., Kranas, D., Cholidis, P., Anastasiadou, C., & Tsoupras, A. (2025). A Comprehensive Review on the Valorization of Bioactives from Marine Animal By-Products for Health-Promoting, Biofunctional Cosmetics. Marine Drugs, 23(8), 299. https://doi.org/10.3390/md23080299








